Abstract
The apparent oral clearance (CL/F, mL/min) of busulfan was measured in 279 adolescent and adult patients. Significant (P< .05) determinants of CL/F by linear regression were: actual body weight (BW; r2 = 0.300), body surface area (BSA; r2 = 0.277), adjusted ideal body weight (AIBW; r2 = 0.265), and ideal body weight (IBW; r2= 0.173); whereas body mass index (BMI), height, age, gender, and disease were less important predictors. CL/F (mL/min) for normal weight patients (BMI, 18 to 27 kg/m2) was 16.2% lower (P< .001) than for obese patients (BMI, 27 to 35 kg/m2). Thus, expressing CL/F relative to BW did not eliminate statistically significant differences between normal and obese patients. However, busulfan CL/F expressed relative to BSA (110 ± 24 v 110 ± 24 mL/min/m2, P = 1.0) or AIBW (3.04 ± 0.65 v 3.19 ± 0.67 mL/min/kg, P = .597) were similar in normal and obese patients. Non-Hodgkin’s lymphoma patients (n = 10) had approximately 32% lower mean busulfan CL/F expressed relative to BW, BSA, or AIBW compared with patients with chronic myelogenous leukemia (n = 73). Routine dosing on the basis of BSA or AIBW in adults and adolescents does not require a specific accommodation for the obese. However, dosing based on BSA may be improved by considering CL/F differences in certain diseases. Adjusting dose for body size or disease does not diminish interpatient variability sufficiently to obviate plasma level monitoring in many indications.
BUSULFAN IS A BIFUNCTIONAL alkylating agent commonly used in preparative regimens before hematopoietic stem cell transplantation for treatment of various malignancies and inherited disorders. In hematopoietic stem cell transplantation, outcome at a fixed mg/kg dose has been related to area under the plasma concentration-time curve (AUC) and average steady-state concentration (CSS). Excessively high busulfan AUC or CSS is associated with an increase in hepatic veno-occlusive disease, low levels are associated with a higher relapse rate in patients with chronic myelogenous leukemia (CML), and lower levels are associated with graft rejection in allogeneic transplantation.1-4 Both AUC and CSS are inversely proportional to apparent oral clearance (CL/F) and are directly proportional to dose.
In many instances, busulfan is administered orally on a fixed mg/kg basis without dosage adjustment to achieve target busulfan plasma concentrations. The practice of normalizing doses to body size diminishes variability in clearance among patients.5However, the problem faced in dosing obese patients is choosing the appropriate measure of body size with which to calculate dose, because actual body weight is intuitively questioned. Previously, we found that among the commonly used body size parameters (ie, actual body weight [BW], ideal body weight [IBW], adjusted body weight [AIBW], and body surface area [BSA]), expressing busulfan CL/F relative to BSA produced the lowest coefficient of variation in 42 children and adults,3 although AIBW performed nearly as well. The body size measure that produces the minimum variability in CL/F is the most appropriate for the calculation of dose because it will minimize variability in the resultant AUC or CSS in comparison to alternative measures of body size.
Disease has also been linked to alterations in busulfan pharmacokinetics. Previous studies have shown that the disposition of busulfan is altered in children with inherited disorders. Children with lysosomal storage diseases have been reported to have longer elimination half-lifes, and a trend towards higher CL/F compared with children with immune deficiencies, acute leukemias, and malignant lymphohistiocytosis.6 Children with inherited genetic disorders have been reported to have enhanced elimination half-lifes after the first dose of busulfan and elevated busulfan CL/F compared with children with leukemias,7 although patient numbers were small.
The only known pathway for the elimination of busulfan involves glutathione (GSH) conjugation to form γ-glutamyl-β-(S-tetrahydrothiophenium ion) alanyl-glycine (THT+). Busulfan is uncommon in this respect, because there are few other drugs that are primarily eliminated by GSH conjugation. We have found that human cytosolic glutahione S-transferase (GST) catalyzes THT+ formation,8 and that GSTA1-1, the major liver GST, is the predominant GST isoform in busulfan conjugation.9 Less than 2% of an oral busulfan dose is excreted unchanged.10 The effect of obesity on GSTA1-1 activity is unstudied.
The purpose of this report is to compare busulfan CL/F in obese and normal patients treated for various diseases to provide a pharmacokinetic rationale for the appropriate dosing of busulfan, and to gain insight on the activity of GSTA1-1 in obesity and disease. We used a base of 279 adult and adolescent patients undergoing hematopoietic stem cell transplantation at the Fred Hutchinson Cancer Research Center (Seattle, WA) between January 1992 and December 1996, in whom busulfan CL/F had been measured on at least two occasions during conditioning for hematopoietic stem cell transplantation.
MATERIALS AND METHODS
Patients.
Records collected as part of routine clinical busulfan monitoring between January 1992 and December 1996 at the Fred Hutchinson Cancer Research Center were examined retrospectively. Inclusion required that the patients were in treatment protocols that stipulated busulfan monitoring. These patients represent about 32% of all patients treated with a busulfan-based conditioning regimen during that time period. The patients provided informed consent for the pharmacokinetic analysis. All patients with complete information (consisting of age, dose, disease, height, weight, dose five CL/F, and dose nine CL/F) were included in the analysis, and individuals less than 12-years old (due to age-dependence in busulfan CL/F3 11) or greater than 60-years old were excluded. The final database contained 279 patients who received 0.44 to 1.8 mg/kg oral busulfan tablets every 6 hours for 4 days as part of their transplant preparative regimen. No other cytotoxic agents or irradiation were administered immediately before or concomitantly with busulfan. Patients received phenytoin for seizure prophylaxis. The diseases treated included acute myelogenous leukemia (AML; n = 60), breast cancer (BrCa; n = 55), (CML; n = 73), myelodysplastic syndrome (n = 49), multiple myeloma (MM; n = 25), non-Hodgkin’s lymphoma (NHL; n = 10), and ovarian cancer (n = 7).
Determination of CL/F.
Blood samples were collected just before and 60, 120, 180, 240, and 360 minutes after the administration of busulfan. Plasma busulfan concentrations were determined by gas chromatography with mass selective detection, and mean CL/F was calculated for the 5th and 9th doses of busulfan as previously described.3 Body size estimates were calculated using the following equations in which height is measured in cm and weight in kg.
Patients were classified by BMI: underweight, BMI < 18 kg/m2; normal, BMI 18 to 26.9 kg/m2; obese, BMI 27 to 35 kg/m2, and severely obese, BMI > 35 kg/m2. BMI and the percentage of BW relative to IBW are correlated (BMI = 0.2 (%IBW) + 2.10, r2 = 0.907), such that a BMI of 27 corresponds to 125% BW/IBW.
Statistical analysis.
All statistical comparisons were performed using SPSS version 7.5 (SPSS Inc, Chicago, IL). In univariable linear regression analysis, the relationship between CL/F (mL/min) and each of the following individual patient variables was assessed: age, AIBW, BMI, BSA, height, IBW, gender, disease, and BW. One-way analysis of variance (ANOVA) was used to compare differences in body size-normalized CL/F among different disease categories with the Bonferroni correction for multiple comparisons. The Levene statistic was used to test for homogeneity of variance.
RESULTS
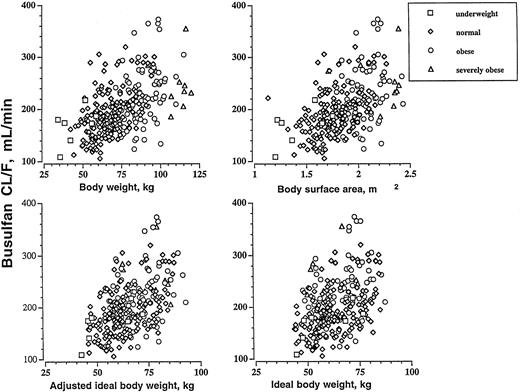
In univariable linear regression analysis, the regression coefficients for absolute busulfan CL/F and each of the following variables were: BW (r2 = 0.300), BSA (r2 = 0.277), AIBW (r2 = 0.265), IBW (r2 = 0.173), height (r2 = 0.164), BMI (r2 = 0.176), and age (r2 = 0.019). Figure 1 shows the relationship between absolute busulfan CL/F and BW, BSA, AIBW, and IBW. All correlations were statistically significant (P < .001; except for age, P = .022). This analysis suggests that BW, BSA, and AIBW are comparable single predictors of CL/F.
The relationship between busulfan CL/F (mL/min) and BW, BSA, IBW, and AIBW in underweight (BMI < 18 kg/m2; n = 7), normal (BMI 18 to 26.9 kg/m2; n = 173), obese (BMI = 27 to 35 kg/m2; n = 89), and severely obese (BMI > 35 kg/m2; n = 10) patients.
The relationship between busulfan CL/F (mL/min) and BW, BSA, IBW, and AIBW in underweight (BMI < 18 kg/m2; n = 7), normal (BMI 18 to 26.9 kg/m2; n = 173), obese (BMI = 27 to 35 kg/m2; n = 89), and severely obese (BMI > 35 kg/m2; n = 10) patients.
Busulfan CL/F is listed in Table 1 for patients in four BMI categories: underweight, normal, obese, and severely obese. The ratio of BW:IBW for each respective BMI category was: 78.4% ± 7.4% (range, 65.6% to 88.1%), underweight; 106% ± 12% (range, 76.4% to 138%), normal; 136% ± 13% (range, 117% to 166%), obese; and 177% ± 23% (range, 151% to 215%), severely obese. Statistically significant differences in absolute (mL/min) CL/F between genders were noted within normal and obese categories. (We did not test for gender differences in the underweight and severely obese patients due to the small sample size of these BMI categories (n = 7 and n = 10, respectively.) Males had a higher absolute (mL/min) CL/F than females in both normal and obese patients.
Busulfan CL/F* in Underweight (BMI <18 kg/m2), Normal (BMI = 18 to 26.9 kg/m2), Obese (BMI = 27 to 35 kg/m2), and Severely Obese Patients (BMI >35 kg/m2)
| . | Underweight . | Normal . | Obese . | Severely Obese . | ||||
|---|---|---|---|---|---|---|---|---|
| Mean . | Male . | Female . | Mean . | Male . | Female . | Mean . | Mean . | |
| n | 7 | 75 | 98 | 46 | 43 | 10 | ||
| Age, years | 23.2 ± 12.7 | 39.3 ± 12.2 | 41.7 ± 11.8 | 40.7 ± 12.0 | 44.2 ± 10.3 | 43.8 ± 9.2 | 44.0 ± 9.7 | 47.0 ± 11.6 |
| CL/F, mL/min† | 169 ± 34 | 209 ± 48 | 175 ± 361-153 | 190 ± 45 | 240 ± 59 | 206 ± 381-153 | 223 ± 53 | 250 ± 47 |
| mL/min/kg BW† | 3.84 ± 0.87 | 2.88 ± 0.62 | 2.91 ± 0.62 | 2.90 ± 0.62 | 2.60 ± 0.62 | 2.52 ± 0.48 | 2.56 ± 0.55 | 2.30 ± 0.51 |
| mL/min/m2 ‡ | 119 ± 22 | 111 ± 23 | 106 ± 23 | 108 ± 23 | 114 ± 27 | 107 ± 20 | 110 ± 24 | 113 ± 26 |
| mL/min/kg AIBW‡ | 3.11 ± 0.54 | 2.91 ± 0.63 | 3.08 ± 0.63 | 3.01 ± 0.63 | 3.10 ± 0.74 | 3.28 ± 0.63 | 3.19 ± 0.69 | 3.41 ± 0.79 |
| . | Underweight . | Normal . | Obese . | Severely Obese . | ||||
|---|---|---|---|---|---|---|---|---|
| Mean . | Male . | Female . | Mean . | Male . | Female . | Mean . | Mean . | |
| n | 7 | 75 | 98 | 46 | 43 | 10 | ||
| Age, years | 23.2 ± 12.7 | 39.3 ± 12.2 | 41.7 ± 11.8 | 40.7 ± 12.0 | 44.2 ± 10.3 | 43.8 ± 9.2 | 44.0 ± 9.7 | 47.0 ± 11.6 |
| CL/F, mL/min† | 169 ± 34 | 209 ± 48 | 175 ± 361-153 | 190 ± 45 | 240 ± 59 | 206 ± 381-153 | 223 ± 53 | 250 ± 47 |
| mL/min/kg BW† | 3.84 ± 0.87 | 2.88 ± 0.62 | 2.91 ± 0.62 | 2.90 ± 0.62 | 2.60 ± 0.62 | 2.52 ± 0.48 | 2.56 ± 0.55 | 2.30 ± 0.51 |
| mL/min/m2 ‡ | 119 ± 22 | 111 ± 23 | 106 ± 23 | 108 ± 23 | 114 ± 27 | 107 ± 20 | 110 ± 24 | 113 ± 26 |
| mL/min/kg AIBW‡ | 3.11 ± 0.54 | 2.91 ± 0.63 | 3.08 ± 0.63 | 3.01 ± 0.63 | 3.10 ± 0.74 | 3.28 ± 0.63 | 3.19 ± 0.69 | 3.41 ± 0.79 |
Data are mean ± SD.
Statistical comparisons of CL/F among different BMI categories are made in Table 2.
There were no statistically significant differences in the mean CL/F expressed relative to BSA or AIBW among underweight, normal, obese, and severely obese patients.
Denotes a statistically significant difference (P < .05) for the mean CL/F in males and females within a BMI category.
We compared the mean CL/F among underweight, normal, obese, and severely obese patients using one-way ANOVA (Table 2). Absolute (mL/min) CL/F was elevated in obese (17%) and severely obese (32%) patients compared with normal patients, P < .05, but there was no statistical difference in CL/F for normal and underweight patients. CL/F relative to BW (mL/min/kg) was 12% and 21% lower in obese and severely obese patients compared with normal patients, respectively, and 32% higher in underweight patients compared with normal patients. There was not a statistically significant difference in CL/F relative to BSA (mL/min/m2) or AIBW (mL/min/kg AIBW) among underweight, normal, obese, and severely obese patients. We compared busulfan CL/F as a function of age by decade (Table 3). There was no age-dependence in busulfan CL/F when expressed relative to BSA or AIBW.
Statistical Comparisons of Table 1 Mean Busulfan CL/F Among Underweight, Normal, Obese, and Severely Obese Patients
| BMI Category (1) . | BMI Category (2) . | % Difference in Absolute CL/F* (mL/min) . | P . | % Difference in CL/F/BW* (mL/min/kg) . | P . |
|---|---|---|---|---|---|
| Underweight | Normal | −12.4 | 1.000 | 24.5 | .000 |
| Obese | −32.0 | .021 | 33.3 | .000 | |
| Severely obese | −47.9 | .004 | 40.1 | .000 | |
| Normal | Underweight | 11.1 | 1.000 | −32.4 | .000 |
| Obese | −17.4 | .000 | 11.7 | .000 | |
| Severely obese | −31.6 | .001 | 20.7 | .014 | |
| Obese | Underweight | 24.2 | .021 | −50.0 | .000 |
| Normal | 14.8 | .000 | −13.3 | .000 | |
| Severely obese | −12.1 | .571 | 10.2 | 1.000 | |
| Severely obese | Underweight | 32.4 | .004 | −67.0 | .000 |
| Normal | 24.0 | .001 | −26.1 | .014 | |
| Obese | 10.8 | .571 | −11.3 | 1.000 |
| BMI Category (1) . | BMI Category (2) . | % Difference in Absolute CL/F* (mL/min) . | P . | % Difference in CL/F/BW* (mL/min/kg) . | P . |
|---|---|---|---|---|---|
| Underweight | Normal | −12.4 | 1.000 | 24.5 | .000 |
| Obese | −32.0 | .021 | 33.3 | .000 | |
| Severely obese | −47.9 | .004 | 40.1 | .000 | |
| Normal | Underweight | 11.1 | 1.000 | −32.4 | .000 |
| Obese | −17.4 | .000 | 11.7 | .000 | |
| Severely obese | −31.6 | .001 | 20.7 | .014 | |
| Obese | Underweight | 24.2 | .021 | −50.0 | .000 |
| Normal | 14.8 | .000 | −13.3 | .000 | |
| Severely obese | −12.1 | .571 | 10.2 | 1.000 | |
| Severely obese | Underweight | 32.4 | .004 | −67.0 | .000 |
| Normal | 24.0 | .001 | −26.1 | .014 | |
| Obese | 10.8 | .571 | −11.3 | 1.000 |
The percent difference in CL/F = ( BMI category (1) − BMI category (2)/BMI category (1)) × 100.
Body Mass Index and Busulfan CL/F (Mean ± SD) as Functions of Age
| Busulfan CL/F . | Age, years (n) . | ||||
|---|---|---|---|---|---|
| 12-20 (16) . | 21-30 (26) . | 31-40 (63) . | 41-50 (104) . | 51-60 (70) . | |
| mL/min/m2 BSA | 116 ± 23 | 108 ± 24 | 108 ± 25 | 109 ± 23 | 110 ± 24 |
| mL/min/kg AIBW | 3.19 ± 0.58 | 3.01 ± 0.72 | 2.99 ± 0.61 | 3.11 ± 0.65 | 3.13 ± 0.71 |
| Busulfan CL/F . | Age, years (n) . | ||||
|---|---|---|---|---|---|
| 12-20 (16) . | 21-30 (26) . | 31-40 (63) . | 41-50 (104) . | 51-60 (70) . | |
| mL/min/m2 BSA | 116 ± 23 | 108 ± 24 | 108 ± 25 | 109 ± 23 | 110 ± 24 |
| mL/min/kg AIBW | 3.19 ± 0.58 | 3.01 ± 0.72 | 2.99 ± 0.61 | 3.11 ± 0.65 | 3.13 ± 0.71 |
Busulfan CL/F was compared among disease categories after expressing busulfan CL/F relative to BW, AIBW, and BSA (Table 4). There was a statistical difference when comparing the mean CL/F expressed relative to BW in patients with NHL to those with AML (2.15 ± 0.22 v 2.82 ± 0.66 mL/min/kg, P < .045, respectively), and NHLv CML (2.15 ± 0.22 v 2.92 ± 0.70 mL/min/kg,P < .007, respectively). Significant differences in mean busulfan CL/F expressed relative to BSA were found in patients with NHL in comparison to those with CML (87.9 ± 12.3 v 116 ± 25 mL/min/m2, P < .006, respectively). The mean CL/F expressed relative to AIBW was statistically significantly different in patients with NHL compared with those with BrCa (2.41 ± 0.42v 3.15 ± 0.62 mL/min/kg, P < .017, respectively), NHL v CML (2.41 ± 0.42 v 3.20 ± 0.70 mL/min/kg,P < .006, respectively), and NHL v MM (2.41 ± 0.42 v 3.24 ± 0.61 mL/min/kg, P < .012, respectively).
Disease-Dependent Differences in Busulfan CL/F Expressed Relative to BW, BSA, or AIBW
| Busulfan CL/F Expressed Relative to: . | Disease (1) . | Disease (2) . | %, Mean Difference* . | P . |
|---|---|---|---|---|
| BW (mL/min/kg) | NHL | AML | −31.2 | .045 |
| NHL | CML | −36.0 | .007 | |
| BSA (mL/min/m2) | NHL | CML | −32.0 | .006 |
| AIBW (mL/min/kg) | NHL | BrCa | −31.1 | .017 |
| NHL | CML | −33.1 | .006 | |
| NHL | MM | −34.8 | .012 |
| Busulfan CL/F Expressed Relative to: . | Disease (1) . | Disease (2) . | %, Mean Difference* . | P . |
|---|---|---|---|---|
| BW (mL/min/kg) | NHL | AML | −31.2 | .045 |
| NHL | CML | −36.0 | .007 | |
| BSA (mL/min/m2) | NHL | CML | −32.0 | .006 |
| AIBW (mL/min/kg) | NHL | BrCa | −31.1 | .017 |
| NHL | CML | −33.1 | .006 | |
| NHL | MM | −34.8 | .012 |
Busulfan CL/F expressed relative to BW, AIBW, or BSA, relative to BW, BSA, or AIBW was compared among patients with AML (n = 60), BrCa (n = 55), CML (n = 73), myelodysplastic syndrome (n = 49), MM (n = 25), non-Hodgkin’s lymphoma (n = 10), and ovarian cancer (n = 7). Statistical comparisons were made using one-way ANOVA with the Bonferroni correction for multiple comparisons. Only the statistically significant results were listed in the table.
We sought an effect of elevations in liver function tests (ie, alkaline phosphatase, alanine aminotransferase, and aspartate aminotransferase) and kidney function tests (ie, blood urea nitrogen and creatinine) on busulfan CL/F expressed relative to BSA. The normal range for each test was: alkaline phosphatase, 50 to 120 U/L; alanine aminotransferase, 10 to 35 U/L; aspartate aminotransferase, 20 to 48 U/L; blood urea nitrogen, 5 to 20 mg/dL; and creatinine, 0.1 to 1.1 mg/dL. We found that elevations in liver and kidney function tests were not significant predictors of changes in CL/F relative to BSA (P > .14). Also, 15 patients included in this analysis were undergoing a second transplant (at least 3 months after the first transplant). There was no difference in the mean CL/F relative to BSA for patients undergoing first versus second transplant (109 ± 23 v 107 ± 22 mL/min/m2, P = .732, respectively).
DISCUSSION
The major finding was that although CL/F varies among BMI classes when expressed relative to BW, there are no differences in busulfan CL/F among BMI classes when CL/F is expressed relative to AIBW or BSA (Tables 1 and 2). Patients with NHL (n = 10) had lower busulfan CL/F expressed relative to BW, BSA, or AIBW compared with patients with CML (Table 4).
The current clinical practice at most transplant centers is to dose busulfan relative to body weight or adjusted ideal body weight, although there is a dilemma about what to do in the obese. BSA or AIBW-adjusted dosing will eliminate differences in busulfan CL/F among normal and obese patients, but it does not eliminate disease-specific differences. There were no differences in busulfan CL/F expressed relative to BSA or AIBW in underweight patients compared with obese and severely obese patients. Therefore, these measures of body size are appropriate for calculating dose in any adult. The data suggest that it may be prudent to lower the dose/m2 or dose/kg AIBW in NHL patients if it were desired to produce equivalent mean AUC or CSS in all patients.
The relationship between busulfan CSS and a variety of outcomes has been examined. Busulfan CSS was related to regimen-related toxicity (RRT) and graft rejection in 42 patients with a variety of diseases undergoing hematopoietic stem cell transplantation.3 Severe-grade 3-4 RRT was only observed in patients with CSS > 900 ng/mL. The incidence of graft rejection for those receiving HLA partially matched related or unrelated donor grafts with a busulfan CSS < 600 ng/mL was 7 in 9, whereas graft rejection in patients with CSS > 600 ng/mL was 1 in 7. Based on these findings, the therapeutic window for busulfan is CSS of 600 to 900 ng/mL (which corresponds to mean AUC of 900 to 1,350 μmol/L × minute) over 16 doses for these patients.3 More recent findings suggest that tolerance to busulfan may vary by disease. CML patients tolerate busulfan CSS > 900 ng/mL without severe-grade 3-4 RRT. 4 In addition, the incidence of relapse was significantly higher in CML patients with busulfan CSS levels below the median for the entire group of 45 patients (917 ng/mL).
Interpatient variability in busulfan CL/F is large and is an important determinant of transplantation outcome. In the studies just cited (in which patients received a constant mg/kg dose), inappropriate busulfan AUC was the single most important determinant of toxicity, rejection, or relapse. The coefficients of variation for busulfan CL/F expressed relative to AIBW and BSA when including all patients in this study were both 21.3%. Thus, given the frequency with which busulfan AUC will exceed thresholds associated with undesired outcomes even when dose is adjusted for AIBW or BSA, busulfan level monitoring is still required in certain situations.
As mentioned previously, busulfan is eliminated by GSH conjugation catalyzed by GST. The results of this study suggest that total GSTA1-1 activity is most closely related to BSA or AIBW. In general, absolute (mL/min) clearances of xenobiotics (ie, caffeine, phenytoin, desmethyl-diazepam, carbamazepine, midazolam, and antipyrine) eliminated primarily by hepatic P450 oxidative metabolism (phase-I enzymes) are similar in obese and lean subjects,12-17whereas absolute clearances of drugs eliminated primarily by phase-II enzymes (ie, phenol sulfotransferase, glucuronyl transferase) are higher in obese subjects compared with lean controls.18-21The findings with busulfan are similar to those with other drugs eliminated by phase-II enzymes (GST is a phase-II enzyme).
Interestingly, NHL patients had lower busulfan CL/F when expressed relative to BW, BSA, or AIBW compared with patients with CML, whose CL/F relative to BW, BSA, and AIBW was 4.5%, 6.0%, and 3.8% higher than the mean of all patients, respectively. The reason for a lower CL/F in NHL is not clear, but it does not seem to be prior chemotherapy. For example, 85% of CML patients received hydrea, and 34% alpha-interferon. For AML, 80% of patients received cytosine arabinoside. In contrast, NHL patients received a range of therapies. NHL patients that did not receive prior chemotherapy (3 in 10) had a similar CL/F compared with those that did (5 in 10; 80.3 ± 7.0v 88.8 ± 13.0 mL/min/m2, P = .28, respectively). Data on prior chemotherapy were not available for two patients.
After the standard 1 mg/kg (BW) busulfan dose, a patient with CL/F of 2.9 mL/min/kg (the mean value for patients with BMI between 18 and 27 kg/m2) will have a CSS of 960 ng/mL (which corresponds to an AUC of 1,400 μmol/L × minute). NHL patients administered a 1 mg/kg dose will have a CSS of 1,290 ng/mL respectively, (which corresponds to AUC of 1,890 μmol/L × minute, respectively). Previous studies have identified the threshold for severe toxicity to be CSS of 900 to 1,000 ng/mL (AUC of 1,350 to 1,500 μmol/L × minute).1-3 Thus, current data suggest that the apparent reduction in busulfan CL/F in NHL patients could result in enhanced toxicity after a fixed 1 mg/kg dose. However, the small number (n = 10) of NHL patients included in this analysis suggests that this issue should be investigated further.
In conclusion, absolute busulfan CL/F is elevated in obesity. Expressing CL/F relative to AIBW or BSA eliminated mean differences in CL/F among underweight, normal, obese, and severely obese patients. There appears to be a potentially important difference between NHL patients and those with CML in busulfan CL/F expressed relative to BW, BSA, or AIBW. Even when expressed relative to BSA or AIBW, interpatient variability in busulfan CL/F expressed relative to any measure of body size is large relative to the therapeutic window in certain indications.3 4 The need for adjusting busulfan dose based on AUC or CSS measured in the individual patient remains in certain settings regardless of body size measure.
Supported in part by National Institutes of Health Grant (CA 18029).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal