Abstract
Genetic studies in animal models of microcytic anemia and biochemical studies of transport have implicated the Nramp2gene in iron transport. Nramp2 generates two alternatively spliced mRNAs that differ at their 3′ untranslated region by the presence or absence of an iron-response element (IRE) and that encode two proteins with distinct carboxy termini. Antisera raised against Nramp2 fusion proteins containing either the carboxy or amino termini of Nramp2 and that can help distinguish between the two Nramp2 protein isoforms (IRE: isoform I; non-IRE: isoform II) were generated. These antibodies were used to identify the cellular and subcellular localization of Nramp2 in normal tissues and to study possible regulation by dietary iron deprivation. Immunoblotting experiments with membrane fractions from intact organs show that Nramp2 is expressed at low levels throughout the small intestine and to a higher extent in kidney. Dietary iron starvation results in a dramatic upregulation of the Nramp2 isoform I in the proximal portion of the duodenum only, whereas expression in the rest of the small intestine and in kidney remains largely unchanged in response to the lack of dietary iron. In proximal duodenum, immunostaining studies of tissue sections show that Nramp2 protein expression is abundant under iron deplete condition and limited to the villi and is absent in the crypts. In the villi, staining is limited to the columnar absorptive epithelium of the mucosa (enterocytes), with no expression in mucus-secreting goblet cells or in the lamina propria. Nramp2 expression is strongest in the apical two thirds of the villi and is very intense at the brush border of the apical pole of the enterocytes, whereas the basolateral membrane of these cells is negative for Nramp2. These results strongly suggest that Nramp2 is indeed responsible for transferrin-independent iron uptake in the duodenum. These findings are discussed in the context of overall mechanisms of iron acquisition by the body.
IRON IS AN ESSENTIAL element for all forms of life, and living organisms have developed sophisticated means to recycle, scavenge, and recruit iron from their environment.1,2 Excessive accumulation of iron can be potentially harmful through the generation of free radicals and other toxic substances.3 In mammals, there is no physiological excretion system for iron, which is normally lost from the organism by nonspecific mechanisms such as cell desquamation. Therefore, body iron levels appear to be primarily regulated at the level of absorption and through efficient storage.4,5 In mammals, nutritional iron absorption (both heme and nonheme iron) occurs primarily in the intestine.6,7 Heme iron constitutes only a small fraction of the available dietary iron, but it is highly available for absorption.8,9 On the other hand, the absorption of nonheme iron is low and markedly regulated in the first part of the duodenum,6,7 in which the acidic pH promotes solubilization of iron transformed to Fe2+ by ferrireductase10and ascorbate.11 Inorganic iron absorption occurs as a three-step process: (1) uptake by the enterocytes from the lumen across the apical membrane, (2) intracellular transport, and (3) transfer across the basolateral membrane into plasma. It has been suggested that the rate-limiting step for absorption of inorganic iron is uptake of the ingested metal across the brush border membrane of the enterocyte.12 Uptake of ferrous iron by the enterocyte is an energy-dependent and carrier-mediated process (saturation kinetics, temperature dependence, and sensitivity to proteases),13,14and a large number of candidates for iron binding proteins and/or transport activities have been suggested by labeling, cross-linking, or direct transport measurements in brush border membrane vesicles.15 The recent identification of genes mutated in animal models of deficit in iron metabolism has shed considerable light on the proteins possibly involved in iron transport in the intestine as well as peripheral tissues. The mk mutant mouse strain16,17 and the Belgrade (b) rat18 are two rodent models of iron deficiency, both exhibiting a severe microcytic hypochromic anemia marked by a severe defect in iron absorption by intestinal cells and in erythroid iron use. Moreover, studies in vivo have shown that this anemia cannot be corrected by increased dietary iron19,20 or by direct iron injection,21 suggesting also a second block in iron uptake by red blood cell precursors and other peripheral tissues. It has recently been demonstrated that the Nramp2 (natural resistance-associated macrophage protein 2) gene is mutated in both themk and b animal models.22,23 Indeed, both the mk mouse and the b rat have been shown to carry the same mutation at Nramp2, ie, a glycine to arginine (Gly185Arg) substitution in one of the predicted transmembrane domains (TM4) of the protein.22,23 In addition, studies in transiently transfected HEK293T cells have shown that overexpression of the wild-type but not the mk/b mutant variant of Nramp2 stimulates cellular iron uptake.23,24 Finally, studies in Xenopus laevis oocytes have suggested that Nramp2 may act as a pH-dependent divalent cation transporter, functioning by a proton symport mechanism.25
The Nramp2 gene was initially identified as encoding a protein highly similar to the natural resistance-associated macrophage protein 1 (Nramp1).26,27 Naturally occurring28,29 and experimentally induced mutations30 at Nramp1abrogate natural resistance to infection with unrelated intracellular micro-organisms such as Salmonella,Leishmania, and Mycobacteria in mice.31,32Likewise, polymorphic variants at the human NRAMP1 locus are associated with differential susceptibility of humans to tuberculosis and leprosy in endemic areas of disease.33,34 Nramp1 and Nramp2 are highly hydrophobic integral membrane glycoproteins composed of 12 transmembrane (TM) domains that possess several structural characteristics of ion channels and transporters.35,36 As opposed to Nramp1, which is expressed exclusively in mononuclear phagocytes such as tissue macrophages,37,38Nramp2 mRNA expression is more ubiquitous and has been detected in most tissues and cell types analyzed27,39; however, it is higher in brain, thymus, proximal intestine, kidney, and bone marrow.25 cDNA cloning and sequencing experiments have indicated that Nramp2 produces two alternatively spliced transcripts generated by alternative use of two 3′ exons encoding distinct C-termini of the protein as well as distinct 3′ untranslated regions (UTRs).40 Interestingly, oneNramp2 mRNA contains an iron-responsive element (IRE) in its 3′UTR. The IRE is an RNA secondary structure present in the 5′- or the 3′-UTR of animal mRNAs encoding proteins involved in iron metabolism.41,42 In iron-replete cells, iron-regulatory proteins interact with IREs to either enhance the stability or inhibit translation of the tagged RNAs, thus regulating the amount of protein expressed.43,44 The second Nramp2splice isoform (non-IRE) encodes a protein (isoform II) in which the C-terminal 18 amino acids of the IRE form (isoform I) are replaced by a novel 25 amino acids segment and codes for a distinct 3′ UTR lacking the IRE. Gunshin et al25 have observed that Nramp2 mRNA expression is regulated by dietary iron.
Immunolocalization studies with protein-specific antibodies have shown that Nramp1 is expressed in macrophages in the late endosomal/lysosomal compartment.45 Upon phagocytosis, Nramp1 is recruited to the membrane of the phagosome,45 where it may act to modulate the intravesicular cation content to affect intracellular microbial replication.46 On the other hand, the absence of isoform-specific anti-Nramp2 antibodies has so far precluded the identification of the organ and cell types expressing the protein. Likewise, the cellular and subcellular localization in normal tissues of the two Nramp2 protein isoforms as well as their possible regulation by iron remain unknown. These aspects of Nramp2 need to be better characterized to understand the role of this protein in dietary iron uptake, possible trans-epithelial transport of iron, and iron metabolism in peripheral tissues. In the current study, we have generated affinity-purified, isoform-specific anti-Nramp2 antibodies and have determined the organ-specific, cell-specific, as well as subcellular distribution of the Nramp2 isoforms in normal tissues. We have also analyzed the regulation of these two isoforms by dietary iron.
MATERIALS AND METHODS
Animals.
Normal inbred mouse strain 129sv were initially obtained from Taconic Farms (Germantown, NY) and subsequently bred and handled in our animal care facility according to the rules and regulations of the Canadian Council on Animal Care. For iron depletion studies, animals were fed a low iron diet (ICN, Costa Mesa, CA) for a period of 8 weeks, whereas control animals were fed an identical diet supplemented with 3% ferric phosphate (ICN).
Cell culture and transfections.
Chinese hamster ovary (CHO) cells LR7347 were grown in α-minimum essential medium (α-MEM) supplemented with 10% fetal calf serum, 2 mmol/L L-glutamine, 50 U/mL penicillin, and 50 μg/mL streptomycin. All media and media supplements were purchased from GIBCO/BRL (Grand Island, NY). An expression plasmid was constructed45 using the mammalian expression vector pMT2 containing the entire coding sequence of mouse Nramp1 cDNA modified at its carboxy terminus by the in-frame addition of four consecutive antigenic peptide epitopes (EQKLISEEDL) derived from the human c-Myc protein. In a similar manner, a c-Myc–tagged Nramp2 expression plasmid was constructed by introducing the c-Myc–taggedNramp2 cDNA from plasmid pBluescript48 in mammalian expression vector pMT2. pMT2 uses SV40 and adenovirus regulatory sequences to overexpress exogenous cDNAs and is introduced into mammalian cells by cotransfection with pSV2neo to allow selection of cotransfectants in geneticin (G418). Plasmids were introduced in CHO LR73 cells by transfection, using the calcium phosphate coprecipitation method.49 Individual G418R cell clones were isolated, expended in culture, and analyzed for recombinant Nramp1 and Nramp2 protein expression by immunofluorescence and immunoblotting.
Microsomal membrane fractions preparation.
Crude membrane fractions from homogenized tissues and various CHO-transfected cell clones were prepared according to a previously described procedure.50 Kidney, liver, spleen, and portions of the gastrointestinal system including colon were removed immediately after death, were frozen in liquid nitrogen, and were ground to a fine powder using mortar and pestle precooled on carbonic ice. CHO cells were grown to 70% confluency and harvested in cold phosphate-buffered saline (PBS)-citrate. Tissue powder or CHO cell pellets were then resuspended in 10 mL/g of tissue of a solution consisting in 0.25 mol/L sucrose/0.03 mol/L histidine (pH 7.2) supplemented with 2 mmol/L EDTA, 0.1 mg/mL phenylmethylsulfonyl fluoride, 2 μg/mL leupeptin, 1 μg/mL pepstatin, and 2 μg/mL aprotinin. Tissues and cells were homogenized using a glass potter with a tight fitting teflon pestle rotated at 1,300 revolutions/min. The homogenate was then centrifuged at 6,000g for 15 minutes. The sediment was resuspended several times in the original volume of sucrose-histidine solution and centrifuged again at 6,000g for 15 minutes. The combined supernatants were then centrifuged at 80,000g for 30 minutes and the pellet corresponding to the microsomal fraction was resuspended in sucrose/histidine buffer and stored frozen at −80°C until use. Protein concentration of the various membrane fractions was determined by the Bradford assay (Bio-Rad, Hercules, CA).
Preparation of Nramp immunogens.
The fusion protein containing the amino terminal domain (region 1-54) of Nramp1 fused in-frame to glutathione-S-transferase (GST) was constructed and used to generate anti-Nramp1 (mN1-NT) antibodies, as previously described.51 For producing polyclonal antisera against the two protein isoforms of Nramp2 (IRE: isoform I; non-IRE: isoform II), a fusion protein was produced containing GST fused to a peptide segment corresponding to the amino terminus (residues 1-73) region of Nramp2. To generate a specific antibody against the isoform II, a GST fusion protein containing the carboxy terminal coding region (residues 532-568) of the Nramp2 non-IRE mRNA was constructed. Nucleotide sequences of final constructs were verified before production of the proteins in Escherichia coli.52GST-chimeras were purified over glutathione-agarose, analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and excised from the gel after light staining with Coomassie blue. Antisera were raised in New Zealand White rabbits using purified protein (0.1 mg per rabbit per injection) emulsified in Freund’s complete or incomplete adjuvant. Rabbits were boosted at 6-week intervals, and blood (10 mL) was obtained by arterial puncture 11 days after each boost. Serum was isolated and kept frozen at −20°C.
Affinity purification of anti-Nramp antisera.
The same strategy was used for affinity purification of anti-Nramp1 and anti-Nramp2 antibodies and consisted in using the same Nramp peptide segments fused to dihydrofolate reductase (DHFR). Briefly, each pGEX-Nramp construct (see above) was digested with EcoRI, and the resulting overhangs were repaired using the Klenow fragment of DNA polymerase I (Pharmacia, Uppsala, Sweden) before digestion with the BamHI and ligation into Bgl II- andSma I-digested pQE40 bacterial expression vector (Qiagen, Mississauga, Ontario, Canada). The in-frame (His)6-DHFR-Nramp1 or Nramp2 fusion protein constructs were transformed into E coli strain M15(pREP4) for expression (Qiagen). Purification of DHFR chimeras was performed on Ni-NTA agarose according to experimental conditions suggested by the manufacturer (Qiagen). The polyclonal antiserum fractions were then purified by a preparative immunoblot procedure.53 The fusion proteins were loaded onto a 12% SDS-polyacrylamide gel using a preparative comb and transferred to a polyvinylidene fluoride membrane (PVDF; westran; Schleicher & Schuell, Keene, NH). The band corresponding to a fusion protein was localized on the membrane by Ponceau S staining (Sigma, St Louis, MO), excised, cut in small pieces, and incubated for 2 hours at room temperature with 5% skim milk powder in PBS/0.2 % Tween 20 to block nonspecific sites. PVDF membrane fragments were further incubated 16 hours at 4°C with the immune serum diluted 2:3 in PBS. After 5 washes with PBS, bound antibodies were eluted with 0.2 mol/L glycine, pH 2.2, for 3 minutes. The pH of the eluate was quickly neutralized by adding 1 mol/L Tris base to pH ∼7.5, and bovine serum albumin (BSA) and glycerol were added to a final concentration of 0.1% and 50%, respectively. Aliquots of the purified sera were kept at −20°C.
Immunoblotting.
Crude membrane preparations from tissues (100 μg) or CHO cells (25 μg) were dissolved in Laemmli buffer and incubated for 30 minutes at room temperature before SDS-PAGE (10% polyacrylamide) and transfer to PVDF membranes. Similar loading and transfer of proteins was verified by staining the blots with Ponceau S (Sigma). PVDF membranes were preincubated with blocking solution (0.02% Tween20, 5% skim milk in PBS) for 2 hours at room temperature, followed by incubation with primary antibodies for 16 hours at 4°C in blocking solution. For immunoblotting of tissue membrane extracts, primary antibodies were used at the following dilutions: rabbit anti-Nramp1 (1/200), rabbit anti-Nramp2 NT (1/100), rabbit anti-Nramp2 CT (1/75), rat monoclonal anti-mouse transferrin receptor (1/250; Biosource International, Camarillo, CA), and rabbit polyclonal anti-biliary glycoprotein 1 (Bgp1; 1/4,000; provided by Dr N. Beauchemin, McGill University, Montreal, Quebec, Canada). For immunoblotting of CHO membrane extracts, purified anti-Nramp 1 and anti-Nramp 2 as well as mouse monoclonal anti-cMyc (9E10) were used at the 1/100 dilution. After incubation with the primary antibody and washing with PBS + 0.2% Tween20, membranes were incubated with peroxidase labeled anti-rat, anti-rabbit, or anti-mouse secondary antibodies (1/10,000; Amersham, Arlington Heights, IL) for 1 hour at room temperature, and the signal was visualized by ECL (Amersham). After ECL detection, some membranes were incubated at 50°C for 30 minutes in stripping solution (100 mmol/L β2-mercaptoethanol, 2% SDS, 62.5 mmol/L Tris-HCl, pH 6.8) and then reprobed with a different primary antibody.
Immunohistochemistry.
Tissues were fixed in Bouin’s solution for 72 hours at room temperature. They were then dehydrated in a series of ethanol (3 times for 20 minutes: 70%, 95%, and 100%), ethanol/xylene (1/1), and xylene solutions, followed by embedding in paraffin. Five-micrometer sections were cut and mounted with gelatin on glass slides. Before labeling, sections were deparaffinized in xylene and rehydrated in a graded series of ethanol baths (100%, 95%, 70%, 50%, and 30%). Endogenous peroxidase activity in the deparaffinized sections was blocked with ethanol 70%-peroxide 1% solution. Sections were then incubated in PBS/100 mmol/L glycine, rinsed in PBS, and blocked for at least 1 hour at room temperature in PBS containing 1% BSA (Fraction V; fatty acid free; Boehringer Mannheim, Indianapolis, IN) and 10% normal goat serum (GIBCO). Incubation with the primary antibody diluted in blocking solution was performed in a wet chamber overnight at 4°C. Immunohistochemical staining of fixed paraffin-embedded sections was performed using the peroxidase/antiperoxidase procedure. After incubation with the primary antibody, three washes in PBS, and incubation with the secondary antibody (swine antirabbit IgG, 1:100; DAKO), subsequent rabbit peroxidase-antiperoxidase immunostaining (PAP; 1:100; DAKO) was shown using 3′-diaminobenzidine tetrahydrochloride (DAB) in solution. Sections were then counterstained with 0.1% methylene blue in PBS. Finally, sections were dehydrated again in ethanol and xylene and mounted in Permount. For immunofluorescence, after incubation with the primary antibodies, sections were further incubated with an anti-rabbit secondary antibody conjugated to Cy3/Rhodamine (Jackson Immunochemicals, West Grove, PA). Sections were then washed with PBS and directly mounted in ImmuMount (Shandon, Pittsburgh, PA). Immunofluorescence was analyzed with a Nikon microscope using the 100× oil immersion objective.
RESULTS
Production and characterization of isoform-specific anti-Nramp2 antisera.
To further study localization and regulation of the Nramp2 protein in normal tissues, we aimed at generating specific anti-Nramp2 antisera that would not react with Nramp1. In addition, it has been previously observed that the Nramp2 gene can generate by alternative splicing two Nramp2 mRNAs and two corresponding polypeptides that differ markedly at their 3′ untranslated region (presence or absence of iron-response element [IRE]) and at their carboxyl termini, respectively (Fig 1A). For clarity, we have designated proteins encoded by the IRE-containing and non-IRE containing Nramp2 mRNAs, Nramp2 isoform I and isoform II, respectively. Thus, we attempted to raise antisera that could also distinguish the two Nramp2 protein variants. For this, we have used two fusion proteins as immunogens in rabbits. The first one comprised the first 73 amino terminal residues of Nramp2 (NT, identical in both proteins), and antibodies raised against this protein should recognize Nramp2 isoforms I and II. The second immunogen comprised residues 532-568 from the carboxy terminus of Nramp2 encoded by the non-IRE containing mRNA (CT), and antibodies against this sequence should be specific for isoform II (Fig 1A). Using both antibodies in parallel should allow one to monitor the expression of either isoform in test cells and tissue samples.
Detection of the Nramp1 and Nramp2 cMyc-tagged proteins in CHO cell membranes. (A) Schematic representation of the two mRNAs corresponding to the IRE-containing form and to the non-IRE form ofNramp2 generated by alternative splicing of a 3′ end exon (see Lee et al40). The two mRNAs harbor distinct 3′ untranslated region (3′UTR) and encode two polypeptides with distinct carboxy termini (hatched boxes). The mN2 (NT) antibody was raised against an amino terminal segment of Nramp2 that is common to the two Nramp2 protein isoforms (solid boxes). The mN2 (CT) antibody was raised against the carboxy terminal extremity of the protein encoded by the Nramp2 non-IRE mRNA (Nramp2 isoform II). (B) Crude membrane extracts from untransfected CHO cells (lanes 1, 3, 5, 7, 9, 11, 13, and 15), from Nramp1 transfectants (lanes 10, 12, 14, and 16), and from Nramp2 isoform II transfectants (lanes 2, 4, 6, and 8) were separated by SDS-PAGE on a 10% acrylamide gel followed by transfer to PVDF membranes. Immunodetection was with affinity-purified mNramp1 [mN1 (NT); lanes 1, 2, 9, and 10], mN2 (NT; lanes 3, 4, 11, and 12), and mN2 (CT; lanes 5, 6, 13, and 14) antibodies and monoclonal anti-cMyc antibody 9E10 (lanes 7, 8, 15, and 16), as described in Materials and Methods. The positions and sizes (in kilodaltons) of molecular weight markers are shown.
Detection of the Nramp1 and Nramp2 cMyc-tagged proteins in CHO cell membranes. (A) Schematic representation of the two mRNAs corresponding to the IRE-containing form and to the non-IRE form ofNramp2 generated by alternative splicing of a 3′ end exon (see Lee et al40). The two mRNAs harbor distinct 3′ untranslated region (3′UTR) and encode two polypeptides with distinct carboxy termini (hatched boxes). The mN2 (NT) antibody was raised against an amino terminal segment of Nramp2 that is common to the two Nramp2 protein isoforms (solid boxes). The mN2 (CT) antibody was raised against the carboxy terminal extremity of the protein encoded by the Nramp2 non-IRE mRNA (Nramp2 isoform II). (B) Crude membrane extracts from untransfected CHO cells (lanes 1, 3, 5, 7, 9, 11, 13, and 15), from Nramp1 transfectants (lanes 10, 12, 14, and 16), and from Nramp2 isoform II transfectants (lanes 2, 4, 6, and 8) were separated by SDS-PAGE on a 10% acrylamide gel followed by transfer to PVDF membranes. Immunodetection was with affinity-purified mNramp1 [mN1 (NT); lanes 1, 2, 9, and 10], mN2 (NT; lanes 3, 4, 11, and 12), and mN2 (CT; lanes 5, 6, 13, and 14) antibodies and monoclonal anti-cMyc antibody 9E10 (lanes 7, 8, 15, and 16), as described in Materials and Methods. The positions and sizes (in kilodaltons) of molecular weight markers are shown.
Hyperimmune antisera were generated in rabbits and the anti-Nramp2 Ig fraction was purified by affinity chromatography (see Materials and Methods). The specificity of the two antisera was tested by immunoblotting using membrane fractions from CHO cells and from CHO cells that have been transfected and overexpress either Nramp1 or isoform II of Nramp2. In addition, these transfected proteins contain a cMyc epitope tag fused in-frame at their C-terminus that allows one to verify the specificity of the signal obtained by immunoblotting using an anti-cMyc monoclonal antibody (9E10). Results from these analyses are shown in Fig 1B. The 9E10 antibody specifically detects a single protein species of 80 to 90 kD in the Nramp1-transfected cells (Fig 1B, lane 16) and a species of 90 to 100 kD in the Nramp2-transfected cells (Fig 1B, lane 8). These immunoreactive bands are absent in membrane extracts from untransfected CHO controls (Fig 1B, lanes 1, 3, 5, 7, 9, 11, 13, and 15). In extracts from Nramp1 transfectants, the affinity-purified Nramp1 antiserum [mN1 (NT)] recognizes a single protein of apparent molecular mass 80 to 90 kD (Fig 1B, lane 10). Both affinity-purified anti-Nramp2 antibodies raised against the N terminus [mN2 (NT)] or the C terminus [mN2 (CT)] fusions detect a band of apparent mobility 90 to 100 kD in membranes from Nramp2-transfected cells (Fig 1B, lanes 4 and 6, respectively). The electrophoretic mobility characteristics of Nramp proteins detected with affinity-purified anti-Nramp antisera were very similar to that of the species detected by the anti-cMyc antibody in the same cell extracts. Finally, no cross-reactivity was seen between the anti-Nramp1 and the anti-Nramp2 antibodies (compare lanes 2, 4, and 6 with lanes 10, 12, and 14, respectively). Together, these results clearly establish that our affinity-purified anti-Nramp1 and anti-Nramp2 antisera are protein specific.
Tissue expression of Nramp2 and regulation by dietary iron.
In normal mouse tissues, Nramp2 mRNA is present in low abundance in most tissues and cell types analyzed.27Northern blot analysis of RNA from rat tissues also showed broad expression of Nramp2 (DCT1), but also identified upregulation of Nramp2 in response to depletion of dietary iron.25 However, the organ- and cell-specific expression of the two Nramp2 protein isoforms as well as their subcellular distribution remain unknown. This issue was investigated using the two anti-Nramp2 antisera. Considering both the previous mRNA expression data and the known gastrointestinal site of iron absorption, we restricted the analysis to a few organs (small intestine [I], colon [C], kidney [K], spleen [S], and liver [L]; Fig 2). We also investigated the possible role of dietary iron depletion on Nramp2 protein expression. For these studies, mice were fed either a low iron diet (−Fe) or the same diet supplemented with iron (+Fe; Fig 3). After 8 weeks, mice were killed and organs were collected. Two-centimeter sections corresponding to the proximal duodenum (I1), distal duodenum (I2), distal ileum (I3), and colon (C) were dissected (Fig 2), and microsomal membrane fractions were isolated by centrifugation after tissue homogenization. These fractions were then separated by gel electrophoresis and analyzed with the two anti-Nramp2 antisera (NT and CT), as well as with the anti-Nramp1 antiserum (Fig3A, B, and C, respectively). In these experiments, membrane fractions from CHO cells and CHO transfectants expressing either Nramp1 or Nramp2 isoform II were included as controls (Fig 3, lanes 15, 16, and 17).
Dissection scheme for the gastrointestinal tract. Different segments of the gastrointestinal tract were dissected from mice fed a low iron diet or fed a normal diet. The first part of the small intestine was divided into two pieces, I1 and I2, corresponding to the proximal and distal parts of the duodenum, respectively. The distal part of the small intestine, the ileum (I3), as well as the colon (C) were also dissected.
Dissection scheme for the gastrointestinal tract. Different segments of the gastrointestinal tract were dissected from mice fed a low iron diet or fed a normal diet. The first part of the small intestine was divided into two pieces, I1 and I2, corresponding to the proximal and distal parts of the duodenum, respectively. The distal part of the small intestine, the ileum (I3), as well as the colon (C) were also dissected.
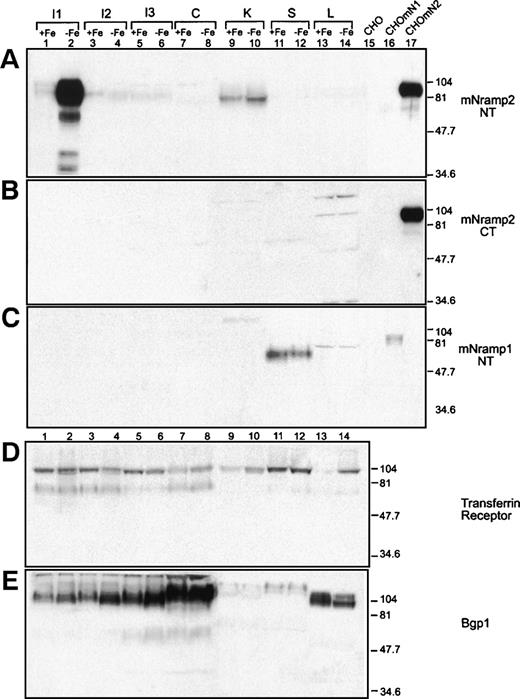
Effect of dietary iron depletion on Nramp protein expression in normal tissues. Organs were harvested from mice maintained on low iron diet (−Fe) or on the same diet but supplemented with iron (+Fe). One hundred micrograms of microsomal membrane fractions isolated from proximal duodenum (I1), distal duodenum (I2), ileum (I3), colon (C), kidney (K), spleen (S), and liver (L) were resolved on a 10% acrylamide gel, transferred to a PVDF membrane, and analyzed by immunoblotting. To control the specificity of the anti-Nramp antibodies, 25 μg of membrane proteins from control CHO cells (CHO) or CHO transfectants expressing either a cMyc-tagged Nramp1 (CHOmN1) or a cMyc-tagged Nramp2 isoform II (CHOmN2) were included. Immunoblotting was performed with antibodies raised against Nramp2 N-terminus (A; mNramp2 NT), Nramp2 C-terminus (B; mNramp2 CT), Nramp1 (C; mNramp1 NT), TfR (D), and Bgp1 proteins (E). The positions and sizes (in kilodaltons) of molecular weight markers are shown. The exposure times of (A) and (B) were adjusted to produce a similar reference signal by the two antisera against the protein expressed in transfected CHO cells.
Effect of dietary iron depletion on Nramp protein expression in normal tissues. Organs were harvested from mice maintained on low iron diet (−Fe) or on the same diet but supplemented with iron (+Fe). One hundred micrograms of microsomal membrane fractions isolated from proximal duodenum (I1), distal duodenum (I2), ileum (I3), colon (C), kidney (K), spleen (S), and liver (L) were resolved on a 10% acrylamide gel, transferred to a PVDF membrane, and analyzed by immunoblotting. To control the specificity of the anti-Nramp antibodies, 25 μg of membrane proteins from control CHO cells (CHO) or CHO transfectants expressing either a cMyc-tagged Nramp1 (CHOmN1) or a cMyc-tagged Nramp2 isoform II (CHOmN2) were included. Immunoblotting was performed with antibodies raised against Nramp2 N-terminus (A; mNramp2 NT), Nramp2 C-terminus (B; mNramp2 CT), Nramp1 (C; mNramp1 NT), TfR (D), and Bgp1 proteins (E). The positions and sizes (in kilodaltons) of molecular weight markers are shown. The exposure times of (A) and (B) were adjusted to produce a similar reference signal by the two antisera against the protein expressed in transfected CHO cells.
In mice fed on a normal diet (+ Fe), the anti-Nramp2 antiserum directed against the N-terminus [mN2 (NT)] and recognizing both Nramp2 isoforms detected a low level of Nramp2 protein expression (80 to 90 kD) in the two duodenum fractions (Fig 3A, lanes 1 and 3), as well as in the ileum fraction (Fig 3A, lane 5) and to a higher extent in the kidney (Fig 3A, lane 9). No Nramp2 protein expression was detected in colon (Fig 3A, lane 7), spleen, and liver (Fig 3A, lanes 11 and 13). After prolonged exposure, a low level of Nramp2 expression was also detected in colon, spleen, and liver (data not shown). The specificity of the antiserum was confirmed by the presence of the immunoreactive band seen only in the Nramp2 CHO transfectant (Fig 3A, compare lanes 15 and 16 with lane 17). After diet-induced iron depletion (−Fe), Nramp2 protein expression was dramatically enhanced (by a factor of 50- to 100-fold) in the first part of the small intestine (I1) corresponding to the proximal duodenum (Fig 3A, lane 2). This strong upregulation of Nramp2 protein expression appeared only in the first 2 cm of the duodenum and was not seen in more distal portions of the small intestine (Fig 3A, lanes 4 and 6) or in the colon (Fig 3A, lane 8), spleen (Fig 3A, lane 12), and liver (Fig 3A, lane 14). Two additional immunoreactive species of faster electrophoretic mobility were also detected in the duodenum sample from iron-deprived animals. Because these protein species are not detected in any of the other tissue samples analyzed and because they are of lower apparent molecular mass than that of the predicted peptide backbone of Nramp2, they most likely represent partial degradation products from the mature protein. Nramp2 protein expression was also increased by iron depletion in the kidney (2 independent experiments), but only by a factor of twofold (Fig 3A, lanes 9 v 10). Together, these results identify strong upregulation of Nramp2 protein expression in proximal duodenum in response to dietary-induced iron depletion.
To gain information into which of the two Nramp2 isoforms is upregulated by dietary iron, an immunoblot containing the same membrane fractions was probed with the anti-Nramp2 [mN2 (CT)] antiserum directed against isoform II (Fig 3B). The anti-Nramp2 (CT) antiserum did not detect specific immunoreactive products in any of the tissues analyzed, either under normal or low iron diet conditions. The absence of immunoreactive bands did not appear to result from lack of reactivity of the anti-Nramp2 (CT) antiserum, because a strong signal was seen in membrane fractions from CHO transfectants expressing Nramp2 isoform II (Fig 3B, lane 17). The signal obtained with the anti-Nramp2 CT antiserum in Nramp2 CHO transfectants was adjusted to that produced by the anti-Nramp2 NT antiserum in the same sample (compare lanes 17 in Fig 3A v B) to facilitate comparison between other lanes of the two panels. The strong iron-induced upregulation of Nramp2 in the proximal duodenum, together with the apparent absence of Nramp2 isoform II expression in this tissue, strongly suggest that it is the isoform I of Nramp2 that is expressed and regulated in the proximal intestine. The observed constitutive and iron-inducible expression of Nramp2 in duodenum is consistent with the known site of iron and other divalent cations uptake in the gut.11 13
Additional controls were included (Fig 3C, D, and E). First, expression of Nramp1 was investigated in the same tissue panel, using a protein specific anti-Nramp1 antiserum.51 Nramp1 expression was detected only in spleen microsomal fractions (Fig 3C, lanes 11 and 12), in agreement with the known expression of Nramp1 mRNA in reticuolendothelial organs28 and the known phagocyte-specific expression of the Nramp1 protein.45Nramp1 protein expression in spleen was not influenced by the dietary iron status of the mice (Fig 3C, lanes 11 and 12).
Expression of the transferrin receptor (TfR) was studied in the same samples (Fig 3D). TfR was expressed as an approximately 90-kD species present in the three intestinal segments analyzed, in the colon, in the kidney, and also in the spleen. However, TfR was expressed only at very low levels in liver (Fig 3D, lane 13). Upon iron depletion, a small increase in TfR expression was noted in colon (lanes 7 v 8) and kidney (lanes 9 v 10), whereas clear induction was seen in the liver (lanes 13 v 14).
Finally, a control was included to ascertain that protein degradation during sample preparation did not contribute to differences in Nramp2 protein expression in different tissues and in response to iron depletion. Biliary glycoproteins (Bgps) are a group of surface glycoproteins abundantly expressed in epithelial cells of the gastrointestinal tract, including small intestine, colon, and also liver.54 An anti-Bgp1 antiserum was used to monitor Bgp in the various membrane fractions (Fig 3E). Abundant Bgp expression was seen in all intestinal segments (lanes 1 through 8) as well as in the liver (lanes 13 and 14), whereas low level expression was seen in kidney (lanes 9 and 10) and spleen (lanes 11 and 12). The levels of Bgp in these tissues were not influenced by the dietary status of the mice. This Bgp expression pattern is in agreement with that previously published55-57 and indicates that protein degradation did not contribute significantly to the differential expression of Nramp2 isoforms observed in normal tissues and in response to dietary iron.
Thus, (1) the robust induction of Nramp2 seen in duodenum under low iron diet and (2) the noted increase in TfR expression in liver in response to low iron diet, together with (3) decreased plasma ferritin levels measured in low iron diet animals (128 ± 20 μg/L, mean ± SE of 3 mice), compared with levels measured in control animals (225 ± 34 μg/L, mean ± SE of 3 mice), indicate that the diet used indeed resulted in iron depletion in these mice.
Cellular and subcellular localization of the Nramp2 protein in epithelial cells of the intestinal villi.
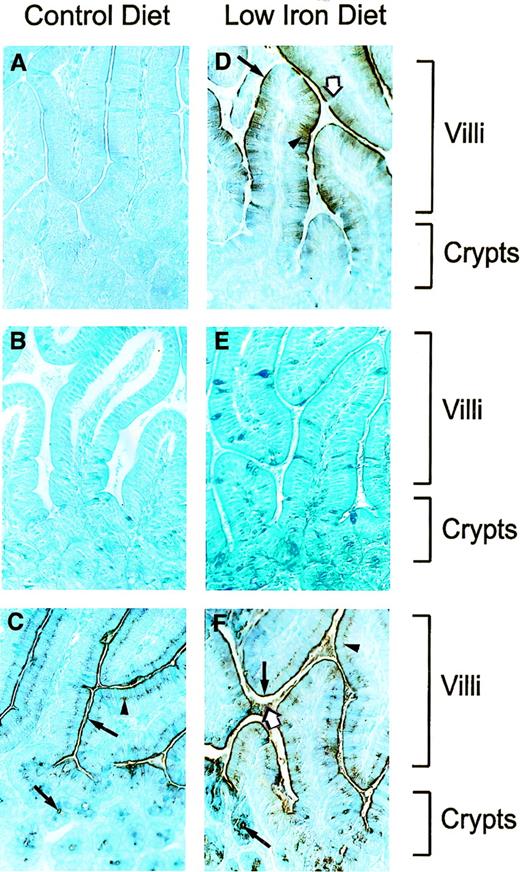
The cellular and subcellular sites of expression of Nramp2 in the proximal portion of the duodenum were investigated by immunostaining of histological sections obtained from mice fed normal or iron-depleted diets. Freshly dissected tissues were fixed in Bouin’s solution and embedded in parafilm, and sections were analyzed with either normal preimmune serum, anti-Nramp2 (NT) antiserum, or anti-Bgp1 antiserum, followed by counterstaining with methylene blue (Fig 4). In mice fed a normal diet, we were unable to detect a positive Nramp2 staining in any of the structures examined (Fig 4A). This was somehow anticipated, considering the relatively low level of Nramp2 noted in these tissues by Western blot analyses (Fig 3, lane 1). However, in tissue sections from mice fed a low iron diet, a very intense Nramp2 immunostaining was observed (Fig4D). This Nramp2 staining was detected in villi only and was absent from the crypts of Lieberkuhn located more proximal and near the muscularis mucosae (Fig 4D). In addition, in several individual villi analyzed, Nramp2 staining seemed to be most intense in the most luminal half of the villi. In villi, staining was restricted to columnar epithelial cells (enterocytes) and was absent from mucus-secreting goblet cells (Fig 4D, white arrow). No Nramp2 staining was evident in the different cell types and structures of the central internal portion of the villus (lamina propria). In epithelial absorptive cells, staining was most intense at the luminal surface of the epithelium, possibly associated with the brush border (microvilli; Fig 4D, arrow). In several villi examined, Nramp2 staining was reduced in a region at the tip of the villi, possibly corresponding to the zone of exfoliating epithelial cells. The Nramp2 staining observed in these sections was specific and was not seen in control sections incubated with preimmune serum (Fig 4E). Staining of additional sections with the anti-Nramp2 (CT) antiserum specific for isoform II failed to detect a signal in intestinal villi (data not shown), in agreement with immunoblotting results (Fig 3B) and strongly suggesting that it is the Nramp2 isoform I that is prominently expressed at that site. Anti-Bgp 1 antibody was used as a positive control on these sections (Fig 4C and F), because it is known that biliary glycoproteins are expressed at the surface of the brush border of epithelial cells of the villi.57 This antibody indeed produced strong immunostaining at the brush border of epithelial cells (Fig 4C and F; villi, arrows) which was similar to that seen for Nramp2 (Fig 4D). Bgp1 was also detected in the supranuclear intracellular region of the epithelial cells (Fig 4C and F, arrowheads) in the luminal space itself (Fig 4F, white arrow), as well as in the crypts of Lieberkuhn (Fig 4C and F, arrows) and in goblet cells of the villi. The latter four staining patterns are clearly distinct from that observed for Nramp2. Finally, Bgp1 staining was independent of iron dietary status of the animals.
Immunohistochemical staining for Nramp2 in the intestine. Tissue sections were prepared as described in Materials and Methods and were immunostained with polyclonal rabbit antimouse Nramp2 (NT) antibody (A and D), preimmune serum (B and E), and polyclonal rabbit anti-mouse Bgp1 antibody (C and F). Sections were counterstained with methylene blue, before microscopic examination and photography. Low magnification (400×) of histological sections of proximal duodenum (I1; Fig 2) from mice fed with a control (A, B, and C) or low iron (D, F, and E) diet. Position of villi and crypts are identified. Arrows in (D) identify Nramp2 brush border staining (arrow), intracellular staining (arrowhead), or negative goblet cells (white arrow). In (C) and (F), arrows identify Bgp1 staining in the brush border and in the crypts (arrow), in the supranuclear intracellular region (arrowhead), and in the lumen (white arrow).
Immunohistochemical staining for Nramp2 in the intestine. Tissue sections were prepared as described in Materials and Methods and were immunostained with polyclonal rabbit antimouse Nramp2 (NT) antibody (A and D), preimmune serum (B and E), and polyclonal rabbit anti-mouse Bgp1 antibody (C and F). Sections were counterstained with methylene blue, before microscopic examination and photography. Low magnification (400×) of histological sections of proximal duodenum (I1; Fig 2) from mice fed with a control (A, B, and C) or low iron (D, F, and E) diet. Position of villi and crypts are identified. Arrows in (D) identify Nramp2 brush border staining (arrow), intracellular staining (arrowhead), or negative goblet cells (white arrow). In (C) and (F), arrows identify Bgp1 staining in the brush border and in the crypts (arrow), in the supranuclear intracellular region (arrowhead), and in the lumen (white arrow).
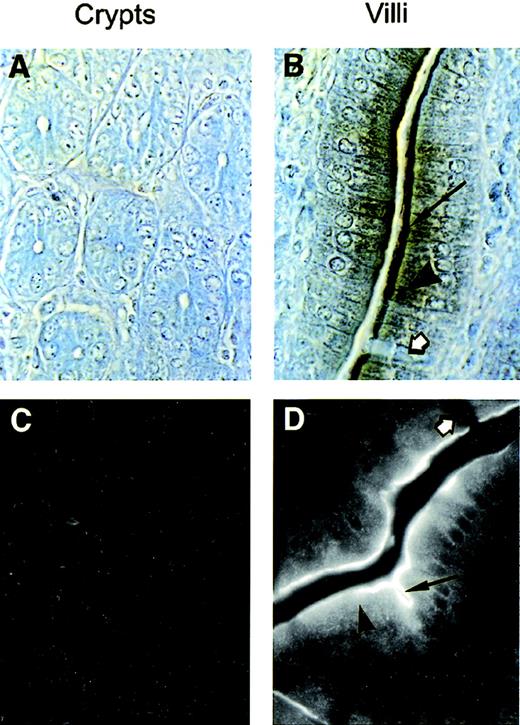
Subcellular localization of Nramp2 was examined further by immunohistochemical staining and by immunofluorescence under higher (1,000×) magnification (Fig 5). Results in Fig 5 confirm that Nramp2 expression is restricted to the villi portion of the intestinal epithelium (Fig 5B and D), whereas both the internal lamina propria of the villi and the crypts of Lieberkuhn are negative for Nramp2 (Fig 5A and C). These high magnification pictures also confirm that only absorptive epithelial cells and not goblet cells (Fig 5B and D, white arrows) express Nramp2. They also show that, although Nramp2 is most concentrated at the brush border of the epithelial cells (Fig 5B and D; arrow), staining can also be observed as a punctate intracellular pattern in the apical half portion of the cells (Fig 5B and D; arrowhead). The latter staining pattern suggests that Nramp2 may also be expressed in a subcellular membranous compartment as well. This staining was not an artifact of the Cy3 fluorophore used and was also observed with a rhodamine-coupled secondary antibody (data not shown) and by immunochemical staining (Fig5B). In addition, this staining was not seen in the crypts (Fig 5C) or in animals fed normal diet (data not shown).
Subcellular localization of Nramp2 in enterocytes. Tissue sections from the proximal duodenum (I1; Fig 2) from mice maintained on a low iron diet were analyzed for Nramp2 expression. Immunochemistry (A and B) or immunofluorescence (C and D) were performed on sections using the anti-Nramp2 NT antibody. Crypts of Lieberkuhn (A and C) and villi (B and C) are shown. Nramp2 staining was observed in the brush border (arrow) and intracellularly (arrowhead), whereas goblet cells (white arrow) and lamina propria remain negative. (Original magnification × 1,000.)
Subcellular localization of Nramp2 in enterocytes. Tissue sections from the proximal duodenum (I1; Fig 2) from mice maintained on a low iron diet were analyzed for Nramp2 expression. Immunochemistry (A and B) or immunofluorescence (C and D) were performed on sections using the anti-Nramp2 NT antibody. Crypts of Lieberkuhn (A and C) and villi (B and C) are shown. Nramp2 staining was observed in the brush border (arrow) and intracellularly (arrowhead), whereas goblet cells (white arrow) and lamina propria remain negative. (Original magnification × 1,000.)
Together, these results establish that, under iron-deplete conditions, Nramp2 is abundantly expressed in the brush border of absorptive epithelial cells of the duodenum.
DISCUSSION
Nramp2 was cloned originally by cross-hybridization to theNramp1 gene.27,28Nramp1 is expressed in macrophages and mutations in this gene cause susceptibility to infection with intracellular parasites.30,51 The Nramp1 protein is an integral membrane phosphoglycoprotein expressed in the lysosomal compartment of macrophages. It is targeted to the membrane of the phagosome after phagocytosis, where it is believed to modify the intravesicular milieu of the phagosome to affect in situ microbial replication.45,46 Nramp1 and Nramp2 share 78% sequence similarity with highly conserved primary sequence motifs and secondary structures (12 TM domains) that define a large protein family conserved throughout the eukaryotic and prokaryotic kingdoms.36Recent studies have indicated that Nramp2 plays an important role in divalent cations transport, including iron. (1) Nramp2 is homologous to the SMF yeast genes family of Mn2+transporters,58 and Nramp2 can functionally complement aSMF1/SMF2 null mutant.48 (2) Nramp2 has also been identified in expression cloning studies in Xenopus laevis oocytes as DCT1, a pH-dependent divalent cation proton cotransporter.25 (3) Nramp2is mutated in two rodent models of hypochromic, microcytic anemia, the mk mouse,22 and the brat.23 The inability to correct the microcytic anemia in mk and b animals by dietary iron19,20 or iron injections21 has suggested that Nramp2 may be involved not only in intestinal iron uptake but also in iron metabolism in peripheral tissues.
Nramp2 mRNA is ubiquitously expressed,27 but is abundant in kidney and intestine25 and is dramatically upregulated by iron starvation in the latter tissue.25 TheNramp2 gene encodes two mRNAs generated by alternative splicing of two 3′ terminal exons.40 The two mRNAs code for two proteins with distinct carboxy termini and also have different 3′ untranslated regions in their respective mRNAs, with one showing an IRE. However, the tissue, cellular and subcellular localization of the Nramp2 protein isoforms I (IRE) and II (non-IRE) have not yet been determined and need to be elucidated to better understand the role of these two proteins in iron metabolism in the intestine and in peripheral tissues. In the current study, we have generated an antiserum against the N-terminus of Nramp2 that is identical in both Nramp2 isoforms and another one against the C-terminus of isoform II (Fig 1A). We show that these antisera are specific and do not cross-react with the closely related Nramp1 protein (Fig 1B). Furthermore, the simultaneous use of these two antisera facilitated the analysis of both isoforms in immunoblotting, immunochemistry, and immunofluorescence studies.
The expression of the two Nramp2 protein isoforms was monitored in a subset of organs and tissues that are important for iron uptake and storage or that were previously known to express Nramp2 mRNA. In tissues and in CHO transfectants controls, Nramp2 was expressed as a broad immunoreactive species of molecular mass 80 to 100 kD. This is larger than that predicted from cDNA sequencing (60 kD). We have recently shown that this is caused by extensive N-linked glycosylation of the protein.59 In general, the isoform II of Nramp2 could not be detected by our antibody in these tissues by immunoblotting (Fig 3) and immunostaining (data not shown). The lack of immunoreactive isoform II expression in gut tissues was somewhat surprising and could be explained by one of three possibilities: (1) isoform II is not expressed in these tissues; (2) it is expressed but only at low levels and our antiserum is not reactive enough to detect it; and (3) it is expressed but posttranslationally modified in such a way that it is no longer recognized by the antiserum. Although the antiserum directed against isoform II is indeed less reactive than that directed against the amino terminus, the following observations suggest that it is clearly not isoform II that is upregulated in the duodenum. First, the anti-isoform II antiserum detects the protein expressed in CHO cells (Figs 1 and 3). Secondly, the antibody recognizes isoform II expressed in other cell types such as macrophages (J774, RAW) and erythroleukemia cells (MEL; F.C.-H., unpublished data). On the other hand, isoform I was detected by our antiserum in membrane fractions from duodenum, proximal intestine, and from kidneys. In addition, expression of isoform I was dramatically upregulated by dietary iron starvation. This regulation was specific for the duodenum and was not seen in other tissues positive for expression (Fig 3). In the duodenum, Nramp2 isoform I is expressed in the mucosa, is localized to the villi, and is absent in the crypts. In villi, it is expressed in the apical two thirds part and is restricted to absorptive epithelial cells (absent in goblet cells), where it is concentrated at the brush border (Figs 4 and 5). Moreover, Nramp2 protein expression was highest in the proximal duodenum and decreased more distally (data not shown). In the rat, it has been shown that DCT1 (Nramp2) mRNA expression is highest in the duodenum and also decreases more distally.25This proximal to distal gradient of Nramp2 expression in the duodenum and the localization of Nramp2 at the brush border of the enterocytes are in agreement with the known physiological site of ferrous iron uptake in the gut, which is mostly limited to the brush border of the proximal intestine.13,14 In addition, the location of the Nramp2 protein restricted to the proximal part of the duodenum is compatible with the demonstrated pH-dependence divalent cation transport by Nramp2,25 in which a relatively acidic pH of the gastric juice60 could provide the gating mechanism for Nramp2 activation. Finally, the localization of Nramp2 to the brush border of the villi is in agreement with a carrier-mediated process for ferrous iron uptake and with the large body of published data documenting the presence of specific iron-binding proteins15,61 and iron transport activities in brush border membrane preparations.11 62
Iron homeostasis is maintained primarily by controlling intestinal iron absorption.4,5 This is a complicated process performed by the enterocyte that involves conversion of iron to a transportable form, transport across the apical membrane of the epithelium, intracellular movement to the basal pole, and transport across the basolateral membrane and ultimately across endothelial cells into the blood stream.63 Thus, iron has to traverse several biological barriers in the form of cell membranes to which it is fairly impermeant, and the carrier-mediated process seems to play a key role in this journey. For example, in the unicellular eukaryoteSaccharomyces cerevisiae (yeast), up to 6 distinct transport activities for iron have been functionally identified,64 and there is no reason to believe that this number would be lower in mammals. In the duodenal lumen, ferric iron is converted to ferrous iron by the action of ferrireductase.11 Conversion of Fe(III) to Fe(II) has been shown to be a prerequisite for intestinal iron absorption by mice and humans,10 and inhibition of ferrireductase in Caco-2 cells reduces iron transport.11,65 The transport of iron across the apical pole of the enterocyte has generally been believed to be a carrier-mediated process.61,66 Teichmann and Stremmel67 have described a saturable, temperature-dependent, iron transport activity (FeIII) in brush border membrane vesicles. These investigators were able to isolate a putative iron transporter as a membrane glycoprotein of 160 kD, and composed of a trimer of a 54-kD monomeric unit.67 More recently, Ikeda-Moore et al15 described low- and high-affinity binding sites for Fe(II) present in solubilized brush border membrane (BBM) of rat intestine and isolated three proteins (143, 100, and 77 kD) with iron binding activity. The microcytic anemia phenotype of rat and mouse mutants at Nramp222,23 and the known biochemical properties of Nramp2,25 together with the localization of Nramp2 to the brush border of the duodenum presented in this report, clearly establish that Nramp2 is the membrane transporter responsible for apical iron entry into enterocytes. Nramp2 is specific for Fe(II) and does not use Fe(III) as a substrate,25suggesting that Nramp2 only transports Fe(II) at that site and is thus functionally linked to ferrireductase activity in the lumen. The proton gradient (with exterior positive) required for activation of Nramp2 and for the proton cotransport of iron25 by Nramp2 may be provided by the relatively acidic environment of the proximal portion of the duodenal lumen.
The intracellular trafficking of iron in enterocytes to reach the basolateral membrane is the least understood aspect of iron uptake. It has been suggested that, once released in the cytoplasm, Fe(II) may bind to specific chelators or iron chaperones to shuttle to the baso-lateral membrane.64 Conrad et al68 have proposed a model in which iron would bind to mucin in the intestinal lumen, then transfer to integrin at the cell surface, and then transfer to the intracellular protein mobilferrin. However, a precise role for these proteins in iron transport has yet to be demonstrated. The transport of iron across the basolateral membrane is most likely a carrier-mediated process. Indeed, studies in everted membrane vesicles from duodenum of the mouse mutant sla (sex-linked anemia) indicate that mucosal acquisition of iron is normal in this mutant, whereas iron export from these cells would be impaired.69,70 The recent chromosomal assignment of thesla mutation,71 together with the noted absence of Nramp2 expression at the basolateral membrane, suggests that Nramp2 is not involved in this process. Furthermore, biochemical and genetic studies in copper-deficient animals72,73 have pointed at the serum copper-containing protein ceruloplasmin (ferroxidase activity) as an important component of iron release from the enterocyte.74 Recently, Vulpe et al75 have identified a gene in the sla region that codes for a protein presenting 50% sequence similarity with the mouse ceruloplasmin, including conservation of multiple copper binding sites. This protein is highly expressed in mouse intestine. Interestingly, in slamice, this gene shows an in-frame deletion of 194 amino acids, probably resulting in a nonfunctional product. The investigators conclude that this new protein is a transmembrane-bound ferroxidase necessary for iron export from the intestine.
Finally, the iron overload syndrome hereditary hemochromatosis (HH) has been shown to be caused by a mutation in a novel HFE gene (HLA-H), a member of the major histocompatibility complex (MHC) class I family.76 This syndrome is characterized by a defect in regulation of iron absorption that, ultimately, leads to iron deposition in several organs.77 A similar phenotype has been observed in a mouse mutant bearing a null allele at the β2-microglobulin locus, and β2M−/− mice have been proposed as an animal model for the study of HH. Recent studies78 have suggested that the defect observed in β2M−/− mice (inability to downregulate intestinal iron absorption) could reflect a regulatory role of β2M/HFE on Nramp2 or on the sla gene product or alternatively on the activity or expressivity of ferrireductase.
We have noted dramatic upregulation of the Nramp2 protein expression in the duodenal mucosa by dietary iron. These findings are in agreement with the upregulation of Nramp2 RNA previously detected by Northern blotting in the same tissue.25 This dramatic upregulation of Nramp2 mRNA and protein may occur through the presence of the IRE element in the 3′ UTR of the Nramp2mRNA or alternatively may be caused by transcriptional activation of the gene. We do not have direct evidence to support either, and discussion of this regulation can only be speculative at this time. However, many mRNAs encoding proteins involved in metabolism of iron contain IREs in their UTRs that mediate changes in protein levels in response to iron availability through posttranscriptional mechanisms.43 Under conditions of low iron availability, IRE-binding proteins are available for binding to IREs. Binding of proteins to IREs in the 5′UTR of mRNAs such as that encoding ferritin causes a decreased translation of the message. Conversely, protein binding to IREs in the 3′UTR of mRNAs like the one encoding the transferrin receptor causes an increased half-life of the mRNA. This allows for the coordinate regulation of iron uptake (TfR) and storage (ferritin) in response to iron availability. Interestingly, we note that the iron depletion protocol used here had no effect on TfR expression in the intestine, whereas it caused upregulation only in liver. The lack of TfR regulation by dietary iron in the intestine is surprising but has been previously reported.79 Nevertheless, we must consider that the IRE present in Nramp2 mRNA may function to regulate expression of Nramp2 protein in response to iron. This is strongly suggested by the observation that it is the Nramp2 isoform I that is upregulated in the duodenal mucosa (Fig 3) and by the recent report that both IRP1 and IRP2 bind to the hairpin structure in the 3′end of the Nramp2 IRE mRNA.80 Gunshin et al25 have observed a gradient of Nramp2 mRNA expression in the intestinal mucosa, from high in the crypts and at the base of the villi, to progressive attenuation of expression towards the distal part of the villi, to complete absence of expression in the distal third of the villi. On the other hand, we have observed somewhat of an opposite pattern of Nramp2 protein expression, with no expression in the crypts and at the base of the villi, and increasing expression from the proximal half to the distal half of the villi (Fig 3). This suggests that Nramp2 mRNA expression may be turned on in precursor cells of the crypts in response to iron starvation. The mRNA continues to accumulate during maturation of the cells along the structure of the villus, at which point Nramp2 isoform I protein begins to accumulate to become maximum in the most mature cells of the villi. Interestingly, Oates et al81 reported a similar observation for ferritin gene expression in intestine of rats with varying iron stores. In iron-deficient and iron-loaded rats, ferritin mRNA was seen at highest levels in the epithelial cells of the crypt and at lower levels in villus epithelial cells. However, the ferritin protein was not seen in the crypt region but was seen with increasing staining in the apical two thirds of the villus cells of control and iron-loaded rats, but not in iron-deficient rats. Therefore, it is tempting to speculate that expression of ferritin and Nramp2 genes and proteins may be regulated in somewhat opposite manner in response to body iron level but through a common mechanism. In undifferentiated crypt cells, the Nramp2 and ferritin genes are transcribed, but the messages may not be translated. During and after differentiation of the enterocytes, Nramp2 and ferritin expression could then be regulated posttranslationally in response to the size of iron stores.
Finally, we noted only a modest increase in Nramp2 isoform I expression in the kidney, in response to depletion of dietary iron. This suggests that some component of the IRE or other regulatory pathway required for Nramp2 isoform I expression is missing in kidney tissue or that only a subset of Nramp2 positive cells in the kidney are responsive to iron regulation and, consequently, Nramp2 isoform I overexpression. In addition, we cannot exclude the possibility that regulation ofNramp2 mRNA expression may be at the transcriptional level in the kidney and does not involve participation of the IRE element itself. Finally, Nramp2 has been shown to transport a number of divalent cations in addition to iron. Thus, it is also possible that regulation of Nramp2 protein expression in the kidney involve additional mechanisms associated with metabolisms of these other cations. The answers to these questions await the identification of the cell type and subcellular localization of the Nramp2 protein in kidney and further characterization of its transcriptional or posttranscriptional regulation.
ACKNOWLEDGMENT
The authors are indebted to Dr N. Beauchemin (McGill University) for the generous gifts of the anti-Bgp1 antiserum and to Dr E. Roux (McGill University) for helpful advice and guidance in the immunochemistry studies.
Supported by a research grant to P.G. from NIAID (Grant No. AI35237). S.G. is supported by a studentship from the Medical Research Council of Canada (MRC). P.G. is an International Research Scholar of the Howard Hughes Medical Institute and is a senior scientist of the MRC.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to P. Gros, PhD, Department of Biochemistry, McGill University, 3655 Drummond, Room 907, Montreal, Quebec, Canada H3G-1Y6; e-mail: gros@med.mcgill.ca.

![Fig. 1. Detection of the Nramp1 and Nramp2 cMyc-tagged proteins in CHO cell membranes. (A) Schematic representation of the two mRNAs corresponding to the IRE-containing form and to the non-IRE form ofNramp2 generated by alternative splicing of a 3′ end exon (see Lee et al40). The two mRNAs harbor distinct 3′ untranslated region (3′UTR) and encode two polypeptides with distinct carboxy termini (hatched boxes). The mN2 (NT) antibody was raised against an amino terminal segment of Nramp2 that is common to the two Nramp2 protein isoforms (solid boxes). The mN2 (CT) antibody was raised against the carboxy terminal extremity of the protein encoded by the Nramp2 non-IRE mRNA (Nramp2 isoform II). (B) Crude membrane extracts from untransfected CHO cells (lanes 1, 3, 5, 7, 9, 11, 13, and 15), from Nramp1 transfectants (lanes 10, 12, 14, and 16), and from Nramp2 isoform II transfectants (lanes 2, 4, 6, and 8) were separated by SDS-PAGE on a 10% acrylamide gel followed by transfer to PVDF membranes. Immunodetection was with affinity-purified mNramp1 [mN1 (NT); lanes 1, 2, 9, and 10], mN2 (NT; lanes 3, 4, 11, and 12), and mN2 (CT; lanes 5, 6, 13, and 14) antibodies and monoclonal anti-cMyc antibody 9E10 (lanes 7, 8, 15, and 16), as described in Materials and Methods. The positions and sizes (in kilodaltons) of molecular weight markers are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/12/10.1182_blood.v93.12.4406/4/m_blod41221001w.jpeg?Expires=1770545347&Signature=J7rU4lDVOjllMPT1MRGdBX0pLszc5ja0RfUM76wADrRRv3kufgTZerraGdNBgKr3moCEcyWuln30aCxjMykLVQ7XHIxGA4JTEqRe9maTJyrYN9a8yAqlOpXaffrnCBkNiF9UBzUAdzilJeJLhCxtZwkDEkJ1Ol8oFJHyQDFYY7VBJhlyXVvVtVBxZZx26ILPk5KYmtV6WjVTeyPVSvl~-mtI0FTo9naaVeOkao7EbNaGi6RNFtly15PKmg26x4Apbmc3ue1wY~ScWSsXJHY8b5Wf76m2iX98Y3Nc~wMtNhkzEdDAJ2rEmYiG8zwzfJpxhpMcQfmHLndUPHRMzM00qQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal