Abstract
We previously reported that the mouse GATA-2 gene is regulated by two alternative promoters (Minegishi et al, J Biol Chem, 273:3625, 1998). Although the more proximal IG (general) promoter is active in almost all GATA-2–expressing cells, the distal IS (specific) promoter activity was selectively detected in hematopoietic tissues but not in other mesodermal tissues. We report here in vivo analysis of the GATA-2 locus and its regulatory characteristics in hematopoietic tissues of transgenic mice. Transgenes containing 6 or 7 kbp of sequence flanking the 5′ end of the IS first exon direct expression of β-galactosidase or green fluorescent protein (GFP) reporter genes specifically to the para-aortic splanchnopleura, aorta-gonads, and mesonephros (AGM) region, and in the neural tissues. In situ hybridization analysis showed that reporter gene expression specifically recapitulates the endogenous expression profile of GATA-2 in these tissues. The flk-1, CD34, c-kit, and CD45 antigens were identified in the GFP-positive cells from the AGM region and fetal liver, indicating that GATA-2 is expressed in immature hematopoietic cells. Deletion of 3.5 kbp from the 5′ end of the 6.0 kbp IS promoter construct, including one of the DNase I hypersensitive sites, completely abolished hematopoietic expression. These experiments describe an early developmental GATA-2 hematopoietic enhancer located between 6.0 and 2.5 kbp 5′ to the IS exon.
HEMATOPOIESIS IS REGULATED, in part, by a network of lineage-restricted as well as ubiquitous transcription factors. In particular, the GATA transcription factor family has been shown to play prominent regulatory roles in multiple hematopoietic lineages.1,2 The founding member of the family, GATA-1, is essential for the terminal differentiation of erythroid cells, megakaryocytes, mast cells, and eosinophils.3-8 GATA-1 is also expressed from an alternative promoter and first exon in Sertoli cells of the testis.9-11 Acting at an earlier step than GATA-1, GATA-2 has been shown to be essential for early involvement in the regulatory network controlling hematopoietic progenitors.12 In gene targeting experiments, the loss of GATA-2 in mice was shown to confer embryonic lethality with severe anemia at 10-days postcoitus (dpc), and the affected embryos manifested a broad hematopoietic deficit.12 In vitro differentiation of GATA-2 (-/-) ES cells showed that GATA-2 is required for the proliferation and/or survival of definitive hematopoietic progenitors and mast cells.12
The expression of GATA-2 gene is common in hematopoietic lineages in all vertebrates.13-19 GATA-2 was originally cloned from a chicken reticulocyte complementary DNA (cDNA) library,13 and has been shown to be expressed in a wide variety of tissues (eg, endothelial cells, brain, sympathetic neurons, fibroblasts, kidney, liver, ovary, lung, and cardiac muscle).13,18,20 Within hematopoietic cell lineages, GATA-2 is expressed in the stem and/or progenitor cell fraction,14,21-23 as well as in immature erythroid cells, mast cells, eosinophils, and megakaryocytes.23-27 In Xenopus, GATA-2 is known to be expressed as a maternal messenger RNA (mRNA) in oocytes, and zygotic expression of GATA-2 mRNA starts in the ventral mesoderm, the initial site of hematopoiesis in the frog.15,16,28,29 This restricted expression of theGATA-2 gene is under the positive control of BMP-4 and the negative regulatory influence of activin and noggin.15,30In zebrafish, GATA-2 is expressed in the yolk syncytial layer and the intermediate cell mass, both of which contain hematopoietic progenitors.17 19 These data strongly imply GATA-2 as an important factor in the ontogeny of hematopoietic cell development.
We have recently found that the mouse GATA-2 (mGATA-2) gene is regulated by two promoters.31 Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis showed that the proximal (IG) promoter, a counterpart to the previously reported Xenopus32and human33 GATA-2 promoters, was generally used in adult tissues that express GATA-2 protein.31 In contrast, the distal (IS) promoter was activated specifically in hematopoietic but not in other mesodermal tissues.31 When examined in transient transfection assays, both the IS and IG promoters were regulated by regions within 100 bp from their respective transcription start sites.31 When more extensive regions of the locus were examined, no additional transcriptional activities were shown.31 Thus the data suggested that activities determined using the transient transfection assay might be somewhat different from those that must be operative in vivo.
To understand the regulatory mechanisms controlling GATA-2 gene expression in hematopoietic stem cells and progenitors, we set out to examine the regulatory activity of the mGATA-2 genomic locus in vivo using transgenic mice. Here we focused on the role of the GATA-2 IS promoter in early hematopoietic development. The results show that a discrete hematopoietic element, lying between 2.5 and 6.0 kbp 5′ to the IS promoter, directs GATA-2 gene expression in the para-aortic splanchnopleura and aorta, gonads, and mesonephros (AGM) region, in which definitive hematopoiesis is believed to commence.34, 35
MATERIALS AND METHODS
In situ hybridization analysis.
Digoxygenin (DIG)-labeled riboprobes used for both the whole-mount and tissue section in situ hybridization analyses were prepared following the manufacturer’s recommendations (Boehringer Mannheim, Mannheim, Germany). Antisense and sense probes were generated from a Bluescript plasmid (Stratagene, La Jolla, CA) containing a partial mGATA-2 cDNA (nt 170 to 871)36 using T3 or T7 RNA polymerase, respectively. To reduce nonspecific binding, these probes did not contain the zinc finger region of mGATA-2, which is highly conserved among the GATA factors.
Mapping of DNase I hypersensitive sites.
Nuclei were isolated from P815 mastocytoma cells (generously provided by Dr Atushi Ichikawa, Kyoto University, Kyoto, Japan) and digested with serial dilution of DNase I. DNA was extracted from the treated nuclei and Southern blot hybridization was performed as described previously.37 Probes used were a PstI-SacI fragment (probe a; 0.6 kbp) and a NotI-BamHI fragment (probe b; 0.6 kbp), both of which contain the IS exon.
mGATA-2 transgene constructs.
Various mGATA-2 genomic fragments lying 5′ to theNotI site in the IS exon were ligated into the XhoI site of pSVβ (Clontech, Palo Alto, CA) containing β-galactosidase (LacZ) gene. These plasmids, designated p6.0ISLacZ, p2.5ISLacZ, and p0.65ISLacZ, were injected into fertilized murine eggs. A genomic promoter fragment extending from a HindIII site (7.0 kbp 5′ to the IS exon) to the NotI site, as well as a DNA fragment containing the splice donor and acceptor sequences of pSVβ, were ligated to the green fluorescent protein (GFP) cDNA in pCMX-SAH/Y145F plasmid (the kind gift from Dr Kazuhiko Umesono, Kyoto University). A SacI-EcoRV fragment from this plasmid intermediate was then introduced into the SacI and NotI sites of pSVβ. This final construct, referred to as p7.0ISGFP, additionally contained the polyadenylation consensus sequence of pSVβ.
Transgenic mice.
Linearized plasmids were purified by NACS PREPAC (GIBCO BRL, Rockville, MD), adjusted to 5 ng/mL and injected into mouse oocytes as described.10 Transgene integration was confirmed by PCR of genomic DNA from mouse tails. The primers used were: IS primer, 5′-ACAAAAGCGGCTGTCTGCGCGACG-3′; IG primer, 5′-CACCCCTATCCCGTGAATCCG-3′; LacZ primer, 5′-GCAACGAAAATCACGTTCTTGT-3′; GFP primer, 5′-TGCCGTCGTCCTTGAAGAAGATG-3′. The transgenes copy numbers were estimated by Southern blot analysis.10 LacZ staining and RT-PCR analysis were performed as previously described.31,38 Fluorescence-activated cell sorting (FACS) was performed as previously described,31,38 using FACS Vantage and FACS Caliber equipment (Becton Dickinson, Mountain View, CA). Anti–flk-1 antibody (Avas12)39 was kindly provided by Dr Shin-ichi Nishikawa (Kyoto University), and was biotinylated using EZ-link NHS-LC-Biotin (PIERCE, Rockford, IL). Phycoerythrin (PE)-conjugated anti–c-kit, anti-Sca1 and anti-CD45 antibodies, biotin-conjugated anti-CD34 and anti-Ter119 antibodies, and streptavidin allophycocyanine (APC) were purchased from PharMingen (San Diego, CA).
RESULTS
Expression of GATA-2 in hematopoietic tissues.
To begin to elucidate the mechanisms that regulate mGATA-2 gene expression in vivo, in situ hybridization analyses were performed. Embryos at early developmental stages were analyzed by whole-mount in situ hybridization using both sense and antisense mGATA-2 probes. At 7.5 dpc, the expression of GATA-2 mRNA was first detected in the lateral mesoderm, adjacent to the primitive streak, as well as in the extraembryonic mesoderm (Fig 1A; panel B shows a sense probe control). GATA-2 transcripts were also abundant in the ectoplacental cone (data not shown) and para-aortic splanchnopleura of 9.5 dpc embryos (Fig 1C; panel D shows a sense probe control). We also observed the positive signals in the branchial arch and neural tissues. Evaluation of coronal sections of 9.5 and 10.5 dpc embryos (Fig 1E and F, respectively) allowed us to further localize the cellular distribution of GATA-2 mRNA in the splanchnopleura and the mesoderm of the AGM region. GATA-2 expression was evident in rounded cells bordering the dorsal aorta (Fig 1F). These results suggested that mGATA-2 could be expressed in definitive hematopoietic precursors34,35 40 from the very earliest developmental stages. However, we failed to detect GATA-2 mRNA in the yolk sac, which is the site of the developing primitive hematopoietic system (data not shown).
In situ hybridization analysis of GATA-2 mRNA expression in early mouse embryos. GATA-2 mRNA expression was observed in the lateral mesoderm adjacent to primitive streak of 7.5 dpc embryo (A and B) and AGM region in 9.5 dpc embryo (C and D) with antisense probe (A and C), but not with sense probe (B and D). Arrowhead in panel A indicates the lateral mesoderm, and in panel C an arrow shows the splanchnopleura. Panels E and F are coronal sections of 9.5 and 10.5 dpc embryos analyzed with the antisense probe. Abbreviations are a, dorsal aorta; m, mesonephros; n, neural tube. Original magnifications are ×50 (E) and ×25 (F).
In situ hybridization analysis of GATA-2 mRNA expression in early mouse embryos. GATA-2 mRNA expression was observed in the lateral mesoderm adjacent to primitive streak of 7.5 dpc embryo (A and B) and AGM region in 9.5 dpc embryo (C and D) with antisense probe (A and C), but not with sense probe (B and D). Arrowhead in panel A indicates the lateral mesoderm, and in panel C an arrow shows the splanchnopleura. Panels E and F are coronal sections of 9.5 and 10.5 dpc embryos analyzed with the antisense probe. Abbreviations are a, dorsal aorta; m, mesonephros; n, neural tube. Original magnifications are ×50 (E) and ×25 (F).
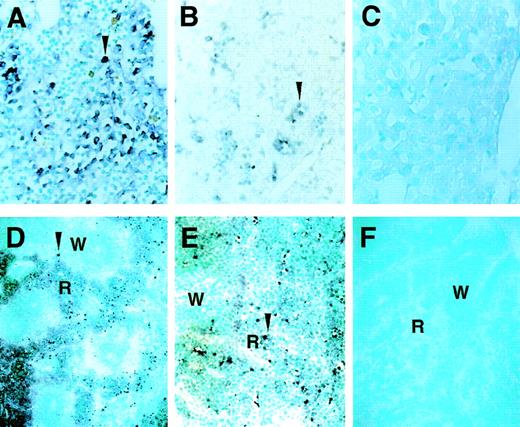
We next examined the expression of GATA-2 in the fetal liver and postnatal spleen, which are two major definitive hematopoietic tissues, by in situ hybridization. GATA-2 mRNA was easily detectable in 12.5 dpc fetal livers in the section (Fig 2A). Evaluation of GATA-2 expressing cells indicated that GATA-2 expressing cells constituted 9.4% of total fetal liver cells at 12.5 dpc (Table 1). The number of cells that expressed GATA-2 declined postnatally (Fig 2B and C and Table 1), concomitant with waning hematopoietic activity in the liver. GATA-2 signals were also observed in the red pulp of the neonatal mouse spleen, another hematopoietic organ (Fig 2D and E). In the spleens of 5-day old pups, approximately 2% of the cells in the red pulp were positive for GATA-2 expression. It is noteworthy that GATA-2 mRNA was detected only in the red pulp, but not the white pulp, of the spleen (Fig 2D). Because the white pulp consists principally of lymphocytes,41 this observation is consistent with the previous conclusion that GATA-2 is an important factor for myeloid lineage differentiation, but perhaps less so for later lymphoid differentiation.42 GATA-2–expressing cells were scarcely detectable in the adult spleen (data not shown).
Hematopoietic cell-specific expression of GATA-2 mRNA in the liver and spleen of embryos and neonates. The expression of GATA-2 mRNA in the fetal liver of 12.5 dpc embryo (A) as well as in the liver of 3-day (B) or 7-day (C) postnatal pups was analyzed by in situ hybridization analysis on tissue sections. In 5-day old pups, GATA-2 mRNA-positive cells were observed in the red pulp but not in the white pulp (R and W, respectively) of the spleen (D and E). The results with antisense (A-E) and sense (F) probes are shown. Arrowheads in panels A, B, D, and E indicate positive cells. Original magnifications are ×64 (A-C, E) and ×20 (D and F).
Hematopoietic cell-specific expression of GATA-2 mRNA in the liver and spleen of embryos and neonates. The expression of GATA-2 mRNA in the fetal liver of 12.5 dpc embryo (A) as well as in the liver of 3-day (B) or 7-day (C) postnatal pups was analyzed by in situ hybridization analysis on tissue sections. In 5-day old pups, GATA-2 mRNA-positive cells were observed in the red pulp but not in the white pulp (R and W, respectively) of the spleen (D and E). The results with antisense (A-E) and sense (F) probes are shown. Arrowheads in panels A, B, D, and E indicate positive cells. Original magnifications are ×64 (A-C, E) and ×20 (D and F).
mGATA-2 Expression in Mouse Hematopoietic Organs
| Tissue . | Stage . | Positive Cells/ Total Cells (%) . |
|---|---|---|
| Fetal Liver | 12.5 dpc | 9.4 |
| Liver | 7 days postnatal | 0 |
| Spleen | 5 days postnatal | 2.4 |
| Tissue . | Stage . | Positive Cells/ Total Cells (%) . |
|---|---|---|
| Fetal Liver | 12.5 dpc | 9.4 |
| Liver | 7 days postnatal | 0 |
| Spleen | 5 days postnatal | 2.4 |
One thousand cells per section were counted at high magnification (×200) after in situ hybridization. Those labeled with digoxigenin were scored separately to determine the percentage of GATA-2–expressing cells.
From these in situ analyses, we also found that GATA-2 was expressed in a variety of other embryonic and adult tissues. Amongst these sites, GATA-2 mRNA was most abundantly expressed in neural tissues, especially in the anterior horn of the developing spinal cord and dorsal root ganglia in 17.5 dpc embryo (Fig 3A), as well as in the cerebral cortex (Fig 3B) and Purkinje cells in the cerebellum (Fig 3C) of the adult mouse.
GATA-2 mRNA expression in neuronal cells. GATA-2 was expressed in anterior horn of the spinal cord and the dorsal root ganglia of a 17.5 dpc embryo (A). In 12-week-old adult mice, GATA-2 was expressed in the cerebral cortex (B) and Purkinje cells of the cerebellum (arrowhead; C). Original magnifications are ×10 (A), ×64 (B), and ×16 (C).
GATA-2 mRNA expression in neuronal cells. GATA-2 was expressed in anterior horn of the spinal cord and the dorsal root ganglia of a 17.5 dpc embryo (A). In 12-week-old adult mice, GATA-2 was expressed in the cerebral cortex (B) and Purkinje cells of the cerebellum (arrowhead; C). Original magnifications are ×10 (A), ×64 (B), and ×16 (C).
Three major DNase I hypersensitive sites surround the mGATA-2 IS exon.
DNase I hypersensitive sites are generally considered to be strong primary candidates for transcription regulatory regions.1 37 To locate potential regulatory regions within the mGATA-2 gene locus, we first performed DNase I hypersensitivity assays using nuclei from P815 mouse mastocytoma cells, which express abundant GATA-2. DNase I hypersensitive sites were detected at 2.7 and 0.4 kbp 5′ to the IS exon (shown as 3.1 and 0.8 kbp fragments in Fig 4A and B), as well as one located 3.0 kbp 3′ to the IS exon (shown as a 3.0 kbp fragment in Fig 4C). Several weaker bands were also detected in these experiments (Fig 4A and B).
DNase I hypersensitive sites in the upstream region of the IS and IG exons of GATA-2 gene. The top panel shows the position of DNase I hypersensitive sites in the 5′ region of the locus. Long arrows represent major hypersensitive sites and short arrows represent minor sites. Probe positions are also shown (a and b). Genomic DNA treated with DNase I were digested byKpnI/SacI (A),XhoI/SacI (B), orNotI/SalI (C), and hybridized with probe a (A and B) or probe b (C). Two major hypersensitive sites were found in the upstream region of the IS exon and two minor sites were also found at 1.8 and 1.5 kbp upstream from the SacI site. The SacI site is 0.4 kbp downstream from the transcription start site of IS exon. One major hypersensitive site exists in 3.0 kbp downstream from the NotI site in the IS exon (C).
DNase I hypersensitive sites in the upstream region of the IS and IG exons of GATA-2 gene. The top panel shows the position of DNase I hypersensitive sites in the 5′ region of the locus. Long arrows represent major hypersensitive sites and short arrows represent minor sites. Probe positions are also shown (a and b). Genomic DNA treated with DNase I were digested byKpnI/SacI (A),XhoI/SacI (B), orNotI/SalI (C), and hybridized with probe a (A and B) or probe b (C). Two major hypersensitive sites were found in the upstream region of the IS exon and two minor sites were also found at 1.8 and 1.5 kbp upstream from the SacI site. The SacI site is 0.4 kbp downstream from the transcription start site of IS exon. One major hypersensitive site exists in 3.0 kbp downstream from the NotI site in the IS exon (C).
GATA-2–directed LacZ reporter gene expression in hematopoietic cells.
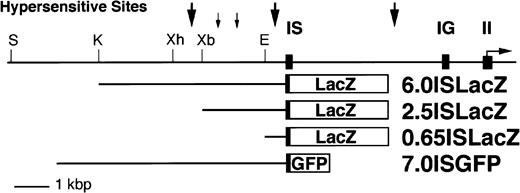
To test whether any of the DNase I hypersensitive sites identified in the previous section have transcriptional regulatory activity, we generated a series of reporter plasmids for transgenic analysis (Fig 5). We first analyzed lines bearing a transgene in which a 6.0 kbp genomic fragment 5′ to the IS exon was ligated to the LacZ gene (Fig 5).
Reporter constructs used in the transgenic assays. Three different LacZ reporter constructs, and one GFP construct, were prepared to study the IS promoter mediated transcriptional activity of the mGATA-2 gene. The open boxes represent the LacZ orGFP gene and closed boxes represent mGATA-2 gene exons. Abbreviations for the restriction enzyme sites are E, EcoRI; K,KpnI; S, SalI; Xb, XbaI; Xh, XhoI.
Reporter constructs used in the transgenic assays. Three different LacZ reporter constructs, and one GFP construct, were prepared to study the IS promoter mediated transcriptional activity of the mGATA-2 gene. The open boxes represent the LacZ orGFP gene and closed boxes represent mGATA-2 gene exons. Abbreviations for the restriction enzyme sites are E, EcoRI; K,KpnI; S, SalI; Xb, XbaI; Xh, XhoI.
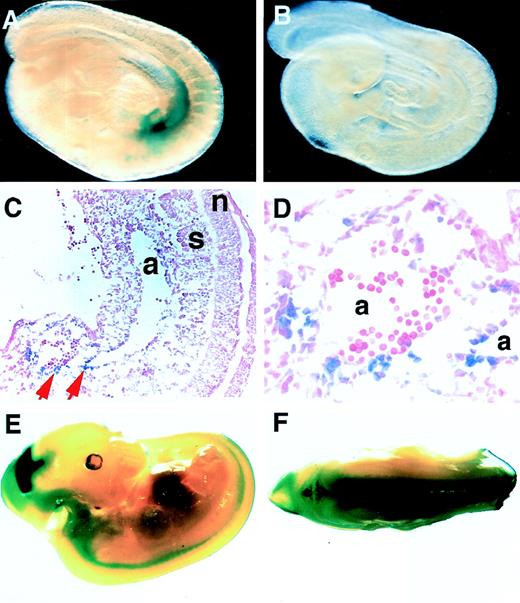
Whole-mount staining of 9.5 dpc embryos from 6.0ISLacZ line 869 showed that LacZ activity was detected in the para-aortic splanchnopleura (Fig 6A; Fig 6B shows a nontransgenic littermate). Two of five 6.0ISLacZ transgenic lines displayed similar expression (Table 2). When this transgenic embryo was sectioned saggitally, LacZ-positive cells were present in the aortic wall (Fig 6C and D, arrows). Because only two out of five 6.0ISLacZ lines showed LacZ activity in the splanchnopleura, the transcriptional activity of this region may be insufficient to overcome the suppressive environment at the transgene integration sites (Table2).
Hematopoietic and neural tissue-specific transcription in the 6.0ISLacZ transgenic mouse. Whole-mount LacZ staining of 9.5 (A and B) and 11.5 (E and F) dpc embryos with 6.0ISLacZ transgene (line 869) is shown. Panel B shows a transgene-negative littermate (9.5 dpc), and C and D is a sagittal section of A. Panel D is a higher magnification of C. Original magnifications are ×25 (C), and ×200 (D). Panel F is a dorsal view of the embryo in panel E, showing that LacZ reporter-positive cells reside in the anterior horn of the spinal cord. Upper limb buds were eliminated to show the staining in the trunks. Abbreviations: a, dorsal aorta; s, somites; n, neural tube.
Hematopoietic and neural tissue-specific transcription in the 6.0ISLacZ transgenic mouse. Whole-mount LacZ staining of 9.5 (A and B) and 11.5 (E and F) dpc embryos with 6.0ISLacZ transgene (line 869) is shown. Panel B shows a transgene-negative littermate (9.5 dpc), and C and D is a sagittal section of A. Panel D is a higher magnification of C. Original magnifications are ×25 (C), and ×200 (D). Panel F is a dorsal view of the embryo in panel E, showing that LacZ reporter-positive cells reside in the anterior horn of the spinal cord. Upper limb buds were eliminated to show the staining in the trunks. Abbreviations: a, dorsal aorta; s, somites; n, neural tube.
Summary GATA-2 Transgene Expression in Embryonic Hematopoietic and Adult Neuronal Tissues
| Construct . | Line . | Copy No. . | Para-aortic Splanchnopleura (9.5 dpc) . | Fetal Liver (11.0 dpc) . | CNS* . |
|---|---|---|---|---|---|
| 6.0ISLacZ | 385 | 1-2 | + | − | + |
| 396 | 1-2 | − | − | + | |
| 670 | 3-5 | − | − | − | |
| 869 | 1-2 | + | − | + | |
| 989 | 3-5 | − | − | − | |
| 2.5ISLacZ | 259 | 1-2 | − | − | + |
| 270 | 3-5 | − | − | + | |
| 363 | >10 | − | − | + | |
| 678 | 3-5 | − | − | − | |
| 760 | 3-5 | − | − | + | |
| 763 | 3-5 | − | − | + | |
| 0.65ISLacZ | 70 | 3-5 | − | − | + |
| 381 | 1-2 | − | − | + | |
| 7.0ISGFP | 398 | 3-5 | + | + | n.d.† |
| 982 | 1-2 | + | + | n.d.† |
| Construct . | Line . | Copy No. . | Para-aortic Splanchnopleura (9.5 dpc) . | Fetal Liver (11.0 dpc) . | CNS* . |
|---|---|---|---|---|---|
| 6.0ISLacZ | 385 | 1-2 | + | − | + |
| 396 | 1-2 | − | − | + | |
| 670 | 3-5 | − | − | − | |
| 869 | 1-2 | + | − | + | |
| 989 | 3-5 | − | − | − | |
| 2.5ISLacZ | 259 | 1-2 | − | − | + |
| 270 | 3-5 | − | − | + | |
| 363 | >10 | − | − | + | |
| 678 | 3-5 | − | − | − | |
| 760 | 3-5 | − | − | + | |
| 763 | 3-5 | − | − | + | |
| 0.65ISLacZ | 70 | 3-5 | − | − | + |
| 381 | 1-2 | − | − | + | |
| 7.0ISGFP | 398 | 3-5 | + | + | n.d.† |
| 982 | 1-2 | + | + | n.d.† |
No ectopic expression was observed in any of the lines.
Adult central nervous system.
Not determined.
We also examined LacZ expression in 11.5 dpc embryos of 6.0ISLacZ line 869. On whole-mount staining, LacZ reporter activity was found in neuronal cells and in the liver and heart of these embryos (Fig 6E and F). Although the embryonic liver and heart appeared to be positively stained in this embryo, signals could not be detected in the sections (data not shown). We had previously observed similar low-level transgene expression in the embryonic liver and heart.20
Because the regulatory elements controlling GATA-2transcription in hematopoietic and neural tissues appeared to reside within the 6.0ISLacZ transgene, we focused on further defining these elements by generating two smaller reporter gene constructs (Fig 5). Examination of 9.5 and 11.0 dpc litters from six lines of 2.5ISLacZ and two lines of 0.65ISLacZ transgenic mice indicated that all hematopoietic tissues were negative for transgene expression (Table 2). The 3.5 kbp differing between the two largest transgenes also contains one of the strong DNase I hypersensitive sites (see Fig 4). Taken together, these results indicate that a GATA-2 hematopoietic enhancer, which is active in the splanchnopleura of 9.5 dpc embryos, resides within the 3.5 kbp KpnI/XbaI fragment defining the 5′ boundaries of the 6.0 and 2.5 kbp IS-LacZ transgenes.
The IS promoter contributes to GATA-2 expression in the para-aortic splanchnopleura.
To further characterize the regulatory influence of this distal promoter, GFP reporter transgenic mice were prepared that contained 7.0 kbp flanking the IS first exon (7.0ISGFP, see Fig 5). The use of the GFP reporter enabled us to sensitively analyze live hematopoietic cells. Two lines of 7.0ISGFP mice (398 and 982) displayed expression that was similar to that observed in the 6.0ISLacZ transgenic lines in both hematopoietic as well as neural tissues.
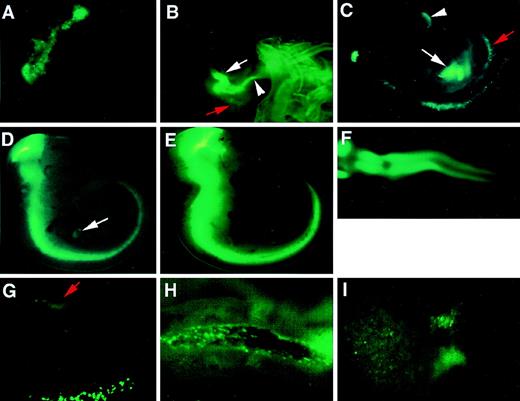
In 8.5 dpc 7.0ISGFP transgenic embryos, a ring of green fluorescence was observed in the yolk sac (Fig 7A). We previously observed a similar expression pattern when a LacZ reporter gene was placed under the control of a GATA-1 transgene.10Thus, the expression profiles of GATA-2 and GATA-1genes appeared to overlap at this developmental stage. However, although GATA-1-LacZ gene expression persists and expands after 8.5 dpc, the number of GATA-2–directed GFP-positive cells markedly diminished in the yolk sac blood islands after this stage (data not shown).
Hematopoietic and neural tissue-specific transcription in the 7.0ISGFP transgenic mouse. Whole-mount analysis of GFP expression in 7.0ISGFP transgenic mouse embryos (line 398). GFP-positive cells were identified in the 8.5 dpc yolk sac (A) and 9.0 (B), 9.5 (C), 10.5 (D), and 11.5 (E) dpc embryos. Green fluorescence-positive cells are observed in para-aortic splanchnopleura (red arrows), liver rudiment (white arrows), and vitelline vessels (arrowheads). In panel E , the GFP brightly positive cells are in the ventral side of the neural tube. Panel F shows a dorsal view of the embryo in panel E. These embryos were also analyzed after dissection. GFP-positive cells are observed in para-aortic splanchnopleura in 9.5 dpc embryo (G) and in or near the dorsal aortic wall of 10.5 dpc embryo (H). GFP-positive cells are also observed in 11.5 dpc fetal liver (I). Upper limb buds were eliminated to show the staining in the trunks.
Hematopoietic and neural tissue-specific transcription in the 7.0ISGFP transgenic mouse. Whole-mount analysis of GFP expression in 7.0ISGFP transgenic mouse embryos (line 398). GFP-positive cells were identified in the 8.5 dpc yolk sac (A) and 9.0 (B), 9.5 (C), 10.5 (D), and 11.5 (E) dpc embryos. Green fluorescence-positive cells are observed in para-aortic splanchnopleura (red arrows), liver rudiment (white arrows), and vitelline vessels (arrowheads). In panel E , the GFP brightly positive cells are in the ventral side of the neural tube. Panel F shows a dorsal view of the embryo in panel E. These embryos were also analyzed after dissection. GFP-positive cells are observed in para-aortic splanchnopleura in 9.5 dpc embryo (G) and in or near the dorsal aortic wall of 10.5 dpc embryo (H). GFP-positive cells are also observed in 11.5 dpc fetal liver (I). Upper limb buds were eliminated to show the staining in the trunks.
Within the embryo proper, GFP staining was first visible in the para-aortic splanchnopleura and liver rudiment at 9.0 (Fig 7B) and 9.5 (Fig 7C) dpc, which concurs with the 6.0ISLacZ transgenic analysis (see Fig 6A). GFP expression was not detected earlier than 8.5 dpc in the embryo (data not shown). Interestingly, we also detected GFP-positive cells in the vitelline vessels (Fig 7B and C, arrowheads) of 9.0 and 9.5 dpc transgenic embryos. This observation suggests possible circulation-mediated transfer of hematopoietic progenitors between the yolk sac and the embryo proper. In the 10.5 dpc embryo, the liver rudiment also contained GFP-positive cells (Fig 7D, arrow), but the fluorescence was much weaker than that in neural tissues. In 11.5 dpc embryos, GFP expression in neural tissues was again most prominent, showing excellent coincidence with the 6.0ISLacZ analysis (Fig 7E and F; compared with Fig 6D and E). In contrast, GFP-positive cells were not as obvious in the AGM region nor in the fetal liver in the whole-embryo analysis (Fig 7E).
The low frequency of the GFP-positive cells in hematopoietic tissues of 10.5 and 11.5 dpc transgenic embryos might reflect the limit of whole-embryo analysis. We therefore dissected these transgenic embryos to visualize cellular resolution of green fluorescence. We found GFP-positive cells in 9.5 dpc para-aortic splanchnopleura (Fig 7G, arrow), 10.5 dpc AGM region (Fig 7H), and 11.5 dpc fetal liver (Fig7I). An important finding here was the identification of green fluorescence-positive cells in or near the dorsal aortic wall (Fig 7H), consistent with the in situ hybridization analysis (Fig 1F) and analysis of the 6.0ISLacZ transgenic mouse (Fig 6C).
To determine frequency of these GFP-positive cells, the yolk sac, AGM, and the fetal liver rudiment were dissected from the 10.5 dpc transgenic embryos. Single-cell suspensions were then analyzed by FACS. GFP-positive cells were found to constitute 0.5% of the yolk sac cells (Fig 8A), 1% to 5% of total liver cells, and 0.7% to 5% of total AGM cells at this stage (data not shown). In contrast to the 10.5 dpc AGM, GFP-positive cells represented only 0.25% of the total cells recovered from 13.5 dpc transgenic embryo livers (data not shown), suggesting that the expression of GFP reporter gene in this line is either subject to position effect or that some activating cis-acting elements are still missing in this transgene construct.
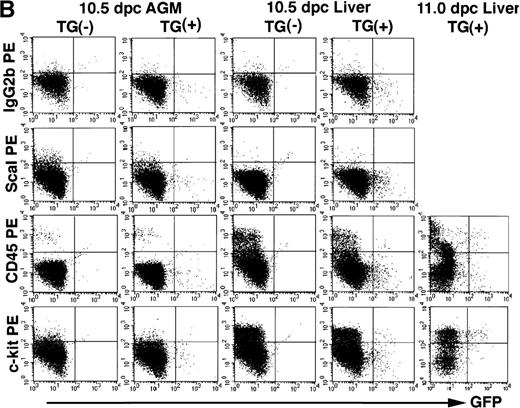
GFP-positive cells show cell surface markers characteristic to hematopoietic progenitors. The 7.0ISGFP transgenic mouse embryos (line 398) were analyzed. Yolk sac from 10.5 dpc embryos (A), AGM regions from 10.5 dpc embryos (B and C), and liver rudiments from 10.5 and 11.0 dpc embryos (B and C) were dissected, and single-cell suspensions from the tissues were analyzed by FACS directly (A) or after staining with monoclonal antibodies (B and C). PE-conjugated anti-Sca1, anti-CD45, and anti–c-kit antibodies (B), and biotin-conjugated anti-CD34, anti–flk-1, and anti-Ter119 antibodies and streptoavidine-APC were used (C). IgG2b antibody was used as a control.
GFP-positive cells show cell surface markers characteristic to hematopoietic progenitors. The 7.0ISGFP transgenic mouse embryos (line 398) were analyzed. Yolk sac from 10.5 dpc embryos (A), AGM regions from 10.5 dpc embryos (B and C), and liver rudiments from 10.5 and 11.0 dpc embryos (B and C) were dissected, and single-cell suspensions from the tissues were analyzed by FACS directly (A) or after staining with monoclonal antibodies (B and C). PE-conjugated anti-Sca1, anti-CD45, and anti–c-kit antibodies (B), and biotin-conjugated anti-CD34, anti–flk-1, and anti-Ter119 antibodies and streptoavidine-APC were used (C). IgG2b antibody was used as a control.
We next stained 10.5 dpc AGM and liver cells (line 398) with antibodies recognizing cell surface markers, and performed FACS analysis. A fraction of the GFP-positive cells in the liver and AGM were stained with the anti-CD45 (common leukocyte antigen) or anti–c-kit antibodies (Fig 8B, compare upper right boxes of TG(+) with TG(-)). GFP/CD45 and GFP/c-kit double-positive cells are more abundant in 10.5 and 11.0 dpc fetal liver than in the 10.5 dpc AGM region. The 10.5 dpc AGM contained GFP/CD34 double-positive cells, whereas the 10.5 dpc liver cells contained GFP/CD34 and GFP/flk-1 double-positive cells (Fig 8C). We were unable to detect GFP/Ter119 (mature erythroid marker) double-positive cells (Fig 8C). In the 11.0 dpc fetal liver, approximately 40% of the GFP-positive cells are also immunoreactive with anti-CD45 antibody (Fig 8B). These data thus indicate hematopoietic progenitors are clearly among the GATA-2–directed GFP positive cells.
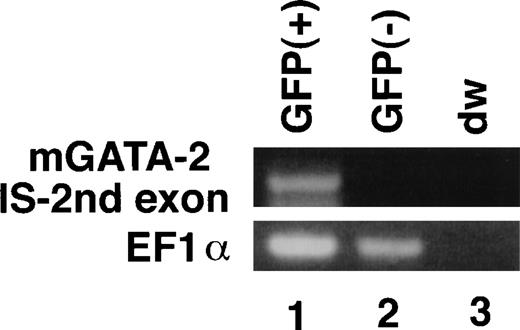
We then sorted the GFP-positive and -negative fetal liver cells from 11.5 dpc transgenic embryos and extracted RNA from both cell fractions. To assess endogenous GATA-2 gene expression, a promoter-specific RT-PCR analysis was performed. The assay detected PCR products containing the IS exon sequence in the GFP-positive fraction, but not in the GFP-negative fraction (Fig9), indicating that the 7.0 kbp gene regulatory region has an activity to recapitulate the IS promoter activity in 11.5 dpc hematopoietic progenitors.
GFP-expression recapitulates endogenous IS promoter activity. GFP-positive and -negative cell fractions were sorted by FACS from the liver of 11.5 dpc embryo of 7.0ISGFP transgenic mouse. Endogenous expression of the IS exon of mouse GATA-2 gene was analyzed using RT-PCR. The primer pairs amplifying the elongation factor (EF) 1 mRNA was used as a control.38
GFP-expression recapitulates endogenous IS promoter activity. GFP-positive and -negative cell fractions were sorted by FACS from the liver of 11.5 dpc embryo of 7.0ISGFP transgenic mouse. Endogenous expression of the IS exon of mouse GATA-2 gene was analyzed using RT-PCR. The primer pairs amplifying the elongation factor (EF) 1 mRNA was used as a control.38
The IS promoter contributes to neural expression of GATA-2.
The IS promoter was also used to direct GATA-2 neural expression. LacZ activity was observed in the adult brain of 6.0ISLacZ, 2.5ISLacZ, and 0.65ISLacZ transgenic mice, the expression profiles of these transgenes were generally consistent with that determined by the in situ hybridization analysis (Table 2, Fig 3). The neural expression of GFP was also detected in the 7.0ISGFP transgenic embryos (see Fig 7D to F). Dissection of transgenic 11.5 dpc embryos showed that GFP was expressed in the ventral portion of neural tube, consistent with the results of the in situ hybridization analysis (Fig 3A). In a higher magnification of a 9.5 dpc embryo, large filamentous GFP-positive cells are visible in the dorsal region (Fig 7G). These results suggest that a positive neural regulatory element is located within the 0.65 kbp region, lying immediately 5′ to the IS exon, in contrast to the regulation ofGATA-2 gene expression in hematopoietic cells (see above). These data thus show that the elements regulating the usage ofGATA-2 gene IS promoter activity in hematopoietic as well as neural cells are under the regulation of multiple regulatory elements.
DISCUSSION
Transgenic mouse analysis is an effective tool for defining the position and identity of cis-acting elements that function in restricted tissue types or at different stages of development.10 To identify hematopoietic regulatory elements for the GATA-2 gene, we examined the activity of various segments of the mGATA-2 gene. In this in vivo analysis, we never detected ectopic expression of the reporter genes, so tissues in which these activities were observed are likely to be sites in which the regulatory regions in the transgene constructs exert their normal activity. Concurrent in situ analyses underscored this contention. The cis-regulatory elements that direct GATA-2 expression in the AGM region and neural tissues were identified here, although these genomic sequences alone appeared to be insufficient to fully recapitulate the endogenous expression profile of the GATA-2gene.
In this study, we identified GATA-2–expressing cells in the para-aortic splanchnopleura in 9.5 dpc and AGM region in 10.5 dpc mouse embryos. It is significant to note that the definitive hematopoietic lineage was shown to arise from mesodermal cells in these regions of the embryo.34,35 These hematopoietic cells of 10.5 dpc AGM region retain both spleen colony forming activity and long-term reconstitution ability in lethally irradiated mice.34 In this regard, GATA-2 and SCL/tal-1 mRNAs were reportedly expressed in lineage marker-negative (Lin−) cells isolated from 7.0 dpc early- to mid-primitive streak stage embryos when cocultured with stromal cells for 5 to 6 days, and this fraction contains the earliest detectable multipotent progenitors.43GATA-2–positive cell lines derived from this culture system display long-term reconstituting activity of the lymphohematopoietic system in lethally irradiated mice.43 Hematopoietic stem cells in the AGM region and liver of mouse embryos have been found in the c-kit+/CD34+ double-positive fraction.44 Cells expressing flk-1 in 10.5-11.5 dpc AGM region have been shown to generate both hematopoietic and angiopoietic cells, so that these cells are termed hemangioblasts, or hematogenic endothelial cells.45,46 The present study of 7.0ISGFP transgenic mouse embryos showed that the GATA-2-GFP–positive hematopoietic cell fraction contains CD34, c-kit, and flk-1–positive cells. Additionally, we previously showed that GATA-2 mRNA is expressed in the Lin−/c-kit+/Sca1+fraction of adult bone marrow cells, which contains the highest percentage of long-term reconstituting hematopoietic stem cells.14,31 Taken together, these observations suggest that GATA-2 activity is a common feature of hematopoietic stem cells and progenitors.22-24 31
We found in this analysis that GATA-2 mRNA is first detected in the extra-embryonic and lateral plate mesoderm, and later in the para-aortic splanchnopleura, the AGM region, and the liver rudiment, during mouse development. GFP expression was also detected in the early yolk sac cells, and diminished in later stages. These observations are consistent with a previous report concluding that GATA-2 mRNA is expressed in the late primitive streak stage mesoderm, and is then downregulated in yolk sac mesodermal cells.40 On the other hand, both the expression of GATA-1 and GATA-1 gene regulatory region-directed LacZ reporter genes are detected abundantly in the visceral yolk sac blood islands.10 The 9.5 to 10.5 dpc yolk sac hematopoietic cells of GATA-2 knockout mice show impaired hematopoietic ability, but 15% to 50% of the normal number of primitive erythrocytes are recovered from GATA-2 (−/−) embryos.12 These observations suggest that GATA-2 is required only at the beginning of hematopoietic cell differentiation in yolk sac hematopoiesis, and that a requirement for GATA-2 at this stage may be compensated to some extent, or even circumvented, by other factors.
Previous transient transfection assays indicated that both IS and IG promoter activities are regulated by regions lying within 100 bp from their respective transcription start sites, and these assays failed to identify any additional transcriptional regulatory requirements in other regions of the gene.31 In contrast, transgenic mice bearing reporters that contain additional upstream sequence showed reporter gene expression in hematopoietic tissues. The most straightforward way to explain this discrepancy is to invoke chromatin or nucleosome remodeling activity,47-49 which might be detectable only in chromatin-associated (ie, integrated) transgenes. Indeed, specific cis-acting elements have been defined with the ability to ensure an open chromatin configuration.50, 51 An alternative explanation for this disparity is that hematopoietic stem cells, or early progenitors, are by nature very difficult to mimic in transformed cell culture assays, and therefore enhancer activities that function only in a very specific subpopulation of early hematopoietic cells might be difficult to reproduce in cell culture.
The transcriptional activity of the 6.0 and 7.0 kbp sequences upstream of the IS promoter was detected in the ventral central nervous system. In this regard, it is interesting to note that GATA-2 is expressed during pituitary organogenesis.52, 53 The expression of GATA-2 in the embryonic pituitary gland is reported to be under the control of BMP-2/4 signals,53 which are also regulators of mesodermal development.54,55 BMP-4 has been shown to stimulate the expression of GATA-2 and induce hematopoietic development in Xenopus.30 Therefore, the possibility exists that the transcription of IS-initiated GATA-2 transcription may be under the influence of BMP-2/4 signaling.
In summary, we have shown here that the expression of GATA-2 in hematopoietic cells and neural tissues is regulated by discrete genomic regions that direct GATA-2 gene IS promoter-directed expression in transgenic mice. The identification of a regulatory region that can direct para-aortic splanchnopleura and AGM expression of theGATA-2 gene may enable targeted expression of various factors to hematopoietic stem cells in vivo. Furthermore, this information may enable us to isolate specific hematopoietic progenitor cells, perhaps including the most primitive stem cells, using the GATA-2 gene regulatory region, and we are currently exploring this exciting prospect.
ACKNOWLEDGMENT
We thank Drs Kim-Chew Lim, Ken-ichi Yagami, Naomi Kaneko, Hozumi Motohashi, Takahiko Hara, Yousuke Mukouyama, and Hiromitsu Nakauchi for critical reading of the manuscript and experimental assistance. We also thank Drs Kazuhiko Umezono, Shin-ichi Nishikawa, and Atushi Ichikawa for generous gifts of pCMX-SAH/Y145F plasmid, anti–flk-1 antibody and P815 cell line, respectively.
N.M. and J.O. contributed equally to this work
Supported in part by a research grant from the NIH (GM28896; J.D.E.); Grants-in-Aid from the Ministry of Education, Science, Sports, and Culture; CREST; and the Japanese Society for Promotion of Sciences (JSPS Research for the Future Project).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal