Abstract
Calcium is accumulated from the cytosol into the endoplasmic reticulum by sarco-endoplasmic reticulum calcium transport ATPase (SERCA) enzymes. Because calcium stored in the endoplasmic reticulum is essential for cell growth, differentiation, calcium signaling, and apoptosis and because different SERCA enzymes possess distinct functional characteristics, in the present report we explored SERCA expression during in vitro differentiation of the human myeloid/promyelocytic cell lines HL-60 and NB4 and of freshly isolated acute promyelocytic leukemia cells. Two SERCA species have been found to be coexpressed in these cells: SERCA 2b and another isoform, SERCAPLIM, which is recognized by the PLIM430 monoclonal antibody. Induction of differentiation along the neutrophil granulocytic lineage by all-trans retinoic acid or cyclic AMP analogs led to an increased expression of SERCAPLIM, whereas the expression of the SERCA 2b isoform was decreased. The modulation of SERCA expression was manifest also on the mRNA level. Experiments with retinoic acid receptor isoform-specific retinoids indicated that SERCA expression is modulated by retinoic acid receptor -dependent signaling. SERCA expression of retinoic acid-resistant cell variants was refractory to treatment. Differentiation along the monocyte/macrophage lineage by phorbol ester resulted in an increased expression of both SERCA isoforms. In addition, when cells were treated by phorbol ester in the presence of the glucocorticoid dexamethasone, a known inhibitor of monocyte differentiation, a selective blockage of the induction of SERCAPLIM was observed. Altered SERCA expression modified the functional characteristics of calcium transport into the endoplasmic reticulum. These observations show for the first time that the modulation of calcium pump expression is an integral component of the differentiation program of myeloid precursors and indicate that a lineage-specific remodelling of the endoplasmic reticulum occurs during cell maturation. In addition, these data show that SERCA isoforms may serve as useful markers for the study of myeloid differentiation.
ACCUMULATION OF CALCIUM ions from the cytosol into the endoplasmic reticulum (ER) is accomplished by various sarco-endoplasmic reticulum calcium transport ATPase (SERCA) enzymes.1 Because calcium stored in the endoplasmic reticulum is required for second messenger-induced calcium mobilization2-4 as well as for the posttranslational processing of nascent proteins in the ER lumen,5,6 calcium pumping into this organelle is essential for a large array of cell functions. The direct inhibition of SERCA activity by cell permeable drugs such as thapsigargin can induce cell activation leading to differentiation,7,8 growth arrest,9apoptosis,10-15 or enhanced human immunodeficiency virus (HIV) production,16 depending on cell type, indicating that SERCA activity represents an important control mechanism of various types of cell activation. Direct functional association of a SERCA enzyme with Bcl-2, resulting in the modulation of the apoptotic potential of the cell, has also been reported.17
The expression and alternative RNA splicing of the three known human SERCA genes is tissue-dependent and developmentally regulated. The SERCA 1a and 1b isoenzymes are expressed in adult and neonatal skeletal muscle, respectively.18 Whereas SERCA 2a is expressed in cardiac muscle, SERCA 2b has been found ubiquitously in all nonmuscle cell types studied so far.19,20 Cells of hematopoietic origin coexpress SERCA 2b, recognized by the IID8 antibody and another SERCA-type calcium pump, termed SERCAPLIM, recognized by the PLIM430 monoclonal antibody.21,22 Recent data on the tissue distribution of SERCA 3 mRNA23,24 and analysis of the recognition pattern by PLIM430 of recombinant SERCA 3 proteins25 suggest that SERCAPLIM corresponds to an alternatively spliced SERCA 3 variant, the SERCA 3b isoform. Interestingly, and in accordance with the presence of a GATA motif in the SERCA 3 promoter,26 the expression of the SERCAPLIM enzyme appears to be restricted to the hematopoietic lineage.21 In addition, the expression levels of the two coexpressed SERCA isoenzymes vary depending on blood cell type,21 and the two enzymes are associated with functionally distinct subcompartments of the ER27 and possess distinct biochemical and pharmacological characteristics such as calcium affinity24 or sensitivity to inhibitors.21 Taken together, these observations suggest that the two SERCA enzymes play functionally specialized, distinct roles within the same cell.
Myeloid differentiation is accompanied by the acquisition of new signaling, as well as effector functions, such as responsiveness to bacterial endotoxin, to chemotactic peptides, or to growth factors and chemokines, phagocytosis, or respiratory burst formation. Given that cellular calcium homeostasis and calcium-dependent signaling are intimately involved in these processes,28-30 intracellular calcium transport may be significantly remodelled during differentiation.
The NB4 promyelocytic31 and HL-60 myeloblastic32 leukemia cell lines offer a very useful model to study in vitro myeloid differentiation. Upon treatment with all-trans retinoic acid (ATRA), dimethylsulfoxide (DMSO), or cAMP analogs, these cells readily undergo terminal neutrophil granulocytic differentiation.31-35 ATRA exerts a wide range of effects on cell proliferation and differentiation.36These activities are mediated by at least two distinct classes of nuclear receptors37-39: the retinoic acid receptors (RARs), which include RARα, RARβ, and RARγ; and the retinoid X receptors (RXRs), which include RXRα, RXRβ, and RXRγ. RARs display high affinity towards ATRA as well as 9-cis-retinoic acid,38 whereas RXRs have 9-cis-retinoic acid as a natural ligand.40 After the binding of retinoids, RAR and RXR homodimers or heterodimers regulate the expression of specific genes, coding for proteins involved in differentiation and/or growth arrest.41 In basal conditions, NB4 and HL-60 cells express RARα and RXRs, whereas expression of RARβ and RARγ is not observed.38,39,42 ATRA-induced differentiation of HL-60 is essentially mediated by RARα,35 whereas in NB4 cells, which have been derived from an acute promyelocytic leukemia (APL) patient,31 differentiation may also be mediated by the PML-RARα oncogenic protein.43 In NB4 or fresh APL cells, ATRA administered at pharmacological concentrations (10−6 mol/L) circumvents the differentiation blockage and induces the maturation of the cells along the granulocytic lineage.44 The maturation of NB4 cells can be induced also by other cyto-differentiating agents, such as cell permeable cAMP analogs.34 This cell line represents an in vitro model generally predictive for the behavior of freshly isolated APL blasts upon culturing with ATRA, retinoic acid derivatives, or other compounds. This finding is of clinical importance, because differentiation therapy using retinoids is a very successful clinical modality in the treatment of APL in vivo.44,45 However, ATRA monotherapy may be complicated by the emergence of a differentiation-resistant malignant cell population.46 47
In addition to their granulocytic differentiation potential, phorbol ester treatment induces a monocyte/macrophage-like phenotype in HL-60 cells12,29,48,49 that can be further modulated by glucocorticoids.50-52 This bilineage differentiation potential and the availability of various ATRA-resistant clonal derivatives53-56 of HL-60 and NB4 represent an interesting in vitro system for the study of phenotypic changes occuring upon drug-induced myeloid differentiation.
To gain insight into the involvement of SERCA enzymes in myeloid differentiation, in differentiation-induction therapy of APL, and in ATRA resistance, in the present report we investigated the expression of the two SERCA isoforms during in vitro differentiation of HL-60 and NB4 cells and their differentiation-defective variants and of primary APL cells.
MATERIALS AND METHODS
Cells.
HL-60 cells57 were obtained from the ATCC (Rockville, MD). The ATRA-resistant HL-60 variant53 was a generous gift of Dr Robert Gallagher (Montefiore Medical Center, Bronx, NY). NB4 cells, as well as the NB4-R1 and R2 variants, were described previously.31 54-56 All cells were grown in RPMI-1640 medium (GIBCO-BRL Paisley, UK) supplemented with glutamax-I, 2 mmol/L glutamine, and 10% heat-inactivated fetal calf serum at 37°C in a humidified atmosphere containing 5% carbon dioxide.
Chemicals.
ATRA, phorbol 12-myristate 13-acetate (PMA), 8-(4-chlorophenylthio)-adenosine 3′:5′-cyclic monophosphate (CTP-cAMP), dexamethasone, and nitroblue tetrazolium (NBT) were purchased from Sigma-Aldrich (St Louis, MO). TTNPB, Ro 41-5253, and Ro 61-8432 were kindly provided by Dr M. Klaus (Hoffman-la Roche, Basel, Switzerland). AM580 and CD2019 were synthetized by CIRD-Galderma (Sophia Antipolis, Valbonne, France). SR 11237 was kindly provided by Dr H. Gronemeyer (IGBMC, Strasbourg, Marseille, France). The Bear-1 and the BU15 monoclonal antibodies directed against CD11b (integrin αM subunit) and CD11c (integrin αX-chain), respectively, as well as isotype-matched control antibody and fluorescein-conjugated antimouse IgG were obtained from Coulter/Immunotech (Marseille, France) and were used for flow cytometry according to the instructions of the manufacturer. The IID8 anti-SERCA 2 antibody was purchased from BioMol (Plymouth Meeting, PA) and the PLIM430 antibody was purified from hybridoma supernatant by protein-A chromatography.
Induction of cell differentiation.
Before the experiments, exponentially growing cells were harvested and resuspended in the above-described medium at a density of 2 × 105 cells/mL. Retinoids, PMA, or dexamethasone were added to the cells from concentrated stock solutions in DMSO. The amount of DMSO vehicle added to the cells did not exceed 0.1%, was included in control experiments, and did not interfere with the assays. In experiments in which retinoic acid was used in combination with antagonists, these were preincubated with the cells for 1 hour before the addition of retinoic acid. Differentiation of cells was assessed by measuring NADPH-oxydase activity using NBT in the presence of PMA over a period of 30 minutes at 37°C (as described earlier58), by staining for naphtyl-acetate esterase using a commercially available kit obtained from Sigma-Aldrich, and by light microscopy of May-Grünwald-Giemsa–stained cytospin preparations. Fluorescence-activated cell sorting (FACS) analysis of CD11b and CD11c antigen expression of differentiating cells was performed on a FACS-Calibur flow cytometer (Becton Dickinson, Mountain View, CA) by standard protocols.
Primary APL cells.
After we received informed consent, primary APL cells were obtained by Ficoll gradient centrifugation from bone marrow aspirates of 5 newly diagnosed APL patients presenting an initial percentage of blasts of more than 80%. Cells were resuspended in complete medium at a density of 106 cells/mL and treated for 1 week with 0.1 μmol/L ATRA. Differentiation of the cells was evaluated by NBT reduction and by microscopical examination. The presence of the t(15;17) translocation in the cells was confirmed by reverse transcriptase-polymerase chain reaction (RT-PCR) amplification of the PML-RARα fusion transcript.59
Immunoblotting.
After treatment cell counts and viabilities were determined, cells were washed once with PBS, resuspended in cold 5% trichloroacetic acid, and kept at 4°C for 1 hour. The precipitate was then centrifuged for 15 minutes at 12,000g at 4°C, the pellet was dissolved in electrophoresis sample buffer as described,60 and the protein concentration of the lysate was determined. Five microliter to 25 μL samples containing 20 μg cellular protein per well were run on sodium dodecyl sulfate (SDS)-polyacrylamide gels, electroblotted onto nitrocellulose, and immunostained as described previously.60 Luminograms were quantitated using an LKB laser densitometer.60
RNA isolation and RT-PCR.
Total RNA was isolated from cells using the RNAPlus solution according to the manufacturers’ instructions (Quantum Bioprobe, Montreuil sous Bois, France). Reverse transcription of 500 ng total RNA was performed essentially as described,23 with the modifications as follows: RT reaction was used as template for PCR reaction in a 50 μL reaction mixture including PCR buffer, 2 or 1.5 mmol/L MgCl2 (for SERCA 2b or SERCA 3b, respectively), 0.15 μmol/L primers, and 1.5 U of AmpliTaq DNA polymerase. The reaction was heated to 94°C for 3 minutes and 10 cycles of touchdown PCR were performed as described previously61 to increase the specificity of priming during the initial cycles of amplification. PCR was thereafter performed as described23 for 18 and 20 amplification cycles for SERCA 2b and SERCA 3b, respectively. One amplification cycle consisted of 1 minute at 94°C; 1 minute at 55°C or 58°C for SERCA 2b and SERCA 3b, respectively; and 1 minute at 72°C. The last extension step at 72°C was performed for 7 minutes. As an internal control, RT-PCR amplification of glyceraldehyde 3-phosphate dehydrogenase (G3PDH) was performed. The following primers were used: SERCA 2b: forward primer, 5′ TCA TCT TCC AGA TCA CAC CGC T 3′ located at nt 2861-2882, and reverse primer, 5′ TCA AGA CCA GAA CAT ATC GC 3′, corresponding to the inverse complementary sequence of nt 3110-3129 of the human SERCA 2b sequence19; and SERCA 3b: forward primer, 5′ GAG TCA CGC TTC CCC ACC ACC 3′ located at nt 2674-2694, and reverse primer, 5′ GGC TCA TTT CTT CCG GTG TGG TC 3′, corresponding to the nucleotide stretch located at nt 3058-3080 of the human SERCA 3b sequence.26 Amplification products were separated on 1.5% agarose gels, blotted onto Hybond Z+ nylon membranes (Quantum Bioprobe), and visualized by Southern blotting using the Amersham ECL 3′-oligolabeling and detection system according to the instructions of the manufacturer (Amersham, Little Chalfont, UK). The following oligonucleotide probes were used for Southern detection: SERCA 2b: nt 2956-2992 of the human SERCA 2 cDNA sequence19; SERCA 3b: nt 3017-3057 of the human SERCA 3 cDNA sequence26; and G3PDH: nt 328-357 of the human cDNA sequence.62 Prehybridization (30 minutes) and hybridization (2 hours) were performed at 42°C with 5 ng/mL labeled oligonucleotide probes. Membranes were then washed twice at 50°C in 0.5× SSC, 0.1% SDS for 15 minutes and chemiluminescent signal was detected with Hyperfilm ECL (Amersham). The molecular mass of the obtained amplification products corresponded to that calculated based on the published sequences. Moreover, the identity of the SERCA amplification products was also confirmed by direct sequencing (performed by Eurogentec, Seraing, Belgium).
Membrane preparation and calcium transport.
HL-60 cells grown for 4 days in the presence or absence of 1 μmol/L ATRA were harvested by centrifugation, washed once with 160 mmol/L KCl, 17 mmol/L HEPES-K (pH 7.0), and lysed by 100 strokes in a teflon-glass homogenizer on ice in a lysis buffer containing 10 mmol/L KCl, 10 mmol/L HEPES-K (pH 7.0), 50 μmol/L EDTA, 50 μmol/L EGTA, 100 μmol/L dithiothreitol, 0.1 mg/mL aprotinin, 0.1 mg/mL Bowman-Birk trypsin-chymotrypsin inhibitor, 0.2 mg/mL soybean trypsin inhibitor, 0.125 mg/mL leupeptin, and 0.05 mg/mL pepstatin-A. The cell lysate was centrifuged at 1,600g for 10 minutes at 4°C. Supernatant was then centrifuged at 100,000g for 1 hour at 4°C. The obtained pellet was resuspended in a buffer containing 30 mmol/L KCl, 17 mmol/L HEPES-K (pH 7.0), and 0.2 mmol/L dithiothreitol, aliquoted, frozen immediately in liquid nitrogen, and kept at −80°C. Calcium influx into membrane vesicles prepared from control and ATRA-treated HL-60 cells was measured at 37°C for 3 minutes by rapid filtration, as described earlier.27 The transport medium contained 200 μg membrane protein per milliliter, 119 mmol/L KCl, 43 mmol/L HEPES-K (pH 7.2), 2 mmol/L MgCl2, 1 mmol/L dithiothreitol, 0.01 mg/mL aprotinin, 0.01 mg/mL leupeptin, 100 μmol/L CaCl2 (labeled with45Ca2+), and 110 μmol/L EGTA (to obtain 1.3 μmol/L free Ca2+ concentration). The free calcium concentration was superior to the calcium affinities of both SERCA 2 and SERCA 3 enzymes (0.2 and 1.1 μmol/L, respectively),63permitting the simultaneous measurment of calcium transport activity of both enzymes. Under these conditions, Ca2+ uptake by HL-60 membrane vesicles was linear for 6 to 8 minutes. To measure Ca2+ uptake by SERCAPLIM, the membrane vesicles were preincubated with 30 μg/mL purified PLIM430 antibody (which inhibits the transport activity of SERCAPLIMselectively27,64) for 30 minutes before initiating Ca2+ uptake by the addition of 0.5 mmol/L ATP, as described.27 Ca2+ uptake by contaminating plasma membrane-type calcium pumps was determined by preincubating the vesicles at 37°C for 10 minutes with 1 μmol/L thapsigargin (an inhibitor of total SERCA transport activity that is inactive on plasma membrane-type calcium pumps) before the addition of ATP. To calculate the total SERCA-dependent Ca2+ uptake, thapsigargin-resistant Ca2+ uptake was substracted from total Ca2+ uptake values.
Experiments shown here were performed three or more times (specified in the figure legends) and are presented as the means ± standard error of the mean (SEM).
RESULTS
Time course of the modulation of SERCA expression in ATRA-treated HL-60 cells.
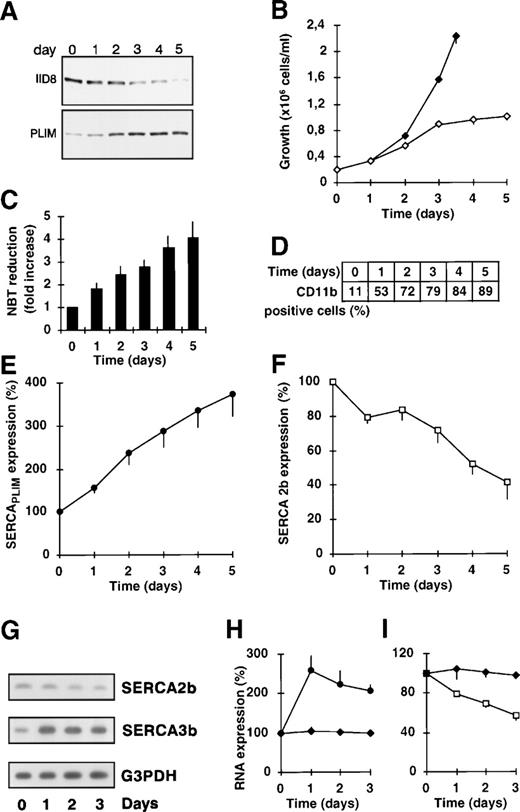
As shown in Fig 1A, E, and F, treatment of HL-60 cells with 1 μmol/L ATRA for 5 days resulted in an approximately fourfold (3.72- ± 0.46-fold; n = 7) overexpression of SERCAPLIM and a concomitant downregulation (to 41.6% ± 8.7%; n = 7) of the expression of the SERCA 2b isoform, as detected by the PLIM430 and the IID8 antibodies, respectively. During this treatment, and in accordance with data in the literature,35the cells underwent terminal granulocytic differentiation as reflected by the aquisition of NADPH oxidase activity measured by NBT reduction (Fig 1C), induction of CD11b expression (Fig 1D), appearence of U-shaped cell nuclei and accumulation of granules in the cytosol (not shown), and growth arrest (Fig 1B). Similar results were obtained on SERCAPLIM expression also in NB4 cells (see later).
Time course of the modulation of calcium pump expression in ATRA-treated HL-60 cells. HL-60 cells were treated with 1 μmol/L ATRA during 5 days and calcium pump expression was determined by discriminating monoclonal antibodies. In parallel, cell differentiation was detected by NBT reduction, CD11b expression, and growth arrest. (A) Immunostaining for SERCA 2b and SERCAPLIM with the IID8 and PLIM430 antibodies, respectively. (B) Inhibition of cell proliferation by ATRA. (◊) ATRA-treated cells; (⧫) untreated cells. (C) NADPH oxidase activity of the cells measured by NBT reduction. Data presented are the mean ± SE of 7 experiments. (D) Induction of CD11b expression by ATRA-treated cells. (E and F) Densitometric analysis of SERCAPLIM and SERCA 2b expression, respectively. (G, H, and I) Estimation of the relative abondance of SERCA mRNA species in HL-60 cells during ATRA-induced differentiation. RNA was isolated from HL-60 cells treated with 1 μmol/L ATRA, and SERCA mRNA was amplified by RT-PCR using isoform-specific oligonucleotide primers. As an internal control, RT-PCR using G3PDH-specific primers was used. (H) (•) SERCA 3b. (I) (□) SERCA 2b; (⧫) G3PDH. Data presented are the mean ± SEM of 3 experiments.
Time course of the modulation of calcium pump expression in ATRA-treated HL-60 cells. HL-60 cells were treated with 1 μmol/L ATRA during 5 days and calcium pump expression was determined by discriminating monoclonal antibodies. In parallel, cell differentiation was detected by NBT reduction, CD11b expression, and growth arrest. (A) Immunostaining for SERCA 2b and SERCAPLIM with the IID8 and PLIM430 antibodies, respectively. (B) Inhibition of cell proliferation by ATRA. (◊) ATRA-treated cells; (⧫) untreated cells. (C) NADPH oxidase activity of the cells measured by NBT reduction. Data presented are the mean ± SE of 7 experiments. (D) Induction of CD11b expression by ATRA-treated cells. (E and F) Densitometric analysis of SERCAPLIM and SERCA 2b expression, respectively. (G, H, and I) Estimation of the relative abondance of SERCA mRNA species in HL-60 cells during ATRA-induced differentiation. RNA was isolated from HL-60 cells treated with 1 μmol/L ATRA, and SERCA mRNA was amplified by RT-PCR using isoform-specific oligonucleotide primers. As an internal control, RT-PCR using G3PDH-specific primers was used. (H) (•) SERCA 3b. (I) (□) SERCA 2b; (⧫) G3PDH. Data presented are the mean ± SEM of 3 experiments.
Estimation of SERCA mRNA in ATRA-treated HL-60 cells.
Recent data in the literature indicate that SERCAPLIMrecognizes the SERCA 3b splice variant, which is expressed preferentially in cells of hematopoietic origin. To estimate the modulation of SERCA mRNA levels during myeloid differentiation, we designed RT-PCR systems for the amplification of SERCA 2b and SERCA 3b and compared the relative mRNA levels in control and ATRA-treated HL-60 cells. As shown Fig 1G, H, and I, whereas SERCA 2b mRNA levels progressively decreased to 50% of the control value during ATRA treatment, SERCA 3b mRNA was induced approximately 2.5-fold. These data indicate that the modulation of SERCA expression during ATRA-induced differentiation of HL-60 cells is controlled, at least in part, on the transcriptional level. As an internal control, G3PDH was also amplified. mRNA levels of this constitutively expressed housekeeping enzyme did not change significantly during the treatments. Whereas SERCA 2b levels decreased steadily during the treatment, SERCA 3b mRNA peaked at day 1 posttreatment and remained elevated thereafter. The differences between the time course of the induction of SERCA 3b mRNA and protein are probably due to the combined effect of time required for mRNA translation and posttranslational processing of nascent SERCA 3b, to differences of half lives of mRNA and protein, and to the fact that, during treatment, cells assume growth arrest, permitting accumulation of newly formed SERCA protein within the cell.
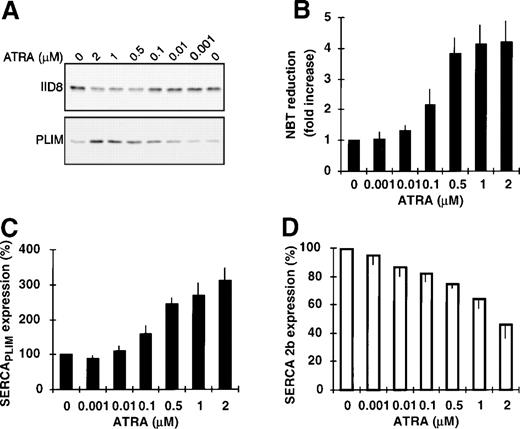
Concentration-dependent modulation of SERCA expression by ATRA and cAMP.
HL-60 cells were exposed for 4 days to various concentrations of ATRA and SERCA expression was determined by immunoblotting. In agreement with in vitro as well as in vivo data in the literature and as determined by NBT reduction here (Fig 2B), cell differentiation was induced at submicromolar to low micromolar concentrations of ATRA. This result was accompanied by the induction of the expression of SERCAPLIM (Fig 2A and C) and the downmodulation of the expression of SERCA 2b (Fig 2A and D) in the same concentration range. Retinoic acid treatment did not modify expression levels of either SERCA isoform in the ATRA-resistant65K-562 myelogenous leukemia cells (not shown).
Concentration dependence of ATRA-induced modulation of calcium pump expression. HL-60 cells were treated for 4 days with various concentrations of ATRA and calcium pump expression; NBT reduction by the cells was also determined. (A) Immunostaining for SERCA 2b and SERCAPLIM with the IID8 and PLIM430 antibodies, respectively. (B) NADPH oxidase activity of the cells as measured by NBT reduction. (C and D) Densitometric analysis of SERCAPLIM and SERCA 2b expression. Data represent the mean ± SEM of 5 experiments.
Concentration dependence of ATRA-induced modulation of calcium pump expression. HL-60 cells were treated for 4 days with various concentrations of ATRA and calcium pump expression; NBT reduction by the cells was also determined. (A) Immunostaining for SERCA 2b and SERCAPLIM with the IID8 and PLIM430 antibodies, respectively. (B) NADPH oxidase activity of the cells as measured by NBT reduction. (C and D) Densitometric analysis of SERCAPLIM and SERCA 2b expression. Data represent the mean ± SEM of 5 experiments.
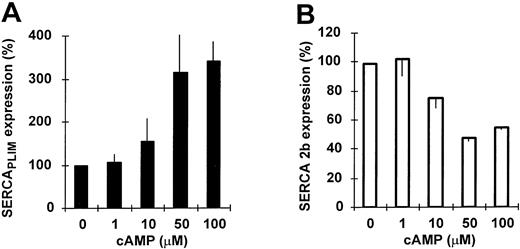
The modulation of calcium pump expression could be obtained by using other established inducers of granulocytic differentiation such as 9-cis-retinoic acid and DMSO (not shown) or by cAMP analogs (Fig 3A and B).
Modulation of calcium pump expression by cAMP. HL-60 cells were treated with various concentrations of the cell-permeable cAMP analog CTP-cAMP for 4 days and SERCA expression was determined. (A and B) Concentration dependence of the modulation of SERCAPLIM and SERCA 2b expression by cAMP, respectively. Data represent the mean ± SEM of 3 experiments.
Modulation of calcium pump expression by cAMP. HL-60 cells were treated with various concentrations of the cell-permeable cAMP analog CTP-cAMP for 4 days and SERCA expression was determined. (A and B) Concentration dependence of the modulation of SERCAPLIM and SERCA 2b expression by cAMP, respectively. Data represent the mean ± SEM of 3 experiments.
Modulation of calcium transport function by ATRA-induced differentiation.
To gain insight into the functional consequences on cellular calcium homeostasis of the modulation of SERCA expression during differentiation, we investigated ATP-driven active calcium transport into microsomal membrane preparations obtained from untreated and retinoic acid-differentiated HL-60 cells. Calcium uptake was determined in the absence or presence of thapsigargin (an inhibitor of total SERCA activity, ie, SERCA 2b plus SERCAPLIM) or in the presence of purified PLIM430 antibody. This antibody inhibits calcium transport by its cognate antigen, SERCAPLIM selectively, and therefore can be used as a functional probe in the analysis of calcium transport.27 64 As shown in Table 1, in membranes prepared from undifferentiated cells, PLIM430-inhibitable calcium transport accounted for 32% of total SERCA-dependent calcium accumulation. Although total SERCA-dependent calcium transport did not change significantly after differentiation (0.55 v 0.57 nmol Ca2+/mg membrane protein), in membranes prepared from retinoic acid-differentiated cells, PLIM430-inhibitable transport was increased to 60%. Increased SERCAPLIM expression combined with the concomitant decrease of SERCA 2b expression thus resulted in an approximately twofold shift towards calcium uptake into the SERCAPLIM-associated calcium pool, indicating that the modification of SERCA protein levels upon differentiation results in the modification of calcium transport function as well.
Calcium Uptake Into Microsomal Membrane Vesicles Prepared From Control and ATRA Differentiated HL-60 Cells
| . | Calcium Uptake (pmol Ca2+/mg membrane protein ± SEM) . | |
|---|---|---|
| Untreated Cells . | ATRA-Treated Cells . | |
| Total SERCA-dependent Ca2+uptake | 554 ± 13 | 572 ± 43 |
| SERCA 2b-dependent Ca2+ uptake | 375 ± 13 | 222 ± 46 |
| SERCAPLIM-dependent Ca2+ uptake | 181 ± 6 | 346 ± 21 |
| . | Calcium Uptake (pmol Ca2+/mg membrane protein ± SEM) . | |
|---|---|---|
| Untreated Cells . | ATRA-Treated Cells . | |
| Total SERCA-dependent Ca2+uptake | 554 ± 13 | 572 ± 43 |
| SERCA 2b-dependent Ca2+ uptake | 375 ± 13 | 222 ± 46 |
| SERCAPLIM-dependent Ca2+ uptake | 181 ± 6 | 346 ± 21 |
Calcium transport by SERCA 2b and SERCAPLIM into membrane vesicles prepared from untreated and ATRA-treated HL-60 cells was determined. Although total SERCA-dependent calcium uptake did not change significantly during differentiation, calcium transport into the SERCAPLIM-associated calcium pool increased from 32% of total SERCA-dependent calcium accumulation to 60% after ATRA treatment.
Effect of synthetic retinoids on SERCA expression.
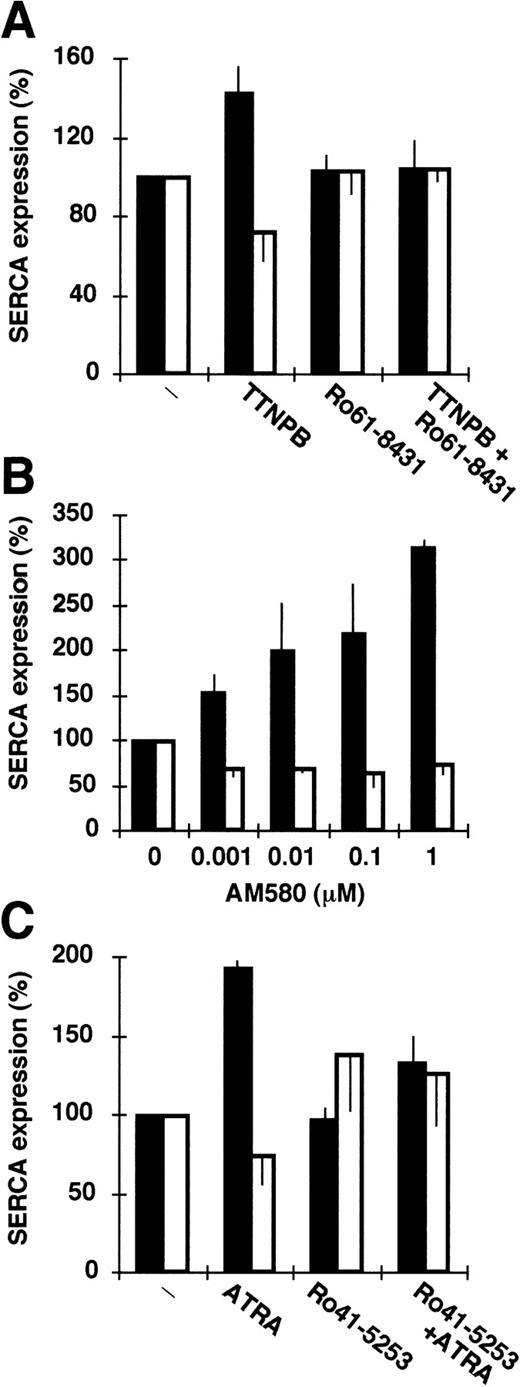
To define the retinoic acid receptor isoforms involved in the modulation of calcium pump expression, we treated HL-60 and NB4 cells with activators and antagonists of different specificities towards the various retinoic acid receptor subtypes for 4 days. As shown in Fig 4, TTNPB (a pan-RAR agonist66), similarly to ATRA, induced both SERCAPLIM expression (Fig 4A) and granulocytic differentiation (assessed by NBT reduction; not shown). As expected, Ro-618431 (a pan-RAR antagonist67) inhibited the effect (Fig 4A). On the other hand, SR 11237 (a pan-RXR agonist68) was without effect on NB4 cells (not shown). These results suggest that the RAR family rather than the RXR family is involved in the modulation of SERCA expression. The RARα selective agonist AM58042induced SERCA expression (as well as differentiation) in the submicromolar range (Fig 4B), whereas the RARβ specific agonist CD201942 was without effect on these cells (not shown). Ro 41-5253 (an RARα-selective antagonist69) could inhibit the effect of ATRA (Fig 4C). Taken together, these data indicate that the modulation of SERCA expression by ATRA proceeds via RARα-dependent signaling.
Treatment of HL-60 and NB4 cells by retinoids in the presence of retinoic acid receptor antagonists. (▪) SERCAPLIM; (□) SERCA 2b. (A) Treatment of NB4 cells by 0.1 μmol/L TTNPB (a pan-RAR agonist) in the presence or absence of 10 μmol/L Ro61-8431 (a pan-RAR antagonist). (B) Treatment of NB4 cells with various concentrations of AM580 (an RAR selective agonist). (C) Treatment of HL-60 cells with 0.1 μmol/L ATRA in the presence or absence of 10 μmol/L Ro41-5253 (an RAR specific antagonist). Data represent the mean ± SEM of 3 experiments.
Treatment of HL-60 and NB4 cells by retinoids in the presence of retinoic acid receptor antagonists. (▪) SERCAPLIM; (□) SERCA 2b. (A) Treatment of NB4 cells by 0.1 μmol/L TTNPB (a pan-RAR agonist) in the presence or absence of 10 μmol/L Ro61-8431 (a pan-RAR antagonist). (B) Treatment of NB4 cells with various concentrations of AM580 (an RAR selective agonist). (C) Treatment of HL-60 cells with 0.1 μmol/L ATRA in the presence or absence of 10 μmol/L Ro41-5253 (an RAR specific antagonist). Data represent the mean ± SEM of 3 experiments.
Modulation of calcium pump expression by ATRA in fresh APL cells.
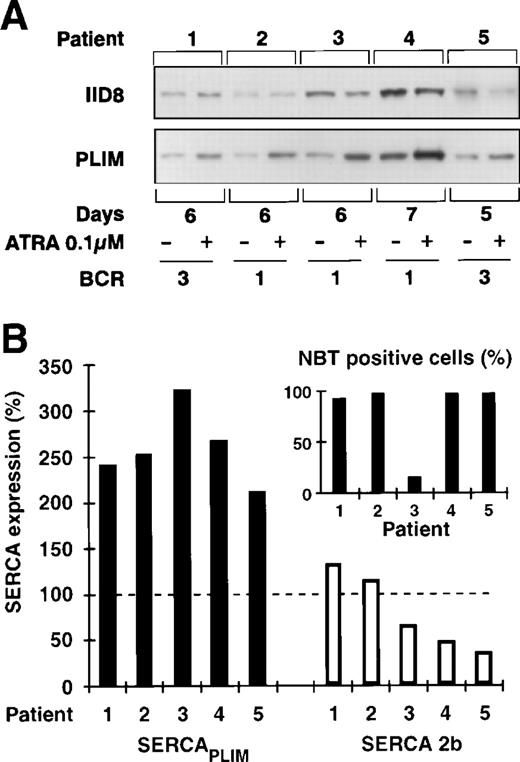
APL blasts isolated from 5 newly diagnosed patients were treated with 0.1 μmol/L ATRA, and calcium pump expression and cell differentiation was determined. As shown in Fig 5, ATRA treatment specifically induced an approximately threefold overexpression of SERCAPLIM in all 5 cases, whereas the expression of SERCA 2b decreased or did not change significantly. During treatment, the cells underwent terminal granulocytic differentiation, as detected by NBT reduction and morphological examination.
Effect of ATRA treatment on the SERCA expression of fresh APL cells. Freshly isolated APL blasts were treated by 0.1 μmol/L ATRA over 7 days and SERCA expression; cell differentiation was also determined. (A) Immunoblot analysis of SERCA 2b and SERCAPLIM expression. (B) Modulation of SERCA expression upon ATRA treatment. One hundred percent refers to SERCA expression levels in untreated cells. (▪) SERCAPLIM; (□) SERCA 2b. Treatment resulted in a marked induction of SERCAPLIMexpression. (Inset) The percentage of NBT-positive cells after ATRA treatment.
Effect of ATRA treatment on the SERCA expression of fresh APL cells. Freshly isolated APL blasts were treated by 0.1 μmol/L ATRA over 7 days and SERCA expression; cell differentiation was also determined. (A) Immunoblot analysis of SERCA 2b and SERCAPLIM expression. (B) Modulation of SERCA expression upon ATRA treatment. One hundred percent refers to SERCA expression levels in untreated cells. (▪) SERCAPLIM; (□) SERCA 2b. Treatment resulted in a marked induction of SERCAPLIMexpression. (Inset) The percentage of NBT-positive cells after ATRA treatment.
Lack of modulation of SERCA expression in ATRA-resistant cells.
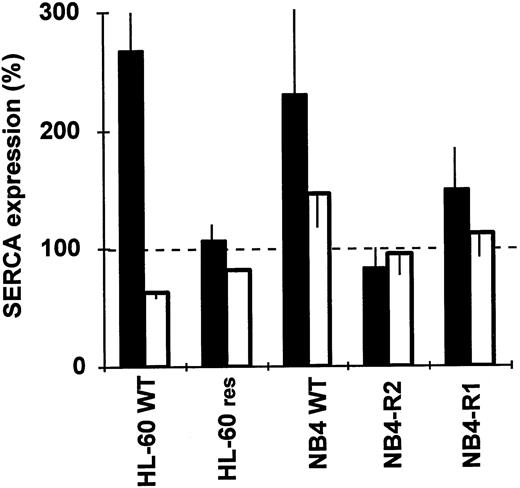
Continuous ATRA therapy in APL patients after complete remission may be complicated by the emergence of a malignant cell population that fails to differentiate upon ATRA treatment. The expression of SERCA enzymes in differentiation-resistant variants of HL-60 and NB4 cells was not modified by ATRA. Whereas in wild-type cells ATRA induced a significant overexpression of SERCAPLIM, in ATRA-resistant HL-60RES and NB4-R2 cells (Fig6), SERCA expression levels as well as NADPH oxidase activity (not shown) remained essentially unchanged upon ATRA treatment. In NB4-R1 cells, ATRA treatment resulted in a very modest induction of SERCAPLIM (Fig 6). This is in agreement with previous data indicating that NB4-R1 cells undergo a very limited differentiation compared with wild-type NB4 cells55 under the experimental conditions used.
SERCA expression in ATRA-resistant HL-60 and NB4 cells. Wild-type and ATRA-resistant HL-60 and NB4 cells were treated with 1 μmol/L of ATRA for 4 days and SERCA expression was determined. (▪) SERCAPLIM; (□) SERCA 2b. Data are the mean ± SEM of 3 experiments.
SERCA expression in ATRA-resistant HL-60 and NB4 cells. Wild-type and ATRA-resistant HL-60 and NB4 cells were treated with 1 μmol/L of ATRA for 4 days and SERCA expression was determined. (▪) SERCAPLIM; (□) SERCA 2b. Data are the mean ± SEM of 3 experiments.
Modulation of SERCA expression during differentiation of HL-60 cells towards macrophage-like cells.
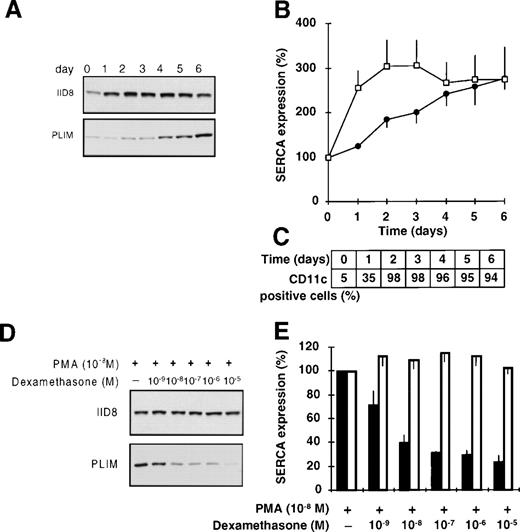
Upon PMA treatment, HL-60 cells display a mature macrophage-like phenotype.48 As shown in Fig 7A and B and in contrast with ATRA-induced granulocytic differentiation, in which a selective induction of SERCAPLIM expression is seen (Fig 1), PMA treatment of HL-60 cells resulted in the induction of the expression of both SERCA isoforms. This was accompanied by immediate growth arrest, assumption of an adherent phenotype, and the induction of the expression of nonspecific esterase (all cells becoming positive upon microscopical examination starting at day 1, as compared with less than 5% in untreated cells), and a strong induction of CD11c expression (Fig 7C).
Differentiation of HL-60 cells towards macrophage-like cells by PMA. HL-60 cells were treated with 10−8 mol/L PMA for 6 days and SERCA expression was determined. (A) Immunoblot staining for SERCA 2b and SERCAPLIM using the IID8 and the PLIM430 antibodies, respectively. (B) Densitometric analysis of SERCA expression in PMA-treated cells. (□) SERCA 2b; (•) SERCAPLIM. (C) Induction of CD11c expression by PMA-treated HL-60 cells. (D and E) Selective inhibition of the PMA-induced overexpression of SERCAPLIM by dexamethasone. HL-60 cells were treated for 5 days with 10−8 mol/L PMA in the presence of various concentrations of the glucocorticoid dexamethasone. (D) Immunostaining for SERCA 2b and SERCAPLIM using the IID8 and PLIM430 monoclonal antibodies, respectively. (E) Densitometric analysis of the expression levels of (□) SERCA 2b and (▪) SERCAPLIM in cells treated with PMA and dexamethasone (100% refers to SERCA expression of cells treated with PMA only). Data represent the mean ± SEM of 6 experiments.
Differentiation of HL-60 cells towards macrophage-like cells by PMA. HL-60 cells were treated with 10−8 mol/L PMA for 6 days and SERCA expression was determined. (A) Immunoblot staining for SERCA 2b and SERCAPLIM using the IID8 and the PLIM430 antibodies, respectively. (B) Densitometric analysis of SERCA expression in PMA-treated cells. (□) SERCA 2b; (•) SERCAPLIM. (C) Induction of CD11c expression by PMA-treated HL-60 cells. (D and E) Selective inhibition of the PMA-induced overexpression of SERCAPLIM by dexamethasone. HL-60 cells were treated for 5 days with 10−8 mol/L PMA in the presence of various concentrations of the glucocorticoid dexamethasone. (D) Immunostaining for SERCA 2b and SERCAPLIM using the IID8 and PLIM430 monoclonal antibodies, respectively. (E) Densitometric analysis of the expression levels of (□) SERCA 2b and (▪) SERCAPLIM in cells treated with PMA and dexamethasone (100% refers to SERCA expression of cells treated with PMA only). Data represent the mean ± SEM of 6 experiments.
Because dexamethasone has been described to impair the differentiation of monocytes,50-52 we investigated the effect of this glucocorticoid on PMA-induced SERCA expression of HL-60 cells. When PMA treatment was performed in the presence of dexamethasone, the induction of SERCAPLIM was selectively and efficiently inhibited by submicromolar concentrations of the glucocorticoid (Fig 7D and E). Dexamethasone, when applied alone, did not have an appreciable effect on SERCA expression.
DISCUSSION
The data presented in this report show for the first time that intracellular calcium pump expression is modulated during myeloid differentiation in a lineage-specific manner. Granulocytic differentiation resulted in increased expression of SERCAPLIM, whereas SERCA 2b expression was decreased. This phenomenon was manifest on the protein as well as on the mRNA level. Observations made with agonists and antagonists of distinct selectivity towards different retinoic acid receptors indicated that the modulation of calcium pump expression is linked to RARα-dependent signaling. The modulation of SERCA expression was a differentiation-associated phenomenon, because in differentiation-defective HL-60 and NB4 derivatives, the same treatment failed to modulate SERCA expression significantly. The modulation of calcium pump expression during differentiation could also be demonstrated in primary APL cells. Changes in SERCA protein expression resulted in the modification of calcium transport function, as reflected by a significant shift in calcium accumulation into SERCAPLIM- versus SERCA 2b-associated calcium pool in differentiated cells, as compared with untreated control.
In contrast to granulocytic differentiation, phorbol ester-induced differentiation towards the monocyte/macrophage lineage resulted in the simultaneous induction of the expression of both SERCA isoforms. In addition, when the cells were treated with phorbol ester in combination with the glucocorticoid dexamethasone, a marked and selective blockage of the induction of SERCAPLIM was observed. This is in agreement with data in the literature indicating the presence of a putative glucocorticoid-responsive element in the SERCA 3 promoter,26 with the observation that PMA induces the expression of the glucocorticoid receptor in HL-60 cells,70and with the observation that dexamethasone inhibits the expression of markers of monocytic differentiation and impairs macrophage function.50-52
The endoplasmic reticulum consists of a dynamically interconnected membrane network71,72 that is involved in several distinct functions, such as protein posttranscriptional modification, sorting, secretion,5,6 and calcium-dependent signal transduction.3,4 In structural terms, ER multifunctionality is reflected by the uneven distribution of ER-associated proteins within the organelle,73-75 forming functionally distinct regions. In particular, regions possessing distinct densities of SERCA proteins and D-myo-inositol 1,4,5-trisphosphate (IP3) receptors have been described in nonmuscle cells,73 and the association of distinct SERCA isoenzymes with IP3 mobilizable and IP3-resistant calcium pools has been observed.27,76 77 Taken together, these observations strongly suggest that the ER comprises connected but functionally specialized subcompartments.
The different homeostatic, synthetic, and signaling systems of the ER undergo a profound reorganization in structural as well as functional terms when a proliferating, undifferentiated cell undergoes differentiation and assumes a mature, quiescent phenotype. Moreover, differentiation is associated with the acquisition of new cellular structures involved in functions characteristic of the differentiated cell. Several functions of the differentiated granulocyte and monocyte-macrophage, such as respiratory burst, phagocytosis, degranulation, responsiveness to chemotactic peptides, cytokines, or chemokines, as well as apoptosis, are calcium dependent.11,15,28-30 78 The acquisition of these functions during cell differentiation implies a complex and cell-type–specific remodelling and de novo synthesis of calcium-dependent signaling and effector systems and structures involved. This is demonstrated in the present report by the complex and specific modifications of the expression levels of endomembrane calcium transport ATPases during differentiation.
The cell-type–dependent and isoform-specific modulation of SERCA expression during granulocytic or monocyte/macrophage differentiation of promyelocytic cells may have an important modulatory effect on cell activation. The two SERCA isoenzymes possess distinct biochemical characteristics such as calcium affinity79 or sensitivity towards biochemical regulation80 or pharmacological manipulation21 and are located in distinct ER subcompartments.27,75-77 In particular, SERCAPLIM has been shown to be specifically associated with the IP3-sensitive calcium pool.27 Taken together, these data strongly suggest that changes in SERCA isoform expression levels can exert significant effects on the calcium homeostasis and signaling of the cell, even if total endomembrane calcium pump mass or activity is unaltered. In light of data in the literature81 and our own observations (not shown) on ATRA-treated HL-60 cells, indicating that IP3-receptor levels are increased upon granulocytic differentiation, it is tempting therefore to speculate that increased SERCAPLIM expression may reflect the enhanced synthesis by the cell of an IP3-sensitive intracellular calcium storage organelle required for the augmentation of the intensity of extracellular stimulus-dependent calcium mobilization and influx observed in differentiated cells as compared with undifferentiated control,82 whereas increased SERCA 2b expression may be involved in ER functions linked to effector functions such as phagocytosis.
The modulation of calcium pump isoform levels may modify gene expression as well. It has been shown recently that the modification of the frequency and geometry of cytosolic calcium oscillations has a dramatic effect on the activation of calcium-dependent enzymes and transcription factors regulating gene expression.83-87Cytosolic calcium oscillations are maintained by a dynamic interplay of calcium release and elimination mechanisms.88-91 Because the calcium affinity of SERCA 3 is inferior to that of SERCA 2b, overexpression of SERCA 3 may lead to slower calcium elimination, higher resting calcium levels, and an overall modification of the activity of proinflammatory transcription factors such as NF-κB, NF-AT, or Oct/OAP.83-87
Although the elucidation of the intricate interplay of mechanisms regulating ER organellogenesis during differentiation as well as the interaction of different intracellular calcium-dependent signaling systems and calcium pools during cell activation requires further study, the present work indicates that SERCA enzymes are intimately involved in hematopoietic differentiation and that the endoplasmic reticulum undergoes a significant remodelling upon this process. The modulation of the calcium homeostasis of the endoplasmic reticulum may therefore offer new approaches in the pharmacological modulation of cell growth, differentiation, and apoptosis.
ACKNOWLEDGMENT
The authors are indebted to Dr Robert Gallagher for the ATRA-resistant HL-60 cells and to Dr Neville Crawford for the PLIM430 hybridoma. We thank Dr Anabelle Le Grand and Laurent Barbe for their help with flow cytometry. The discussions and support of Dr Sylviane Lévy-Tolédano, Dr Michel Lanotte, Prof Hugues de Thé, and Dr Balàzs Sarkadi are gratefully acknowledged.
This work is dedicated to the memory of Jacques Maclouf.
Supported by the Institut National de la Santé, et de la Recherche Médicale Reseau Est-Ouest No. 4E004B, the Association pour la Recherche sur le Cancer, and the Agence Nationale pour la Recherche sur le SIDA, France. M.G. was supported by a fellowship from the Ligue Nationale contre le Cancer and the Fondazione Italiana per la Ricerca sul Cancro.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Béla Papp, PhD, U. 348 INSERM, Hôpital Lariboisière, 8, rue Guy Patin, 75475 Paris Cedex 10, France; e-mail: bela.papp@inserm.lrb.ap-hop-paris.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal