Abstract
Patients with Kaposi’s sarcoma (KS) have a human herpesvirus-8 (HHV-8) load higher than patients without KS and present a CD8+ T-cell activation with production of Th1-type cytokines both in tissues and peripheral blood mononuclear cells (PBMC). Because in tissues of KS patients detection of inflammatory cytokines (IC) can precede detection of HHV-8 DNA and because signs of immunoactivation and/or dysregulation can precede KS development, we investigated the effect of IC on HHV-8 infection. To achieve this goal, PBMC and purified cell populations from 45 patients with KS and 45 patients at risk of KS were analyzed for HHV-8 DNA and/or gene expression and for cell survival, growth, and phenotype before or after culture with or without the IC increased in KS. The results indicate that PBMC that are polymerase chain reaction (PCR)-positive at day 0 generally loose the virus upon culture. However, the presence of IC maintains HHV-8 DNA load in cultured cells. In addition, IC increase viral load to detectable levels in PBMC from serologically positive patients that were PCR-negative before culture. γ Interferon is sufficient for these effects, whereas tumor necrosis factor and interleukin-6 have little or no activity. The increase of HHV-8 DNA by IC is observed after short-term (7 days) or long-term (28 days) culture of the cells and occurs in one or both of the two circulating cell types that are infected in vivo: B cells and monocytes. In both cases it is associated with lytic gene expression, suggesting that virus reactivation is one of the most likely mechanisms for the effect of IC on virus load. However, IC have also effects on the cells target of HHV-8 infection, because they increase B-cell survival and induce the growth and differentiation of monocytes into KS-like spindle cells with markers of endothelial macrophages. Because cells with markers of endothelial macrophages are present in blood and lesions from KS patients and are infected by HHV-8, these data may explain the high HHV-8 load associated with KS development and suggest that infected monocytes may carry the virus to tissues, transmit the infection, or differentiate in loco in spindle cells with endothelial macrophage markers.
HUMAN HERPESVIRUS-8 (HHV-8) is associated with Kaposi’s sarcoma (KS)1 and its presence in individuals at high risk of KS can predict disease development.2,3 In KS patients, HHV-8 load is higher than in infected individuals without KS4 and the virus is also detected in secretions and uninvolved tissues.5-7Similarly, HHV-8 load increases during lesions progression to the nodular stage.8,9 Thus, virus persistence and replication is associated and, perhaps, required for KS development. However, patients without KS and normal blood donors can also be infected by HHV-8, particularly in geographical areas with a high incidence of KS (eg, Mediterranean countries and Africa).10-15This finding suggested that additional factors cooperate with HHV-8 in KS development.
KS lesions are characterized by angiogenesis, inflammatory cell infiltration, and the presence of spindle-shaped cells that are considered to be the tumor cells of KS. Recent evidence indicates that spindle cells are a mixed cell population dominated by activated endothelial cells and macrophages.16-19 In addition, lesional macrophages express vascular-endothelial cadherin (VE-cadherin), an endothelial cell marker typically expressed by the so-called endothelial macrophages of lymphatic organs.18,20 21
Spindle cells are latently infected by HHV-8,9,22,23whereas lesional mononuclear cells can be lytically infected.24,25 HHV-8 has also been detected in B cells26,27 and in KS-like spindle cell progenitors that are increased in the blood of patients with all forms of KS and in individuals at high risk for KS.28,29 Upon culture, spindle cells of endothelial origin loose the virus,30 whereas macrophagic spindle cells from the lesions and KS-like spindle cell progenitors from the blood are persistently infected.19 29
Inflammatory cells, including CD8+ T cells and CD14+/CD68+ monocytes/macrophages, are abundant in early stage KS lesions and produce inflammatory cytokines (IC).7,16-19,31 These include γ interferon (γIFN), tumor necrosis factor (TNF), interleukin-1 (IL-1), IL-6, and others. High levels of Th1-type cytokines, such as γIFN, are also produced by activated peripheral blood mononuclear cells (PBMC) of patients with the acquired immunodeficiency syndrome (AIDS)-associated KS (AIDS-KS) or classical KS (CKS) as compared with patients with other dermatological disorders.19 Finally, γIFN can be detected in early lesions and uninvolved tissues from KS patients even before HHV-8 detection by the polymerase chain reaction (PCR).7
These findings and the progressive increase of viral load in individuals developing KS suggested that immunodysregulation and production of IC may modify HHV-8 replication, spread, and persistence. We therefore analyzed the effect of IC increased in KS or in individuals at high risk of KS on HHV-8 infection of PBMC from patients with all the epidemiological forms of KS and from individuals at high risk of KS, including homosexual men with AIDS or asymptomatic and posttransplanted individuals that are positive or negative for HHV-8 infection by serology.
MATERIALS AND METHODS
Patients.
Human immunodeficiency virus (HIV)-infected homosexual men with AIDS-KS or with AIDS without KS (NKS-AIDS) or asymptomatic (HIV+), HIV-seronegative patients with CKS or posttransplant KS (PT-KS), or posttransplant patients without KS (PT) were studied. Patients with NKS-AIDS or AIDS-KS were treated with combinations of AZT, D4T, 3TC, ddC, ddI, granulocyte-monocyte colony-stimulating factor (GM-CSF), αIFN, vincristin, bleomycin, or Taxol. Patients with CKS were treated with αIFN or cortisone or were not under therapy. Posttransplant patients with or without KS were treated with a combination of cyclosporin and cortisone. All patients gave their informed consent to participate in the study.
Cytokines and cell cultures.
Conditioned media from activated T cells (TCM) were prepared as previously described.7,19 32-34 The average concentration of cytokines in TCM as determined by enzyme-linked immunosorbent assay (ELISA) is as follows: IL-1α (0.5 ng/mL), IL-1β (3.5 ng/mL), IL-6 (35 ng/mL), TNF-α (0.2 ng/mL), TNF-β (50 pg/mL), GM-CSF (0.4 ng/mL), oncostatin M (0.5 to 1 ng/mL), and γIFN (150 pg/mL). Reconstituted in vitro TCM (RTCM) were prepared by combining recombinant cytokines (Boehringer Mannheim, Mannheim, Germany) at the same concentration described above. Oncostatin M was purchased from R&D Systems (Minneapolis, MN).
PBMC were isolated by Ficoll-Hypaque density gradient centrifugation and seeded in 6-well culture plates (3 to 4 × 106cells/well). Total PBMC were cultured in RPMI 1640 containing 15% fetal calf serum with or without TCM or RTCM (1:4 dilution) or single cytokines. A half volume of fresh medium was added at day 3. TCM or RTCM were added at day 3 and single cytokines were added at day 2 or 4, as specified. For long-term culture experiments, a half volume of fresh medium was added at day 3 every week, as was performed for the short-term cultures. In addition, half of the culture medium was replaced with fresh medium with or without RTCM at the end of each week of culture. Cells eventually present with the medium removed were harvested by low speed centrifugation and readded to the culture. At the end of the coculture, cells were harvested as a bulk or by separating floating and adherent cells counted, and cell viability determined by trypan blue dye exclusion. Adherent cells were all viable at the time of harvesting, whereas bulk PBMC and floating cells showed some level of cell death. The average percentage of dead cells was comparable in PBMC cultured in the absence or presence of TCM or RTCM (unfractionated PBMC: 6% [±4.9%] without TCM or RTCM, 7% [±6.6%] with TCM or RTCM; floating cells: 17% [±19.1%] without TCM or RTCM, 17% [±14.5%] with TCM or RTCM). Statistical analysis of the data (see below) showed that the viability of unfractionated PBMC or floating cells cultured with TCM or RTCM did not differ from that of cells cultured with medium alone (Wilcoxon signed-rank test; unfractionated PBMC, P = .679; floating cells, P = .884).
Primary effusion lymphoma (PEL) cell lines.
Cell purification.
B cells were isolated from PBMC with anti-CD19 and further purified with anti-CD4, anti-CD8, and anti-CD14 antibody-coated beads (Dynal, Oslo, Norway), according to the manufacturer’s instructions. After removal of B cells, T cells were purified with anti-CD4 and anti-CD8 beads. T cells were further purified with a cocktail of anti-CD14 and anti-CD19 beads. Monocytes were isolated from the residual cells by 1 hour of adherence at 37°C on tissue culture plates. Adherent cells were then scraped and further purified with anti-CD4, anti-CD8, and anti-CD19 beads. Cell purification was always monitored by fluorescence-activated cell sorting (FACS) analysis. Freshly isolated PBMC and purified cell populations were counted and viability was determined by trypan blue dye exclusion. Both freshly isolated PBMC and purified cell populations were all viable after isolation.
PCR analysis.
PBMC or derived cell fractions (floating or adherent cells or purified cell populations) were counted and suspended at the same cell density (107 cells/mL) in lysis buffer containing 0.001% Triton (Sigma), 0.0001% sodium dodecyl sulfate (SDS; Sigma), 0.6 mg/mL proteinase K (Sigma), or, alternatively, 0.1% polyoxyethylene 10 lauryl ether (Sigma), and 0.1 mg/mL proteinase K, incubated at 56°C or 65°C, respectively, for 2 hours and heat-inactivated at 94°C for 15 minutes. Because dead cells also contain amplifiable DNA, total cells (viable and dead) were normalized with lysis buffer.
Amounts of lysates corresponding to 105 cells were amplified with primer set 1 (nucleotides 790-810 and 1207-1228 in the KS330 BAM sequence)37 or set 2 (nucleotides 112-130 and 430-453 in the KS631 BAM sequence)37 in 34 consecutive patients. Similarly, primer set 3 (nucleotides 987-1006 and 1200-1219 in the KS330 BAM sequence)37 was used in 56 consecutive patients. Oligonucleotides internal to the amplified sequences were used as probes for PCR product detection. β-Globin primers were 5′-CAA CTT CAT CCA CGT TCA CC-3′ and 5′-GAA GAG CCA AGG ACA GGT AC-3′. PCR conditions with primer set 1 and 2 were as follows: 5 minutes at 94°C, 35 to 45 cycles of denaturation (92°C for 1 minute), anealling (55°C for 2 minutes), and extension (72°C for 2 minutes); 1 mmol/L MgCl2 was included in the reaction mixture. PCR conditions with primer set 3 (35 to 45 cycles) were as described.37 PCR products were blotted on nylon membranes or subjected to liquid hybridization. For liquid hybridization, 10 μL of amplified DNA was mixed with 1 μL of 32P-labeled oligonucleotide and 5 μL of a solution containing 66.7 mmol/L NaCl and 44 mmol/L EDTA; the samples were then subjected to 5 minutes of denaturation at 94°C and 15 minutes of anealling at 55°C. Products were loaded onto 10% nondenaturing acrylamide gels and exposed for 1 to 12 hours. Hybridization of blotted PCR products were performed by standard techniques.
For semiquantitative PCR analysis, cell extracts were serially diluted in a buffer containing 10 mmol/L Tris-HCl (pH 7.8), 0.1 mmol/L EDTA, and highly purified sonicated salmon sperm DNA (50 μg/mL; all from Sigma) as described.36 Dilution factors are indicated in the text. To ascertain that the same relative amount of cells was analyzed, the same extracts were analyzed by serial dilution PCR with primers for β-globin.36
Reverse transcription-PCR (RT-PCR) analysis.
Total RNA was extracted with the RNA assay Mini kit (Qiagen, GmbH, Germany) and further purified with pancreatic DNAse I (Boehringer Mannheim), and purified RNA (0.5 μg) was retrotranscribed with the reverse transcription system kit (Promega, Madison, WI) by incubating the reactions with hexanucleotide random primers for 10 minutes at room temperature, 30 minutes at 42°C, and 30 minutes at 53°C. After heat inactivation of RT, one third of each reaction was subjected to 45 cycles of PCR for VP23 or T0.7, whereas amplification of β-actin was performed with 1/15 of RT-reactions and 40 PCR cycles. Primers set 3 was used for VP23 amplification, and primers RT-22A (CAC CAT TCC TCT CCG CAT TA) and RT-22B (GTC TGC CGA AGT CAG TGC CA) were used for T0.7 amplification with the same cycling conditions. β-Actin was amplified with primers BA1 (CAT GTG CAA GGC CGG CTT CG) and BA4 (GAA GGT GTG GTG CCA GAT TT).
In situ hybridization (ISH).
Cultured PBMC or BCBL-1 cells were harvested, centrifuged, washed twice, suspended in phosphate-buffered saline (PBS), seeded onto silan-coated slides, air-dried, and fixed in 4% buffered paraformaldehyde as described.9 ISH was performed under high stringency conditions with strand-specific35[S]-radiolabeled VP23 RNA hybridization probes (specific activity, ≈109 cpm/μg) transcribed from the plasmid p557-19 as described previously.25
FACS analysis.
Cells were analyzed by FACS38 with mouse monoclonal antibodies conjugated with fluorescein isothiocyanate (FITC) or phycoerythrin (PE): anti-CD3-FITC + anti-CD8-PE or anti-CD20-FITC + anti-CD14-PE (Becton Dickinson, Bedford, MA). Cells stained with FITC- or PE-conjugated isotype-matched antibodies directed against irrelevant epitopes served as negative controls. Living cells were gated based on forward and side scatter parameters. Cells positive for the isotypic controls (ie, nonspecifically stained) were excluded from the gate.
Immunocytochemistry.
PBMC were plated in gelatin-coated 8-well chamber slides (5 × 105 cells/well; Nunc Inc, Naperville, IL) and grown with or without TCM or RTCM (1:4). After 6 or 7 days, floating cells were harvested and adherent cells were washed with PBS without Ca2+ and Mg2+ and fixed (10 minutes) at 4°C with ethanol (80% vol/vol). Floating cells were analyzed by FACS or plated on polylysine-coated chamber slides and fixed as described above. Slides were stained using the alkaline phosphatase antialkaline phosphatase (APAAP) method as described39 with monoclonal antibodies directed against CD4 (1:20), CD8 (1:100), CD19 (1:20), CD20 (1:200), CD14 (1:20), CD31 (1:250), CD68 (1:200), CD45 (1:300), CDla (1:20), VE-cadherin (1:20), FVIII-RA (1:100), and CD34 (1:20) (all from Dako [Glostrup, Denmark], except for CD1a [Becton Dickinson] and VE-cadherin [Coulter Immunotech, Marseille, France]). The antibody directed against CD45 (leukocyte common antigen [LCA]) reacts with all CD45 isoforms. Slides were incubated with the antibody for 2 hours at room temperature or 12 hours at 4°C, washed with Tris-buffered solution, incubated (30 minutes) with rabbit antimouse IgG (1:25; Dako), washed, and incubated (45 minutes) with the APAAP (mouse) complex (1:40; Dako). The reaction was developed with the Fast Red Substrate System (Dako) and slides counterstained with Mayer’s hematoxylin solution (Sigma). The percentage of positive cells in at least 3 high power microscopic fields per slide was expressed as the average and the range of the minimal and maximal values.
Anti–HHV-8 serology.
BCBL-1 cells were treated for 48 hours with TPA (20 ng/mL). Ten microliters of a suspension of 4 × 106 cells/mL was smeared on coverslips, rapidly air-dried, and fixed in acetone/methanol solution for 10 minutes. Fixed smears were incubated successively in two steps of 30 minutes each at 37°C with serially diluted serum samples (in duplicate) and with fluorescein-labeled affinity-purified goat antibodies to human IgG (KPL Lab Inc, Gaithersburg, MD). All of the microscopic examinations were conducted by two different investigators on coded samples in a blinded fashion. An inverse titer of 20 or more was considered positive in the presence of a bright cytoplasmic staining. No correlation was found between Epstein-Barr virus (EBV) and HHV-8 antibody titers by this assay.3 15 Serum samples from 8- to 12-month-old babies and HIV-seronegative KS patients were used as negative and positive controls, respectively.
Statistical analysis.
Ninety-five percent confidence intervals (95%CI) for HHV-8 DNA prevalence in PBMC and HHV-8 seroprevalence were estimated using binomial distribution. The estimated prevalence of HHV-8 DNA or anti–HHV-8 antibodies in KS patients was compared with the prevalence found in non-KS patients using the test on equality of proportions.
The percentages of dead cells (unfractionated PBMC or floating cells) after culture with RPMI or in the presence of RTCM were compared with the Wilcoxon matched-pairs signed-ranks test. This test was used also to evaluate the induction of adherent cell growth and floating cell survival by IC. This was performed by comparing the number of total adherent or viable floating cells present at day 7 of culture in PBMC cultured with TCM/RTCM versus PBMC cultured without IC. In addition, the adherent cell growth induced by IC was estimated after 7 days of culture as the ratio of the number of adherent cells from PBMC cultured with TCM or RTCM and the number of adherent cells from PBMC cultured with medium alone. The increase of survival of floating cells by IC was similarly evaluated as the ratio between the percentage of alive floating cells after 7 days of culture in PBMC cultured in the presence of TCM or RTCM as compared with cells cultured without IC. These ratios were calculated for KS patients and patients at risk of KS and compared throughout the Mann-Whitney test. This test was used also to compare the levels of adherent cell growth among patients that showed (responders) or did not show (nonresponders) an increased PCR signal or conversion to PCR positivity upon culture with RTCM for 7 days.
The Mc-Nemar test for matched dychotomous data was used to calculate the probability that the responses to TCM (see Table 2) or RTCM (see Table 3) were obtained by chance.
One way analysis of variance was applied to analyze the expression of VE-cadherin in adherent cells from PBMC cultured with or without TCM or RTCM.
All statistical analyses were performed using STATA, version 5.0 package (StataCorp Stata Statistical software [release 5.0], College Station, TX).
RESULTS
Effect of cytokines increased in KS on HHV-8 load in PBMC from patients with KS or at risk of KS.
PBMC from 45 patients with KS and 45 individuals at risk of KS were analyzed by PCR with primers amplifying 3 different HHV-8 DNA regions. Sixty-four of these patients were HIV+ homosexual men with AIDS-KS (32 patients), or NKS-AIDS (25 patients) or asymptomatic (HIV+, 7 individuals) not undergoing therapy with HIV-protease inhibitors; and 26 were HIV− individuals with CKS (11 patients), PT-KS (2 patients), or PT (13 patients).
HHV-8 DNA was detected by PCR in 29 (64%) of the 45 KS patients (22 [69%] of the 32 with AIDS-KS, 6 [54%] of the 11 with CKS, and 1 [50%] of the 2 with PT-KS) and in 4 (9%) of the 45 individuals at risk of KS (2 [8%] of the 25 NKS-AIDS patients, 2 [15%] of the 13 PT patients, and in none of the 7 HIV+ individuals; Table 1). Fifty-nine of these patients for whom sera were available were also analyzed for anti–HHV-8 antibodies by IFA and 47 (80%) of the 59 analyzed were found to be positive with the highest seroprevalence (100%) in KS patients (Table 1). The prevalence of both viral DNA and specific antibodies was significantly higher in patients with KS as compared with patients at risk of KS (test on equality of proportions; P < .001; Table 1).
Detection of HHV-8 DNA in PBMC and Presence of Anti–HHV-8 Antibodies in Sera of Patients With KS or at Risk of KS
| Patients . | No. of Patients . | Positive/Analyzed Patients (%) . | |||
|---|---|---|---|---|---|
| HHV-8 DNA (PCR) . | 95% CI . | Anti–HHV-8 Antibodies (IFA) . | 95% CI . | ||
| KS | |||||
| AIDS-KS | 32 | 22/32 (69%) | 50-83.8 | 17/17 (100%) | 80.5-100 |
| CKS | 11 | 6/11 (54%) | 23.4-83.6 | 11/11 (100%) | 71.5-100 |
| PT-KS | 2 | 1/2 (50%) | 1.3-98.7 | 2/2 (100%) | 15.8-100 |
| Total | 45 | 29/45 (64%) | 48.8-78.1 | 30/30 (100%) | 88.4-100 |
| At risk of KS | |||||
| NKS-AIDS | 25 | 2/25 (8%) | 1.0-26.0 | 6/12 (50%) | 21.1-78.9 |
| HIV+ | 7 | 0/7 (0%) | 0-40.1 | 5/7 (71%) | 29.0-96.3 |
| PT | 13 | 2/13 (15%) | 1.9-45.4 | 6/10 (60%) | 26.2-87.8 |
| Total | 45 | 4/45 (9%) | 2.5-21.2 | 17/29 (59%) | 38.9-76.5 |
| Patients . | No. of Patients . | Positive/Analyzed Patients (%) . | |||
|---|---|---|---|---|---|
| HHV-8 DNA (PCR) . | 95% CI . | Anti–HHV-8 Antibodies (IFA) . | 95% CI . | ||
| KS | |||||
| AIDS-KS | 32 | 22/32 (69%) | 50-83.8 | 17/17 (100%) | 80.5-100 |
| CKS | 11 | 6/11 (54%) | 23.4-83.6 | 11/11 (100%) | 71.5-100 |
| PT-KS | 2 | 1/2 (50%) | 1.3-98.7 | 2/2 (100%) | 15.8-100 |
| Total | 45 | 29/45 (64%) | 48.8-78.1 | 30/30 (100%) | 88.4-100 |
| At risk of KS | |||||
| NKS-AIDS | 25 | 2/25 (8%) | 1.0-26.0 | 6/12 (50%) | 21.1-78.9 |
| HIV+ | 7 | 0/7 (0%) | 0-40.1 | 5/7 (71%) | 29.0-96.3 |
| PT | 13 | 2/13 (15%) | 1.9-45.4 | 6/10 (60%) | 26.2-87.8 |
| Total | 45 | 4/45 (9%) | 2.5-21.2 | 17/29 (59%) | 38.9-76.5 |
Freshly isolated PBMC were analyzed for HHV-8 DNA by PCR and sera for anti–HHV-8 antibodies by IFA. 95% CI are shown. Patients with KS show a higher prevalence of both viral DNA and antibodies as compared with patients at risk of KS (test on equality of proportions;P < .001).
To analyze the effect of IC on HHV-8 infection, PBMC from KS and risk individuals were cultured for 6 to 7 days with or without TCM. TCM contain the same IC increased in KS patients and they have been previously used to mimic the IC combination found in the lesions.7,19,32 33 The addition of TCM to PBMC induced a dramatic enhancement of both the intensity of the HHV-8–specific PCR signals and the detection of viral DNA in TCM-cultured PBMC as compared with cells cultured in its absence (Fig1A and Table 2).
(A) Increase of HHV-8 DNA load in PBMC from 2 AIDS-KS patient after culture (days 6 to 7) in the presence of TCM or RTCM or in its absence (RPMI). Shown are the autoradigrams of serial-dilution PCR experiments performed with primers specific for HHV-8 ORF 26 (VP23) and for the human β-globin gene used as a control of the amount of the genomic DNA analyzed. PCR products were hybridized with specific oligonucleoutide probes. Numbers above lanes represent dilution factors (for VP23) or aliquots of cell extracts corresponding to the indicated number of cells (for β-globin), respectively. Cell extracts were diluted in salmon sperm DNA as described in Materials and Methods. (C) Amplification of HHV-8 DNA from PBMC (day 0) and long-term cultures (21 or 28 days) of PBMC from 4 patients with KS (2 AIDS-KS, 2 CKS) and a PT patient. Floating (F) and adherent (A) cells were separately harvested at day 21 or 28 from PBMC cultured in the presence (RTCM) or absence (RPMI) of IC and the same number of cells (105) were analyzed with primer set 3. Negative controls (NC) are PCR reactions performed without DNA template or aliquots of salmon sperm DNA processed with PBMC. Positive controls were made with the indicated numbers of molecules (Mol) of plasmid p557-19. PCR products were transferred to nylon membranes and hybridized to a 32[P]-labeled oligonucleotide probe internal to the amplified sequences. Ethidium bromide stainings showed amplification of β-globin gene sequences from the same specimens. PBMC from the 2 CKS patients and the PT patient were analyzed also at day 7 and 14 with negative results. (D) PCR analysis of HHV-8 DNA with PBMC cultured with or without RTCM, γIFN, TNF, or IL-6. The same cell number was analyzed with primer set 3. NC is the negative control, consisting of either salmon sperm DNA processed with the specimens or PCR reactions lacking DNA template. (a) NKS-AIDS patient; (b through e) AIDS-KS patients; (f) 50 and 5 molecules of a positive control plasmid. PBMC from the patient shown in (a) were cultured for 11 days; PBMC from the other patients were cultured for 3 to 5 days. Fresh RTCM was added at days 0 and 3 of culture; single cytokines were added at days 0 and 2 for the patients shown in (a) and (b) and at days 0 and 4 for the other patients, respectively. γlFN was used at a concentration of 10, 50, or 100 IU/mL, as indicated in parenthesis. TNF was used at 30 ng/mL and IL-6 was used at 100 IU/mL, respectively. Experiments repeated with TNF or IL-6 (at 100 or 1,000 IU/mL) on 2 other patients that responded to RTCM gave similar results. All samples were positive for β-globin amplification, as shown by ethidium bromide staining of the PCR products. (B) Detection of HHV-8 DNA in PBMC (day 0) and in floating (F) or adherent (A) cells from 4 AIDS-KS patients (AIDS-KS), an asymptomatic homosexual man (HIV+), and a PT patient (PT) cultured (6 to 7 days) in the presence of TCM or RTCM or in its absence (RPMI). The same cell number (105) was analyzed with primer set 3. PC are positive controls made with the indicated numbers of molecules of plasmid p557-19. NC is negative control made without adding template DNA. PCR products were transferred to nylon membranes and hybridized to a32[P]-labeled oligonucleotide probe internal to the amplified sequences. Ethidium bromide staining shows amplification of β-globin gene sequences from the same specimens.
(A) Increase of HHV-8 DNA load in PBMC from 2 AIDS-KS patient after culture (days 6 to 7) in the presence of TCM or RTCM or in its absence (RPMI). Shown are the autoradigrams of serial-dilution PCR experiments performed with primers specific for HHV-8 ORF 26 (VP23) and for the human β-globin gene used as a control of the amount of the genomic DNA analyzed. PCR products were hybridized with specific oligonucleoutide probes. Numbers above lanes represent dilution factors (for VP23) or aliquots of cell extracts corresponding to the indicated number of cells (for β-globin), respectively. Cell extracts were diluted in salmon sperm DNA as described in Materials and Methods. (C) Amplification of HHV-8 DNA from PBMC (day 0) and long-term cultures (21 or 28 days) of PBMC from 4 patients with KS (2 AIDS-KS, 2 CKS) and a PT patient. Floating (F) and adherent (A) cells were separately harvested at day 21 or 28 from PBMC cultured in the presence (RTCM) or absence (RPMI) of IC and the same number of cells (105) were analyzed with primer set 3. Negative controls (NC) are PCR reactions performed without DNA template or aliquots of salmon sperm DNA processed with PBMC. Positive controls were made with the indicated numbers of molecules (Mol) of plasmid p557-19. PCR products were transferred to nylon membranes and hybridized to a 32[P]-labeled oligonucleotide probe internal to the amplified sequences. Ethidium bromide stainings showed amplification of β-globin gene sequences from the same specimens. PBMC from the 2 CKS patients and the PT patient were analyzed also at day 7 and 14 with negative results. (D) PCR analysis of HHV-8 DNA with PBMC cultured with or without RTCM, γIFN, TNF, or IL-6. The same cell number was analyzed with primer set 3. NC is the negative control, consisting of either salmon sperm DNA processed with the specimens or PCR reactions lacking DNA template. (a) NKS-AIDS patient; (b through e) AIDS-KS patients; (f) 50 and 5 molecules of a positive control plasmid. PBMC from the patient shown in (a) were cultured for 11 days; PBMC from the other patients were cultured for 3 to 5 days. Fresh RTCM was added at days 0 and 3 of culture; single cytokines were added at days 0 and 2 for the patients shown in (a) and (b) and at days 0 and 4 for the other patients, respectively. γlFN was used at a concentration of 10, 50, or 100 IU/mL, as indicated in parenthesis. TNF was used at 30 ng/mL and IL-6 was used at 100 IU/mL, respectively. Experiments repeated with TNF or IL-6 (at 100 or 1,000 IU/mL) on 2 other patients that responded to RTCM gave similar results. All samples were positive for β-globin amplification, as shown by ethidium bromide staining of the PCR products. (B) Detection of HHV-8 DNA in PBMC (day 0) and in floating (F) or adherent (A) cells from 4 AIDS-KS patients (AIDS-KS), an asymptomatic homosexual man (HIV+), and a PT patient (PT) cultured (6 to 7 days) in the presence of TCM or RTCM or in its absence (RPMI). The same cell number (105) was analyzed with primer set 3. PC are positive controls made with the indicated numbers of molecules of plasmid p557-19. NC is negative control made without adding template DNA. PCR products were transferred to nylon membranes and hybridized to a32[P]-labeled oligonucleotide probe internal to the amplified sequences. Ethidium bromide staining shows amplification of β-globin gene sequences from the same specimens.
Effect of TCM on HHV-8 DNA Detection in Cultured PBMC
| Patients . | PCR-Positive/Total Cultures (%) . | ||||
|---|---|---|---|---|---|
| −TCM . | +TCM . | Increased Signal . | Conversion to Positivity . | Total Responders . | |
| KS | |||||
| AIDS-KS | 6/10 (60%) | 9/10 (90%) | 4/10 (40%) | 3/10 (30%) | 7/10 (70%) |
| At risk of KS | |||||
| NKS-AIDS | 0/7 (0%) | 2/7 (29%) | 0/6 (0%) | 2/7 (29%) | 2/7 (29%) |
| Total | 6/17 (35%) | 11/17 (65%) | 4/17 (23%) | 5/17 (29%) | 9/17 (53%) |
| Patients . | PCR-Positive/Total Cultures (%) . | ||||
|---|---|---|---|---|---|
| −TCM . | +TCM . | Increased Signal . | Conversion to Positivity . | Total Responders . | |
| KS | |||||
| AIDS-KS | 6/10 (60%) | 9/10 (90%) | 4/10 (40%) | 3/10 (30%) | 7/10 (70%) |
| At risk of KS | |||||
| NKS-AIDS | 0/7 (0%) | 2/7 (29%) | 0/6 (0%) | 2/7 (29%) | 2/7 (29%) |
| Total | 6/17 (35%) | 11/17 (65%) | 4/17 (23%) | 5/17 (29%) | 9/17 (53%) |
PBMC from some of the individuals shown in Table 1 were cultured for 6 to 7 days with RPMI or TCM and the same cell number (105) was analyzed. Responders are defined as those patients whose PBMC showed either an increase in the PCR signal or converted to PCR positivity with TCM. All patients’ DNA were positive for β-globin amplification. The percentage of patients (AIDS-KS plus NKS-AIDS) that responded to treatment with TCM was significantly increased (Mc-Nemar test for matched dychotomous data;P = .025).
The same effect was also obtained by adding together the same (recombinant) IC at the same concentration as found in TCM (RTCM; Table 3).
Effect of RTCM on HHV-8 DNA Detection in Cultured PBMC and Serological Status of the Patients
| Patients . | PCR-Positive/Total Cultures (%) . | Serology . | |||||
|---|---|---|---|---|---|---|---|
| −RTCM . | +RTCM . | Increased Signal . | Conversion to Positivity . | Total Responders . | Responders . | Nonresponders . | |
| KS | |||||||
| AIDS-KS | 6/19 (31%) | 12/19 (63%) | 5/19 (26%) | 7/19 (37%) | 12/19 (63%)3-150 | 8/8 (100%) | 7/7 (100%) |
| CKS | 0/6 (0%) | 1/6 (17%) | 0/6 (0%) | 1/6 (17%) | 1/6 (17%) | 1/1 (100%) | 5/5 (100%) |
| PT-KS | 0/1 (0%) | 0/1 (0%) | 0/1 (0%) | 0/1 (0%) | 0/1 (0%) | — | 1/1 (100%) |
| Total | 6/26 (23%) | 13/26 (50%) | 5/26 (19%) | 8/26 (31%) | 13/26 (50%) | 9/9 (100%) | 13/13 (100%) |
| At risk of KS | |||||||
| NKS-AIDS | 0/5 (0%) | 0/5 (0%) | 0/5 (0%) | 0/5 (0%) | 0/5 (0%) | — | 3/5 (60%) |
| HIV+ | 0/6 (0%) | 1/6 (17%) | 0/6 (0%) | 1/6 (17%) | 1/6 (17%) | 1/1 (100%) | 4/5 (80%) |
| PT | 0/8 (0%) | 1/8 (12%) | 0/8 (0%) | 1/8 (12%) | 1/8 (12%) | 1/1 (100%) | 5/5 (100%) |
| Total | 0/19 (0%) | 2/19 (10%) | 0/19 (0%) | 2/19 (10%) | 2/19 (10%) | 2/2 (100%) | 12/15 (80%) |
| Patients . | PCR-Positive/Total Cultures (%) . | Serology . | |||||
|---|---|---|---|---|---|---|---|
| −RTCM . | +RTCM . | Increased Signal . | Conversion to Positivity . | Total Responders . | Responders . | Nonresponders . | |
| KS | |||||||
| AIDS-KS | 6/19 (31%) | 12/19 (63%) | 5/19 (26%) | 7/19 (37%) | 12/19 (63%)3-150 | 8/8 (100%) | 7/7 (100%) |
| CKS | 0/6 (0%) | 1/6 (17%) | 0/6 (0%) | 1/6 (17%) | 1/6 (17%) | 1/1 (100%) | 5/5 (100%) |
| PT-KS | 0/1 (0%) | 0/1 (0%) | 0/1 (0%) | 0/1 (0%) | 0/1 (0%) | — | 1/1 (100%) |
| Total | 6/26 (23%) | 13/26 (50%) | 5/26 (19%) | 8/26 (31%) | 13/26 (50%) | 9/9 (100%) | 13/13 (100%) |
| At risk of KS | |||||||
| NKS-AIDS | 0/5 (0%) | 0/5 (0%) | 0/5 (0%) | 0/5 (0%) | 0/5 (0%) | — | 3/5 (60%) |
| HIV+ | 0/6 (0%) | 1/6 (17%) | 0/6 (0%) | 1/6 (17%) | 1/6 (17%) | 1/1 (100%) | 4/5 (80%) |
| PT | 0/8 (0%) | 1/8 (12%) | 0/8 (0%) | 1/8 (12%) | 1/8 (12%) | 1/1 (100%) | 5/5 (100%) |
| Total | 0/19 (0%) | 2/19 (10%) | 0/19 (0%) | 2/19 (10%) | 2/19 (10%) | 2/2 (100%) | 12/15 (80%) |
PBMC from some of the individuals shown in Table 1 were cultured for 6 to 7 days with RPMI or RTCM and the same cell number (105) was analyzed by PCR. Responders are defined as those patients whose PBMC showed either an increase in the PCR signal or converted to PCR positivity with RTCM. All patients’ DNA were positive for β-globin amplification. HHV-8 seroprevalence of patients that responded or not to RTCM is also shown. The percentages of responders to RTCM was slightly lower as compared with TCM, particularly for AIDS-KS patients. The percentage of patients (AIDS-KS plus NKS-AIDS) that responded to treatment with RTCM was significantly higher than that who responded without RTCM (Mc-Nemar test for matched dychotomous data; P < .01).
One AIDS-KS patient was PCR-positive only in PBMC cultured without RTCM.
Patients whose PBMC showed an enhancement of the PCR signal or a conversion to PCR positivity with TCM or RTCM (Tables 2 and 3) were defined as responders. In total, 24 (39%) of the 62 patients analyzed were found to be responders. These included 19 (65%) of the 29 AIDS-KS patients analyzed (9 showed an increased PCR signal and 10 showed a conversion to PCR positivity); 1 (17%) of the 6 CKS patients; and 4 (15%) of the 26 patients without KS (2 NKS-AIDS, 1 HIV+, and 1 PT patients) examined (all of these patients showed a conversion to PCR positivity; Tables 2 and 3). In contrast, all of the other patients analyzed remained PCR-negative except for 1 AIDS-KS patient that was PCR-negative after culture with RTCM but positive in its absence. The enhancement of the PCR-signal and the conversion to PCR-positivity were found to be significantly associated with the exposure to IC (Mc-Nemar test for matched dychotomous data; TCM:P = .025; RTCM: P < .01; Tables 2 and 3).
The increase of the PCR signal was quantitated by serial dilution PCR in 2 AIDS-KS patients. This showed in both patients a 10-fold increase of HHV-8 DNA load in cells cultured with RTCM as compared with cells cultured in its absence (Fig 1A). In addition, the sera from the patients that were found to convert to PCR positivity and that were available for the analysis were found to be all positive for anti–HHV-8 antibodies (Table 3).
These data indicated that IC increase HHV-8 load and allow virus detection in cultured PBMC.
Increase of HHV-8 viral load induced by IC in floating and adherent cells from short-term and long-term PBMC cultures.
To further examine the effect of IC on HHV-8 infection and to gain information on the target cell types, viral load was analyzed in fresh PBMC (day 0) and in cells cultured for 6 to 7 days with or without IC that were harvested in toto or after separation in floating and adherent cells. This analysis was performed for 33 HHV-8–seropositive patients with or without KS that were either PCR-positive or PCR-negative at day 0 (Table 4).
Maintenance of the PCR Signal or Induction of PCR-Conversion Upon Culture With TCM or RTCM in PBMCs or in PBMC-Derived Adherent or Floating Cells From PCR-Positive or -Negative Patients
| Cell Population . | Maintenance of PCR Signal With IC4-150 (patients positive at day 0) . | PCR-Conversion With IC4-151 (patients negative at day 0) . | |
|---|---|---|---|
| KS Patients‡ (N = 12) . | KS Patients4-153 (N = 9) . | Risk Individuals4-153 (N = 12) . | |
| Unfractionated cells4-155 | 1/3 (33%) | 1/2 (50%) | 0/3 (0%) |
| Floating cells | 3/9 (33%) | 0/7 (0%) | 1/9 (11%) |
| Adherent cells | 3/8 (37%) | 2/7 (28%) | 2/9 (22%) |
| Responders | 5/12 (42%) | 3/9 (33%) | 2/12 (17%) |
| Presence of anti–HHV-8 Ab | 12/12 (100%) | 9/9 (100%) | 12/12 (100%) |
| Cell Population . | Maintenance of PCR Signal With IC4-150 (patients positive at day 0) . | PCR-Conversion With IC4-151 (patients negative at day 0) . | |
|---|---|---|---|
| KS Patients‡ (N = 12) . | KS Patients4-153 (N = 9) . | Risk Individuals4-153 (N = 12) . | |
| Unfractionated cells4-155 | 1/3 (33%) | 1/2 (50%) | 0/3 (0%) |
| Floating cells | 3/9 (33%) | 0/7 (0%) | 1/9 (11%) |
| Adherent cells | 3/8 (37%) | 2/7 (28%) | 2/9 (22%) |
| Responders | 5/12 (42%) | 3/9 (33%) | 2/12 (17%) |
| Presence of anti–HHV-8 Ab | 12/12 (100%) | 9/9 (100%) | 12/12 (100%) |
PBMC from serologically positive patients were analyzed at day 0 and after culture for 6-7 days with or without RTCM. Cells were harvested in toto (unfractionated) or by separating floating and adherent cells. All cells were counted and the same number of cells (105) was analysed by PCR.
Maintenance of the PCR signal by IC was defined as the presence of HHV-8 DNA upon culture in TCM or RTCM as compared with RPMI for patients whose PBMC were already positive at day 0. These included all patients that after culture in RPMI showed a loss of viral DNA or a reduced PCR signal as compared with TCM/RTCM.
Induction of PCR conversion by IC was defined as the detection of HHV-8 DNA in cells cultured with TCM or RTCM as compared with RPMI-negative cultures for the patients that were PCR-negative at day 0, but serologically positive.
Including 9 AIDS-KS and 3 CKS.
Including 6 AIDS-KS, 2 CKS, 1 PT-KS, 2 NKS-AIDS, 5 HIV+, and 5 PT patients.
Cultured PBMC from 3 patients with AIDS-KS, 1 with CKS, and 3 PT patients were harvested as unfractionated cells.
Without IC, a dramatic reduction or loss of the HHV-8 PCR signal occurred after culture of unfractionated PBMC or in both floating or adherent cells from patients whose PBMC were positive at day 0. By contrast, cultures treated with IC maintained HHV-8 DNA in unfractionated PBMC or in adherent or floating cells (Fig 1B and Table4). It is noteworthy that IC increased viral DNA load and allowed virus detection in PBMC and adherent or floating cells from seropositive patients whose PBMC were negative at day 0 (Fig 1B and Table 4). Only a few or no CD14+ cells were present in the floating populations at the time of harvesting (see Fig 4); therefore, it is highly unlikely that detached adherent cells accounted for the results obtained with the floating cell fraction.
To determine whether IC can maintain the virus in culture for a prolonged period of time, PBMC from 5 HHV-8 seropositive patients (2 AIDS-KS, 2 C-KS, and 1 PT patient) were cultured with or without RTCM for 3 to 4 weeks, and adherent and floating cells were separately analyzed for the presence of HHV-8 DNA at day 21 or 28. For those patients that had enough cells, PCR was also performed at earlier time points. Three of these patients (1 with AIDS-KS and the 2 CKS patients) were PCR-positive at day 0 and the other 2 were negative. Two of the 3 patients that were PCR-positive at day 0 (both CKS patients) turned negative at day 7 and day 14; however, they became positive again in the adherent or floating cells after long-term culture (28 days) in the presence of RTCM but not in its absence (Fig 1C). One of the 2 patients negative at day 0 (the PT patient) remained negative at day 7 and 14 but turned positive in the adherent cell fraction after long-term culture with RTCM but not in its absence. For the other patients, the virus was lost (1 AIDS-KS) or remained undetectable (Fig 1C).
These data suggested that IC can reactivate HHV-8 upon short-term or long-term culture, that this can occur in both floating or adherent cells, and that it may require a chronic exposure to IC for detection.
Detection of HHV-8 DNA in PBMC after culture with γIFN.
To identify the cytokine(s) responsible of the effect of TCM or RTCM on HHV-8 infection, γIFN, TNF, or IL-6, which are the most prominent cytokines present in TCM and increased in KS patients, were added to PBMC of patients with or without KS. The addition of γIFN at 10 or 50 IU/mL but not at higher concentrations augmented viral DNA load in all 4 of the patients analyzed (Fig 1D, a through d), mimicking the effect of TCM or RTCM. Three of these patients showed a conversion to PCR positivity and 1 showed an increased PCR signal, respectively (Fig 1D). In contrast, TNF and IL-6 had little or no activity at the concentrations used (Fig 1D). Thus, γIFN plays a key role in the effects of TCM or RTCM on HHV-8 infection.
Induction of virus reactivation by IC.
To determine whether the effect of IC on HHV-8 viral load was due to virus reactivation, latent (T0.7) and lytic (VP23) viral gene expression were analyzed in 6 patients with AIDS-KS at both day 0 and upon 2 days of culture with or without IC. Three of the 6 patients were negative for RNA expression by RT-PCR in all samples, including that from day 0, although viral DNA was present in fresh PBMC (day 0). The other 3 patients analyzed showed expression of T0.7 but not VP23 at day 0, and 2 of these showed both lytic and latent viral gene expression upon culture. This was found to be augmented or induced by IC in both floating or adherent cells (Fig 2A). In particular, both patients showed lytic VP23 gene expression in adherent cells in the presence of RTCM but not in its absence. Floating cells from 1 patient showed lytic gene expression in RPMI; however, the expression was augmented by IC (Fig 2A). For an additional patient with a very high viral load, it was possible to perform an in situ hybridization analysis using a probe specific for the lytic VP23 mRNA.25 This was found to be expressed in cells with the typical morphology of lymphocytes and monocytes but only upon culture with RTCM (Fig 2B). Thus, IC appear to promote viral reactivation in both floating or adherent cells (Fig 2A and B).
(A) Detection of latent and lytic viral gene expression in total PBMC at day 0 and in floating or adherent cells cultured with or without IC from 2 patients with AIDS-KS. (a) RT-PCR and hybridization for T0.7; (b) RT-PCR and hybridization for VP23; (c) RT-PCR and hybridization for T0.7 in the absence of reverse transcriptase; (d) RT-PCR and ethidium bromide staining for human β-actin. NC are negative controls, including Jurkat cells and PCR reactions without DNA template. RT− indicates β-actin RT-PCR reactions performed from the same samples of RNA in the absence of reverse transcriptase. The same number of RTCM-treated and untreated cells were processed and RNA normalized by spectrophotometric determination. (B) ISH detection of HHV-8 lytic (Vp23) gene expression in PBMC from an AIDS-KS patient after culture with RTCM. ISH of (A) PBMC cultured for 72 hours without RTCM (original magnification × 25) and (B) corresponding dark field; (C) PBMC cultured for 72 hours with RTCM (original magnification × 25), arrows point to ISH-positive cells and (D) corresponding dark field; (E) PBMC from a patient not infected by HHV-8 (original magnification × 25) and (F) corresponding dark field; (G) higher magnification showing positive cells with monocytic morphology; a cell with lymphocyte morphology is negative. Several microscopic fields were analyzed and a similar density of positive cells was present in cells cultured in the presence of RTCM. In contrast, cells cultured in the presence of RPMI were always negative. Hybridization to a latency-associated HHV-8 gene probe could not be performed, because cells were not sufficient.
(A) Detection of latent and lytic viral gene expression in total PBMC at day 0 and in floating or adherent cells cultured with or without IC from 2 patients with AIDS-KS. (a) RT-PCR and hybridization for T0.7; (b) RT-PCR and hybridization for VP23; (c) RT-PCR and hybridization for T0.7 in the absence of reverse transcriptase; (d) RT-PCR and ethidium bromide staining for human β-actin. NC are negative controls, including Jurkat cells and PCR reactions without DNA template. RT− indicates β-actin RT-PCR reactions performed from the same samples of RNA in the absence of reverse transcriptase. The same number of RTCM-treated and untreated cells were processed and RNA normalized by spectrophotometric determination. (B) ISH detection of HHV-8 lytic (Vp23) gene expression in PBMC from an AIDS-KS patient after culture with RTCM. ISH of (A) PBMC cultured for 72 hours without RTCM (original magnification × 25) and (B) corresponding dark field; (C) PBMC cultured for 72 hours with RTCM (original magnification × 25), arrows point to ISH-positive cells and (D) corresponding dark field; (E) PBMC from a patient not infected by HHV-8 (original magnification × 25) and (F) corresponding dark field; (G) higher magnification showing positive cells with monocytic morphology; a cell with lymphocyte morphology is negative. Several microscopic fields were analyzed and a similar density of positive cells was present in cells cultured in the presence of RTCM. In contrast, cells cultured in the presence of RPMI were always negative. Hybridization to a latency-associated HHV-8 gene probe could not be performed, because cells were not sufficient.
Identification of the blood cells infected in vivo by HHV-8.
The effect of IC on HHV-8 infection may be due to direct effects on the virus but also to effects on the cells target of HHV-8 infection. Therefore, experiments were designed to identify the cell types infected in vivo. In the previous experiment, PBMC from the AIDS-KS patient that was analyzed by ISH showed viral gene expression mostly in cells with a bean-shaped nucleus that is typical of monocyte-macrophages (Fig 2B). However, infected lymphocytes were also detected. Therefore, B cells, T cells, and monocytes were purified from PBMC of 5 patients (2 AIDS-KS, 2 NKS-AIDS, and 1 HIV+) and analyzed by PCR. HHV-8 DNA was detected in B cells and monocytes from HIV-infected homosexual men with or without KS or AIDS and in T cells from a late stage AIDS-KS patient (Fig 3). HHV-8 DNA was also detected in monocytes or B cells from a NKS-AIDS patient and an HIV+ homosexual man, respectively, whose PBMC were negative by PCR (Fig 3 and data not shown). In total, 3 (60%) of 5 B-cell fractions, 3 (60%) of 5 monocyte fractions, and 1 (20%) T-cell fraction from the 5 patients were PCR-positive. Thus, both B cells and monocytes can be infected by HHV-8 in vivo.
Detection of HHV-8 DNA in purified cell populations from PBMC of homosexual men with HIV infection. Aliquots of cell lysates (105 cells) were subjected to PCR with primer set 3.PCR products were analyzed by Southern blot hybridization with a32P-labeled oligonucleotide probe internal to the amplified sequences. Shown are the results with B cells (B), monocytes (M), or T cells (T) from 2 AIDS-KS and 2 NKS-AIDS patients. Cell populations were purified as described and purification was monitored by FACS.All samples were positive for β-globin amplification (data not shown). An HIV+ patient was positive in B cells (data not shown). The NKS-AIDS and the HIV+ patients were positive for anti–HHV-8 Ab by IFA.
Detection of HHV-8 DNA in purified cell populations from PBMC of homosexual men with HIV infection. Aliquots of cell lysates (105 cells) were subjected to PCR with primer set 3.PCR products were analyzed by Southern blot hybridization with a32P-labeled oligonucleotide probe internal to the amplified sequences. Shown are the results with B cells (B), monocytes (M), or T cells (T) from 2 AIDS-KS and 2 NKS-AIDS patients. Cell populations were purified as described and purification was monitored by FACS.All samples were positive for β-globin amplification (data not shown). An HIV+ patient was positive in B cells (data not shown). The NKS-AIDS and the HIV+ patients were positive for anti–HHV-8 Ab by IFA.
Effect of cytokines increased in KS on the survival and growth of the cells target of HHV-8 infection.
To evaluate the effects of IC on the cell types infected by HHV-8 in vivo, PBMC were cultured for 6 to 7 days with or without TCM or RTCM. Floating and adherent cells were separately harvested, counted, and analyzed by FACS or immunocytochemistry and compared with the PBMC of origin (day 0).
The number of viable (survived) floating cells after culture for 6 or 7 days in the presence or absence of IC did not differ (Wilcoxon signed-rank test; P = .345), however, FACS analysis of PBMC (day 0) and of floating cells from the cultured PBMC indicated that IC increased B-cell survival (Fig 4A). In contrast, CD8+ and CD4+ T cells, CD8+ natural killer cells, and non-T cells decreased with time and at a similar rate with or without IC (Fig 4A and data not shown).
(A) FACS analysis of PBMC at day 0 and PBMC-derived floating cells cultured for 6 days without TCM (RPMI, day 6) or with TCM (TCM, day 6). T cells were doubly stained for CD3 and CD8 to obtain the percentage of CD8+ T cells (CD3+/CD8+), CD4+ T cells (CD3+/CD8−), CD8+ NK cells (CD3−/CD8+), and non-T cells (CD3−/CD8−). Staining with anti-CD14 and anti-CD20 antibodies was used to identify monocyte and B cells, respectively (left side). Shown are representative examples from 2 patients (upper and lower panels, respectively). Similar results were also obtained with floating cells from other 3 AIDS-KS patients and 1 asymptomatic HIV+ individual analyzed by immunocytochemistry (data not shown). (B) Phenotypic characterization of adherent cells obtained from PBMC of an AIDS-KS patient cultured for 6 days with TCM and analyzed by immunohistochemistry (APAAP method). (a) A representative negative control (isotype-matched control Ab) of adherent cells counterstained by hematoxylin with the typical spindle morphology is shown (original magnification × 400). (b through d) Representative examples of staining for CD68 (b; original magnification × 400), CD14 (c; original magnification × 1,000), and VE-cadherin (d; original magnification × 400) are presented. Nearly 100% of the cells present in these fields showed a specific red staining. The average percentage of positive cells from different fields for all the patients analyzed is shown in Table 5.
(A) FACS analysis of PBMC at day 0 and PBMC-derived floating cells cultured for 6 days without TCM (RPMI, day 6) or with TCM (TCM, day 6). T cells were doubly stained for CD3 and CD8 to obtain the percentage of CD8+ T cells (CD3+/CD8+), CD4+ T cells (CD3+/CD8−), CD8+ NK cells (CD3−/CD8+), and non-T cells (CD3−/CD8−). Staining with anti-CD14 and anti-CD20 antibodies was used to identify monocyte and B cells, respectively (left side). Shown are representative examples from 2 patients (upper and lower panels, respectively). Similar results were also obtained with floating cells from other 3 AIDS-KS patients and 1 asymptomatic HIV+ individual analyzed by immunocytochemistry (data not shown). (B) Phenotypic characterization of adherent cells obtained from PBMC of an AIDS-KS patient cultured for 6 days with TCM and analyzed by immunohistochemistry (APAAP method). (a) A representative negative control (isotype-matched control Ab) of adherent cells counterstained by hematoxylin with the typical spindle morphology is shown (original magnification × 400). (b through d) Representative examples of staining for CD68 (b; original magnification × 400), CD14 (c; original magnification × 1,000), and VE-cadherin (d; original magnification × 400) are presented. Nearly 100% of the cells present in these fields showed a specific red staining. The average percentage of positive cells from different fields for all the patients analyzed is shown in Table 5.
IC had even more striking effects on adherent cells, because they induced the development of spindle-shaped cells (Fig 4B, a) in the majority of the cultures, although in AIDS-KS patients they were already detectable without IC. IC also induced the growth of these cells (Wilcoxon signed-rank test; P < .0001). Adherent cells from patients with KS or AIDS showed the highest proliferative response (average of 3.1- ± 2.0-fold growth induction by day 6 of culture for 16 KS patients versus 3.0- ± 4.0-fold for 14 NKS-AIDS patients, 1.6- ±0.59-fold for 4 HIV+ individuals, and 1.4- ± 0.89-fold for 7 PT patients, respectively).
To verify whether the effect of IC on HHV-8 DNA load in adherent cells was or was not related to the proliferation of latently infected cells, the growth of adherent cells from these patients was compared with the PCR results in both the presence and absence of IC. However, no significant association of cell growth with the maintenance of the PCR signal or the conversion to PCR positivity was detected (data not shown; Mann-Whitney test; all patients, P = .241; KS patients,P = .257). This confirmed that the increase of viral load observed in this cell population is due to virus reactivation and not to the proliferation of latently infected cells.
IC induce circulating spindle cell progenitors to differentiate in endothelial macrophages that are present in KS lesions.
As expected, adherent cells induced by IC did not express markers of T and B lymphocytes (CD8, CD19, and CD20), whereas they expressed the leukocyte common antigen (LCA/CD45), markers of tissue macrophages such as CD68 (Fig 4B, b), and markers of monocytic cells (CD31 and CD14; Fig4B, c), both with or without IC (Table 5). In addition, CD1a, a marker of dendritic cells, was detected in 1 HIV+ patient and its expression was increased upon culture with IC (Table 5). It is noteworthy that untreated cells from AIDS-KS patients, but not from the other groups, also expressed VE-cadherin, a marker of endothelial cells and endothelial macrophages (Fig 4B, d, and Table 5). In addition, VE-cadherin expression was induced by IC in adherent cells from all groups of patients (Table 5). Because staining for endothelial cell markers such as factor VIII-related antigen (FVIII-RA) and CD3432 remained negative (Table 5), these cells were identified as endothelial macrophages.21 Thus, PBMC-derived adherent cells are of monocytic origin and IC induce their proliferation and differentiation toward tissue macrophages and spindle-like endothelial macrophages. It is noteworthy that these same cells have been found to be increased in the blood of KS patients20,28,29 and that they are present in KS lesions18,19; in both cases, they are infected by HHV-8 and maintain the virus upon culture.19 29
Immunocytochemical Analysis of PBMC-Derived Adherent Cells Cultured With or Without TCM or RTCM
| Culture Condition . | Average % of Positive Cells (min-max values) . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4 . | CD8 . | CD19 . | CD14 . | CD68 . | CD31 . | CD34 . | CD45 . | CD1a . | VE-Cadherin . | FVIII-RA . | |
| RPMI | 3 | 0 | 0 | 17 | 81.3 | 94 | 0 | 97.6 | 0.75-150 | 35-151 | 0 |
| (0-13) | (0-77) | (60-100) | (90-97) | (96-100) | (0-8) | (0-17) | |||||
| N = 7 | N = 3 | N = 8 | N = 7 | N = 4 | N = 3 | N = 4 | N = 5 | N = 12 | N = 15 | N = 3 | |
| TCM or RTCM | 10 | 0.3 | 0 | 13.6 | 90 | 95 | 0 | 100 | 1.95-150 | 225-151 | 0 |
| (1-70) | (0-1) | (0-60) | (86-95.5) | (91-100) | (100-100) | (0-21) | (0-87) | ||||
| N = 11 | N = 6 | N = 6 | N = 7 | N = 4 | N = 3 | N = 4 | N = 5 | N = 1 | N = 15 | N = 3 | |
| Culture Condition . | Average % of Positive Cells (min-max values) . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4 . | CD8 . | CD19 . | CD14 . | CD68 . | CD31 . | CD34 . | CD45 . | CD1a . | VE-Cadherin . | FVIII-RA . | |
| RPMI | 3 | 0 | 0 | 17 | 81.3 | 94 | 0 | 97.6 | 0.75-150 | 35-151 | 0 |
| (0-13) | (0-77) | (60-100) | (90-97) | (96-100) | (0-8) | (0-17) | |||||
| N = 7 | N = 3 | N = 8 | N = 7 | N = 4 | N = 3 | N = 4 | N = 5 | N = 12 | N = 15 | N = 3 | |
| TCM or RTCM | 10 | 0.3 | 0 | 13.6 | 90 | 95 | 0 | 100 | 1.95-150 | 225-151 | 0 |
| (1-70) | (0-1) | (0-60) | (86-95.5) | (91-100) | (100-100) | (0-21) | (0-87) | ||||
| N = 11 | N = 6 | N = 6 | N = 7 | N = 4 | N = 3 | N = 4 | N = 5 | N = 1 | N = 15 | N = 3 | |
Average percentage of adherent cells positive for CD4, CD8, CD19, CD14, CD68, CD31, CD34, CD45, CD1a, VE-cadherin, and FVIII-RA expression by immunocytochemistry after 7 days of culture with TCM or RTCM or growth medium (RPMI). Values are the average of determinations from 8 KS patients, 4 NKS-AIDS patients, and 5 HIV+individuals. Range of determinations with cells from different patients are indicated in parentheses. The results of each determination are from the counting of at least 3 high power macroscopic fields per slide from 1 or more experiments with cells from the same patients. CD19 staining was also confirmed with anti-CD20 antibodies.
Abbreviation: N, number of patients.
Expressed only by 1 HIV+ individual before and after TCM treatment.
VE-cadherin was expressed in RPMI only by KS patients (average, 7%; N = 8) and this was statistically significant (P = .00963) as determined by analysis of variance. VE-cadherin expression was induced by TCM or RTCM in patients from all groups (asymptomatic [N = 3] from 0% to 1%, NKS-AIDS [N = 4] from 0% to 13%, and KS [N = 8] from 7% to 34%), and the effect of TCM or RTCM was statistically significant (P = .028).
DISCUSSION
Previous evidence suggested that IC, particularly of the Th1-type, may act as triggering factors in KS development. IC, and in particular γIFN, appear to initiate KS development. In fact, they induce endothelial cells to acquire the features of KS spindle cells cultured in vitro or present in primary lesions and promote the formation of KS-like lesions after injection in mice.7,32,34,40-42 The development of KS-like lesions is mediated by basic fibroblast growth factor and vascular endothelial growth factor, two angiogenic factors that are highly expressed in KS and whose production is induced by IC.34,39,41-43 IC also promote the expression of the receptors for the HIV-l Tat protein that acts as a progression factor in KS development33,39,44 and increases HHV-8 load.45 IC also support the establishment and the long-term growth of lesional KS spindle cells of both endothelial and macrophage origin.7,19,34 40
Consistent with these data, the administration to KS patients of γIFN, TNF, or IL-2 has resulted in KS progression or onset.46,47 In agreement with these findings are also data indicating that immunoactivation or immunodysregulation and production of IC are common in individuals at a high risk of KS, including homosexual men even before HIV-1 infection,7,19,32,40,48,49African individuals from areas at high incidence of KS,49,50 or elderly men of the Eastern Mediterranean area that are preferentially affected by CKS.51,52 All of these individuals have a high HHV-8 seroprevalence3,11,15 and present a CD8 T-cell activation.7,19,32,40,49,52CD8+ T cells are one of the most important source of γIFN and other IC in KS patients.7 19
We have shown here that the same IC maintain or increase HHV-8 DNA load in cultured PBMC from patients with KS or at risk of KS. These effects occur on both B cells and monocytes that are the two main circulating cell types infected in vivo.
Specifically, IC can maintain HHV-8 DNA in PBMC that are PCR-positive at day 0 or can increase viral DNA load to detectable levels in PBMC that are PCR-negative before culture. One or the other of these two effects were observed in 24 (39%) of 62 patients analyzed; hence, many, although not all, patients can respond to IC. Quantitation of HHV-8 DNA load indicates that IC can increase viral load up to 10-fold as compared with cells cultured in their absence.
The results of the long-term culture experiments also show that HHV-8 can be maintained at undetectable levels for long periods of time in PBMC cultured with IC, but viral load increases to detectable levels after chronic exposure to IC. Furthermore, IC induce the expression of HHV-8 lytic genes in cultured PBMC to levels detectable by RT-PCR or ISH.
These effects of IC are observed in both floating and/or adherent cells from cultured PBMC. Consistent with these data, B cells and monocytes were found to be the two main cell types infected in vivo. The data, therefore, indicate that IC increase or maintain HHV-8 infection and have similar effects in both cell types.
Although other cytokines may contribute to the viral effects of TCM or RTCM on PBMC, γIFN appears to be sufficient to maintain and to increase HHV-8 load. Because γIFN is the earliest and most abundant IC detectable in KS,7 these data suggest that it may be key to both HHV-8 load and persistence and to the development of KS.
The mechanisms responsible for the increase of viral load induced by IC may be several, including the reactivation of HHV-8 infection and effects on the survival of B cells and on the growth and differentiation of monocyte/macrophages or to a combination of these effects. However, several lines of evidence suggest that the major mechanisms of IC is the reactivation of HHV-8 infection in these cell types.
Although IC induced the growth of adherent cells, this was not significantly associated with the increase of the PCR signal or with the conversion to PCR positivity. These data strongly argue against the preferential growth of a pre-existing population(s) of latently infected cells. In addition, both adherent and/or floating cells turned PCR-positive upon several weeks of culture in the presence of IC but the same cells were found to be PCR-negative at day 0 and/or after culture for shorter periods of times. This is strongly suggestive of viral reactivation induced by the chronic exposure to IC, and it is similar to the reactivation of human cytomegalovirus (HCMV) observed in PBMC after a prolonged allogeneic stimulation.53
Moreover, IC induced the expression of the lytic VP23 RNA in both floating cells (lymphocytes) and adherent cells (monocytes). Adherent cells from all the patients analyzed expressed VP23 only in the presence of IC; in contrast, although IC increased lytic gene expression also in floating cells, reactivation was already observed simply by culturing the cells. In contrast, only the latency-associated T0.7 mRNA was detected in PBMC at day 0, in agreement with previous work showing that PBMC from KS patients can harbor either lytic or latent HHV-8 genomic forms.4 Although additional studies are required, these data indicate that IC induced HHV-8 lytic replication in cells from PBMC. Work is in progress to study in a greater detail the effect of IC on the expression of HHV-8 latency-associated and lytic genes. The effects of IC on HHV-8 gene expression appear to be specific for PBMC, because they were undetectable in chronically infected PEL cell lines.35 54In fact, no significant differences in latent or lytic viral gene expression were observed in BCBL-1 cells cultured for 2 days in the presence or absence of RTCM or single cytokines, including γIFN, TNFα, and IL-6, by both RT-PCR and in situ hybridization (data not shown). Moreover, in contrast to PBMC, RTCM inhibited in a dose-dependent fashion the expression of the lytic T1.1 HHV-8 nuclear RNA expressed by cells undergoing spontaneous virus reactivation in BC-1 cells, a PEL cell line doubly infected by HHV-8 and EBV (data not shown).
The effects of IC on B-cell survival and adherent cell growth and differentiation may suggest that specific changes induced by IC in the phenotype of the infected cells may be required for the reactivation of HHV-8 infection in PBMC. In particular, the differentiation of adherent cells toward the endothelial-macrophage phenotype may be key for HHV-8 reactivation not only in vitro but also in vivo. In fact, these cells are expanded in the blood of KS patients and are present in KS lesions.18,20,28 29
These and recent data on monocyte-macrophages in KS lesions7,18,24,25 also suggest a crucial role of this cell type for the localization of the virus into tissues. In particular, because B cells are rare or absent in KS lesions, circulating monocytes may recruit the virus into tissues and, upon exposure to IC, they may undergo lytic infection and transmit the virus to neighbor cells. Alternatively, they may differentiate into macrophages and spindle-like endothelial macrophages with a latent infection and a high viral load as observed in vivo in lesional spindle cells.9,22,23 The recruitment of these cells in tissues and in KS lesions is driven by the expression of adhesion molecules in the vascular endothelium that is activated by IC.7,17,19,32,39,40 55 Therefore, it is tempting to speculate that the link between immunoactivation, IC production, and HHV-8 infection in KS development is related to the recruitment, growth, and differentiation of HHV-8–infected circulating monocytes.
ACKNOWLEDGMENT
The authors thank E. Trwniszewska, H.S. Chang, R. Humphrey, T. Merced-Galindez (National Institutes of Health, National Cancer Institute), and A. Schreier (Max-Planck-lnstitut für Biochemie) for technical help and A. Lippa and F.M. Regini for editorial assistance.
P.M. and S.C. contributed equally to this work.
Supported by Italian grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC), Progetto Sangue, and the IX AIDS project from the Ministry of Health; by the European Concerted Action “Pathogenesis of AIDS-KS”; and by a grant from the Deutsche Forschungsgemeinschaft (SFB 464).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Barbara Ensoli, MD, PhD, Laboratory of Virology, Istituto Superiore di Sanità, Viale Regina Elena 299, 00161 Rome, Italy; e-mail: ensoli@virus1.net.iss.it.

![Fig. 1. (A) Increase of HHV-8 DNA load in PBMC from 2 AIDS-KS patient after culture (days 6 to 7) in the presence of TCM or RTCM or in its absence (RPMI). Shown are the autoradigrams of serial-dilution PCR experiments performed with primers specific for HHV-8 ORF 26 (VP23) and for the human β-globin gene used as a control of the amount of the genomic DNA analyzed. PCR products were hybridized with specific oligonucleoutide probes. Numbers above lanes represent dilution factors (for VP23) or aliquots of cell extracts corresponding to the indicated number of cells (for β-globin), respectively. Cell extracts were diluted in salmon sperm DNA as described in Materials and Methods. (C) Amplification of HHV-8 DNA from PBMC (day 0) and long-term cultures (21 or 28 days) of PBMC from 4 patients with KS (2 AIDS-KS, 2 CKS) and a PT patient. Floating (F) and adherent (A) cells were separately harvested at day 21 or 28 from PBMC cultured in the presence (RTCM) or absence (RPMI) of IC and the same number of cells (105) were analyzed with primer set 3. Negative controls (NC) are PCR reactions performed without DNA template or aliquots of salmon sperm DNA processed with PBMC. Positive controls were made with the indicated numbers of molecules (Mol) of plasmid p557-19. PCR products were transferred to nylon membranes and hybridized to a 32[P]-labeled oligonucleotide probe internal to the amplified sequences. Ethidium bromide stainings showed amplification of β-globin gene sequences from the same specimens. PBMC from the 2 CKS patients and the PT patient were analyzed also at day 7 and 14 with negative results. (D) PCR analysis of HHV-8 DNA with PBMC cultured with or without RTCM, γIFN, TNF, or IL-6. The same cell number was analyzed with primer set 3. NC is the negative control, consisting of either salmon sperm DNA processed with the specimens or PCR reactions lacking DNA template. (a) NKS-AIDS patient; (b through e) AIDS-KS patients; (f) 50 and 5 molecules of a positive control plasmid. PBMC from the patient shown in (a) were cultured for 11 days; PBMC from the other patients were cultured for 3 to 5 days. Fresh RTCM was added at days 0 and 3 of culture; single cytokines were added at days 0 and 2 for the patients shown in (a) and (b) and at days 0 and 4 for the other patients, respectively. γlFN was used at a concentration of 10, 50, or 100 IU/mL, as indicated in parenthesis. TNF was used at 30 ng/mL and IL-6 was used at 100 IU/mL, respectively. Experiments repeated with TNF or IL-6 (at 100 or 1,000 IU/mL) on 2 other patients that responded to RTCM gave similar results. All samples were positive for β-globin amplification, as shown by ethidium bromide staining of the PCR products. (B) Detection of HHV-8 DNA in PBMC (day 0) and in floating (F) or adherent (A) cells from 4 AIDS-KS patients (AIDS-KS), an asymptomatic homosexual man (HIV+), and a PT patient (PT) cultured (6 to 7 days) in the presence of TCM or RTCM or in its absence (RPMI). The same cell number (105) was analyzed with primer set 3. PC are positive controls made with the indicated numbers of molecules of plasmid p557-19. NC is negative control made without adding template DNA. PCR products were transferred to nylon membranes and hybridized to a32[P]-labeled oligonucleotide probe internal to the amplified sequences. Ethidium bromide staining shows amplification of β-globin gene sequences from the same specimens.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/12/10.1182_blood.v93.12.4044/4/m_blod41239001aw.jpeg?Expires=1769916155&Signature=eprsucF9Vg3axjKNl7mx4mfcXoQKpDnHcAgo3er4p~G7vQRHyDjnOy4R52WNdvE0vE0D~nw8yNVqyWZ4M5g2o9kGnnyRix2moNUTOeGxvxbrc5sPTaCw4sKBT51D00dN7AcxkEB~Jav7t~95B9l3CRin3FaclEukIggZrBtjDYudiZx0NdiWgTf1QXaDysqfjsQvPOmPuiZm7I73x1j17~q-eJq~qVGDflKACyFByT27j6wAxCROg4PJVm7~eQP4ghxuvK2iUIcV7qXrF6tlQgLGT6Wa2~lIJWRWS7ohy7UZ8llWBwc-8ValM2mAbd3YfatRyKXugNKGUyA3YncxGg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. (A) Increase of HHV-8 DNA load in PBMC from 2 AIDS-KS patient after culture (days 6 to 7) in the presence of TCM or RTCM or in its absence (RPMI). Shown are the autoradigrams of serial-dilution PCR experiments performed with primers specific for HHV-8 ORF 26 (VP23) and for the human β-globin gene used as a control of the amount of the genomic DNA analyzed. PCR products were hybridized with specific oligonucleoutide probes. Numbers above lanes represent dilution factors (for VP23) or aliquots of cell extracts corresponding to the indicated number of cells (for β-globin), respectively. Cell extracts were diluted in salmon sperm DNA as described in Materials and Methods. (C) Amplification of HHV-8 DNA from PBMC (day 0) and long-term cultures (21 or 28 days) of PBMC from 4 patients with KS (2 AIDS-KS, 2 CKS) and a PT patient. Floating (F) and adherent (A) cells were separately harvested at day 21 or 28 from PBMC cultured in the presence (RTCM) or absence (RPMI) of IC and the same number of cells (105) were analyzed with primer set 3. Negative controls (NC) are PCR reactions performed without DNA template or aliquots of salmon sperm DNA processed with PBMC. Positive controls were made with the indicated numbers of molecules (Mol) of plasmid p557-19. PCR products were transferred to nylon membranes and hybridized to a 32[P]-labeled oligonucleotide probe internal to the amplified sequences. Ethidium bromide stainings showed amplification of β-globin gene sequences from the same specimens. PBMC from the 2 CKS patients and the PT patient were analyzed also at day 7 and 14 with negative results. (D) PCR analysis of HHV-8 DNA with PBMC cultured with or without RTCM, γIFN, TNF, or IL-6. The same cell number was analyzed with primer set 3. NC is the negative control, consisting of either salmon sperm DNA processed with the specimens or PCR reactions lacking DNA template. (a) NKS-AIDS patient; (b through e) AIDS-KS patients; (f) 50 and 5 molecules of a positive control plasmid. PBMC from the patient shown in (a) were cultured for 11 days; PBMC from the other patients were cultured for 3 to 5 days. Fresh RTCM was added at days 0 and 3 of culture; single cytokines were added at days 0 and 2 for the patients shown in (a) and (b) and at days 0 and 4 for the other patients, respectively. γlFN was used at a concentration of 10, 50, or 100 IU/mL, as indicated in parenthesis. TNF was used at 30 ng/mL and IL-6 was used at 100 IU/mL, respectively. Experiments repeated with TNF or IL-6 (at 100 or 1,000 IU/mL) on 2 other patients that responded to RTCM gave similar results. All samples were positive for β-globin amplification, as shown by ethidium bromide staining of the PCR products. (B) Detection of HHV-8 DNA in PBMC (day 0) and in floating (F) or adherent (A) cells from 4 AIDS-KS patients (AIDS-KS), an asymptomatic homosexual man (HIV+), and a PT patient (PT) cultured (6 to 7 days) in the presence of TCM or RTCM or in its absence (RPMI). The same cell number (105) was analyzed with primer set 3. PC are positive controls made with the indicated numbers of molecules of plasmid p557-19. NC is negative control made without adding template DNA. PCR products were transferred to nylon membranes and hybridized to a32[P]-labeled oligonucleotide probe internal to the amplified sequences. Ethidium bromide staining shows amplification of β-globin gene sequences from the same specimens.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/12/10.1182_blood.v93.12.4044/4/m_blod41239001cw.jpeg?Expires=1769916155&Signature=veyLRDOnkpSlgkVHd3pGk-CXtKJga4dskocr~rYlh74W4BvFzrgCP3ALFN7TEWOxB09fYqJo3b6G6QfHMLs4T~dzFp-~MF6zf7R7TIWhCKO22q3CsOA29Tu0mA~oo5PikTGvstH-k6~NretagbTNZHdOgevba~49LKppgAbgWq2t7eWNS87wYuNkhmeARqCd~qnBWkHJHD94s7WyGUP3y92nKW66gSFgFupG~OOxQ90fp0vI4mYYHpJ6-RCuFWJ2qDiQpjm41l0TKIEX5FmMWrXjdyUK42NGuxqa4euj8RrWuMijhwjUV5JZfhtgm~004aCZWH26TaxIP0PKl-8N5Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. (A) Increase of HHV-8 DNA load in PBMC from 2 AIDS-KS patient after culture (days 6 to 7) in the presence of TCM or RTCM or in its absence (RPMI). Shown are the autoradigrams of serial-dilution PCR experiments performed with primers specific for HHV-8 ORF 26 (VP23) and for the human β-globin gene used as a control of the amount of the genomic DNA analyzed. PCR products were hybridized with specific oligonucleoutide probes. Numbers above lanes represent dilution factors (for VP23) or aliquots of cell extracts corresponding to the indicated number of cells (for β-globin), respectively. Cell extracts were diluted in salmon sperm DNA as described in Materials and Methods. (C) Amplification of HHV-8 DNA from PBMC (day 0) and long-term cultures (21 or 28 days) of PBMC from 4 patients with KS (2 AIDS-KS, 2 CKS) and a PT patient. Floating (F) and adherent (A) cells were separately harvested at day 21 or 28 from PBMC cultured in the presence (RTCM) or absence (RPMI) of IC and the same number of cells (105) were analyzed with primer set 3. Negative controls (NC) are PCR reactions performed without DNA template or aliquots of salmon sperm DNA processed with PBMC. Positive controls were made with the indicated numbers of molecules (Mol) of plasmid p557-19. PCR products were transferred to nylon membranes and hybridized to a 32[P]-labeled oligonucleotide probe internal to the amplified sequences. Ethidium bromide stainings showed amplification of β-globin gene sequences from the same specimens. PBMC from the 2 CKS patients and the PT patient were analyzed also at day 7 and 14 with negative results. (D) PCR analysis of HHV-8 DNA with PBMC cultured with or without RTCM, γIFN, TNF, or IL-6. The same cell number was analyzed with primer set 3. NC is the negative control, consisting of either salmon sperm DNA processed with the specimens or PCR reactions lacking DNA template. (a) NKS-AIDS patient; (b through e) AIDS-KS patients; (f) 50 and 5 molecules of a positive control plasmid. PBMC from the patient shown in (a) were cultured for 11 days; PBMC from the other patients were cultured for 3 to 5 days. Fresh RTCM was added at days 0 and 3 of culture; single cytokines were added at days 0 and 2 for the patients shown in (a) and (b) and at days 0 and 4 for the other patients, respectively. γlFN was used at a concentration of 10, 50, or 100 IU/mL, as indicated in parenthesis. TNF was used at 30 ng/mL and IL-6 was used at 100 IU/mL, respectively. Experiments repeated with TNF or IL-6 (at 100 or 1,000 IU/mL) on 2 other patients that responded to RTCM gave similar results. All samples were positive for β-globin amplification, as shown by ethidium bromide staining of the PCR products. (B) Detection of HHV-8 DNA in PBMC (day 0) and in floating (F) or adherent (A) cells from 4 AIDS-KS patients (AIDS-KS), an asymptomatic homosexual man (HIV+), and a PT patient (PT) cultured (6 to 7 days) in the presence of TCM or RTCM or in its absence (RPMI). The same cell number (105) was analyzed with primer set 3. PC are positive controls made with the indicated numbers of molecules of plasmid p557-19. NC is negative control made without adding template DNA. PCR products were transferred to nylon membranes and hybridized to a32[P]-labeled oligonucleotide probe internal to the amplified sequences. Ethidium bromide staining shows amplification of β-globin gene sequences from the same specimens.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/12/10.1182_blood.v93.12.4044/4/m_blod41239001dw.jpeg?Expires=1769916155&Signature=3lrYCjGFZF1cJmiDcQrd105GuimnKqZOsRHl6z9VqlzZsseoJpZF8y9S2emX~XRrU3egGxn~t-rc1rWYBsLzbHK2dJQTg4B7bmir1rx-CAcQgc2UAZinbghvX76Gt3hOaD8u4PJLLvzXZBBwBliyE5aTMiIPOBGCEHOxULAPo3XMgSkAQT9RZEcDuSgcOoG1guQWPtXrX2MjsGFnpgZIvt46BTkqeV1WFU3O4B~PmODvrxHPxtDPrV1m~cVcfwC4-l4HD0dsSpTYdwEBMPrUefksWja~XlGydtsAEcKieST7zjJC5GOPfdmZi-Diw8edE261kc9Yk-u~xFsEES~rcw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. (A) Increase of HHV-8 DNA load in PBMC from 2 AIDS-KS patient after culture (days 6 to 7) in the presence of TCM or RTCM or in its absence (RPMI). Shown are the autoradigrams of serial-dilution PCR experiments performed with primers specific for HHV-8 ORF 26 (VP23) and for the human β-globin gene used as a control of the amount of the genomic DNA analyzed. PCR products were hybridized with specific oligonucleoutide probes. Numbers above lanes represent dilution factors (for VP23) or aliquots of cell extracts corresponding to the indicated number of cells (for β-globin), respectively. Cell extracts were diluted in salmon sperm DNA as described in Materials and Methods. (C) Amplification of HHV-8 DNA from PBMC (day 0) and long-term cultures (21 or 28 days) of PBMC from 4 patients with KS (2 AIDS-KS, 2 CKS) and a PT patient. Floating (F) and adherent (A) cells were separately harvested at day 21 or 28 from PBMC cultured in the presence (RTCM) or absence (RPMI) of IC and the same number of cells (105) were analyzed with primer set 3. Negative controls (NC) are PCR reactions performed without DNA template or aliquots of salmon sperm DNA processed with PBMC. Positive controls were made with the indicated numbers of molecules (Mol) of plasmid p557-19. PCR products were transferred to nylon membranes and hybridized to a 32[P]-labeled oligonucleotide probe internal to the amplified sequences. Ethidium bromide stainings showed amplification of β-globin gene sequences from the same specimens. PBMC from the 2 CKS patients and the PT patient were analyzed also at day 7 and 14 with negative results. (D) PCR analysis of HHV-8 DNA with PBMC cultured with or without RTCM, γIFN, TNF, or IL-6. The same cell number was analyzed with primer set 3. NC is the negative control, consisting of either salmon sperm DNA processed with the specimens or PCR reactions lacking DNA template. (a) NKS-AIDS patient; (b through e) AIDS-KS patients; (f) 50 and 5 molecules of a positive control plasmid. PBMC from the patient shown in (a) were cultured for 11 days; PBMC from the other patients were cultured for 3 to 5 days. Fresh RTCM was added at days 0 and 3 of culture; single cytokines were added at days 0 and 2 for the patients shown in (a) and (b) and at days 0 and 4 for the other patients, respectively. γlFN was used at a concentration of 10, 50, or 100 IU/mL, as indicated in parenthesis. TNF was used at 30 ng/mL and IL-6 was used at 100 IU/mL, respectively. Experiments repeated with TNF or IL-6 (at 100 or 1,000 IU/mL) on 2 other patients that responded to RTCM gave similar results. All samples were positive for β-globin amplification, as shown by ethidium bromide staining of the PCR products. (B) Detection of HHV-8 DNA in PBMC (day 0) and in floating (F) or adherent (A) cells from 4 AIDS-KS patients (AIDS-KS), an asymptomatic homosexual man (HIV+), and a PT patient (PT) cultured (6 to 7 days) in the presence of TCM or RTCM or in its absence (RPMI). The same cell number (105) was analyzed with primer set 3. PC are positive controls made with the indicated numbers of molecules of plasmid p557-19. NC is negative control made without adding template DNA. PCR products were transferred to nylon membranes and hybridized to a32[P]-labeled oligonucleotide probe internal to the amplified sequences. Ethidium bromide staining shows amplification of β-globin gene sequences from the same specimens.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/12/10.1182_blood.v93.12.4044/4/m_blod41239001bw.jpeg?Expires=1769916155&Signature=1l2TeJHthZG1DFdJek6Z1CP6heybayCCDgLZM6HZKzv-r7ldrOF0u7tvysTkVyhZ066PB55Zr3NcuPhocFPjlndo7c053x0FrWNTjPuWIiHru7heQjuP84RV2-i63nnHi8GiIyDN3WZW3gIqAtb2JWDxyiRXTZha19dKS9MBA7PQEHWMGasN-1vDOS4LX7j0LCzF8KJ~6yIfUMeB9RSleayjVrjOrYDY0~q2Heu27tr3JZooNC3L~xSARzzYJzOZIRmRwwjw-eJ5WY3IYw6dER1HvZCl4rKo-M2C8JqS3AKokPDIqrQbOZS6DfW9D-ofoMhFx8EYOZq8M-9Gf9Pswg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
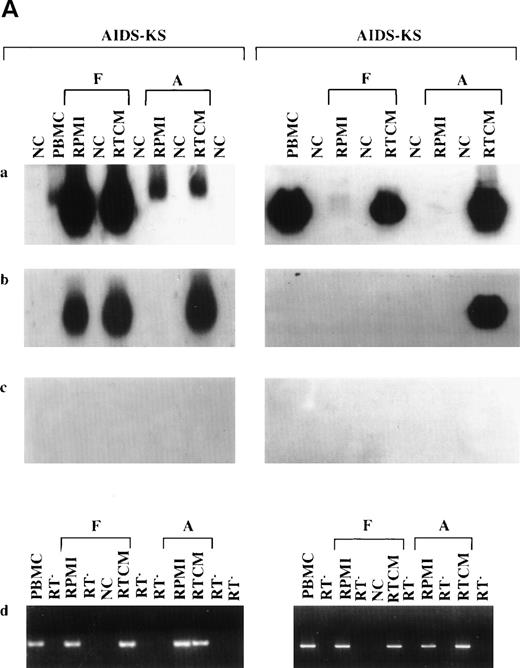
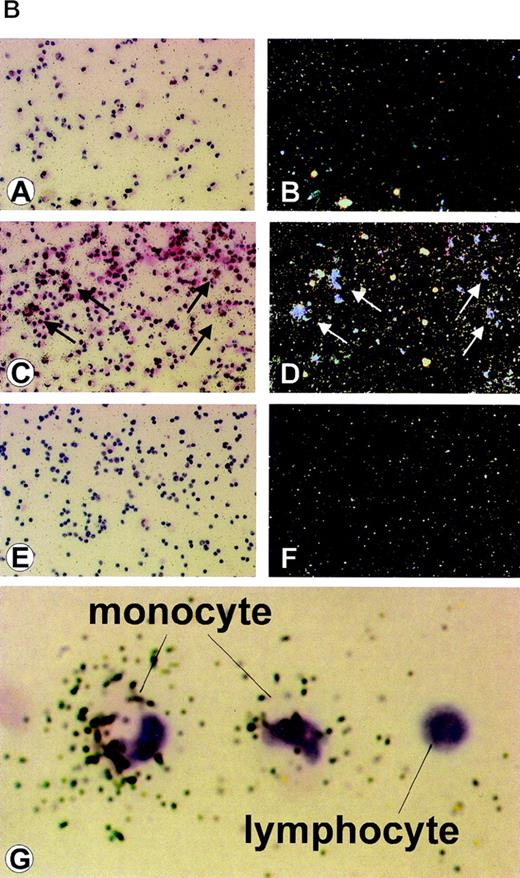

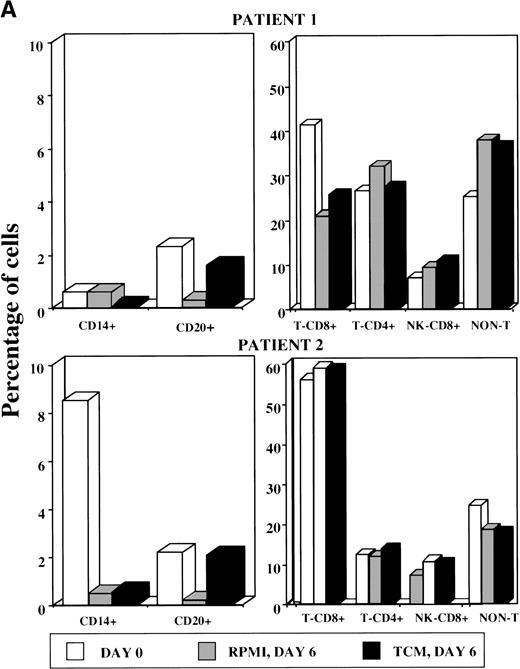
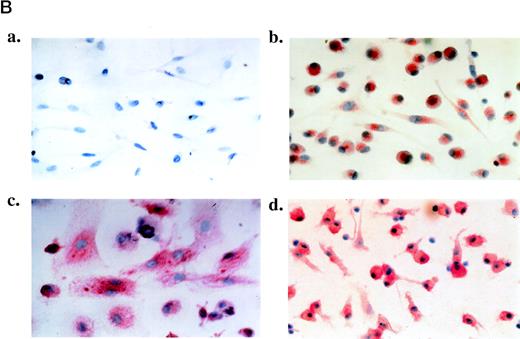
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal