Abstract
Expression of p21 and p27 cyclin-dependent kinase inhibitors is associated with induced differentiation and cell-cycle arrest in some hematopoietic cell lines. However, it is not clear how these inhibitors are expressed during normal hematopoiesis. We examined various human hematopoietic colonies derived from cord blood CD34+cells, bone marrow, and peripheral blood cells using a quantitative reverse transcription-polymerase chain reaction assay, immunochemistry, and/or Western blot analysis. p21 mRNA was expressed increasingly over time in all of the colonies examined (granulocytes, macrophages, megakaryocytes, and erythroblasts), whereas p27 mRNA levels remained low, except for erythroid bursts. Erythroid bursts expressed both p21 and p27 mRNAs with differentiation but expressed neither protein, whereas both proteins were expressed in megakaryocytes and peripheral blood monocytes. In bone marrow, p21 was immunostained almost exclusively in a subset of megakaryocytes and p27 protein was present in megakaryocytes, plasma cells, and endothelial cells. In megakaryocytes, reciprocal expression of p27 to Ki-67 was evident and an inverse relationship between p21 and Ki-67 positivities was also present, albeit less obvious. These observations suggest that a complex lineage-specific regulation is involved in p21 and p27 expression and that these inhibitors are involved in cell-cycle exit in megakaryocytes.
EUKARYOTIC CELL-CYCLE progression is regulated by the cyclin-dependent kinases (CDKs), the activities of which are controlled by multiple mechanisms such as cyclin binding, CDK phosphorylation and dephosphorylation, and binding of CDK inhibitors (CKIs).1 Of the two known families of CKIs, one is the Cip/Kip family, consisting of p21Cip1/Waf1/Sdi1 (p21), p27Kip1 (p27), and p57Kip2, and the other is the INK4 family, which consists of p16INK4a, p15INK4b, p18INK4c, and p19INK4d.2
Among these CKIs, p21 and p27 are implicated in terminal differentiation and proliferation in nonhematopoietic and hematopoietic cells. For example, p21 expression is induced by MyoD during skeletal muscle differentiation,3 and p21 expression in mouse embryo correlates with terminal differentiation of skeletal muscle, cartilage, skin, and nasal epithelium.4 p27 protein is expressed in a transient wave in developing myotomes of the mouse embryo.5p27 protein progressively accumulates in oligodendrocyte precursor cells as they proliferate and is present at high levels in oligodendrocytes.6 In the gastrointestinal tract, p21 expression and proliferation are inversely related.7,8 p27 is deeply involved in regulating normal T- and B-lymphocyte proliferation.9-11 There is a mutually exclusive pattern of staining for Ki-67 and p27 in human reactive lymphatic tissues,12,13 whereas p21 is positive only in a few lymphoid cells in lymph nodes.7
In addition to the role as CKIs, p21 and p27 have functions of promoting the association of CDK4 (or CDK6) with the D-type cyclins and targeting CDK4 and cyclin D1 to the nucleus.14p21-containing cyclin/CDK complexes exist in both catalytically active and inactive forms.15 Monomeric p21 and p27 promotes the assembly of active kinase complexes and, at higher stoichiometric ratios, they inhibit CDK activity.14,16 17
Hematopoiesis is a process characterized by proliferation and differentiation of cells derived from a small number of hematopoietic stem cells.18 Terminal differentiation is often associated with loss of proliferation potentials and the final exit from cell cycle. p21 expression is triggered by multiple differentiation-inducing agents in various hematopoietic cell lines: HL-60,19-24K562,20,21 U937,20,21,25 CMK,26UT-7,27 and MEG-01s.28 p27 expression is also induced by differentiation-inducing agents in some hematopoietic cell lines: HL-60,23 U937,29 and MEG-01s.28 However, cell-cycle controls can be abnormal in cell lines as the result of cellular transformation mechanisms. Information on p21 and p27 expression in normal hematopoiesis is scanty30 and how p21 and p27 are involved in normal hematopoiesis remains to be clarified.
In the present study, we directed our attention to expression patterns of p21 and p27 in normal (primary) hematopoietic cells and terminally differentiated blood cells. The correlation of cell proliferation with p21 and/or p27 expression was given focus and the expression of Ki-67 antigen was also examined. We obtained evidence that the expression of p21 and p27 is regulated in a lineage-specific manner and that both proteins are highly expressed, particularly in megakaryocytes. In megakaryocytes, p27 and probably p21 proteins are involved in terminal exit from the cell cycle.
MATERIALS AND METHODS
Cytokines.
Recombinant human granulocyte colony-stimulating factor (G-CSF) was provided by Chugai Pharmaceutical Co Ltd (Tokyo, Japan), recombinant human interleukin-6 (IL-6) was from Ajinomoto Co Ltd (Kawasaki, Japan), recombinant human stem cell factor (SCF) and recombinant human IL-3 were from Amgen Biologicals (Thousand Oaks, CA), and recombinant human erythropoietin (EPO) and thrombopoietin (TPO) were provided by Kirin Brewery Co Ltd (Tokyo, Japan).
Antibodies.
Antibodies used for immunostaining were as follows: anti-p21 (6B6; Pharmingen, San Diego, CA), anti-p27 (F-8; Santa Cruz Biotechnology, Inc, Santa Cruz, CA), anti-p53 (DO-7; DAKOPATTS A/S, Glostrup, Denmark), and antihuman CD41 (5B12; DAKOPATTS A/S) monoclonal antibodies (MoAbs), which were used at 1:50 dilution. Another anti-p27 MoAb (G173-524; Pharmingen) was used at 1:400 dilution. Antihuman glycophorin A (JC159, DAKOPATTS A/S) and anti-Ki-67 (MIB-1; Immunotech, Marseille, France) MoAbs were used at 1:100 dilution. Rabbit antihuman von Willebrand factor (vWF; A082; DAKOPATTS A/S) was used at 1:3,200 dilution. Rabbit antihuman κ and λ light chains (A191 and A193; DAKOPATTS A/S) was used at 1:25,000 dilution. Anti-p21 (6B6) and anti-p27 (G173-524) MoAbs, at 1:250 dilution, were also used in Western blot analysis.
CD34+ cell preparation.
Human umbilical cord blood was obtained during normal full-term deliveries, after the acquisition of written informed consent. Mononuclear cells (MNCs) were separated by Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden) density gradient centrifugation after depletion of phagocytes with silica (Immuno-Biological Laboratories Co, Ltd, Fujioka, Japan).31 CD34+ cells were purified from the MNCs, using Dynabeads M-450 CD34 and DETACHaBEAD CD34 (Dynal, Oslo, Norway). Flow cytometric analysis showed that 85% to 95% of the cells separated were CD34+.
Clonal cell culture.
For erythroid bursts, granulocyte colonies, and macrophage colonies, purified CD34+ cells were incubated at concentrations of 250 cells/mL in methylcellulose culture, as reported.32 33In brief, 1 mL of culture mixture contained cells, α-medium (GIBCO BRL Life Technologies, Inc, Grand Island, NY), 0.9% methylcellulose (Shinetsu Chemical, Tokyo, Japan), 30% heat-inactivated fetal bovine serum (FBS; HyClone, Logan, UT), 1% crystallized and deionized fraction V bovine serum albumin (BSA; Sigma, St Louis, MO), 0.05 mmol/L 2-mercaptoethanol (Wako Pure Chemical Industries, Osaka, Japan), 100 ng/mL SCF, 20 ng/mL IL-3, 80 ng/mL IL-6, 10 ng/mL G-CSF, and 2 U/mL EPO. For megakaryocyte colonies, 1 mL of culture mixture contained 1,000 cells, α-medium, 0.9% methylcellulose, 30% platelet-poor plasma from a healthy adult volunteer, 1% BSA, 0.05 mmol/L 2-mercaptoethanol, 100 ng/mL SCF, and 5 ng/mL TPO. Individual colonies, lifted under direct microscopic visualization, were suspended in 200 μL of phosphate-buffered saline (PBS) with 30% FBS. Half of the cell suspension was spun in a cytocentrifuge (Cytospin 2; Shandon Southern Instruments, Sewickley, PA) at 500 rpm for 5 minutes and processed for May-Grünwald Giemsa staining. The other half was directly subjected to RNA extraction.
Suspension culture.
One milliliter of culture mixture containing 10,000 to 15,000 purified CD34+ cells, α-medium (GIBCO BRL), 20% heat-inactivated FBS (HyClone), 1% BSA, with or without 100 ng/mL SCF and 80 ng/mL IL-6 was incubated in a 24-well or 48-well tissue culture plate (Becton Dickinson Labware, Lincoln Park, NJ) at 37°C in a humidified atmosphere with 5% CO2.
Preparation of normal human peripheral blood (PB) monocytes, lymphocytes, and granulocytes and bone marrow (BM) MNCs.
PB was obtained from healthy adult volunteers with informed consent. BM aspirates from patients with non-Hodgkin’s lymphoma, without BM involvement, were obtained after the acquisition of informed consent. MNCs of PB or BM were prepared by Ficoll-Paque density gradient centrifugation. PB MNCs were suspended in RPMI1640 medium (GIBCO BRL) supplemented with 10% of heat-inactivated FBS (BioWhittaker, Walkersville, MD) and 60 mg/L kanamycin (Meiji Seika Kaisha, Ltd, Tokyo, Japan) and incubated in an MSP plate (Japan Immunoresearch Laboratories, Co, Ltd, Takasaki, Japan) at 37°C in a humidified atmosphere with 5% CO2 for 1 hour. Nonadherent cells were used as lymphocytes. After four PBS washes, the plate was incubated at 4°C for 30 minutes with ice-cold MSP-E buffer supplied by the manufacturer. The adherent cells were detached by pipetting and used as monocytes. Cytochemically, the lymphocytes were 95% to 96% pure and monocytes were 88% to 89% pure (data not shown). PB granulocytes were prepared from the pellet of Ficoll-Paque density gradient centrifugation. Cells in the pellet were suspended in 3% dextran/PBS and left standing for 30 minutes at room temperature for erythrocyte sedimentation. Cells in the supernatant were resuspended in 0.2% NaCl for 1 minute on ice for hemolysis, followed by the addition of 1.6% NaCl. After pelleting, cells were collected in PBS. Granulocytes usually accounted for greater than 95%.
Cell lines.
A human leukemic cell line of pre-B phenotype, Nalm-6,34was a gift from Dr T. Nakamura (First Department of Internal Medicine, University of Tokyo, Tokyo, Japan). A human megakaryoblastic leukemia cell line, MEG-01s, was a gift from Drs M. Ogura (Aichi Cancer Center, Nagoya, Japan) and H. Saito (First Department of Internal Medicine, Nagoya University, Nagoya, Japan).35 Cells were passaged in RPMI1640 medium supplemented with 10% FBS (BioWhittaker) and 60 mg/L kanamycin at 37°C in a humidified atmosphere with 5% CO2.
RNA preparation and reverse transcription (RT).
Cells were directly subjected to the acid guanidinium thiocyanate-phenol-chloroform (AGPC) method36 with 20 μg yeast tRNA (GIBCO BRL) as a carrier and the extracted RNA was suspended in 30 μL diethyl pyrocarbonate (Sigma)-treated water, boiled for 1 minute, and stored at −80°C until analysis. One sixth of RNA (5 μL) was placed in 20 μL of 1× RT buffer (10 mmol/L Tris-HCl, 50 mmol/L KCl, 4 mmol/L MgCl2, 10 mmol/L dithiothreitol, pH 8.3) with 250 μmol/L of each deoxyribonucleoside triphosphate (dNTP), 1 μmol/L oligo(dT)15 (Boehringer Mannheim, Tokyo, Japan), 20 U rRNasin ribonuclease inhibitor (Promega Co, Madison, WI), 200 U Moloney murine leukemia virus (M-MLV) reverse transcriptase (GIBCO BRL) and was incubated at 37°C for 30 minutes. Total RNAs of the cell lines, Nalm-6 and MEG-01s, and normal BM MNCs were extracted by the AGPC method without yeast tRNA, and cDNA was synthesized with oligo(dT)15 from total RNA, as described.37
Quantitative reverse transcription-polymerase chain reaction (RT-PCR) assay.
Primers used are listed in Table 1. Schematic presentation of design of the PCR primers is depicted in Fig 1A. Expected sizes of the PCR products are as follows: p21, 369 bp; p27, 469 bp; and β-actin, 640 bp. The primers were synthesized by Greiner Japan (Tokyo, Japan). An aliquot (1 μL) of cDNA was placed in 20 μL of 1× PCR buffer (10 mmol/L Tris-HCl, 50 mmol/L KCl, 1.5 mmol/L MgCl2, pH 8.3) with 200 μmol/L of each dNTP, 2 μCi [α-32P]dCTP, primers (p21AS, p27AS, βS, and p27S at 200 nmol/L and βASp27S at 2 nmol/L), and 0.5 U recombinant Taq DNA polymerase (Takara, Kyoto, Japan). Reaction parameters were 94°C for 1 minute (first cycle, 5 minutes), 62°C for 2 minutes, and 72°C for 3 minutes (last cycle, 10 minutes). After the indicated cycles were performed, 5 μL of each PCR product was separated on a 4.5% polyacrylamide gel followed by autoradiography. An optical scanner was used and densitometrical analysis was made using NIH Image software (NIH, Bethesda, MD). As negative controls, water instead of cDNA or the products of the RT reactions without reverse transcriptase were subjected to PCR and we confirmed no false-positive reaction.
Primers Used for PCR Amplification
| Name . | Sequence . | Primer Length (bp) . | GC (%) . | Tm (°C) (0.05 mol/L Na)* . |
|---|---|---|---|---|
| p27S | 5′-CCTCTTCGGCCCGGTGGAC-3′ | 19 | 73.7 | 54.6 |
| p21AS | 5′-CCGTTTTCGACCCTGAGAG-3′ | 19 | 57.9 | 48.1 |
| p27AS | 5′-TCTGCTCCACAGAACCGGC-3′ | 19 | 63.2 | 50.3 |
| βS | 5′-AAGAGAGGCATCCTCACCCT-3′ | 20 | 55.0 | 48.7 |
| βASp27S | 5′-CCTCTTCGGCCCGGTGGACGGAAGGAAGGCTGGAAG-3′ | 36 | 66.7 | 68.5 |
| Name . | Sequence . | Primer Length (bp) . | GC (%) . | Tm (°C) (0.05 mol/L Na)* . |
|---|---|---|---|---|
| p27S | 5′-CCTCTTCGGCCCGGTGGAC-3′ | 19 | 73.7 | 54.6 |
| p21AS | 5′-CCGTTTTCGACCCTGAGAG-3′ | 19 | 57.9 | 48.1 |
| p27AS | 5′-TCTGCTCCACAGAACCGGC-3′ | 19 | 63.2 | 50.3 |
| βS | 5′-AAGAGAGGCATCCTCACCCT-3′ | 20 | 55.0 | 48.7 |
| βASp27S | 5′-CCTCTTCGGCCCGGTGGACGGAAGGAAGGCTGGAAG-3′ | 36 | 66.7 | 68.5 |
Tm was calculated using the formula: Tm (°C) = 81.5 + 16.6 (Log [molar Na+]) + 41 (%GC) − 675/primer length.
Quantitative RT-PCR for relative expression levels of p21 and p27 with endogenously expressed β-actin used as an internal control. (A) Schematic presentation of primer setting on p21, p27, and β-actin sequences. Thick lines indicate coding regions and thin lines represent truncated noncoding regions. Thick arrows indicate primers used in the PCR. The common primer (p27S) is derived from the identical region between p21 and p27 sequences, except for one mismatch in the p21 sequence. The composite primer (βASp27S) consisted of p27S in the 5′ part and β-actin sequence in the 3′ part. The common primer (p27S) is shared in amplification of the three PCR products, whereas p21AS, p27AS, and βS are specific to p21, p27, and β-actin sequences, respectively. (B) Autoradiographs showing kinetics of the PCR. An aliquot (5 μL, 0.1 μg RNA equivalent) of cDNA synthesized from Nalm-6 cells RNA was placed in 100 μL of PCR reaction volume with [-32P]dCTP (10 μCi) and was subjected to PCR as described in Materials and Methods. An aliquot (5 μL) of the reaction mixture was taken at the indicated cycle. Arrows indicate the PCR products corresponding to p21, p27, and β-actin. (C) [-32P]dCTP incorporation was plotted on a logarithmic scale over number of cycles of PCR. Each symbol denotes the following: (•) β-actin; (○) p27; (▵) p21. (D) Comparison between Northern analysis and RT-PCR. RNAs extracted from MEG-01s cells stimulated with TPA at 10 nmol/L were subjected to RT-PCR and to Northern analysis. The gel for PCR products was dried and exposed to an x-ray film for 17 hours at −80°C with an intensifying screen. The membrane for Northern analysis of β-actin and p21 was exposed to x-ray films at −80°C with intensifying screens for 1 day and 7 days, respectively. p27 mRNA was hardly detected by Northern analysis (data not shown). (E) The amount of p21 transcripts standardized to β-actin determined by Northern analysis and by RT-PCR. The signal ratio of p21 over β-actin at time 0 hour was defined as 1 U and the amounts of p21 transcripts standardized to β-actin are plotted on a logarithmic scale over time. Each symbol denotes the following: (○) Northern analysis; (•) RT-PCR.
Quantitative RT-PCR for relative expression levels of p21 and p27 with endogenously expressed β-actin used as an internal control. (A) Schematic presentation of primer setting on p21, p27, and β-actin sequences. Thick lines indicate coding regions and thin lines represent truncated noncoding regions. Thick arrows indicate primers used in the PCR. The common primer (p27S) is derived from the identical region between p21 and p27 sequences, except for one mismatch in the p21 sequence. The composite primer (βASp27S) consisted of p27S in the 5′ part and β-actin sequence in the 3′ part. The common primer (p27S) is shared in amplification of the three PCR products, whereas p21AS, p27AS, and βS are specific to p21, p27, and β-actin sequences, respectively. (B) Autoradiographs showing kinetics of the PCR. An aliquot (5 μL, 0.1 μg RNA equivalent) of cDNA synthesized from Nalm-6 cells RNA was placed in 100 μL of PCR reaction volume with [-32P]dCTP (10 μCi) and was subjected to PCR as described in Materials and Methods. An aliquot (5 μL) of the reaction mixture was taken at the indicated cycle. Arrows indicate the PCR products corresponding to p21, p27, and β-actin. (C) [-32P]dCTP incorporation was plotted on a logarithmic scale over number of cycles of PCR. Each symbol denotes the following: (•) β-actin; (○) p27; (▵) p21. (D) Comparison between Northern analysis and RT-PCR. RNAs extracted from MEG-01s cells stimulated with TPA at 10 nmol/L were subjected to RT-PCR and to Northern analysis. The gel for PCR products was dried and exposed to an x-ray film for 17 hours at −80°C with an intensifying screen. The membrane for Northern analysis of β-actin and p21 was exposed to x-ray films at −80°C with intensifying screens for 1 day and 7 days, respectively. p27 mRNA was hardly detected by Northern analysis (data not shown). (E) The amount of p21 transcripts standardized to β-actin determined by Northern analysis and by RT-PCR. The signal ratio of p21 over β-actin at time 0 hour was defined as 1 U and the amounts of p21 transcripts standardized to β-actin are plotted on a logarithmic scale over time. Each symbol denotes the following: (○) Northern analysis; (•) RT-PCR.
Northern analysis.
RNAs extracted from MEG-01s cells stimulated with 12-o-tetradecanoylphorbol-13-acetate (TPA; Sigma) at 10 nmol/L were subjected to Northern analysis, as described.28
Immunocytochemical study.
For immunostaining of p21, p27, and Ki-67, cytosmears were fixed with 10% formaldehyde in PBS at room temperature for 10 minutes. After a wash in PBS, the cytosmears were treated with 100% methanol for 10 minutes at −20°C and washed twice with Tris-buffered saline (TBS). For immunostaining of CD41 and glycophorin A, cytosmears were air-dried for 2 hours, fixed with 100% acetone at 4°C for 1 minute, and washed with TBS twice. Primary antibody in 3% BSA/TBS was incubated overnight at 4°C in a humidified chamber. After washing with TBS, cells were stained with universal DAKO APAAP kit (DAKOPATTS A/S), as described by the manufacturer, and were counterstained with hematoxylin.
Western blot analysis.
Cells were lyzed with 1× sample buffer (60 mmol/L Tris-HCl, 2% sodium dodecyl sulfate [SDS], 0.1 mol/L dithiothreitol, pH 6.8) and boiled for 5 minutes. Protein concentration was determined by spectrophotometry using BCA Protein Assay Reagent (Pierce, Rockford, IL).38 A total of 30 μg protein per lane was loaded on 15% SDS-polyacrylamide gel and subjected to Western blot analysis, as described.28
Immunohistochemical study.
Human BM aspirates were retrieved from the archives of the Department of Pathology, the Branch Hospital, University of Tokyo, School of Medicine. These materials had been fixed in formalin and embedded in paraffin using standard methods. Three-micrometer–thick sections were dried on MAS-coated glass slides (Matsunami Glass Ind, Ltd, Kishiwada, Japan), deparaffinized with xylene, and soaked in PBS. Slides were transferred to citrate buffer (pH 6.0) and heated in a microwave oven 5 times for 5 minutes. After cooling at room temperature for 1 hour and two washes in TBS, the slides were incubated overnight at 4°C with the indicated primary antibody diluted in 3% BSA/TBS in a humidified chamber. After washing with TBS, staining with universal DAKO APAAP kit was performed as described by the manufacturer. Nuclei were counterstained with hematoxylin. For negative controls, mouse IgG was used. For p27, a human reactive lymph node sample served as a positive control.
Sequential double immunohistochemical staining.
Sequential double immunohistochemical staining was performed using the alkaline-phosphatase/anti-alkaline-phosphatase (APAAP) procedure, followed by the avidin-biotinylated peroxidase complex (ABC) method. After the above-mentioned antigen retrieval, the slides were incubated for 10 minutes in a 3% H2O2 solution in PBS for blocking endogenous peroxidase. After the APAAP procedure, the slides were treated three times for 30 minutes with 0.1 mol/L glycine-HCl buffer (pH 2.2). After three washes in PBS, the slides were covered with normal serum contained in a Vectastain ABC kit (Vector Laboratories, Burlingame, CA) for 30 minutes at room temperature and then incubated overnight at 4°C with the indicated antibody in a humidified chamber. The sections were stained using the Vectastain ABC kit, as described by the manufacturer. Peroxidase activity was visualized by applying diaminobenzidine chromogen, containing 0.015% H2O2. For negative controls, mouse IgG served as a substitute for the primary antibody or the primary antibody was omitted.
RESULTS
Quantitative RT-PCR.
To monitor p21 and p27 mRNAs expression levels in a small amount of material such as a colony generated from a CD34+ cell, we first developed a quantitative RT-PCR assay for relative expression levels of p21 and p27 mRNAs with endogenously expressed β-actin used as an internal control. To circumvent difficulties in conventional competitive RT-PCR assays, coamplification of targets (p21 and p27) and control (β-actin) in a tube was designed (Fig 1A). Simple coamplification of control and target DNAs can correct for variation in tube-to-tube amplification efficiency if the data are obtained during the exponential phase of the PCR reaction. However, β-actin is expressed at a level much higher than those of the target genes and comparable coamplification can be difficult because of rapid saturation of control reactions (data not shown). Therefore, we designed a PCR using a composite primer at a low concentration to reduce the β-actin signals at a constant ratio to the level comparable to the target signals.
We designed a common primer, p27S, derived from a homologous sequence between p21 and p27 and a composite primer, βASp27S, the 3′ part of which is derived from the sequence of β-actin and the 5′ part of which is the same as the common primer, p27S. The βASp27S primer is used in the amplification of β-actin cDNA, but not p21 or p27, because there is a large region of β-actin–specific sequence in the 3′ part. The three specific primers for p21, p27, and β-actin are p21AS, p27AS, and βS, respectively. Amplification of β-actin cDNA must be initiated with βS and βASp27S, and then p27S takes the place of βASp27S, because we use βASp27S at a lower concentration. Final products for β-actin were generated by virtue of p27S and βS. We confirmed the identity of the PCR products by direct sequencing (data not shown). We found exponential amplification phases of three genes overlapped to 26 cycles with a cDNA from cell lines used as a template (Fig1B and C) and to 32 cycles with a cDNA from cells of colonies and PB used as a template (data not shown). Therefore, PCR was run for 21 cycles for analysis of cell lines and for 30 cycles for cells from colonies and PB.
To further confirm the quantitative nature of the PCR, we compared the RT-PCR assay with Northern analysis using RNAs extracted from MEG-01s cells stimulated with TPA. The addition of 10 nmol/L TPA upregulated p21 mRNA expression and induced cell cycle arrest at the G1-S boundary in MEG-01s cells.28 As shown in Fig 1D and E, the two methods gave similar results and the quantitative nature of the PCR was reconfirmed.
p21 and p27 expressions in colonies generated from human cord blood CD34+ cells.
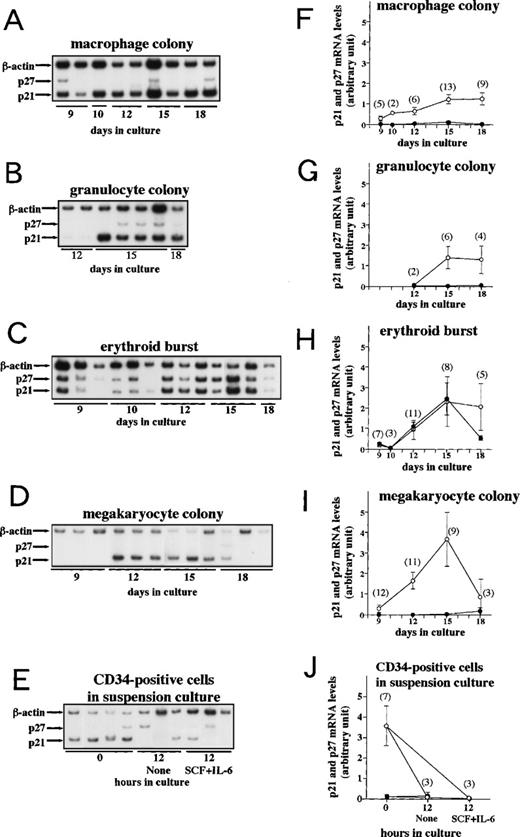
We monitored p21 and p27 mRNA expression levels in various colonies derived from CD34+ cells using the above-mentioned quantitative RT-PCR method. A total of 116 colonies were analyzed. Figure 2 shows the time course of relative expression levels of p21 and p27 mRNAs. In all four lineages (granulocytes, macrophages, megakaryocytes, and erythroblasts), p21 mRNA expression increased over time up to day 15. The increased levels of p21 mRNA in erythroid bursts and megakaryocyte colonies were similar to those observed in differentiated MEG-01s cells. p27 mRNA levels remained low during the observed period in all lineages except erythroid bursts. The gradual increase of p27 mRNA up to day 15 was evident only in erythroid bursts.
p21 and p27 mRNA expression during clonal culture of CD34+ cells. Human umbilical cord CD34+cells were incubated in methylcellulose culture containing cytokines or in suspension culture as described in Materials and Methods. From day 9 to 18, the indicated colonies were harvested for RNA extraction and cytosmeared for lineage confirmation (see Table 2). Purified CD34+ cells and cells in suspension culture of CD34+ cells were harvested for RNA extraction. RT-PCR was performed as described in Materials and Methods and each PCR product was separated on a 4.5% polyacrylamide gel followed by autoradiography. (A through E) Autoradiographs of RT-PCR products. Arrows indicate the PCR products corresponding to p21, p27, and β-actin. (F through J) p21 and p27 mRNA expression levels in the indicated colonies are standardized to β-actin determined by densitometry and are plotted over time in culture. The mean ± SE (bar) is shown. (○) p21; (•) p27. Numbers of colonies analyzed are denoted in parentheses. Seven cord blood samples were used for these studies.
p21 and p27 mRNA expression during clonal culture of CD34+ cells. Human umbilical cord CD34+cells were incubated in methylcellulose culture containing cytokines or in suspension culture as described in Materials and Methods. From day 9 to 18, the indicated colonies were harvested for RNA extraction and cytosmeared for lineage confirmation (see Table 2). Purified CD34+ cells and cells in suspension culture of CD34+ cells were harvested for RNA extraction. RT-PCR was performed as described in Materials and Methods and each PCR product was separated on a 4.5% polyacrylamide gel followed by autoradiography. (A through E) Autoradiographs of RT-PCR products. Arrows indicate the PCR products corresponding to p21, p27, and β-actin. (F through J) p21 and p27 mRNA expression levels in the indicated colonies are standardized to β-actin determined by densitometry and are plotted over time in culture. The mean ± SE (bar) is shown. (○) p21; (•) p27. Numbers of colonies analyzed are denoted in parentheses. Seven cord blood samples were used for these studies.
We also examined p21 and p27 mRNA levels in purified CD34+cells. The p27 mRNA levels were low, but these cells expressed unexpectedly high levels of p21 mRNA that were decreased after 12 hours of incubation in suspension culture with or without additional cytokines (Fig 2E and J). Because the elevated levels of p21 mRNA might be related to the purification process, whether they reflect in vivo levels remains to be investigated.
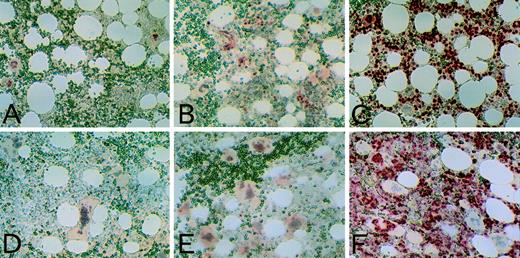
We next immunocytochemically examined p21 and p27 protein expression in these colonies on day 15 of the culture from 7 and 6 subjects, respectively. We confirmed cell lineages by May-Grünwald Giemsa staining and immunostaining with anti-CD41 and anti-glycophorin A antibodies (Table 2). As shown in Fig 3 and Table 2, some megakaryocytes and macrophages were positive for p21, although there were few p21-positive erythroblasts and granulocytes. p21 staining was always restricted to nuclei and with none in the cytoplasm. Some megakaryocytes were positive for p27 (Fig 3), although there were few p27-positive erythroblasts, macrophages, and granulocytes. Therefore, the proteins expression did not necessarily correlate with their mRNA levels.
Positivity of Immunostaining in Cells of Colonies on Day 15
| . | p21 . | p27 . | Ki-67 . | GpA . | CD41 . |
|---|---|---|---|---|---|
| Macrophage colony | 33.7 ± 5.8 (7) | 3.1 ± 0.5 (6) | 13.6 ± 3.8 (4) | ND | ND |
| Granulocyte colony | 4.2 ± 0.5 (5) | 1.2 ± 0.6 (5) | 17.0, 20.2 (2) | ND | ND |
| Erythroid burst | 1.3 ± 0.5 (7) | 2.1 ± 0.9 (6) | 27.2 ± 6.1 (4) | 84.6, 85.6 (2) | ND |
| Megakaryocyte colony | 11.0, 15.6 (2) | 12.6, 41.7 (2) | 21.0 (1) | ND | 82.1, 98.4 (2) |
| . | p21 . | p27 . | Ki-67 . | GpA . | CD41 . |
|---|---|---|---|---|---|
| Macrophage colony | 33.7 ± 5.8 (7) | 3.1 ± 0.5 (6) | 13.6 ± 3.8 (4) | ND | ND |
| Granulocyte colony | 4.2 ± 0.5 (5) | 1.2 ± 0.6 (5) | 17.0, 20.2 (2) | ND | ND |
| Erythroid burst | 1.3 ± 0.5 (7) | 2.1 ± 0.9 (6) | 27.2 ± 6.1 (4) | 84.6, 85.6 (2) | ND |
| Megakaryocyte colony | 11.0, 15.6 (2) | 12.6, 41.7 (2) | 21.0 (1) | ND | 82.1, 98.4 (2) |
Values are percentages. The mean ± standard error is shown in case of more than 2 experiments. Numbers of successful experiments are denoted in parentheses.
Abbreviation: ND, not done.
p21 and p27 expression in cells of colonies. Cells of colonies on day 15 of the culture generated from human cord blood CD34+ cells were immunostained using anti-p21 antibody, 6B6 (A through E), and anti-p27 antibody, F-8 (F through J). Immunostaining was performed using the APAAP method as described in Materials and Methods. Nuclei were counterstained with hematoxylin. Positive cells showed red nuclear staining. (A and F) megakaryocytes, (B and G) erythroblasts, (C and H) macrophages, (D and I) granulocytes, and (E and J) MEG-01s cells harvested 48 hours after the addition of 10 nmol/L TPA (positive control; original magnifications: [A] and [F], ×200; [B] through [E] and [G] through [J], ×50).
p21 and p27 expression in cells of colonies. Cells of colonies on day 15 of the culture generated from human cord blood CD34+ cells were immunostained using anti-p21 antibody, 6B6 (A through E), and anti-p27 antibody, F-8 (F through J). Immunostaining was performed using the APAAP method as described in Materials and Methods. Nuclei were counterstained with hematoxylin. Positive cells showed red nuclear staining. (A and F) megakaryocytes, (B and G) erythroblasts, (C and H) macrophages, (D and I) granulocytes, and (E and J) MEG-01s cells harvested 48 hours after the addition of 10 nmol/L TPA (positive control; original magnifications: [A] and [F], ×200; [B] through [E] and [G] through [J], ×50).
p21 and p27 expressions in PB and BM MNCs.
To confirm the correlation of cells in the colonies and PB cells, we examined PB cells of each lineage by RT-PCR and Western blot analysis using PBs from 4 normal individuals (Fig4). Lymphocytes expressed both p21 and p27 mRNAs, but only a substantial amount of p27 protein as reported by other workers.9 39 Monocytes expressed mainly p21 mRNA and much less p27 mRNA (Fig 4A) and expressed both proteins, albeit weakly (Fig 4B). Consistent with the results of Western analysis, immunocytochemistry showed that both proteins were present in monocytes and that PB lymphocytes expressed only p27 protein (Fig 4D through F and I through K and Table 3). Both proteins were detected in nuclei. In PB granulocytes, p21 or p27 was not detected immunocytochemically (Fig 4G and L).
p21 and p27 expression in PB cells. (A) Autoradiographs of RT-PCR products. Normal BM MNCs (lane 1), PB MNCs (lane 2), lymphocytes (lane 3), and monocytes (lane 4) were analyzed for p21 and p27 mRNA expression by RT-PCR. Arrows indicate the PCR products corresponding to p21, p27, and β-actin. (B) Western blot analysis. Each lane was loaded with 30 μg of total protein from MEG-01s cells harvested 48 hours after 10 nmol/L TPA addition (positive control, lane 1), PB MNCs (lane 2), lymphocytes (lane 3), and monocytes (lane 4). Western blot analyses with anti-p21 (6B6) and anti-p27 (G173-524) antibodies were performed as described in Materials and Methods. Data were confirmed in 3 more independent experiments. Cells were immunostained using anti-p21 antibody, 6B6 (C through G), and anti-p27 antibody, F-8 (H through L). Immunostaining was performed as described in Materials and Methods using the APAAP method. Nuclei were counterstained with hematoxylin. Positive cells showed red nuclear staining. (C and H) MEG-01s cells harvested 24 hours after the addition of 10 nmol/L TPA (positive control), (D and I) PB MNCs, (E and J) lymphocytes, (F and K) monocytes, and (G and L) granulocytes (original magnifications: [C] and [H], ×50; [D] through [G] and [I] through [L], ×200).
p21 and p27 expression in PB cells. (A) Autoradiographs of RT-PCR products. Normal BM MNCs (lane 1), PB MNCs (lane 2), lymphocytes (lane 3), and monocytes (lane 4) were analyzed for p21 and p27 mRNA expression by RT-PCR. Arrows indicate the PCR products corresponding to p21, p27, and β-actin. (B) Western blot analysis. Each lane was loaded with 30 μg of total protein from MEG-01s cells harvested 48 hours after 10 nmol/L TPA addition (positive control, lane 1), PB MNCs (lane 2), lymphocytes (lane 3), and monocytes (lane 4). Western blot analyses with anti-p21 (6B6) and anti-p27 (G173-524) antibodies were performed as described in Materials and Methods. Data were confirmed in 3 more independent experiments. Cells were immunostained using anti-p21 antibody, 6B6 (C through G), and anti-p27 antibody, F-8 (H through L). Immunostaining was performed as described in Materials and Methods using the APAAP method. Nuclei were counterstained with hematoxylin. Positive cells showed red nuclear staining. (C and H) MEG-01s cells harvested 24 hours after the addition of 10 nmol/L TPA (positive control), (D and I) PB MNCs, (E and J) lymphocytes, (F and K) monocytes, and (G and L) granulocytes (original magnifications: [C] and [H], ×50; [D] through [G] and [I] through [L], ×200).
Positivity of Immunostaining in PB Cells
| . | p21 . | p27 . |
|---|---|---|
| Lymphocytes | 0.0 ± 0.0 (5) | 66.1 ± 2.4 (4) |
| Monocytes | 39.9 ± 2.3 (5) | 63.6 ± 3.9 (4) |
| Granulocytes | 0.0 ± 0.0 (5) | 0.0 ± 0.0 (4) |
| . | p21 . | p27 . |
|---|---|---|
| Lymphocytes | 0.0 ± 0.0 (5) | 66.1 ± 2.4 (4) |
| Monocytes | 39.9 ± 2.3 (5) | 63.6 ± 3.9 (4) |
| Granulocytes | 0.0 ± 0.0 (5) | 0.0 ± 0.0 (4) |
The mean ± standard error is shown. Numbers of normal individuals analyzed are denoted in parentheses.
Immunohistochemistry of human BM.
Finally, we immunohistochemically examined p21 and p27 expression in in vivo BM cells from 6 different individuals. Typical results of BM are shown in Figs 5,6, and 7. Because of rarity of megakaryocytes in normal BM, we also used BM from essential thrombocythemia (ET) patients for analysis of megakaryocytes. p21 immunoreactivity was present in the nuclei of a subset of megakaryocytes (Figs 5A and D and 6A). Most cells comprising BM, namely erythroid and myeloid cells, did not stain for p21 antigen. According to double staining using anti-p21 and anti-vWF antibodies, p21 positivity in megakaryocytes in 2 ET patients (31.4% to 42.4%) was similar to 32.0% to 44.7% (n = 2) in normal BM. When we immunohistochemically investigated p53 protein expression in normal BM, we found that normal BM cells, including megakaryocytes, were p53 negative (data not shown). Therefore, p21 may be expressed in megakaryocytes through p53-independent mechanisms, as noted in other cell types.4,19-22,24 40
p21, p27, and Ki-67 staining of BM. Normal BM (A, B, and C) and BM from an ET patient (D, E, and F) was immunostained using anti-p21 antibody, 6B6 (A and D); anti-p27 antibody, G173-524 (B and E); and anti–Ki-67 antibody, MIB1 (C and F). Immunostaining was performed using the APAAP method, as described in Materials and Methods. Positive cells showed red nuclear staining. Although weak cytoplasmic staining in some megakaryocytes was visible when using the MoAb G173-524, it was not reproducible with another antibody F-8 (original magnification × 50).
p21, p27, and Ki-67 staining of BM. Normal BM (A, B, and C) and BM from an ET patient (D, E, and F) was immunostained using anti-p21 antibody, 6B6 (A and D); anti-p27 antibody, G173-524 (B and E); and anti–Ki-67 antibody, MIB1 (C and F). Immunostaining was performed using the APAAP method, as described in Materials and Methods. Positive cells showed red nuclear staining. Although weak cytoplasmic staining in some megakaryocytes was visible when using the MoAb G173-524, it was not reproducible with another antibody F-8 (original magnification × 50).
p21 and Ki-67 expression in megakaryocytes. Serial sections of BM from an ET patient were immunostained for p21 (A) and Ki-67 (B). Positive cells showed red nuclear staining. Arrowheads indicate megakaryocytes: (▪) strongly positive; (▩) moderately positive; (▨) weakly positive; (□) negative (original magnification × 50). (C) Percentage of Ki-67 negative (−) and weakly (+−), moderately (+), and strongly (++) positive megakaryocytes subdivided according to p21 positivity ([−] none, [+−] weak, [+] moderate, [++] strong). Numbers of counted cells are shown in parentheses.
p21 and Ki-67 expression in megakaryocytes. Serial sections of BM from an ET patient were immunostained for p21 (A) and Ki-67 (B). Positive cells showed red nuclear staining. Arrowheads indicate megakaryocytes: (▪) strongly positive; (▩) moderately positive; (▨) weakly positive; (□) negative (original magnification × 50). (C) Percentage of Ki-67 negative (−) and weakly (+−), moderately (+), and strongly (++) positive megakaryocytes subdivided according to p21 positivity ([−] none, [+−] weak, [+] moderate, [++] strong). Numbers of counted cells are shown in parentheses.
p27 and Ki-67 expression in megakaryocytes. Serial sections of BM from an ET patient were immunostained for p27 (A) and Ki-67 (B). Positive cells showed red nuclear staining. Arrowheads indicate megakaryocytes: (▪) strongly positive; (▩) moderately positive; (▨) weakly positive; (□) negative (original magnification × 50). (C) Percentage of Ki-67 negative (−) and weakly (+−), moderately (+), and strongly (++) positive megakaryocytes subdivided according to p27 positivity ([−] none, [+−] weak, [+] moderate). There was no p27 strongly positive megakaryocyte. Numbers of counted cells are shown in parentheses.
p27 and Ki-67 expression in megakaryocytes. Serial sections of BM from an ET patient were immunostained for p27 (A) and Ki-67 (B). Positive cells showed red nuclear staining. Arrowheads indicate megakaryocytes: (▪) strongly positive; (▩) moderately positive; (▨) weakly positive; (□) negative (original magnification × 50). (C) Percentage of Ki-67 negative (−) and weakly (+−), moderately (+), and strongly (++) positive megakaryocytes subdivided according to p27 positivity ([−] none, [+−] weak, [+] moderate). There was no p27 strongly positive megakaryocyte. Numbers of counted cells are shown in parentheses.
Weak to moderate p27 immunoreactivity was evident in nuclei of a subset of megakaryocytes (Figs 5B and E and 7A). Weak cytoplasmic staining in some megakaryocytes was also visible when using the MoAb, G173-524. However, it was not reproducible with another antibody, F-8. Although it is not clear whether the cytoplasmic staining was specific, there might be a significance of cytoplasmic localization of p27 protein.41 Double staining using anti-p27 and anti-vWF antibodies (data not shown) showed that approximately half the number of megakaryocytes expressed p27 protein, both in normal BM (51.35 to 77.9%, n = 2) and in BM from ET patients (42% to 52%, n = 2), although the proportions were somewhat overestimated because of endothelial cells (see below). Some small cells in BM showed strong nuclear staining (Fig 5B and E). Because p27 mRNA levels increased exclusively in erythroid bursts, we examined p27 positivity in erythroid cells. However, glycophorin A-positive cells were negative for p27 (data not shown). Double staining using anti-p27 and anti-κ and -λ light chains antibodies showed the small p27-strongly positive cells to be plasma cells (data not shown); these cells were p21-negative and no Ki-67–positive plasma cells were found, as reported.42 p27 is involved in inhibiting normal B-cell proliferation,11 43 but to our knowledge, there are no reports regarding a role for p27 in normal plasmacytic maturation or in proliferation. It will be of interest to determine if tumor cells of plasma cell dyscrasias show aberrant p27 expression. Vascular endothelial cells and lymphoid aggregates were also p27-positive (data not shown). Findings in the subset of the cells with nuclear staining of p27, using either of the two MoAbs (G173-524 and F-8), were consistent.
We used the anti–Ki-67 antibody, MIB1, as a marker of proliferation. This antibody recognizes an antigen present in the nuclei of continuously cycling cells in G1, S, G2, and M phases, but not in G0 phase,44,45 and is a reliable marker of cell cycling in BM samples.42,46 Most of the cells in BM were positive for Ki-67 (Figs 5C and F, 6B, and 7B), as noted by other investigators.42 To clarify the relationship between cell cycling status and expression of p27 or p21 in in vivo megakaryocytes, serial sections of BM from an ET patient were stained alternately for Ki-67 and p21 (Fig 6) or for Ki-67 and p27 (Fig 7). We scored p21, p27, and Ki-67 immunostaining of each megakaryocyte by the intensity ([−] none, [+−] weak, [+] moderate, or [++] strong). At least 200 cells identified in more than three consecutive sections were counted. Regardless of p21 expression levels, approximately half the number of megakaryocytes expressed no Ki-67 antigen. However, the percentage of Ki-67 strongly positive megakaryocytes decreased with the increase in p21 intensity (Fig 6); thus, there was inverse relationship between p21 and Ki-67 expression in a subset of megakaryocytes. A clear inverse relationship was also noted between p27 and Ki-67 expression (Fig 7). Almost all of the p27-positive megakaryocytes were negative or weakly positive for Ki-67, and almost all of the p27-negative megakaryocytes were moderately or strongly positive for Ki-67. These observations strongly suggest that p27 and probably p21 expression is tightly linked to cell cycling status in megakaryocytes.
DISCUSSION
We made use of quantitative RT-PCR to evaluate p21 and p27 mRNA levels in greater than 100 colonies. The results were reproducible and comparable to findings in Northern analysis. Because each assay is performed in a tube and includes an internal control, a control signal confirms the appropriateness of samples and reaction. The method will be applicable to other genes in multiple samples of small quantities.
According to our assay, p21 mRNA levels were elevated over time in all colonies of the lineage examined. The increased levels were comparable to those of differentiated and cell-cycle–arrested MEG-01s cells (Figs1D and 2). Half lives of p21 transcripts were not altered during differentiation of MEG-01s cells (T.T. and T.M., unpublished data). In other differentiation models, mechanisms for elevation of p21 mRNA were often transcriptional24,26,30,47and p53-independent.4,19-22,24 40 Accordingly, normal BM cells never immunostained for p53. Therefore, p21 mRNA expression during hematopoiesis may be regulated in a p53-independent and lineage-nonspecific manner.
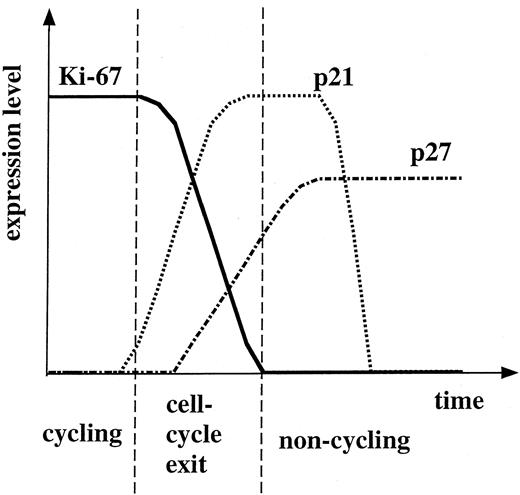
Both p21 and p27 proteins were expressed in a subset of megakaryocytes in normal human BM. An inverse relationship between p27 and Ki-67 suggests that p27 protein is expressed in cell-cycle arrested megakaryocytes. However, reciprocal expression of p21 to Ki-67 was not so obvious as that of p27, although the relationship between p21 and Ki-67 strong positivities was clearly inverse. The presence of p21-negative and Ki-67–negative megakaryocytes suggests that p21 expression is transient compared with continuous p27 expression in cell-cycle arrested cells. The proportion of p21-positive cells among Ki-67 moderately and strongly positive cells was larger than that of p27-positive cells, an observation that may reflect p21 expression earlier than p27 during cell-cycle exit. The hypothetical timing of p21 and p27 expression along the cell-cycle exit of megakaryocytes is shown in Fig 8. p21 may act early and transiently in the course of cell-cycle exit, and p27 may be subsequently upregulated. In support of this, the transient expression of p21 was noted in keratinocytes.48,49 Although the expression of p21 is increased in postmitotic cells immediately adjacent to the proliferative compartment, the expression is decreased in terminally differentiated primary keratinocytes.48 We previously reported that MEG-01s treated with TPA showed megakaryocytic differentiation and expressed both p21 and p27 proteins in association with a G1-phase arrest.28 Both p21 and p27 were present in cyclin E-associated complexes, the histone H1 and Rb kinase activities of which were then inactivated. In this differentiation model, p21 protein expression preceded that of p27 in analysis of synchronized cell population.28 It remains to be elucidated whether p21 and p27 are indeed associated with any cyclin/CDK complex in the course of cell-cycle arrest of normal megakaryocytes.
A hypothesis of the time course of p21, p27, and Ki-67 protein expression in megakaryocytes.
A hypothesis of the time course of p21, p27, and Ki-67 protein expression in megakaryocytes.
Various studies using leukemic cell lines showed that p21 and p27 may be involved in differentiation and polyploidization of megakaryocytes. For example, the ectopic expression of p21 or p27 leads to induction of megakaryocytic differentiation of CMK cells.26Overexpression of p21 results in an increase in ploidy of UT-7 cells, which suggests that p21 may be implicated in polyploidization via suppression of cdc2 activity at mitosis.27 However, reciprocal expression of p21 and p27 to Ki-67 in in vivo megakaryocytes does not support these views. Recent studies using normal megakaryocytes showed that polyploidization of megakaryocytes is due to abortive mitosis due to alterations in the regulation of mitotic exit50,51 and that cdc2 is active in endoreduplicating megakaryocytes.51 Our observations together with these reports suggest that suppression of cdc2 kinase activity by p21 (or p27) is unlikely to be the mechanism of polyploidization in megakaryocytes and that p21 and p27 have functions after endoreduplication is completed.
In mice lacking p21, red and white blood cells in PB are normal,52 although the absolute numbers of marrow progenitors are significantly decreased.53 Meanwhile, in mice lacking p27, complete blood count is normal, although the numbers of hematopoietic progenitors are increased significantly.54Although either p21 or p27 may be dispensable for hematopoietic differentiation, it remains to be determined how hematopoiesis, especially megakaryocytopoiesis, would be affected by knockout of both p21 and p27.
p27 mRNA levels remained low during the observed period in megakaryocyte colonies, even though p27 protein was expressed in a subset of megakaryocytes. This finding is consistent with knowledge that the p27 protein level is regulated mainly by protein degradation steps through the ubiquitin-dependent proteolytic pathway and that p27 mRNA and protein levels do not coincide in many situations.55-57 On the contrary, the elevation of p27 mRNA in erythroid bursts, without detectable proteins in BM erythroblasts, is intriguing. Although p27 regulation is frequently posttranslational, p27 mRNA is indeed upregulated in some situations and is usually accompanied by protein expression.58-60 The elevation of p27 mRNA levels was unique to erythroid lineage and suggests the presence of erythroid-specific regulation of p27 mRNA expression. However, as p27 protein was absent, it may be dispensable in erythropoiesis.
Recently, Dao et al61 reported that CD34+ cells from human BM expressed p27 protein and that reduction in levels of p27 protein using antisense oligonucleotides to p27 coupled with transforming growth factor-β (TGF-β) neutralization induces cell-cycle entry and increases retroviral transduction of primitive human hematopoietic cells. We detected only low levels of p27 mRNA in cord blood CD34+ cells. The difference may be due to posttranslational regulation again. We suppose that primitive hematopoietic cells have elevated p27 protein levels, which are decreased in cycling cells and increased again with cell-cycle exit especially in megakaryocytes, although the mRNA levels remain low.
Steinman et al30 reported that myeloid maturation of umbilical cord blood CD34+ cells is associated with an increase in p21 expression at RNA and protein levels. We observed that p21 mRNA expression increased with time up to day 15 in granulocyte colonies, findings consistent with their report. However, in our system, neither BM myeloid lineage cells nor PB granulocytes were positive for p21 protein and only a few p21-positive cells were detected in granulocyte colonies. Although one would need to exclude the possible expression of p21 protein in granulocytes, p21 protein levels in myeloid cells are much lower than in megakaryocytes.
p21 mRNA expression increased with time in macrophage colonies and some of the macrophages immunostained for p21 antigen. Accordingly, PB monocytes expressed p21 mRNA and protein. Consistent with these observations, several cell lines are seen to express p21 mRNA and protein during monocytic differentiation19-21,25,62 and some alveolar macrophages of the lung are p21-positive.63p27 protein levels are also elevated during monocytic differentiation in some cell lines.23 29 However, the p27 protein level was low in PB monocytes in our study and p27 protein was not detected in macrophages in in vitro colonies.
By way of summary, although p21 mRNA levels increased over time in any lineage of hematopoietic colonies, p21 protein levels were elevated only in limited lineages (megakaryocyte and monocyte/macrophage). p27 protein was detected only in megakaryocytes, monocytes, and lymphoid cells (lymphocytes and plasma cells), whereas p27 mRNA levels were elevated only in erythroid bursts. These observations suggest that complex lineage-specific regulation mechanisms are involved in p21 and p27 protein expression in human hematopoiesis. In addition, reciprocal expression of p21 and p27 to Ki-67 suggests that, in megakaryocytes, both p21 and p27 may be involved in terminal exit from the cell cycle.
ACKNOWLEDGMENT
The authors thank Dr A. Noda for p21 cDNA plasmid, Dr J. Massague for p27 cDNA plasmid, M. Yoshikawa for technical assistance, and M. Ohara for language assistance.
Supported in part by grants from the Ministry of Education, Science, Sports and Culture of Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Toru Motokura, MD, Fourth Department of Internal Medicine, University of Tokyo, School of Medicine, 3-28-6 Mejirodai, Bunkyo-ku, Tokyo 112-8688, Japan.

![Fig. 1. Quantitative RT-PCR for relative expression levels of p21 and p27 with endogenously expressed β-actin used as an internal control. (A) Schematic presentation of primer setting on p21, p27, and β-actin sequences. Thick lines indicate coding regions and thin lines represent truncated noncoding regions. Thick arrows indicate primers used in the PCR. The common primer (p27S) is derived from the identical region between p21 and p27 sequences, except for one mismatch in the p21 sequence. The composite primer (βASp27S) consisted of p27S in the 5′ part and β-actin sequence in the 3′ part. The common primer (p27S) is shared in amplification of the three PCR products, whereas p21AS, p27AS, and βS are specific to p21, p27, and β-actin sequences, respectively. (B) Autoradiographs showing kinetics of the PCR. An aliquot (5 μL, 0.1 μg RNA equivalent) of cDNA synthesized from Nalm-6 cells RNA was placed in 100 μL of PCR reaction volume with [-32P]dCTP (10 μCi) and was subjected to PCR as described in Materials and Methods. An aliquot (5 μL) of the reaction mixture was taken at the indicated cycle. Arrows indicate the PCR products corresponding to p21, p27, and β-actin. (C) [-32P]dCTP incorporation was plotted on a logarithmic scale over number of cycles of PCR. Each symbol denotes the following: (•) β-actin; (○) p27; (▵) p21. (D) Comparison between Northern analysis and RT-PCR. RNAs extracted from MEG-01s cells stimulated with TPA at 10 nmol/L were subjected to RT-PCR and to Northern analysis. The gel for PCR products was dried and exposed to an x-ray film for 17 hours at −80°C with an intensifying screen. The membrane for Northern analysis of β-actin and p21 was exposed to x-ray films at −80°C with intensifying screens for 1 day and 7 days, respectively. p27 mRNA was hardly detected by Northern analysis (data not shown). (E) The amount of p21 transcripts standardized to β-actin determined by Northern analysis and by RT-PCR. The signal ratio of p21 over β-actin at time 0 hour was defined as 1 U and the amounts of p21 transcripts standardized to β-actin are plotted on a logarithmic scale over time. Each symbol denotes the following: (○) Northern analysis; (•) RT-PCR.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/12/10.1182_blood.v93.12.4167/4/m_blod41205001w.jpeg?Expires=1769101560&Signature=fM4owbSozEGD~F7KDurdw6I55g9yLDHTfPoax88ILT-62PKD7m2U5oy41jHgYAghz2EFlK1RlWsC44ssYwRmba6pH8OhxHmjV3VCACgyqdPTQdd4QD7~jtb2dNc--GHdCS9e~tlVgKtc1KxCVk6nNc4LIs3zJNfgYeLsoIaVBEhi4jQSX7hvRxMYCXvWMbLEI5hIjCiOKfJ~zaVmCL4WCzgjdY7j9fQB~9hREdKKThCDajgeLCZTpTVLVPEcdap9BGugO3S9EQQtQH0O~xqx3RSQkSsRHd8wD4qOMhFeT-oveaBW5q7oqQhmTYxEObwUaI7D0h5G3xt332LrnQeK7A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. p21 and p27 expression in cells of colonies. Cells of colonies on day 15 of the culture generated from human cord blood CD34+ cells were immunostained using anti-p21 antibody, 6B6 (A through E), and anti-p27 antibody, F-8 (F through J). Immunostaining was performed using the APAAP method as described in Materials and Methods. Nuclei were counterstained with hematoxylin. Positive cells showed red nuclear staining. (A and F) megakaryocytes, (B and G) erythroblasts, (C and H) macrophages, (D and I) granulocytes, and (E and J) MEG-01s cells harvested 48 hours after the addition of 10 nmol/L TPA (positive control; original magnifications: [A] and [F], ×200; [B] through [E] and [G] through [J], ×50).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/12/10.1182_blood.v93.12.4167/4/m_blod412tan03z.jpeg?Expires=1769101560&Signature=ZpRIa8S8aUWn~qsjEKuZN65Xk6uodyKuKxP2fC0B3vlUwEJcBJoA8LFvoLCQIUT4PUI-7LhN45LX90dvHsqpTKqXDQOtSgATyjuyHJtPSdK6OL1JTW21BUJ9-ppxPYvD1bYgECldLVV-DH5G~Ik2Fhasl2dS3HaQIAJuf5mE4oWAeGufSvNbRIdfqq9R~ZeVEmtdiSgqoR2wJOqKLUqMZkw4mD57oDEOvUEjv9QL0lfCTmpoYWqTWXw98lMfPYTqYplo4DvszVAjODCNRv3OGpzxiYM-bLhPKx~5vssVSIJbwfAv0p1gRoGayjqAs1~LliT4-yO79BnFa~IV38~r-Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. p21 and p27 expression in PB cells. (A) Autoradiographs of RT-PCR products. Normal BM MNCs (lane 1), PB MNCs (lane 2), lymphocytes (lane 3), and monocytes (lane 4) were analyzed for p21 and p27 mRNA expression by RT-PCR. Arrows indicate the PCR products corresponding to p21, p27, and β-actin. (B) Western blot analysis. Each lane was loaded with 30 μg of total protein from MEG-01s cells harvested 48 hours after 10 nmol/L TPA addition (positive control, lane 1), PB MNCs (lane 2), lymphocytes (lane 3), and monocytes (lane 4). Western blot analyses with anti-p21 (6B6) and anti-p27 (G173-524) antibodies were performed as described in Materials and Methods. Data were confirmed in 3 more independent experiments. Cells were immunostained using anti-p21 antibody, 6B6 (C through G), and anti-p27 antibody, F-8 (H through L). Immunostaining was performed as described in Materials and Methods using the APAAP method. Nuclei were counterstained with hematoxylin. Positive cells showed red nuclear staining. (C and H) MEG-01s cells harvested 24 hours after the addition of 10 nmol/L TPA (positive control), (D and I) PB MNCs, (E and J) lymphocytes, (F and K) monocytes, and (G and L) granulocytes (original magnifications: [C] and [H], ×50; [D] through [G] and [I] through [L], ×200).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/12/10.1182_blood.v93.12.4167/4/m_blod412tan04z.jpeg?Expires=1769101560&Signature=265pyLZfsBUSV4FoNfMJjDd8FUlyoTv18Gag52-vFSzY8R~MM~boYCubnmRaFozxyHrvepmP1Jw10XTttc~kDP~IXvIhK7DiFyEvko4QdQtmbgj8EdoiQ68rAuG2Qpr8lhY7E6V5G19tBSaJLIoKd3m5DbXaNWUFPN3hOiYnHNlx2LjbZQtZwralRtY5Qe3w~CA4Oe4WdyBCPd0dfjCtPwVwMReQHx6U5GykS-kGRcPQRJ6B0Y8xBuQ99Juja59JLO3hPldWatUvKj6c43omB4~kRgC4IUDzXvF99Iv-19gyMWwVWF6XGMrm610TIq71TX66EKuNRgeRe3YbCOkq0w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. p21 and Ki-67 expression in megakaryocytes. Serial sections of BM from an ET patient were immunostained for p21 (A) and Ki-67 (B). Positive cells showed red nuclear staining. Arrowheads indicate megakaryocytes: (▪) strongly positive; (▩) moderately positive; (▨) weakly positive; (□) negative (original magnification × 50). (C) Percentage of Ki-67 negative (−) and weakly (+−), moderately (+), and strongly (++) positive megakaryocytes subdivided according to p21 positivity ([−] none, [+−] weak, [+] moderate, [++] strong). Numbers of counted cells are shown in parentheses.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/12/10.1182_blood.v93.12.4167/4/m_blod412tan06z.jpeg?Expires=1769101560&Signature=aAehCp4I1Vo5V96InWwL9XmBYCTUmdfJmZllYmoqMAO5V75LLJSHxRxtsInsFNn1T-yRfugMmPtuJx6hE82ClyjdZ0fkKS15Ou-OVJ2xD4QjGJ3N3etKaAK6aUPVHN9U6HTFs6GLk0IzuqJg37CIZuqlXeUAIgE~sQBmjVzgjIFfbEdEsWA0pGOzLz1FrPBl8zxhCFFEqDu9zlu9jJocPFZwtx0FurGPW-3kx98cZ0zBmofFg41EKJjIJ4H44fZTFq1IUnje8tSmEjik-2jr-5I-TV~ZiuV6CChchChT5jI1JBv27qBx3emdEefc1PTLswGTmhFODOyEQ6Tydk3ZVw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. p27 and Ki-67 expression in megakaryocytes. Serial sections of BM from an ET patient were immunostained for p27 (A) and Ki-67 (B). Positive cells showed red nuclear staining. Arrowheads indicate megakaryocytes: (▪) strongly positive; (▩) moderately positive; (▨) weakly positive; (□) negative (original magnification × 50). (C) Percentage of Ki-67 negative (−) and weakly (+−), moderately (+), and strongly (++) positive megakaryocytes subdivided according to p27 positivity ([−] none, [+−] weak, [+] moderate). There was no p27 strongly positive megakaryocyte. Numbers of counted cells are shown in parentheses.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/12/10.1182_blood.v93.12.4167/4/m_blod412tan07z.jpeg?Expires=1769101560&Signature=JD07MjwXB7rFOMqlft6cJF0rzPFdWq-xTZqeNfOXI1zMmI8gTkrFS6GeU0ftO8x0s3pS2v7z-tjdM3OsxqDTjRy2bJ95J0j2-PSm2qISI4YUkXx~14swW4D9rKyK7MTCclTWG6uQN0DHTtVksit1Umf8Q3FJpGAlnvj1DEsgtx~ovsN-ekt4IJLrKV~3PTopX4HVcvvwF9DWWxrrJgk32w4UxbsQWskyRbMyjyOhofuv7UuHfpCq8T8N6IjskKbd2Qnrt5YoC4KbJ6AfmdaPy9097uqommbJZMbOwyunnjokWKrL2Ck~pmnko5QAqvw2xtepdjBNzq5G~W5IMOUFlQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal