Abstract
Platelets function to protect the integrity of the vascular wall. A subset of platelet activation responses that are especially important for thrombus formation include exposure of phosphatidylserine and release of microparticles, which generate procoagulant surfaces. The resemblance of these platelet activation processes to events occurring in nucleated cells undergoing apoptosis suggests a possible role for caspases, which are major effector enzymes of nucleated cell apoptosis. We demonstrate here the presence of caspase-3 in human platelets and its activation by physiological platelet agonists. Using cell-permeable specific inhibitors, we demonstrate a role for a caspase-3–like protease in the agonist-induced (collagen plus thrombin or Ca2+ ionophore) platelet activation events of phosphatidylserine exposure, microparticle release, and cleavage of moesin, a cytoskeletal-membrane linker protein. The role of caspase-3 in platelet activation is restricted rather than global, because other activation responses, granule secretion, shape change, and aggregation were unaffected by caspase-3 inhibitors. Experiments with two classes of protease inhibitors show that caspase-3 function is distinct from that of calpain, which is also involved in late platelet activation events. These findings show novel functions of caspase and provide new insights for understanding of platelet activation.
PLATELETS ARE THE major players of hemostasis and thrombosis. Among other responses to physiological agonists, activated platelets expose negatively charged phosphotidylserine (PS) on the platelet exterior and release PS-positive microparticles, both of which contribute to fibrin deposition by providing competent surfaces for assembly of coagulation factors and thrombus formation.1 Increased levels of circulating microparticles are associated with various thrombotic disorders, including activated coagulation, transient ischemia, myocardial infarction, and postsurgery in cardiopulmonary bypass patients.2-6 For these reasons, studies to delineate the mechanisms of PS exposure and microparticle release are extremely important for understanding platelet function.
Exposure of PS and release of microparticles occur only within the larger process of platelet activation. Conversely, the activation response of platelets is distinguished by a multiplicity of subevents and regulatory mechanisms. Particularly noteworthy is the sequential nature of the response and the temporal arrangement of individual steps. Several parallels can be discerned between the programmed activation response of platelets and programmed death of nucleated cells—the apoptotic process. Common characteristics include the prominence of coordinated morphological changes and, to a large extent, the quality of irreversibility. In platelet activation, as in apoptosis, transbilayer migration of phosphatidylserine to the outer membrane leaflet is an important event, facilitating interaction with phagocytic cells and, in the case of platelets, generating, in addition, the procoagulant surface. Also, the process of platelet microparticle formation is morphologically similar to the membrane blebbing phase of nucleated cell apoptosis. These shared features prompted us to examine a possible role in platelet activation for caspases, a protease family intimately associated with the programmed death of nucleated cells.
Caspases, which are cysteinyl proteases that cleave after aspartic acid, are key effectors of apoptosis. The family consists of at least 11 enzymes in humans.7-9 In response to apoptotic signals, the proform of an initiator caspase containing an adapter domain, such as caspase-8, -9, or -10, is recruited to a surface membrane complex or a mitochondrial-derived complex, where it is autoproteolytically processed to the two subunit active form. The initially activated caspase processes/activates other procaspases that lack adapter domains, including caspase-3 and -7, and the latter, the effector caspases, incapacitate essential homeostatic pathways by limited cleavage of specific targets.
Another hallmark of nucleated cell apoptosis is cleavage of select cytoskeletal proteins by effector caspases.10,11 We focus in this study on one cytoskeletal protein, moesin, the only member in human platelets12,13 of the ERM (ezrin-radixin-moesin) family proteins, which stabilize surface projections by linking the underlying actin cytoskeleton with the plasma membrane.14,15 In response to agonist, platelet moesin undergoes rapid phosphorylation, localizes transiently to newly formed filopodial/lamellipodial projections,12,16 and subsequently is proteolytically cleaved,16a a reaction expected to terminate moesin’s linker function and facilitate late platelet cytoskeletal changes required for clot retraction and microparticle release. Moesin cleavage requires calpain (Ca2+-activated protease), which is required also for microparticle release17,18; however, pure calpain alone does not cleave moesin,13 suggesting a requirement for a second protease.
The present report breaks new ground by demonstrating the presence and novel function of caspase in human platelets. We identify the zymogen form of the effector caspase, caspase-3, as a component of human platelets and demonstrate that procaspase-3 becomes activated when isolated platelets are stimulated by physiological agonists. A specific cell-permeant inhibitor of caspase-3 is shown to abrogate agonist-induced PS exposure and microparticle release. The caspase inhibitor also prevents cleavage of the structural protein moesin in activated platelets. Comparative experiments with protease inhibitors showed that both caspase and calpain function in agonist-induced late events of platelet activation (PS exposure, microparticle release, and moesin cleavage) and demonstrate that the two proteases have distinct roles.
MATERIALS AND METHODS
Platelet isolation.
Freshly drawn blood from normal healthy donors, who gave written consent, was collected in acid-citrate-dextrose (ACD; NIH formula A) in plastic and fractionated immediately at ambient temperature. Cells were counted using a MAX-M Blood Cell Analyzer (Coulter Corp, Hialeah, FL). The blood was centrifuged at 200g for 12 minutes to separate platelet-rich plasma (PRP). Additional ACD was added (1 part ACD per 3 parts PRP) and platelets were pelleted at 800g for 15 minutes. The platelets were resuspended in platelet buffer (10 mmol/L Tris-hydroxymethyl-methyl-2-aminoethane sulphonic acid [TES], pH 7.2, 136 mmol/L NaCl, 2.6 mmol/L KCl, 0.5 mmol/L NaH2PO4, 2 mmol/L MgCl2, 0.1% glucose, and 0.1% bovine albumin) and, after the addition of ACD (20% of final volume) and prostacyclin (1 μg/mL; Calbiochem, San Diego, CA), were centrifuged at 800g for 10 minutes. The isolated platelets contained no detectable erythrocytes and less than 1 leukocyte per 4,000 platelets. For activation experiments, platelets were suspended in platelet buffer and allowed to recover for 90 minutes at 37°C to ensure their resting state.
Peptidase assay.
Platelets (5 × 108) in 1 mL of platelet buffer with 2 mmol/L CaCl2 and 3 μmol/L A23187 were incubated with stirring in flat-bottom polyethylene vials (14-mm diameter) at 37°C for the indicated time and were lysed by adding 1/3 vol of 4% Triton X-100, 8 mmol/L EGTA, 20 mmol/L dithiothreitol, 200 μg/mL aprotinin, 200 μg/mL benzamidine, and 200 μg/mL leupeptin. The Triton lysates (200 μL) were combined with 0.1 mmol/L of N-acetyl-Asp-Glu-Val-Asp-p-nitro anilide (DEVD-pNA; Enzyme Systems Products, Livermore, CA) or N-acetyl-Tyr-Val-Ala-Asp-p-nitroanilide (YVAD-pNA; Sigma, St Louis, MO). The reactions were incubated for 2 hours in flat-bottom 96-well plates at 37°C with monitoring of OD405 nm. The mean ΔOD per minute was calculated from the linear range of the reaction.
Immunoblotting.
Platelets (5 × 108/mL) were lysed by adding an equal volume of 2% sodium dodecyl sulfate (SDS), 120 mmol/L Tris-HCl, pH 6.8, 4% mercaptoethanol, 100 μg/mL leupeptin, 4 mmol/L EGTA, and 2 mmol/L diisopropyl fluorophosphate (DFP) and heating for more than 3 minutes at 100°C. The lysates were fractionated by SDS-electrophoresis on 8 or 12% polyacrylamide gels (Novex, San Diego, CA).19 Polypeptides were transferred to nitrocellulose at constant 80 mA at approximately 22°C for 16 hours. The membranes were blocked with 2% normal rabbit serum in phosphate-buffered saline with 0.05% Tween-20 for 20 minutes, washed, and incubated for 2 hours with clone 38 moesin monoclonal antibody (MoAb; 100 ng/mL) or C31720 caspase-3 MoAb (250 ng/mL; both from Transduction Laboratories, Lexington, KY), or with caspase-3 MoAb CPP32/p20-E8 (1 μg/mL; Santa Cruz, Santa Cruz, CA) or B27D8 μ-calpain MoAb20 (1 to 10,000 dilution of ascites), or, after blocking with 2% normal goat serum, with rabbit antibodies to caspase-3 (1 to 1,500 dilution; Stratagene, La Jolla, CA). The membranes were washed, incubated with125I-labeled secondary antibody, and exposed to Phosphor-screen. Bands were quantified using Phosphor-Imager Storm 860 and Image Quant v1.1 program (Molecular Dynamics, Sunnyvale, CA). As a control for the detection of active caspase-3, isolated mononuclear cells were lysed at 1.5 × 107/mL in 0.5% NP-40, 10 mmol/L Tris HCl, pH 7.4, 150 mmol/L NaCl, 2 mmol/L DFP, 2 mmol/L EGTA, and 50 μg/mL leupeptin and, after clarification by centrifugation, the lysate was incubated for 1 to 3 hours with 25 μg/mL of granzyme B21 (kindly provided by Dr Zhinan Xia, Center for Blood Research, Boston, MA).
Activation of platelet caspase.
Platelets (5 × 108) in 1 mL of platelet buffer with 2 mmol/L CaCl2 in flat-bottom polyethylene vials were incubated while stirring with 3 μmol/L A23187, 1 U/mL of human thrombin, 10 μg/mL of collagen (native collagen fibrils from equine tendons; Collagen Reagent Horn; Nycomed Arzneimittel GmbH, Munich, Germany), or the combination of thrombin and collagen at 37°C for 20 minutes with stirring. The reaction was terminated by solubilizing with SDS in preparation for immunoblotting.
Treatment with caspase and calpain inhibitors.
For inhibition experiments, carbobenzoxy-Asp(OMe)-Glu(OMe)-Val-Asp(OMe)-fluoromethylketone (DEVD-fmk), carbobenzoxy-Tyr-Val-Ala-Asp(OMe)-fluoromethyl ketone (YVAD-fmk), carbobenzoxy-Phe-Ala-fluoromethylketone (FA-fmk), or diluent was added to platelets during the final 60 minutes of preincubation, and calpeptin and E64d were added during the final 10 minutes. For moesin cleavage experiments, fluoromethylketone stocks were prepared in dimethylsulfoxide, the recommended solvent, or dimethylformamide. For PS exposure experiments, we found that preincubation of platelets with dimethylsulfoxide (0.01% to 1.0%), but not with dimethylformamide, significantly increased A23187-induced exposure of PS (data not shown). Therefore, fluoromethylketone stocks for PS exposure and all other experiments were prepared in dimethylformamide (0.2% final concentration). Using this diluent, incubation of platelets with fluoromethylketones for 1 hour did not alter resting platelet values of any of the parameters studied. Calpeptin and E64d stocks were prepared in ethanol or dimethylformamide.
Platelet activation and flow cytometry.
For PS exposure and microparticle release experiments, 107platelets in 200 μL platelet buffer with 2 mmol/L CaCl2were placed in siliconized 7 × 45 mm glass cuvettes at 37°C in an aggregation meter (DP-247E; Sienco, Morrison, CO). A23187 (1 μmol/L), thrombin (human; 1 U/mL; Sigma), or thrombin (1 U/mL) plus collagen (20 μg/mL) was added from a diluted stock and, after an initial mixing, incubation was continued without stirring at 37°C for 1 to 20 minutes. The platelet suspensions were transferred to an approximately 22°C water bath and, after 1 minute, 50 μL was combined with fluorescein isothiocyanate-labeled annexin V (annexin V-FITC; 1 μg/mL; PharMingen, San Diego, CA) and phycoerythrin (PE)-labeled anti-CD41 (GPIIb) MoAb (150 ng/mL; Coulter/Immunotech, Miami, FL) and incubated for 10 minutes at approximately 22°C. Samples were diluted fivefold with platelet buffer with 2 mmol/L CaCl2 for immediate analysis by flow cytometry.
Samples were acquired and analyzed using a FACS-Calibur flow cytometer and CellQuest software (Becton Dickinson, Mountain View, CA). Particles were gated for CD41+ to distinguish platelets and platelet-derived microparticles from electronic noise. The lower limit of the platelet gate was defined on the forward scatter profile of resting platelets, and CD41+ particles smaller than that were considered microparticles.
To measure α granule secretion, 107 platelets were activated as described above, and the reaction was stopped after 0, 30, or 60 seconds by adding an equal volume of 2% paraformaldehyde. Aliquots of the fixed platelets were incubated for 10 minutes with 1 μg/mL PE-labeled anti-CD62P (clone AC1.2 MoAb; Becton Dickinson, San Jose, CA) and examined by flow cytometry.
To measure aggregation in response to thrombin or A23187, 107 platelets were incubated as described above with stirring, and light transmission was measured as a function of time according to the manufacturer’s instruction.
Moesin degradation assay.
After preincubation of 5 × 108 platelets in 1 mL platelet buffer in flat-bottom polyethylene vials, CaCl2 (2 mmol/L) and A23187 (3 μmol/L) were added and incubation was continued for 10 or 20 minutes at 37°C with stirring. The reaction was terminated by solubilizing with SDS.
Statistical analysis.
The Student’s paired t-test was used to calculate Pvalues.
RESULTS
Detection of caspase-3 in platelets.
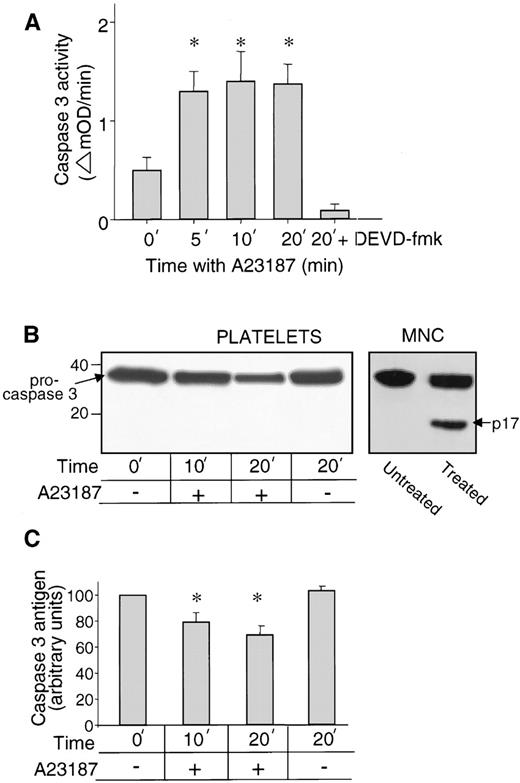
To test for the presence of caspase, isolated platelets were lysed with Triton X-100 and peptidase activity was measured by chromogenic assay with the p-nitroanilid derivative of Asp-Glu-Val-Asp as substrate (DEVD-pNA). Substrates based on DEVD are specific for effector caspases, including caspase-3.7 8 DEVD-pNA cleaving activity was detected at low levels in lysates of resting platelets and was significantly increased on stimulation of platelets with the Ca2+ ionophore A23187 (P < .01), reaching maximal levels at 5 minutes (Fig1A). In parallel assays with YVAD-pNA, a caspase-1 substrate, no activity was detected (data not shown).
Detection of caspase-3 in resting and activated platelets. (A) Caspase-3–like peptidase activity (cleavage of DEVD-pNA) of Triton X-100 lysates of resting platelets (0 time point) and platelets activated by A23187. Data shown are the mean ± SEM (n = 5). Peptidase activity was significantly increased relative to resting platelets after 5, 10, and 20 minutes with A23187 (*P < .01). In the sample 20 min + DEVD, the inhibitor DEVD-fmk was present during platelet activation and peptidase assay. (B) Caspase-3 antigen detected by immunoblot. Resting platelets and platelets activated with A23187 for 10, or 20 minutes were stained with caspase-3–specific MoAb C31720. Molecular weight marker positions are shown on the left and an arrow indicates 32-kD procaspase-3. The lanes MNC (mononuclear cell lysates treated without and with granzyme B) control for the ability to detect active caspase-3, the large subunit of which (p17) is indicated by the arrow on the right. (C). Procaspase-3 antigen quantified by immunoblots. Data shown are the mean ± SEM (n = 4). Platelet content of procaspase-3 antigen was significantly decreased relative to resting platelets after 10 (*P < .02) and 20 minutes (*P <.003) with A23187.
Detection of caspase-3 in resting and activated platelets. (A) Caspase-3–like peptidase activity (cleavage of DEVD-pNA) of Triton X-100 lysates of resting platelets (0 time point) and platelets activated by A23187. Data shown are the mean ± SEM (n = 5). Peptidase activity was significantly increased relative to resting platelets after 5, 10, and 20 minutes with A23187 (*P < .01). In the sample 20 min + DEVD, the inhibitor DEVD-fmk was present during platelet activation and peptidase assay. (B) Caspase-3 antigen detected by immunoblot. Resting platelets and platelets activated with A23187 for 10, or 20 minutes were stained with caspase-3–specific MoAb C31720. Molecular weight marker positions are shown on the left and an arrow indicates 32-kD procaspase-3. The lanes MNC (mononuclear cell lysates treated without and with granzyme B) control for the ability to detect active caspase-3, the large subunit of which (p17) is indicated by the arrow on the right. (C). Procaspase-3 antigen quantified by immunoblots. Data shown are the mean ± SEM (n = 4). Platelet content of procaspase-3 antigen was significantly decreased relative to resting platelets after 10 (*P < .02) and 20 minutes (*P <.003) with A23187.
Because caspase enzymes have overlapping substrate specificities, platelets were also tested for caspase-3 antigen by immunoblotting. The specific anti–caspase-3 MoAb C31720, which recognizes an epitope in the large subunit, detected a 32-kD band in resting platelets, procaspase-3,22 23 the single-chain zymogen form of the molecule (Fig 1B, left lane). On treatment with A23187, the platelet content of procaspase-3 decreased significantly over time (Fig 1B and C; P < .02 at 10 minutes and P < .003 at 20 minutes). However, p17, the large subunit of active caspase-3, was not detected. A positive control for the ability to detect p17 was provided by mononuclear cell lysates treated with granzyme B (Fig 1B, right lane). Similar platelet immunoblot results were obtained also with caspase-3 antibodies from rabbit and with another MoAb (CPP32/p20-E8; data not shown). These findings demonstrate the presence of caspase-3 zymogen in resting platelets and its activation in A23187-treated platelets. The decrease of zymogen combined with the absence of sufficient active caspase-3 for immunoblot detection strongly suggests that the active protease is short lived in platelets.
Physiological agonists activate platelet caspase-3.
Because A23187 is a potent but nonphysiological stimulus, we asked whether processing of platelet caspase-3 zymogen is induced also by physiological agonists. Thrombin, collagen, and the combination thrombin plus collagen were each found to induce processing of procaspase-3 (Table 1). The extent of procaspase-3 processing varied; the order of agonist efficiency was A23187 > thrombin + collagen > either collagen or thrombin.
Activation of Platelet Procaspase-3 by Physiological Agonists
| Agonist . | Procaspase-3 Cleaved (%) . |
|---|---|
| No additive | 0.3 ± 1.8 |
| Thrombin (1 U/mL) | 14.8 ± 2.8 |
| Collagen (10 μg/mL) | 14.2 ± 3.9 |
| Thrombin (1 U/mL) + collagen (10 μg/mL) | 21.0 ± 7.2 |
| A23187 (3 μmol/L) | 30.2 ± 6.3 |
| Agonist . | Procaspase-3 Cleaved (%) . |
|---|---|
| No additive | 0.3 ± 1.8 |
| Thrombin (1 U/mL) | 14.8 ± 2.8 |
| Collagen (10 μg/mL) | 14.2 ± 3.9 |
| Thrombin (1 U/mL) + collagen (10 μg/mL) | 21.0 ± 7.2 |
| A23187 (3 μmol/L) | 30.2 ± 6.3 |
Platelets were incubated with agonist for 20 minutes. Procaspase-3 content was determined by immunoblotting with C31720 MoAb and quantitation of the 32-kD band. Results are expressed as the percentage of decrease (mean ± SEM of 3 experiments) relative to nonactivated starting platelets.
Caspase inhibitor abrogates agonist-induced phosphatidylserine exposure.
We next examined whether caspase is involved in the movement of negatively charged PS from the inner to the outer platelet membrane leaflet, an activation reaction synonymous with generation of the procoagulant surface. Platelets were treated with DEVD-fmk, a cell-permeant inhibitor of caspase-3–like proteases, and then stimulated with agonist. Exposed PS was measured at fixed time points by binding of annexin V-FITC.18 In response to the potent stimulant A23187, exposure of PS was rapid and extensive; 80% ± 4% (n = 4) of platelets became PS positive in 5 minutes (eg, Fig 2A). Incubation with DEVD-fmk substantially inhibited/delayed A23187-induced PS exposure (Fig 2B). At 100 μmol/L, DEVD-fmk caused 74% ± 2% inhibition of PS exposure at 3 minutes and 40% ± 3% at 5 minutes, and FA-fmk, a chemically similar compound lacking caspase inhibitory activity, failed to inhibit PS exposure (Fig 2B). Lower concentrations of DEVD-fmk were also inhibitory, eg, 25 μmol/L inhibited A23187-induced PS exposure by 35% ± 5% at 3 minutes and 52% ± 2% at 5 minutes (n = 3).
Inhibition by the caspase inhibitor DEVD-fmk of PS exposure in platelets activated by A23187 or thrombin plus collagen. Platelets were preincubated for 1 hour with no inhibitor (A), or FA-fmk, or DEVD-fmk (100 μmol/L in [B] and 25 μmol/L in [C]) and were treated for the indicated time with A23187 (1 μmol/L) or thrombin (1 U/mL) plus collagen (20 μg/mL). (A) Platelets without inhibitor treatment. Histograms of annexin V-FITC binding show time-dependent A23187-induced conversion of platelets from PS-negative to PS-positive. (B) Inhibition by DEVD-fmk of A23187-induced PS exposure. The data are the mean percentages ± SEM (n = 4) of annexin V-positive platelets as a function of time with A23187. Note that pretreatment with DEVD-fmk, but not with FA-fmk, significantly inhibited PS exposure (*P < .005 at 3 minutes andP < .009 at 5 minutes). (C) Inhibition by DEVD-fmk of thrombin plus collagen-induced PS exposure (n = 4). Pretreatment with DEVD-fmk significantly inhibited PS exposure (*P < .001 at 5 minutes, P < .008 at 10 minutes, and P < .01 at 20 minutes). The unstimulated baseline values in (B) and (C) (dashed lines) are not significantly different for platelets preincubated without (shown) or with inhibitors.
Inhibition by the caspase inhibitor DEVD-fmk of PS exposure in platelets activated by A23187 or thrombin plus collagen. Platelets were preincubated for 1 hour with no inhibitor (A), or FA-fmk, or DEVD-fmk (100 μmol/L in [B] and 25 μmol/L in [C]) and were treated for the indicated time with A23187 (1 μmol/L) or thrombin (1 U/mL) plus collagen (20 μg/mL). (A) Platelets without inhibitor treatment. Histograms of annexin V-FITC binding show time-dependent A23187-induced conversion of platelets from PS-negative to PS-positive. (B) Inhibition by DEVD-fmk of A23187-induced PS exposure. The data are the mean percentages ± SEM (n = 4) of annexin V-positive platelets as a function of time with A23187. Note that pretreatment with DEVD-fmk, but not with FA-fmk, significantly inhibited PS exposure (*P < .005 at 3 minutes andP < .009 at 5 minutes). (C) Inhibition by DEVD-fmk of thrombin plus collagen-induced PS exposure (n = 4). Pretreatment with DEVD-fmk significantly inhibited PS exposure (*P < .001 at 5 minutes, P < .008 at 10 minutes, and P < .01 at 20 minutes). The unstimulated baseline values in (B) and (C) (dashed lines) are not significantly different for platelets preincubated without (shown) or with inhibitors.
Platelets also expose PS when stimulated by thrombin plus collagen, in which case the response is slower and less extensive; 25% ± 4% of platelets became PS-positive by 20 minutes (Fig 2C, no inhibitor). Pretreatment of platelets with DEVD-fmk, but not with FA-fmk (not shown), significantly inhibited platelet PS exposure induced by thrombin plus collagen (Fig 2C). DEVD-fmk at 25 μmol/L caused 85% ± 6% inhibition at 5 minutes, 45% ± 8% at 10 minutes, and 55% ± 10% inhibition at 20 minutes (Fig 2C). The finding of specific inhibition by DEVD-fmk of PS exposure in response to both A23187 and to thrombin plus collagen strongly indicates that a caspase-3–like enzyme is involved in agonist-induced translocation of platelet PS to the outer membrane leaflet.
Caspase inhibitor abrogates agonist-induced microparticle release.
PS exposure in activated platelets is closely linked with the release of microparticles (Zwaal and Schroit1 and Discussion). The effect of DEVD-fmk on microparticle release was examined using a flow cytometric assay to quantify microparticles (Materials and Methods). Platelet pretreatment with DEVD-fmk, but not with FA-fmk, was found to substantially inhibit/delay microparticle release in response to A23187 (Fig 3A). The extent of inhibition by 100 μmol/L DEVD-fmk was 42% ± 1% at 3 minutes and 32% ± 2% at 5 minutes (Fig 3A). Lower DEVD-fmk concentrations were also inhibitory; 25 μmol/L caused 25% ± 2% inhibition at 3 minutes and 8% ± 3% inhibition at 5 minutes (n = 3). For thrombin plus collagen-treated platelets, microparticle release was also inhibited by DEVD-fmk. DEVD-fmk at 25 μmol/L caused 57% ± 3% inhibition at 5 minutes, 47% ± 4% at 10 minutes, and 58% ± 7% at 20 minutes (Fig3B); FA-fmk had no inhibitory effect (data not shown).
Inhibition by DEVD-fmk, but not by FA-fmk, of microparticle release from platelets stimulated by (A) A23187 or (B) thrombin plus collagen. Platelets were preincubated as indicated for 1 hour with no inhibitor, FA-fmk, or DEVD-fmk (100 μmol/L in [A] and 25 μmol/L in [B]) and were then treated for the indicated time with A23187 (1 μmol/L) or thrombin (1 U/mL) plus collagen (20 μg/mL). Shown are the mean number of released microparticles (MP) ± SEM (n = 4). The unstimulated values (dashed lines) are not significantly different for platelets preincubated without (shown) and with inhibitors. Microparticle release was significantly inhibited in A23187-stimulated platelets at 3 (*P < .001) and 5 minutes (*P < .01) and in thrombin plus collagen-stimulated platelets at 5 (*P < .008), 10 (*P < .01), and 20 minutes (*P < .01).
Inhibition by DEVD-fmk, but not by FA-fmk, of microparticle release from platelets stimulated by (A) A23187 or (B) thrombin plus collagen. Platelets were preincubated as indicated for 1 hour with no inhibitor, FA-fmk, or DEVD-fmk (100 μmol/L in [A] and 25 μmol/L in [B]) and were then treated for the indicated time with A23187 (1 μmol/L) or thrombin (1 U/mL) plus collagen (20 μg/mL). Shown are the mean number of released microparticles (MP) ± SEM (n = 4). The unstimulated values (dashed lines) are not significantly different for platelets preincubated without (shown) and with inhibitors. Microparticle release was significantly inhibited in A23187-stimulated platelets at 3 (*P < .001) and 5 minutes (*P < .01) and in thrombin plus collagen-stimulated platelets at 5 (*P < .008), 10 (*P < .01), and 20 minutes (*P < .01).
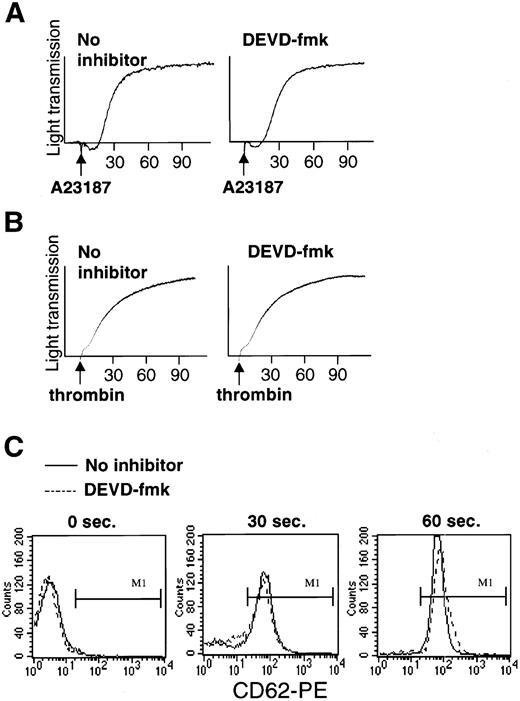
Caspase inhibitor fails to prevent agonist-induced platelet aggregation and secretion of α granules.
Studies were performed to determine whether DEVD-fmk inhibits other platelet activation responses, including the early responses of aggregation and α granule secretion. Aggregometer tracings showed that pretreatment with DEVD (100 μmol/L) did not alter the time course or amplitude of the aggregation response to A23187 (Fig 4A) or to thrombin (Fig 4B). To monitor α granule secretion, we measured surface expression of CD62P (P-selectin), an α granule membrane protein.24Pretreatment with DEVD-fmk failed to alter thrombin-induced upregulation of CD62P. Thirty seconds after thrombin addition, CD62P expression, which was negative on resting platelets, was partially upregulated in both DEVD fmk-pretreated and control platelets and was maximally upregulated in both after 60 seconds (Fig 4C). DEVD also failed to prevent agonist-induced platelet shape change, which was detected in flow cytometry by the alteration of forward and side scatter profiles (data not shown). These findings strongly suggest that caspase-3 is not required for the early platelet activation responses of α granule secretion, shape change, and aggregation.
Pretreatment of platelets with DEVD-fmk (100 μmol/L for 1 hour) does not alter agonist-induced platelet aggregation or granule secretion. (A and B) Absence of effect of DEVD-fmk on platelet aggregation induced by (A) A23187 (1 μmol/L) or (B) thrombin (1 U/mL). (C) Absence of effect of DEVD-fmk on thrombin-induced granule secretion. The histograms show surface expression of the granule membrane marker CD62P, which is absent on resting platelets (left) and upregulated on treatment with thrombin for 30 seconds (middle) or 60 seconds (right). Note that DEVD-fmk pretreatment (dashed lines) did not alter thrombin-induced upregulation of CD62P.
Pretreatment of platelets with DEVD-fmk (100 μmol/L for 1 hour) does not alter agonist-induced platelet aggregation or granule secretion. (A and B) Absence of effect of DEVD-fmk on platelet aggregation induced by (A) A23187 (1 μmol/L) or (B) thrombin (1 U/mL). (C) Absence of effect of DEVD-fmk on thrombin-induced granule secretion. The histograms show surface expression of the granule membrane marker CD62P, which is absent on resting platelets (left) and upregulated on treatment with thrombin for 30 seconds (middle) or 60 seconds (right). Note that DEVD-fmk pretreatment (dashed lines) did not alter thrombin-induced upregulation of CD62P.
Caspase inhibitor abrogates agonist-induced cleavage of platelet moesin.
The effect of DEVD-fmk was also examined on another late platelet activation response, cleavage of the cytoskeletal linker protein moesin. In the absence of inhibitor, A23187 induced cleavage of 60% ± 5% (n = 4) of moesin molecules in 20 minutes (Fig 5A, lanes 1 and 2). Cleavage of moesin was substantially inhibited in platelets pretreated with DEVD-fmk (lane 3). The extent of inhibition of A23187-induced moesin cleavage increased over the DEVD-fmk range of 5, 25, and 50 μmol/L and was complete at 50 and 100 μmol/L (Fig 5B). A23187-induced moesin cleavage was not inhibited in platelets pretreated with FA-fmk and minimally affected in platelets pretreated with YVAD-fmk, a related inhibitor with primary specificity for caspase-1–like protease. These findings strongly suggest that a caspase-3–like enzyme is required for the platelet activation event of moesin cleavage.
Inhibition by DEVD-fmk of A23187-induced cleavage of platelet moesin. (A) Platelets were preincubated for 1 hour without inhibitor or with DEVD-fmk (50 μmol/L) and treated for 20 minutes with A23187. The platelet suspensions were lysed by the addition of SDS (with protease inhibitors). Shown is an immunoblot stained with clone 38 antimoesin MoAb. (B) Quantitation of moesin immunoblots. The methods used are the same as in (A), except that platelets were preincubated with varying concentrations of FA-fmk, YVAD-fmk, and DEVD-fmk. Shown are the mean percentages of inhibition (±SEM, n = 4) of moesin cleavage relative to platelets preincubated without inhibitor.
Inhibition by DEVD-fmk of A23187-induced cleavage of platelet moesin. (A) Platelets were preincubated for 1 hour without inhibitor or with DEVD-fmk (50 μmol/L) and treated for 20 minutes with A23187. The platelet suspensions were lysed by the addition of SDS (with protease inhibitors). Shown is an immunoblot stained with clone 38 antimoesin MoAb. (B) Quantitation of moesin immunoblots. The methods used are the same as in (A), except that platelets were preincubated with varying concentrations of FA-fmk, YVAD-fmk, and DEVD-fmk. Shown are the mean percentages of inhibition (±SEM, n = 4) of moesin cleavage relative to platelets preincubated without inhibitor.
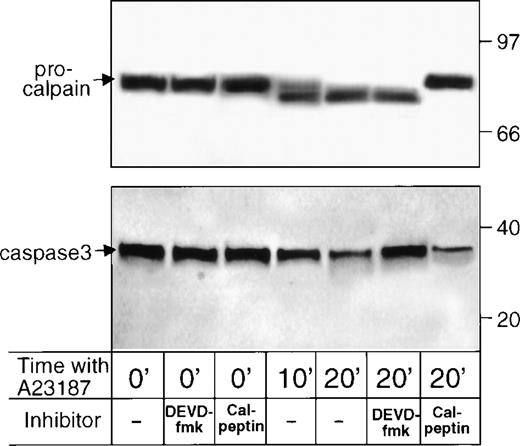
Caspase activation and calpain activation are independent events.
Previous studies demonstrated an important role for another protease, Ca2+-activated neutral protease (calpain), in a subset of platelet activation events. Specific cell permeant reagents, including E64d and calpeptin, which prevent conversion of procalpain to calpain, inhibit agonist-induced cleavage of select platelet cytoskeletal proteins, release of microparticles, and generation of prothrombinase activity.17 18 To determine whether DEVD-fmk alters platelet functions by acting on calpain, the latter protein was measured in DEVD-fmk–pretreated platelets. Reciprocal experiments were performed on calpeptin-pretreated platelets. Neither calpeptin nor DEVD-fmk detectably altered the resting platelet content of μ-procalpain (Fig 6, top panel, first 3 lanes) or procaspase-3 (lower panel, first 3 lanes). Moreover, A23187 induced the conversion of procalpain to calpain in platelets preincubated with DEVD-fmk, but not in calpeptin-pretreated platelets (Fig 6, top panel, last 4 lanes). Similarly, A23187 induced the processing of procaspase-3 in platelets preincubated with calpeptin, but not in DEVD-fmk–pretreated platelets (lower panel, last 4 lanes). These findings indicate that the alteration of platelet function by DEVD-fmk does not rely on acting through calpain; similarly, the effects of calpeptin do not entail acting through caspase-3.
Effects of pretreatment with calpeptin or DEVD-fmk on the content of μ-procalpain/μ-calpain (top panel) and procaspase-3 (lower panel) in resting and A23187-treated platelets. Platelets were preincubated with no inhibitor or calpeptin (50 μg/mL) or DEVD-fmk (25 μg/mL) and were lysed by the addition of SDS, immediately or after stimulation with A23187 for 10 or 20 minutes. Shown are immunoblots of 1.5 × 107 platelets stained with B27D8 anti–μ-calpain (top panel) or C31730 anti–caspase-3 (lower panel) MoAb.
Effects of pretreatment with calpeptin or DEVD-fmk on the content of μ-procalpain/μ-calpain (top panel) and procaspase-3 (lower panel) in resting and A23187-treated platelets. Platelets were preincubated with no inhibitor or calpeptin (50 μg/mL) or DEVD-fmk (25 μg/mL) and were lysed by the addition of SDS, immediately or after stimulation with A23187 for 10 or 20 minutes. Shown are immunoblots of 1.5 × 107 platelets stained with B27D8 anti–μ-calpain (top panel) or C31730 anti–caspase-3 (lower panel) MoAb.
Effects of calpain inhibitor and caspase inhibitor on PS exposure and microparticle release.
To compare the roles of calpain and caspase, platelets were pretreated with calpeptin and examined for agonist-induced PS exposure and microparticle release. Similar to previous reports,17 18calpeptin pretreatment significantly inhibited microparticle release in response to thrombin plus collagen (Fig7B). The extent of inhibition of microparticle release was similar for calpeptin (69% ± 6% inhibition at 20 minutes; Fig 7B) and DEVD-fmk (58% ± 7% inhibition at 20 minutes; Fig 3B), and the inclusion of DEVD-fmk together with calpeptin did not further inhibit residual microparticle release (Fig 7D).
Effects of calpeptin and DEVD-fmk on PS exposure (A and C) and microparticle release (B and D). Platelets were pretreated as indicated with no inhibitor or calpeptin (50 μg/mL) or calpeptin plus DEVD-fmk (25 μg/mL) and stimulated for the indicated times with thrombin plus collagen (1 U/mL; 20 μg/mL). (A) PS exposure induced by thrombin plus collagen was significantly increased by calpeptin at 5, 10, and 20 minutes (*P < .01, P < .001, andP < .002, respectively; n = 4). (C) PS exposure induced by agonist and enhanced by calpeptin was significantly inhibited by DEVD-fmk at 5, 10, and 20 minutes (P < .01, P < .007, and P < .001, respectively). (B) Thrombin plus collagen-induced microparticle release was significantly inhibited by calpeptin at 5, 10, and 20 minutes (P < .02, P < .005, and P < .0003, respectively) and (D) was not further inhibited by DEVD-fmk and calpeptin. Unstimulated values (dashed lines), with the exception of PS exposure after calpeptin pretreatment (shown in [A]), were not significantly different for platelets preincubated without and with inhibitors.
Effects of calpeptin and DEVD-fmk on PS exposure (A and C) and microparticle release (B and D). Platelets were pretreated as indicated with no inhibitor or calpeptin (50 μg/mL) or calpeptin plus DEVD-fmk (25 μg/mL) and stimulated for the indicated times with thrombin plus collagen (1 U/mL; 20 μg/mL). (A) PS exposure induced by thrombin plus collagen was significantly increased by calpeptin at 5, 10, and 20 minutes (*P < .01, P < .001, andP < .002, respectively; n = 4). (C) PS exposure induced by agonist and enhanced by calpeptin was significantly inhibited by DEVD-fmk at 5, 10, and 20 minutes (P < .01, P < .007, and P < .001, respectively). (B) Thrombin plus collagen-induced microparticle release was significantly inhibited by calpeptin at 5, 10, and 20 minutes (P < .02, P < .005, and P < .0003, respectively) and (D) was not further inhibited by DEVD-fmk and calpeptin. Unstimulated values (dashed lines), with the exception of PS exposure after calpeptin pretreatment (shown in [A]), were not significantly different for platelets preincubated without and with inhibitors.
On the other hand, calpeptin pretreatment did not inhibit PS exposure in response to thrombin plus collagen; rather, PS exposure was significantly increased (140% ± 8% increase at 20 minutes of stimulation; Fig 7A). Calpeptin pretreatment also slightly increased spontaneous PS exposure (Fig 7A, 0-minute value). Enhancement of thrombin plus collagen-induced PS exposure was observed also when platelets were pretreated with the chemically unrelated cell permeant calpain inhibitor E64d (data not shown), and enhancement by E64d was previously reported for A23187-induced PS exposure.18Inclusion of DEVD-fmk together with calpeptin in the platelet pretreatment significantly and substantially inhibited PS exposure in response to thrombin plus collagen, including abrogation of the enhancing effect of calpeptin (Fig 7C). Together, these findings show that caspase and calpain both function in specific late events of platelet activation and that the two proteases have distinct functional roles.
DISCUSSION
Our studies with specific reagents demonstrate the presence of at least one caspase family protease in platelets. Peptidase activity corresponding to the effector protease caspase-3 was detected in lysates of resting platelets and was significantly increased in A23187-activated platelets. Specific antibodies identified the zymogen procaspase-3 in resting platelets, and stimulation with agonist induced processing of procaspase-3. Active platelet caspase-3 appears to be short-lived, because it did not accumulate at levels adequate for detection by immunoblot.
Processing/activation of platelet procaspase-3 was induced by several characterized platelet agonists. The order of agonist effectiveness in activating procaspase-3, A23187 > thrombin + collagen > either thrombin or collagen (Table 1), is the same as their order of effectiveness in inducing PS exposure,25,26 microparticle release,25,26 and moesin cleavage.16a
Three agonist-induced platelet activation responses were found to be significantly inhibited by the specific caspase inhibitor DEVD-fmk, which has the greatest specificity for the effector proteases caspase-3 and caspase-7.7 8 DEVD-fmk inhibited A23187-induced cleavage of the cytoskeletal protein moesin in a dose-dependent manner and inhibition was complete at 50 μmol/L. DEVD-fmk inhibited exposure of platelet PS and release of microparticles in response to A23187 and to thrombin plus collagen. The extent of DEVD-fmk inhibition of PS exposure was dose-dependent. The finding of inhibition of these three platelet responses by DEVD-fmk strongly indicates that caspase-3, or a caspase with specificity similar to caspase-3, is required for a subset of late-occurring platelet activation events: moesin cleavage, PS exposure, and microparticle release. In contrast, treatment with DEVD-fmk had no effect on other (earlier) events of platelet activation, including α granule secretion, shape change, and aggregation. Thus, the caspase-3 inhibitor is not a global inhibitor of platelet responses, but rather is directed to a subset of events, including those that are important for the generation of the procoagulant platelet and microparticle surfaces.
Another protease, calpain, was previously shown to be involved in a subset of platelet activation events, not including aggregation, shape change, or secretion,27 and we, therefore, performed parallel testing of calpain inhibitors and caspase inhibitors. These experiments showed that the calpain inhibitor calpeptin and the caspase-3 inhibitor DEVD-fmk are comparably effective in preventing microparticle release in thrombin plus collagen-treated platelets. On the other hand, calpeptin pretreatment substantially increases PS exposure in response to thrombin plus collagen, whereas DEVD-fmk inhibits agonist-induced PS exposure, including abrogating the increase effected by calpeptin.
Whereas DEVD-fmk completely inhibited agonist-induced moesin cleavage, inhibition of PS exposure was only partial. Possibly, low levels of caspase that escape inhibition may suffice to support partial PS exposure, but not moesin cleavage. Alternatively, caspase may be absolutely required for moesin cleavage, but platelets may have caspase-dependent and caspase-independent pathways for activating PS exposure. Support for the latter possibility is provided by the finding that PS exposure in nucleated cells undergoing apoptosis is also abrogated by inhibitors of caspase-3, including DEVD-fmk.28,29 However, in Jurkat T cells, PS exposure is caspase-dependent when apoptosis is induced by Fas ligand, but caspase-independent when the inducing agent is cytolytic granules.30
For rapid exposure of PS, platelets rely on a Ca2+-dependent mechanism, likely involving the recently cloned lipid scramblase, a transmembrane protein with a Ca2+ binding motif that rapidly moves phospholipids between membrane leaflets.31,32 Other requirements for activation of this mechanism are not known. Induction of platelet PS exposure and microparticle release generally occur as linked processes. For example, thrombin, a potent stimulator of aggregation, is a weak inducer of both PS exposure and microparticle release, which are best stimulated by A23187 or thrombin + collagen or collagen in that order of effectiveness.25,26 In Scott syndrome, an inherited bleeding disorder characterized by impaired lipid scramblase activity, ie, failure of agonist-induced PS exposure, patient platelets also fail to release microparticles.33 By strictly regulating intracellular Ca2+ levels34 or through the use of calpain inhibitors, PS exposure can be induced without microparticle release (eg, Fig 7), but agonist-induced microparticle release has not been observed in the absence of PS exposure. Such instances of apparent linkage have led several investigators to hypothesize that PS exposure (loss of lipid asymmetry) is required for microparticle release.1 35
Thus, of the agonist-induced activation events examined in this study, aggregation, shape change, and granule secretion, previously found to be unaffected by calpain inhibition,27 are also unaffected by caspase-3 inhibition (Table 2). PS exposure was inhibited by caspase-3 inhibitor and not by calpain inhibitor; rather, (agonist-induced) PS exposure was substantially enhanced in calpeptin-pretreated platelets. In a recent study, another platelet activation event, filopod extension in response to A23187, was also enhanced in calpeptin-pretreated platelets.36 Finally, moesin cleavage and microparticle release are prevented by either calpain inhibition or caspase-3 inhibition (Table 2).
Summary of Effects of Protease Inhibitors in Platelet Activation Events
| Agonist-Induced Event . | Effect of . | |
|---|---|---|
| DEVD-fmk . | Calpeptin . | |
| α-Granule release | No effect* | No effect27 |
| Shape change | No effect* | No effect27 |
| Aggregation | No effect* | No effect27 |
| PS exposure | Inhibited* | Enhanced18* |
| Filopodia extension | Not examined | Enhanced36 |
| Moesin cleavage | Inhibited* | Inhibited† |
| Microparticle release | Inhibited* | Inhibited17 18* |
| Agonist-Induced Event . | Effect of . | |
|---|---|---|
| DEVD-fmk . | Calpeptin . | |
| α-Granule release | No effect* | No effect27 |
| Shape change | No effect* | No effect27 |
| Aggregation | No effect* | No effect27 |
| PS exposure | Inhibited* | Enhanced18* |
| Filopodia extension | Not examined | Enhanced36 |
| Moesin cleavage | Inhibited* | Inhibited† |
| Microparticle release | Inhibited* | Inhibited17 18* |
Findings of the present study.
Shcherbina et al16a and present study (data not shown).
Together, these findings strongly indicate that caspase-3 functions in agonist-induced activation of the PS exposing mechanism (Table 3), possibly by cleavage/activation of a component of the PS exposing enzyme complex or by cleavage/destruction of an inhibitory/regulatory protein. Based on the findings that PS exposure is potentiated by calpeptin and E64d, we hypothesize that calpain normally terminates the action of the PS exposing system (Table 3). Calpain may act also to terminate filopod extension.36 Whether caspase-3 acts directly at the level of micro particle release cannot be concluded from the present data, because inhibition of PS exposure by DEVD-fmk may suffice to prevent microparticle release. Likewise, the apparent requirement for both calpain and caspase-3 to cleave moesin requires further study. These cumulative findings confirm a direct role for calpain in microparticle release17 18 and demonstrate a distinct role for caspase-3 in activating PS exposure in response to agonist.
Hypothesized Roles of Caspase-3 and Calpain in Platelet Activation Events
| Agonist-Induced Event . | Hypothesized Direct Action of . | |
|---|---|---|
| Caspase-3 . | Calpain . | |
| α-Granule release | − | − |
| Shape change | − | − |
| Aggregation | − | − |
| Activation of PS exposing enzyme | + | − |
| Termination of PS exposing system | − | + |
| Termination of Filopod extension | Not examined | + |
| Microparticle release | (+)3-150 | + |
| Moesin cleavage | (+)3-150 | (+)3-150 |
| Agonist-Induced Event . | Hypothesized Direct Action of . | |
|---|---|---|
| Caspase-3 . | Calpain . | |
| α-Granule release | − | − |
| Shape change | − | − |
| Aggregation | − | − |
| Activation of PS exposing enzyme | + | − |
| Termination of PS exposing system | − | + |
| Termination of Filopod extension | Not examined | + |
| Microparticle release | (+)3-150 | + |
| Moesin cleavage | (+)3-150 | (+)3-150 |
(+) indicates uncertain.
Although best known as effectors of apoptosis, caspases function also in maturation of the cytokines, interleukin-1 by caspase 137,38 and interleukin-16 by caspase 3.39 Also, caspase-3 activity was found in nonapoptotic mitogen-activated T cells,40-42 and a caspase-3–like protease functions in terminal differentiation of lens epithelial cell.43 The present platelet findings represent an additional example of caspase function in a nonapoptotic setting.
Recent studies have identified naturally occurring human caspase inhibitors, the IAP (inhibitors-of-apoptosis) family of protease inhibitors44 and the related tumor cell protein survivin.45 These discoveries suggest pharmacological therapies to induce apoptosis, eg, of tumor cells, or to prevent apoptosis, eg, in neurodegenerative disease. These efforts have relevance to platelet function in coagulation and thrombosis in that agents that inhibit caspases may be useful to prevent pathological thrombotic events. From another perspective, it would be prudent that clinical trials to alter apoptosis by targeting caspases be designed bearing in mind the possible effect of the therapy on platelet function in coagulation and thrombosis.
Altogether, these findings provide a basis for in-depth studies of the molecular mechanisms of caspase family protease action in platelet activation and programmed generation of procoagulant platelets.
ACKNOWLEDGMENT
The authors thank Drs Zhinan Xia and Judy Lieberman for providing granzyme B, Dr Anthony Bretscher for advice, Dr Dianne Kenney for advice and the use of equipment, and Drs John Hartwig and Andrey Prodeus for critical reading of the manuscript. We are grateful to the blood donors for their cooperation.
Supported by National Institutes of Health Grant No. AI39574.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Eileen Remold-O’Donnell, PhD, The Center for Blood Research, 800 Huntington Ave, Boston, MA, 02115; e-mail:remold@cbr.med.harvard.edu.

![Fig. 2. Inhibition by the caspase inhibitor DEVD-fmk of PS exposure in platelets activated by A23187 or thrombin plus collagen. Platelets were preincubated for 1 hour with no inhibitor (A), or FA-fmk, or DEVD-fmk (100 μmol/L in [B] and 25 μmol/L in [C]) and were treated for the indicated time with A23187 (1 μmol/L) or thrombin (1 U/mL) plus collagen (20 μg/mL). (A) Platelets without inhibitor treatment. Histograms of annexin V-FITC binding show time-dependent A23187-induced conversion of platelets from PS-negative to PS-positive. (B) Inhibition by DEVD-fmk of A23187-induced PS exposure. The data are the mean percentages ± SEM (n = 4) of annexin V-positive platelets as a function of time with A23187. Note that pretreatment with DEVD-fmk, but not with FA-fmk, significantly inhibited PS exposure (*P < .005 at 3 minutes andP < .009 at 5 minutes). (C) Inhibition by DEVD-fmk of thrombin plus collagen-induced PS exposure (n = 4). Pretreatment with DEVD-fmk significantly inhibited PS exposure (*P < .001 at 5 minutes, P < .008 at 10 minutes, and P < .01 at 20 minutes). The unstimulated baseline values in (B) and (C) (dashed lines) are not significantly different for platelets preincubated without (shown) or with inhibitors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/12/10.1182_blood.v93.12.4222/4/m_blod41234002x.jpeg?Expires=1765912722&Signature=fO1ymVDFEAaedIsD0mHMG7nHbOrTke5yUOnJfwCfY8x374PEqqeW--VnsT2SLTPsFoYMSbBKsny7V4jF437aVB7xAPx7fnrbxmiCTmEcrSf~PtPjHdox1c7oaSritHPVAS3ZeW0GTqSc5plyYq0L0BHVR4~bX-j8eM39i4A-Jo9~4WZ0VKhSDRic1rMtrAo09~tUDZh8A5GdrxcGw-mki1TU2f1jHC4ISassIz1rXdY-ofNz6aFc98pC5PiI9NTZj6a57q-FDwSEH2E-pCcqFXiV9b41npfxMSvkmwhAI9V1HuTCHmuCKnhePISxUlzTKvSKzwFiF6EmfasmItQlYA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Inhibition by DEVD-fmk, but not by FA-fmk, of microparticle release from platelets stimulated by (A) A23187 or (B) thrombin plus collagen. Platelets were preincubated as indicated for 1 hour with no inhibitor, FA-fmk, or DEVD-fmk (100 μmol/L in [A] and 25 μmol/L in [B]) and were then treated for the indicated time with A23187 (1 μmol/L) or thrombin (1 U/mL) plus collagen (20 μg/mL). Shown are the mean number of released microparticles (MP) ± SEM (n = 4). The unstimulated values (dashed lines) are not significantly different for platelets preincubated without (shown) and with inhibitors. Microparticle release was significantly inhibited in A23187-stimulated platelets at 3 (*P < .001) and 5 minutes (*P < .01) and in thrombin plus collagen-stimulated platelets at 5 (*P < .008), 10 (*P < .01), and 20 minutes (*P < .01).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/12/10.1182_blood.v93.12.4222/4/m_blod41234003x.jpeg?Expires=1765912722&Signature=NYoEkq2gfPBTsvLazmD2OKoDMuI8b-IdrzwmgCsvH2V3vI~9eZbxFSzbv9w9mmmhbiNEVgBOQ7zwwnYE5lCZsl6QfrF-9BDtC16GpqdajHtI8MeExSuYJbDDLI-k~nXR~Y7s7sLI5Ub89xaM5E7sienH-Zg~cCSn72QxjhUjBcK0387kidcF4DSXoJ9QXQMEuvsKF8eEj2H49o~fBVcqkSEI9gbGWdCLBhodHwWaj3JiUr5Z0gAvvEJ-y-IIdzuPVSJqY~aiqMqHBy4c3zSwCwLqdq8LElkomq1shN26L2XuUj~N7sEEGzMlZoENCl3kout1-f~~6Dhq3cnr5egWbw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 7. Effects of calpeptin and DEVD-fmk on PS exposure (A and C) and microparticle release (B and D). Platelets were pretreated as indicated with no inhibitor or calpeptin (50 μg/mL) or calpeptin plus DEVD-fmk (25 μg/mL) and stimulated for the indicated times with thrombin plus collagen (1 U/mL; 20 μg/mL). (A) PS exposure induced by thrombin plus collagen was significantly increased by calpeptin at 5, 10, and 20 minutes (*P < .01, P < .001, andP < .002, respectively; n = 4). (C) PS exposure induced by agonist and enhanced by calpeptin was significantly inhibited by DEVD-fmk at 5, 10, and 20 minutes (P < .01, P < .007, and P < .001, respectively). (B) Thrombin plus collagen-induced microparticle release was significantly inhibited by calpeptin at 5, 10, and 20 minutes (P < .02, P < .005, and P < .0003, respectively) and (D) was not further inhibited by DEVD-fmk and calpeptin. Unstimulated values (dashed lines), with the exception of PS exposure after calpeptin pretreatment (shown in [A]), were not significantly different for platelets preincubated without and with inhibitors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/12/10.1182_blood.v93.12.4222/4/m_blod41234007x.jpeg?Expires=1765912722&Signature=CjVN1LTf-ePofDodEbnE-R8SQm4jOD6n11KHcySJNFsaumBrNFNmd1LER8hi4nxjajSkitWeNZOJGOCICwpxaRXFIDdl0WVMOQ2tYEvtdwXQAKlsY81d89HPBnI3UHXCbUCNaxaCzPD4nZmmCoLuMEbMa1JZmtd3pAyk81WpXY4PA14A1u2ZEtnKIePfqVz0pPMUZMVP64QObJViZqMl~rTPYbJ0HRhKUBaaJiBZKQn8BjVHReiHbLoNkQsTgjGqIoGIxz~LC2-r9gPjM9FUTWHJ6bl51xKfOVDQSM3SBNBR2N1PgAKQCsJEi2nBmSOhYFhwKK8oHRQIAfkwvX8lqw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal