Abstract
The vascular endothelial growth factor (VEGF) receptor-2 (Flk-1) is the first endothelial receptor tyrosine kinase to be expressed in angioblast precursors, and its function is essential for the differentiation of endothelial cells and hematopoietic precursors. We have identified cis-acting regulatory elements of the murineFlk-1 gene that mediate endothelium-specific expression of a LacZ reporter gene in transgenic mice. Sequences within the 5′-flanking region of the Flk-1 gene, in combination with sequences located in the first intron, specifically targeted transgene expression to angioblasts and endothelial cells of transgenic mice. The intronic regulatory sequences functioned as an autonomous endothelium-specific enhancer. Sequences of the 5′-flanking region contributed to a strong, uniform, and reproducible transgene expression and were stimulated by the transcription factor HIF-2. The Flk-1 gene regulatory elements described in this study should allow the elucidation of the molecular mechanisms involved in endothelial cell differentiation and angiogenesis.
THE CARDIOVASCULAR SYSTEM IS THE first functional organ system to be formed in the vertebrate embryo. Endothelial cells, which constitute the inner lining of all blood vessels, differentiate from mesodermal precursor cells, or angioblasts, shortly after gastrulation.1 2 The close association of angioblasts and hematopoietic precursor cells at this developmental stage is thought to reflect their origin from a putative common precursor cell, the hemangioblast. In a process termed vasculogenesis, angioblasts aggregate to form a primitive vascular plexus, and differentiate into endothelial cells. The primitive vascular plexus is then further refined by angiogenesis and remodeling.
Angiogenic growth factors of the vascular endothelial growth factor (VEGF) family and their cognate endothelial receptors, Flt-1 (VEGF receptor-1), Flk-1/KDR (VEGF receptor-2), and Flt-4 (VEGF receptor-3) function as signaling molecules during vascular development.3 Gene targeting experiments have shown that these molecules have an essential function in embryonic vascular development.4 VEGF and the VEGF receptors represent the first endothelial cell-specific signal transduction pathway known to be activated during vascular development.5-8 In particular, Flk-1 appears to play a pivotal role in endothelial cell differentiation and vasculogenesis. Flk-1 is the first endothelial receptor known to be expressed in the primitive mesoderm,9,10 and mice that are homozygously defective forFlk-1 completely lack mature endothelial cells and blood vessels.6,11 Moreover, hematopoiesis is defective in theFlk-1 −/− mice, indicating that Flk-1function is also essential for the differentiation of hematopoietic precursors. This is consistent with the hypothesis that Flk-1is a marker for the hemangioblast.12 Collectively, these data provide evidence that Flk-1 gene activation is a principal event leading to the emergence of the hemangioblastic lineage and the differentiation of endothelial and hematopoietic cells. During later stages of embryonic development Flk-1 is highly expressed on endothelial cells,9,13 but is downregulated in most hematopoietic cells.14 In the adult, Flk-1expression is not detectable in most vascular beds. The strong induction of Flk-1 expression in tumoral endothelial cells is involved in the neovascularization of various human or experimental tumors.15 16 Thus, the understanding of Flk-1 gene regulation represents a key step in the understanding of the mechanisms involved in endothelial lineage differentiation and angiogenesis in development and disease. The identification of the cis-acting elements in the Flk-1 gene that are responsible for its unique expression profile provides a valuable tool for the identification of upstream factors that control the establishment of the hemangioblastic lineage.
We have previously isolated genomic clones that encompass the promoter region of the murine Flk-1 gene, and have performed a functional analysis of the Flk-1 promoter in vitro.17 We17 and others18 showed that Flk-1 5′-flanking sequences confer endothelium-specific expression in transfected endothelial cells. The significance of these cis-acting elements for the developmental expression of the Flk-1 gene in vivo remained unclear. In this report, we have characterized the murine Flk-1 regulatory sequences in transgenic mice. Despite their activity in cultured endothelial cells, 5′-flanking sequences alone could not target expression of a LacZ reporter gene to the endothelium of mouse embryos. However, in combination with sequences from the first intron of theFlk-1 gene, the Flk-1 promoter could specifically drive reporter gene expression in endothelial cells. The transgene expression pattern closely resembled that of the endogenous Flk-1 gene throughout development. The intron sequences conferred endothelium-specific gene expression also to the heterologous herpes simplex virus-thymidine kinase (tk) promoter and fulfilled all the criteria of an autonomous tissue-specific enhancer. The Flk-1promoter sequences were essential for a strong and reproducible transgene expression, and were stimulated by HIF-2α, a basic helix-loop-helix/PAS domain transcription factor that is prominently expressed in the vasculature of embryonic mice.19-21
MATERIALS AND METHODS
DNA sequence analysis.
Restriction fragments of a 12 kb region of the Flk-1 gene ranging from −6.5 kbp to +5.5 kbp relative to the transcription initiation site were subcloned into the pBluescriptII vector (Stratagene, La Jolla, CA). The nucleotide sequence was determined on both strands by using the deoxynucleotide chain termination method on an Applied Biosystems 373 automated sequencer. The nucleotide sequence of the Flk-1 intron enhancer is deposited in the Genbank database (accession number AF061804). The search for potential transcription factor binding sites was performed with the MatInspector software (GBF, Braunschweig, Germany).22
Plasmid construction and transient transfection.
The LacZ reporter vector was generated by inserting the LacZ cassette into the HindIII and BamHI restriction sites of the pGL2-basic plasmid (Promega, Madison, WI), thereby exchanging the luciferase gene against the LacZ cassette. The LacZ cassette was derived from the pβactinSDKLacZ plasmid (a gift from Janet Rossant, Samuel-Lunenfeld-Research Institute, Toronto, Canada).Flk-1 promoter fragments were amplified by the polymerase chain reaction (PCR) as described17 and inserted into theKpnI and HindIII sites of pGL-2. The −4.1 kbp/+299 bp fragment was generated by inserting a Flk-1promoter fragment ranging from −4.1 kbp to −1.9 kbp into the HindIII and EcoRI sites of the pGL2-Basic plasmid that already contained the Flk-1 promoter region from −1.9 kbp to +299 bp, and subsequently exchanging the luciferase gene against the LacZ cassette. The −640 bp/+299 bp promoter fragment was amplified as described17 using the forward primer Flk-640. Flk-640: 5′-GGGGTACCTTCTGGACCGACCCAGCCAGG-3′.
Flk-1 intron fragments were amplified by PCR as described.17 PCR products were digested with BamHI and XhoI or BamHI and SalI and inserted into the BamHI and SalI restriction sites downstream of the LacZ cassette in the modified pGL2-Basic vector that already contained the Flk-1 promoter fragment. A recombinant lambda phage clone, P1617 served as a template for PCR amplification. The following primers were used: 5′-In1fw: 5′-AGGGATCCACTCTTTAGTAGTAAGGCG-3′, 5′-In1rev: 5′-ACCTCGAGACTTGGATGGCAC-3′, 3′-In1fw: 5′-GGGCTATAATTGGTGCCATCC-3′, 3′-In1rev: 5′-GGATGGAGAAAATCGCCAGGC-3′, In2fw: 5′-GTGTGCATTGTTTATGGAAGGG-3′, In2rev: 5′-CATAGACATAAACAGTGGAGGC-3′. The 510 bp intron fragment located between nucleotides +3437 and +3947 was aSwaI/BamHI fragment derived from recombinant phage P1617 and was inserted by blunt-end cloning to the bluntedBamHI site of the modified pGL-2.
Bovine aortic endothelial (BAE) cells, NIH 3T3 cells, and A293 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM)+ supplemented with 10% fetal calf serum. Transient transfections were performed as described17 using 6 μg β-galactosidase reporter vector driven by Flk-1 sequences and 1μg of cytomegalovirus (CMV) promoter-driven luciferase vector in each experiment. Reporter gene assays were performed as described.17 The β-galactosidase activity of each extract was normalized to the respective luciferase activity.
Mouse HIF-1α and HIF-2α complementary DNA (cDNA) clones were isolated from a mouse brain capillary cDNA library23 using standard techniques, and further amplified by PCR as described20 using primer pairs HIF1fw/rev and HIF2 fw/rev. The sequence encoding the FLAG epitope (Eastman-Kodak, Rochester, NY) was included in the reverse oligonucleotide primer. The following primers were used: HIF1fw: 5′-GGGAATTCACCATGAGTTCTGAACGTCGAAAAG-3′, HIF1rev: 5′-AAGCGGCCGCTCATTTATCGTCATCGTCCTTGTA ATCGTTAACTTGATCCAAAGCTCTG-3′, HIF2fw: 5′-GGGATCCGACAATGACAGCTGACAAGGAG-3′, HIF2rev: 5′-AAGCGGCCGCTCATTTATCGTCATCGTCCTTGTAATGGTGGCCTGGTCCA GAGCTC-3′. HIF-1α and HIF-2α expression plasmids were constructed by inserting the cDNAs into the EcoRI andNotI sites of pcDNA3 (Invitrogen, Carlsbad, CA). For cotransfection assays, A293 cells were split 1:2 into 35-mm dishes and transfected 18-hours later with 4 μg of DNA (2 μg ofFlk-1 promoter-driven luciferase plasmid, 1 μg of CMV promoter-driven β-galactosidase expression plasmid, and 1 μg of the HIF-1α or HIF-2α expression plasmids, or pBluescript SKII and pcDNA3 plasmids as a control) using a transfection kit (MBS, Stratagene). After 20 hours, reporter gene activity was measured using the Dual Light Kit (Tropix, Bedford, MA). The luciferase activity of each extract was normalized to the respective β-galactosidase activity. Endogenous background levels of both enzyme activities were determined using extracts from mock-transfected cells and were subtracted. The normalized luciferase activity of the control transfection was arbitrarily set to 1. Each value represents the average of at least six experiments.
Generation and analysis of transgenic mice.
Transgenic mice were generated by microinjection of fertilized mouse oocytes as described.24 Fertilized oocytes were isolated from superovulated C57BL/6 × C3H/He F1 mice, microinjected, and reimplanted into pseudopregnant females of the same hybrid-mouse strain. Mouse embryos were analyzed by whole mount LacZ staining for transgene expression as described.25 Genomic DNA was prepared from unstained embryos or yolk sacs, and genotyping was performed by PCR analysis as described25 using the primer pair LacZP1/LacZP2. LacZP1: 5′-ATCCTCTGCATGGTCAGGTC-3′; LacZP2: 5′-CGTGGCCTGATTCATTCC-3′. For histological analysis, embryos were embedded in paraffin, and 10 μm sections were prepared and counterstained with neutral red.
Cryostat sectioning and LacZ staining of organs from postnatal mice were performed as described.26 Immunofluorescence detection of platelet endothelial cell adhesion molecule-1 (PECAM-1) was performed as described27 using a CY3-conjugated Goat antirat IgG secondary antibody (Jackson Immuno Research Laboratories Inc, West Grove, PA) as recommended by the manufacturer.
RESULTS
Functional analysis of the Flk-1 promoter in transgenic mouse embryos.
We have previously characterized promoter fragments from the murineFlk-1 gene that confer endothelium-specific expression to the firefly luciferase reporter gene in transfected BAE cells.17 Although the Flk-1 promoter sequences extending to −4.1 kbp had the highest cell-type specificity of all fragments tested in vitro, a stronger promoter activity was observed with shorter promoter fragments.17 To investigate whether the Flk-1 gene regulatory regions identified in vitro are also functional in vivo, we generated transgenic mouse embryos carrying a transgene that consists of the LacZ reporter gene under the control of different Flk-1 promoter fragments. Analysis of transgenic embryos was performed at day 10.5 of embryonic development (E10.5). A weak vascular transgene expression was observed in 1 out of 31 transgenic embryos tested that contained a promoter fragment spanning the Flk-1 gene from −1.9 kbp to +299 bp relative to the transcription initiation site (Table1). No endothelium-specific expression was observed with longer (−4.1 kbp/+299 bp) or shorter fragments (−640 bp/+299 bp) (Table 1). Therefore, none of the various Flk-1 promoter fragments mediated a reproducible endothelium-specific reporter gene expression in vivo. From these data we concluded that additional sequences of the Flk-1 gene are required to confer the endogenous expression pattern.
Summary of the In Vivo Activity of Different Flk-1 Constructs
| Construct . | TG . | ES . | ET . | NO . |
|---|---|---|---|---|
| −4100/+299 | 11 | 0 | 3 | 8 |
| −1900/+299 | 31 | 1 | 10 | 20 |
| −640/+299 | 3 | 0 | 1 | 2 |
| −640/+299 // 3′Intron +1677/+3947 | 7 | 6 | 0 | 1 |
| −640/+299 // 3′Intron +3947/+1677 | 4 | 3 | 1 | 0 |
| −640/+299 // 3′Intron +3437/+3947 | 7 | 5 | 0 | 2 |
| −640/+254 // 3′Intron +1677/+3947 | 12 | 8 | 1 | 3 |
| −640/+137 // 3′Intron +1677/+3947 | 13 | 5 | 1 | 7 |
| tk // 3′Intron +1677/+3947 | 15 | 3 | 0 | 12 |
| −5500/+299 // Intron I+II | 3 | 2 | 0 | 1 |
| Construct . | TG . | ES . | ET . | NO . |
|---|---|---|---|---|
| −4100/+299 | 11 | 0 | 3 | 8 |
| −1900/+299 | 31 | 1 | 10 | 20 |
| −640/+299 | 3 | 0 | 1 | 2 |
| −640/+299 // 3′Intron +1677/+3947 | 7 | 6 | 0 | 1 |
| −640/+299 // 3′Intron +3947/+1677 | 4 | 3 | 1 | 0 |
| −640/+299 // 3′Intron +3437/+3947 | 7 | 5 | 0 | 2 |
| −640/+254 // 3′Intron +1677/+3947 | 12 | 8 | 1 | 3 |
| −640/+137 // 3′Intron +1677/+3947 | 13 | 5 | 1 | 7 |
| tk // 3′Intron +1677/+3947 | 15 | 3 | 0 | 12 |
| −5500/+299 // Intron I+II | 3 | 2 | 0 | 1 |
Embryos transgenic for the constructs given above were generated, and LacZ staining and genotyping were performed at E10.5 or E11.5 as described in the Materials and Methods section. Constructs are defined by the position of the promoter or intron fragments in bp relative to the transcription initiation site of the endogenous Flk-1 gene.
Abbreviations: TG, number of transgenic embryos; ES, number of embryos showing endothelial-specific staining; ET, number of embryos showing ectopic staining; NO, number of embryos showing no staining at all.
Identification of endothelium-specific regulatory elements in the first intron of the Flk-1 gene in vitro.
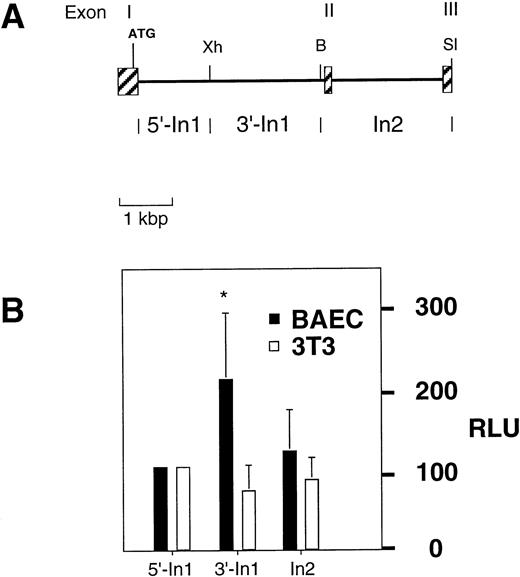
Many tissue-specific gene regulatory elements are located within intronic sequences. To examine whether the first or the second intron of the Flk-1 gene contains sequences that might be able to supplement the function of the Flk-1 promoter in a cell-type–specific manner, we performed reporter gene analysis in transfected cells. Several subfragments of the first two introns (5′-In1, 3′-In1, In2; shown in Fig 1A) were included in β-galactosidase reporter gene constructs together with a 4.4 kbp Flk-1 promoter fragment (−4.1 kbp/+299 bp), and transient transfection was performed in BAE and NIH 3T3 cells. For both cell types, the promoter activity of the reporter gene construct that contained the 5′ region of the first intron (5′-In1) fragment was arbitrarily set to 100 relative light units (RLU). The substitution of this intronic fragment with the 3′ region of the first intron (3′-In1), a 2.3 kbp XhoI/BamHI fragment (+1677 bp/+3947 bp), induced a twofold increase in promoter activity in BAE cells, but not in NIH 3T3 cells (Fig 1B). The second intron (In2), in contrast, did not alter the expression levels significantly (Fig 1B). These results indicate that a positive-acting endothelium-specific element is located in the 2.3 kbp XhoI/BamHI fragment of the firstFlk-1 intron.
Partial structure and functional analysis of the mouseFlk-1 locus. (A) Restriction enzyme map of the region encompassing the first three exons (represented by shaded boxes). Subfragments containing parts of intron 1 or intron 2 are indicated. Abbreviations for restriction enzymes are: B, BamHI, Xh,XhoI, Sl, SalI. (B) β-galactosidase reporter gene assays of various constructs after transient transfection of BAE cells. The intron fragments were tested in combination with a 4.4 kbpFlk-1 promoter fragment spanning the region from −4.1 kbp to +299 bp of the Flk-1 gene. NIH 3T3 cells were used as a reference for nonendothelial cells. Values significantly above (P < .003, Student’s t-test) 100 RLU are marked with an asterisk.
Partial structure and functional analysis of the mouseFlk-1 locus. (A) Restriction enzyme map of the region encompassing the first three exons (represented by shaded boxes). Subfragments containing parts of intron 1 or intron 2 are indicated. Abbreviations for restriction enzymes are: B, BamHI, Xh,XhoI, Sl, SalI. (B) β-galactosidase reporter gene assays of various constructs after transient transfection of BAE cells. The intron fragments were tested in combination with a 4.4 kbpFlk-1 promoter fragment spanning the region from −4.1 kbp to +299 bp of the Flk-1 gene. NIH 3T3 cells were used as a reference for nonendothelial cells. Values significantly above (P < .003, Student’s t-test) 100 RLU are marked with an asterisk.
Endothelium-specific expression mediated by Flk-1 regulatory sequences in vivo.
When the 2.3 kbp XhoI/BamHI fragment of the first intron (3′-In1; +1677 bp/+3947 bp; see above) was tested in combination with the Flk-1 promoter fragment (−640 bp/+299 bp), a reproducible vascular LacZ expression in transgenic E10.5 mouse embryos was observed (Table 1), for example in blood vessels of the head region, in intersomitic vessels, the dorsal aorta, and in the heart anlage (Fig 2A). Sectioning of these embryos confirmed that the β-galactosidase expression was confined to vascular endothelium (data not shown). The intron fragment could also direct endothelium-specific LacZ expression when used in an inverted orientation in the reporter construct (−640 bp/+299 bp//+3947 bp/+1677 bp, Table 1).
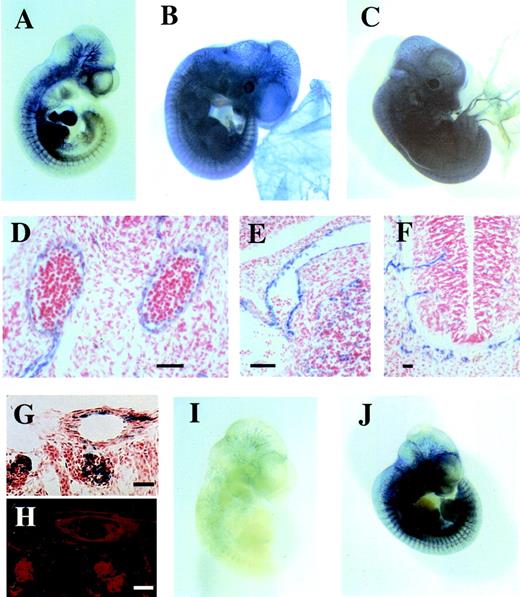
Reporter gene analysis of Flk-1 gene regulatory elements in transgenic mouse embryos. The LacZ reporter gene was fused to regulatory elements derived from the mouse Flk-1 gene and tested for β-galactosidase expression in transgenic mouse embryos. (A) E10.5 transgenic mouse embryo expressing LacZ under the control of a 939bp promoter fragment in combination with a 2.3 kbpXhoI/BamHI fragment of the first intron spanning the region from +1677 bp to +3947 bp of the Flk-1 gene. Most if not all developing vascular structures show β-galactosidase expression, for example the endocardium of the heart, the dorsal aorta, intersomitic vessels or vessels of the developing brain. (B) E11.5 embryo of the transgenic mouse line 2603 that was established with the same construct. (C) E11.5 Flk-1/LacZ knock-in embryo in which the LacZ gene is expressed from the endogenous Flk-1 locus shows a highly similar staining. However, β-galactosidase expression was absent in small blood vessels of the yolk sac. (D to F) Paraffin sections of the embryo from (B) show β-galactosidase expression in the paired dorsal aortae (D), a venous vessel connected with the heart (E), and capillaries invading the neural tube (F). (G) LacZ staining of a 15 μm cryostat section of a P5 transgenic mouse (Line 2603) kidney. (H) Adjacent section of G, immunolabeled with anti-PECAM1 antibody. The LacZ expression in G colocalizes with PECAM1 expression in H. (I) β-galactosidase expression in a transgenic embryo containing thetk promoter in combination with the 2.3 kbpXhoI/BamHI fragment of the Flk-1 first intron. (J) β-galactosidase expression in a transgenic embryo containing a construct with Flk-1 promoter sequences (−640 bp/+299 bp) in combination with a 510 bp fragment of the first intron (+3437 bp to +3947 bp) of the Flk-1 gene. Bars, 100 μm (D to H).
Reporter gene analysis of Flk-1 gene regulatory elements in transgenic mouse embryos. The LacZ reporter gene was fused to regulatory elements derived from the mouse Flk-1 gene and tested for β-galactosidase expression in transgenic mouse embryos. (A) E10.5 transgenic mouse embryo expressing LacZ under the control of a 939bp promoter fragment in combination with a 2.3 kbpXhoI/BamHI fragment of the first intron spanning the region from +1677 bp to +3947 bp of the Flk-1 gene. Most if not all developing vascular structures show β-galactosidase expression, for example the endocardium of the heart, the dorsal aorta, intersomitic vessels or vessels of the developing brain. (B) E11.5 embryo of the transgenic mouse line 2603 that was established with the same construct. (C) E11.5 Flk-1/LacZ knock-in embryo in which the LacZ gene is expressed from the endogenous Flk-1 locus shows a highly similar staining. However, β-galactosidase expression was absent in small blood vessels of the yolk sac. (D to F) Paraffin sections of the embryo from (B) show β-galactosidase expression in the paired dorsal aortae (D), a venous vessel connected with the heart (E), and capillaries invading the neural tube (F). (G) LacZ staining of a 15 μm cryostat section of a P5 transgenic mouse (Line 2603) kidney. (H) Adjacent section of G, immunolabeled with anti-PECAM1 antibody. The LacZ expression in G colocalizes with PECAM1 expression in H. (I) β-galactosidase expression in a transgenic embryo containing thetk promoter in combination with the 2.3 kbpXhoI/BamHI fragment of the Flk-1 first intron. (J) β-galactosidase expression in a transgenic embryo containing a construct with Flk-1 promoter sequences (−640 bp/+299 bp) in combination with a 510 bp fragment of the first intron (+3437 bp to +3947 bp) of the Flk-1 gene. Bars, 100 μm (D to H).
Transgenic mouse lines were generated with this reporter gene construct (−640 bp/+299 bp//+1677 bp/+3947 bp) containing the Flk-1regulatory sequences. Three independent lines were obtained that showed a uniform vascular expression of the reporter gene in the embryo proper and the yolk sac at E11.5, as assessed by whole-mount LacZ staining. One of these lines (2603) was analyzed further (Fig 2B). Sectioning of β-galactosidase–stained E11.5 transgenic embryos showed that reporter gene expression was confined to the endothelium of blood vessels, eg, in the dorsal aorta (Fig 2D), in venous vessels (Fig 2E), in the perineural vascular plexus, and in capillary sprouts invading the neural tube (Fig 2F). The LacZ expression in this line colocalized with the expression of the endothelial marker PECAM1, as confirmed by the parallel immunofluorescence staining with an anti-PECAM1 antibody of adjacent sections (Fig 2G,H). The LacZ staining pattern of these embryos was also compared with heterozygousFlk-1 mutant mouse embryos that express the LacZ gene from the endogenous Flk-1 locus.6 The LacZ staining pattern of transgenic embryos and the knock-in embryos at E11.5 were indistinguishable (Fig 2B,C). These observations showed that the intron sequences in combination with the Flk-1 promoter confer an endothelium-specific expression pattern that closely resembles the expression pattern of the endogenous Flk-1gene.13 28
The first intron of the Flk-1 gene contains an autonomous endothelium-specific enhancer.
To further assess the function of the first Flk-1 intron in endothelium-specific gene expression, we investigated whether the intron sequences can confer endothelium-specific expression to the heterologous tk promoter. This promoter has no intrinsic endothelial cell specificity.26 A LacZ reporter gene construct was generated that contained the tk promoter, in combination with the 2.3 kbp XhoI/BamHI fragment of the first intron (+1677 bp/+3947 bp). Transgenic mouse embryos generated with this construct showed vascular reporter gene expression (Fig 2I). Based on microscopic inspection of whole mount-stained embryos, the β-galactosidase staining observed in these embryos was weaker than in embryos expressing LacZ under the control of the −640 bp/+299 bpFlk-1 promoter in combination with the intron fragment (Fig2A,B). Moreover, the frequency of transgenic mouse embryos expressing this transgene was significantly reduced, when compared with constructs containing the −640 bp/+299 bp Flk-1 promoter in combination with the intron fragment (Table 1). This indicates that thetk promoter lacks positive-acting elements that are present within the Flk-1 promoter. The Flk-1 intron fragment alone, in contrast to the Flk-1 promoter, can reproducibly target reporter gene expression to the endothelium and acts as an autonomous endothelium-specific enhancer.
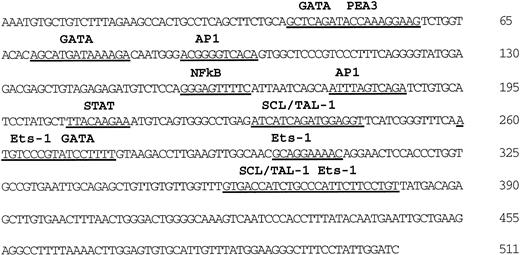
To further characterize the minimal intron sequences that are required for endothelium-specific expression, we analyzed whether shorter intron fragments were also active in combination with the 939 bp promoter region of the Flk-1 gene (−640 bp/+299 bp). By deletion analysis, the intron enhancer was localized to a 510 bp fragment (+3437 bp/+3947 bp) that is located immediately upstream of the second exon. This fragment was sufficient to drive endothelium-specific LacZ expression in transgenic mouse embryos when tested in combination with the Flk-1 promoter (Fig 2J, Table 1). The DNA sequence of this fragment (Fig 3) contains potential binding sites for the GATA and Ets transcription factors, and for Scl/Tal-1, all of which have been proposed to play a role in angiogenesis.29-33
Nucleotide sequence of the Flk-1 intron enhancer and putative transcription factor binding sites. Sequences matching known transcription factor binding sites are underlined. This sequence is deposited in the GeneBank database (accession number AF061804).
Nucleotide sequence of the Flk-1 intron enhancer and putative transcription factor binding sites. Sequences matching known transcription factor binding sites are underlined. This sequence is deposited in the GeneBank database (accession number AF061804).
Flk-1 regulatory sequences target endothelium-specific transgene expression throughout development.
To test whether the regulatory sequences of the Flk-1 promoter and enhancer identified can reproduce the endogenous Flk-1expression pattern throughout development, the LacZ expression pattern of the transgenic mouse line 2603 (Fig 2B) was further analyzed at various stages of embryonic development, at postnatal day 5 (P5) and in the adult (P120). The earliest stage during which transgene expression was examined by whole-mount LacZ staining was at E7.8 (Fig 4A). The analysis of sections of these embryos confirmed that the transgene was expressed in angioblasts of the allantois and the yolk sac (Fig 4B,C). Moreover, transgene expression was restricted to the vascular endothelium at all stages of embryonic development examined (E7.8 to E14.5, data not shown).
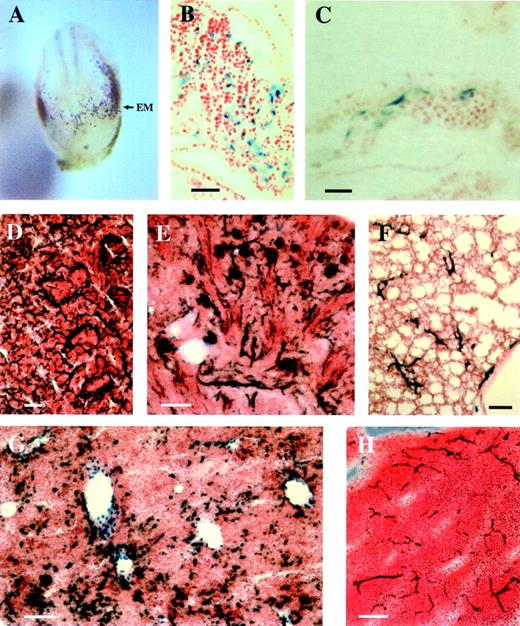
Analysis of transgene expression during early development and in newborn mice in the transgenic mouse line 2603. (A) Frontal view on a whole-mount β-galactosidase–stained E7.8 embryo. The arrow indicates transgene expression in the extraembryonic mesoderm. (B and C) Paraffin sections from the embryo shown in A show transgene expression in endothelial cells of the allantois (B) and the yolk sac (C). (D to H) LacZ staining of spleen (D), kidney (E), lung (F), liver (G), and thymus (H) from a P5 transgenic mouse. (EM) extraembryonic mesoderm. Bars, 25 μm (C), 100 μm (B,D,E,F,G,H).
Analysis of transgene expression during early development and in newborn mice in the transgenic mouse line 2603. (A) Frontal view on a whole-mount β-galactosidase–stained E7.8 embryo. The arrow indicates transgene expression in the extraembryonic mesoderm. (B and C) Paraffin sections from the embryo shown in A show transgene expression in endothelial cells of the allantois (B) and the yolk sac (C). (D to H) LacZ staining of spleen (D), kidney (E), lung (F), liver (G), and thymus (H) from a P5 transgenic mouse. (EM) extraembryonic mesoderm. Bars, 25 μm (C), 100 μm (B,D,E,F,G,H).
LacZ staining was detected in vessels of the spleen, kidney, thymus, liver, and lung from P5 animals (Fig 4D to H). However, LacZ expression was downregulated in most vascular beds of adult animals, except for the spleen (data not shown). These results further support the conclusion that the identified Flk-1 regulatory sequences are sufficient to reproduce most properties of the endogenous Flk-1expression.
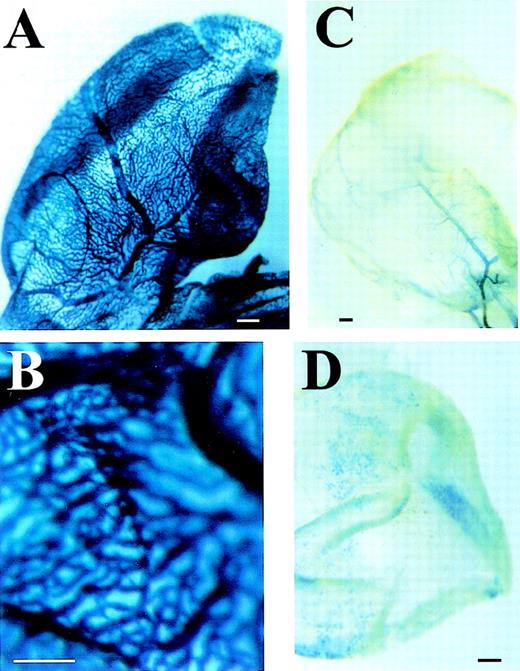
The 5′-UTR of Flk-1 is required for expression in the yolk sac vasculature.
In Flk-1/LacZ knock-in embryos, the LacZ gene is under control of all endogenous regulatory elements except for the regions from bp +137 to bp +299 in the 5′-UTR and approximately the first 600 bp of the first intron.6 In this study we have shown that these intron sequences are not required to generate the strong and uniform endothelial-specific reporter gene expression (Fig 2B and Table1). The uniform vascular LacZ expression in the transgenic yolk sacs (Fig 5A, B) was absent in small vessels of the yolk sacs of the Flk-1/LacZ knock-in embryos (Fig 5C), in which only large yolk sac vessels were stained. This indicates that the region from bp +137 to bp +299 of the Flk-1 5′-UTR is required for uniform Flk-1 expression in yolk sac vessels. To verify this hypothesis, we further analyzed the effect of this deletion in transgenic mice. Deletion of the 5′-UTR sequences from bp +137 to bp +299 in the transgene construct analyzed in Fig 5A resulted in a diminished and incomplete LacZ expression in the yolk sacs of all five transgenic mouse lines tested (Fig 5D), and in a reduced frequency of transgenic embryos expressing LacZ (Table 1, construct −640/+137//3′ Intron+1677/+3947). Replacement of the entireFlk-1 promoter including the 5′-UTR by the tkpromoter in the transgenic construct also nearly abolished LacZ expression in the yolk sac (data not shown), and led to a reduced expression frequency in transgenic embryos (Table 1). Thus, the 5′-UTR might be involved in specifying Flk-1 expression in a subset of endothelial cells in the yolk sac.
The 5′-UTR is required for expression of theFlk-1 gene in the yolk sac vasculature. Transgenic mouse embryos that carry a Flk-1 promoter and 5′-UTR (−640 bp/+299 bp)/enhancer (+1677 bp/+3947 bp) reporter gene construct show a uniform vascular expression in the yolk sac vasculature (A and B). In contrast, the yolk sac of Flk-1/LacZ knock-in embryos that lack part of the 5′-UTR (+137 bp to +299 bp) shows expression only in large collecting vessels that connect with the embryo, but not in the smaller vessels (C). Transgenic yolk sacs that carry a Flk-1 promoter (−640 bp/+137 bp) / enhancer (+1677 bp/+3947 bp) reporter gene construct show a diminished and incomplete vascular LacZ expression (D) when compared with the construct containing the complete 5′-UTR. (A and B) Bar, 500 μm.
The 5′-UTR is required for expression of theFlk-1 gene in the yolk sac vasculature. Transgenic mouse embryos that carry a Flk-1 promoter and 5′-UTR (−640 bp/+299 bp)/enhancer (+1677 bp/+3947 bp) reporter gene construct show a uniform vascular expression in the yolk sac vasculature (A and B). In contrast, the yolk sac of Flk-1/LacZ knock-in embryos that lack part of the 5′-UTR (+137 bp to +299 bp) shows expression only in large collecting vessels that connect with the embryo, but not in the smaller vessels (C). Transgenic yolk sacs that carry a Flk-1 promoter (−640 bp/+137 bp) / enhancer (+1677 bp/+3947 bp) reporter gene construct show a diminished and incomplete vascular LacZ expression (D) when compared with the construct containing the complete 5′-UTR. (A and B) Bar, 500 μm.
The Flk-1 promoter is activated by HIF-2α.
As shown above, the Flk-1 promoter (−640 bp/+299 bp) contains positive-acting regulatory sequences required for a strong and reproducible reporter gene transcription in transgenic mice. This suggests that transcription factors that are specifically expressed in endothelial cells activate the Flk-1 promoter in a cell-type–specific manner.
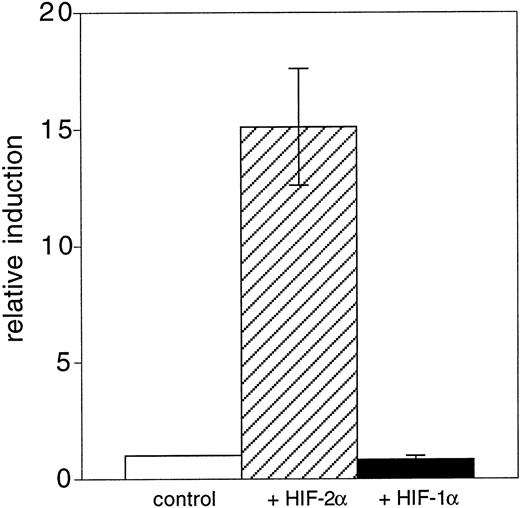
The basic helix-loop-helix PAS-domain transcription factor, HIF-2α (also known as HLF, HRF, or EPAS1), is prominently expressed in endothelial cells during mouse embryonic development19-21and is thus a candidate regulator of Flk-1 expression. To determine if HIF-2α might be involved in the regulation ofFlk-1 gene expression, we cotransfected A293 cells with a luciferase reporter gene construct containing Flk-1 promoter sequences (−640 bp to +299 bp) and an eukaryotic expression vector that contained the mouse HIF-2α cDNA. In comparison to cells transfected with the luciferase reporter construct alone, cotransfection of the HIF-2α construct increased reporter gene activity approximately 15-fold (Fig 6). In contrast, HIF-1α, a close relative of HIF-2α that stimulates the hypoxia-induced transcription of the VEGF gene, failed to stimulate the reporter construct (Fig 6).
HIF-2 stimulates Flk-1 gene expression. A293 cells were cotransfected with a reporter gene construct containingFlk-1 promoter sequences from −640 bp to +299 bp and with expression vectors encoding the murine HIF-1 or HIF-2 cDNAs, respectively. Relative promoter activities were determined as described in the Materials and Methods section. The promoter activity of the control transfection was arbitrarily set to 1.
HIF-2 stimulates Flk-1 gene expression. A293 cells were cotransfected with a reporter gene construct containingFlk-1 promoter sequences from −640 bp to +299 bp and with expression vectors encoding the murine HIF-1 or HIF-2 cDNAs, respectively. Relative promoter activities were determined as described in the Materials and Methods section. The promoter activity of the control transfection was arbitrarily set to 1.
DISCUSSION
The murine Flk-1 receptor is crucial for the differentiation of the hemangioblastic lineage and during embryonic vascular development.1,2,6 Moreover, Flk-1 plays a central role in the regulation of neovascularization in a wide variety of tumors.34,35 To elucidate the basis of its endothelial expression, we have isolated and characterized regulatory sequences of the murine Flk-1 gene that confer endothelium-specific reporter gene expression in transgenic mouse embryos. Transgene expression driven by these sequences was strong, specific, and highly reproducible. Most importantly, we have shown that the isolated sequences were active in early-stage vascular development and may thus represent a clue towards the identification of the molecular mechanisms involved in hemangioblast differentiation and vasculogenesis. Moreover, transgene expression persisted until shortly after birth and was downregulated in adult animals, as it was described for the endogenousFlk-1 gene.13 28
The endothelium-specific expression in transgenic mouse embryos was mediated by a 939 bp fragment of the promoter region only in combination with a fragment of the first intron. 5′-flanking fragments up to −5.5 kbp alone were not sufficient to confer a reproducible endothelium-specific transgene expression. Reproducible endothelium-specific expression was therefore dependent on sequences from the first intron. These sequences also activated the heterologoustk promoter specifically in endothelial cells in vivo, and were active in an orientation-independent manner. Thus, they fulfill the criteria for an autonomous tissue-specific enhancer.
The endothelium-specific enhancer sequences were contained in a 510 bp intron fragment. Up to now, we have not observed endothelium-specific expression with shorter fragments, suggesting that multiple regulatory elements are clustered in this region. Several potential binding sites for transcription factors could be identified therein, including consensus binding sites for c-ets1, PEA3 (an Ets-like transcription factor), GATA transcription factors, and Scl/Tal-1. The c-ets1 transcription factor was proposed to be involved in the early differentiation of endothelial cells from their precursors,33 and c-ets1 is expressed in endothelial cells during tumor vascularization and other forms of angiogenesis in humans.32 Transcription factors of the GATA family are involved in the transcription of genes that are expressed in the hematopoietic and endothelial lineages, such as von Willebrand factor,36 and Scl/Tal-1 has recently been implicated in the regulation of Flk-1 expression in zebrafish.29However, no direct effect of Scl/Tal-1 on Flk-1 expression has been observed so far in mice, although Scl-null mice have vascular defects.30
Recently, analyses of the regulatory elements of other endothelium-specific genes such as von Willebrand factor,37c-ets-1,38 or the endothelial receptorsTie139 and Tie225,26 have been reported. The most uniform vascular expression pattern reported was conferred by regulatory elements of the Tie2 gene. As it is the case for Flk-1, the first intron of the Tie2 gene also contains an autonomous endothelium-specific enhancer with potential binding sites of the Ets and GATA families.26 A major difference between the structural organization of the regulatory elements of the Flk-1 gene and the Tie2 gene is, however, that the Tie2 promoter by itself is active in certain embryonic blood vessels.25 Further studies will show whether common mechanisms are involved in the regulation of various endothelium-specific genes.
Analysis of Flk-1/LacZ knock-in mouse embryos that express the LacZ gene from the endogenous Flk-1 locus has previously shown that the LacZ reporter gene is expressed ubiquitously in the developing vasculature of E7.5 embryos.6 However, we have found that a fragment of the 5′-UTR that is deleted in the knock-in construct is required for reporter gene expression in the yolk sac vasculature during later stages of embryonic development. In addition, deletion of this sequence between nucleotides +136 and +299 of the Flk-15′-UTR in the transgenic construct reduced the expression frequency of the reporter gene. Based on transient transfection analyses in BAE cells, this sequence has been shown to contain a positive-acting, endothelial cell-specific element.17Currently, we are investigating if proteins that specifically bind to the 5′-UTR are involved in endothelial-specific transcription. The Flk-1 promoter appears to contain additional positive-acting regulatory sequences that are required for a strong and reproducible endothelium-specific expression in the embryo proper. This assumption is supported by the observation that the heterologous tkpromoter, when combined with the intron enhancer, showed significantly lower reporter gene expression levels and a reduced expression frequency in transgenic mouse embryos, when compared with the Flk-1 promoter. A positive activity of the Flk-1promoter sequences has already been suggested by our previous studies in transfected BAE cells.17 Based on the stimulation ofFlk-1 promoter activity in vitro and its endothelial expression, it seems likely that HIF-2α regulates the Flk-1promoter in vivo. The involvement of HIF-2α in the regulation ofFlk-1 expression further emphasizes the role of basic helix-loop-helix/PAS-domain transcription factors in the regulation of components of the VEGF signal transduction system and of vascular development. In particular, this has been shown in mouse embryos lacking functional genes for HIF-1α40 or ARNT41 that show defects in vascular development, perhaps due to reduced VEGF expression levels. HIF-2α is expressed most prominently in the endothelium of several developing organs, for example in the brain.20 It seems therefore likely that HIF-2α is involved in the regulation of Flk-1 expression in blood vessels that coexpress both HIF-2α and Flk-1. Interestingly, HIF-2α is also expressed in tissues that express the Flk-1 receptor ligand, VEGF, and has been shown to stimulate VEGF expression.19 Taken together, these observations support our hypothesis that HIF-2α is both an intrinsic and extrinsic regulator of blood vessel growth and function,20 by stimulating both receptor and ligand expression.
Among the endothelial receptor tyrosine kinases identified thus far, Flk-1 is the only receptor whose function is required for the determination of the endothelial lineage. Therefore, the Flk-1gene represents the ideal candidate for studying the transcriptional regulatory mechanisms that are active during the emergence of the endothelial lineage. The observation that the isolated regulatory elements of the Flk-1 gene are active in early-stage vascular development is of great importance for this objective. Knowledge of theFlk-1 gene regulatory sequences is also of great potential relevance for the therapy of certain angiogenesis-dependent diseases, including cancer. Therefore, the study of the regulatory elements involved in the upregulation of Flk-1 expression in the tumor endothelium is particularly relevant for unraveling the mechanisms of tumor angiogenesis. The analysis of Flk-1 gene regulatory elements active in the tumor vasculature will provide a clue to the signaling pathways that could be targeted for antiangiogenic tumor therapy. Finally, the Flk-1 gene regulatory elements will be useful for targeting expression of genes to the vasculature, and the use of the Flk-1 gene regulatory elements in combination with the Cre/loxP system may provide a powerful tool for specifically inactivating genes in the developing vasculature or in tumor endothelium.
ACKNOWLEDGMENT
We thank Dr Janet Rossant for kindly providing the Flk-1/LacZ knock-in mice and the gift of plasmid DNA; Gunnar Teichmann, Michael Walker, and Dr Felix Müller-Holtkamp for generating transgenic mice; Silvia Hennig, Stefanie Pebler, Marie von Reutern, and Carola Wild for DNA sequence analysis; Thorsten Schlaeger for helpful discussions and gift of plasmid DNA; and Dr Christopher Mitchell and Dr Simon Bamforth for carefully reading this manuscript and helpful suggestions.
In memoriam Werner Risau (1953-1998).
Submitted October 15, 1998; accepted February 17, 1999.
A.K. and V.R. have contributed equally to this work.
Supported in part by the SFB 397.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal