Abstract
To develop a new immunotherapy for multiple myeloma, we have generated a monoclonal antibody (MoAb) that detects a human plasma cell-specific antigen, HM1.24. Our previous study has shown that mouse anti-HM1.24 MoAb inhibits the proliferation of human myeloma cells implanted into severe combined immunodeficiency mice. In this report, we evaluated the antitumor activity of the humanized anti-HM1.24 MoAb (IgG1κ), which was constructed by grafting the complementarity-determining regions. In contrast to the parent mouse MoAb, humanized anti-HM1.24 MoAb mediated antibody-dependent cellular cytotoxicity (ADCC) against both myeloma cell lines and myeloma cells from patients in the presence of human peripheral blood mononuclear cells (PBMCs). The PBMCs from untreated myeloma patients exhibited ADCC activity as efficiently as those of healthy donors. Although decreased ADCC activity of PBMCs was observed in patients who responded poorly to conventional chemotherapy, it could be significantly augmented by the stimulation with interleukin-2 (IL-2), IL-12, or IL-15. There was a strong correlation between the percentage of CD16+ cells and ADCC activity in the PBMCs of myeloma patients. Moreover, peripheral blood stem cell collections from myeloma patients contained higher numbers of CD16+ cells than PBMCs and exhibited ADCC activity that was enhanced by IL-2. These results indicate that humanized anti-HM1.24 MoAb has potential as a new therapeutic strategy in multiple myeloma and that treatment of effector cells with immunomodulating cytokines can restore the effect of humanized anti-HM1.24 MoAb in patients with diminished ADCC activity.
MULTIPLE MYELOMA remains an incurable malignancy despite certain advances in chemotherapeutic regimens. Conventional chemotherapy results in a low complete response rate and disease progression usually occurs within a few years.1,2Although myeloablative chemotherapy followed by allogeneic or autologous hematopoietic stem cell transplantation has increased the incidence of complete remission, relapses are still observed.3-5 Thus, new forms of maintenance chemotherapy and/or immunotherapy are needed to eliminate minimal residual disease and improve prognosis.
Antibody-dependent cellular cytotoxicity (ADCC) is one of the most important weapons of the immune response against tumor cells.6,7 This activity is mediated by tumor-specific antibodies and Fc receptor-bearing effector cells, such as natural killer (NK) cells, T lymphocytes, and phagocytes.8,9Importantly, ADCC depends on the cytolytic activity of these effector cells. However, because ADCC activity and/or NK cell function are suppressed in certain cancer patients,9,10 this immunotherapeutic approach has not proved as successful as originally expected. Nevertheless, we and others have observed an increased number and activity of NK cells in the peripheral blood and bone marrow of myeloma patients.11-14 These cells could act as effector cells to kill antibody-coated target cells by an ADCC mechanism. Immunotherapy with tumor cell-specific antibodies, therefore, might be a valuable treatment option for myeloma patients.
Although several researchers have developed plasma cell-specific antibodies,15-20 the application of these antibodies to the immunotherapy of multiple myeloma has not been extensively investigated. Recently, we have generated a mouse monoclonal antibody (MoAb) to a novel plasma cell-specific antigen, termed HM1.24, for the treatment of multiple myeloma.21 HM1.24 is a type II transmembrane protein that has a molecular weight of 29 to 33 kD22 and is expressed selectively on terminally differentiated normal and neoplastic B cells.21Our previous studies have shown that anti-HM1.24 MoAb accumulates in human myeloma xenografts in severe combined immunodeficiency (SCID) mice and induces strong antitumor activity by an ADCC mechanism.23-25 Thus, HM1.24 antigen is an attractive target for the immunotherapy of multiple myeloma and its biological function in normal and myeloma cells is under investigation.
To develop an immunotherapeutic agent for clinical use, we have constructed humanized anti-HM1.24 MoAb (IgG1κ) by grafting the complementarity-determining regions from the parent mouse MoAb to a human MoAb. In this study, we evaluate the antitumor activity of humanized anti-HM1.24 MoAb and the cytolytic activity of various effector cells from myeloma patients. Our results indicate that humanized anti-HM1.24 MoAb can mediate ADCC activity against myeloma cells in the presence of effector cells of patients, providing supportive data for clinical trials of immunotherapy for multiple myeloma and related plasma cell dyscrasias.
MATERIALS AND METHODS
Patients.
The diagnosis and clinical staging of multiple myeloma were performed according to the criteria of Durie and Salmon.26 A total of 45 treated or untreated myeloma patients were included in this study. Their mean age was 65.3 years (range, 40 to 96), with 21 males and 24 females. Clinical stage was distributed as follows: IA, 22%; IIA, 24%; IIIA, 49%; and IIIB, 5%. Monoclonal serum or urine Ig was found in 98% of patients: IgG 62%; IgA, 22%; IgD, 7%; and light chain only, 7%. The κ/λ light chain isotype ratio was 1.4.
Patients were treated with melphalan-prednisone with or without vincristine and anthracyclins. For those patients receiving intermittent chemotherapy, samples were taken at least 4 weeks after the last cycle of chemotherapy. In five patients undergoing autologous peripheral blood stem cell transplantation (PBSCT), PBSCs were mobilized by an intravenous injection of 3 g/m2cyclophosphamide on days 1 and 2 followed by daily subcutaneous administration of 5 μg/kg granulocyte colony-stimulating factor (G-CSF). Three patients received PBSCT and their blood samples were examined 2, 3, or 9 months after PBSCT. Response to treatment was assessed following the criteria defined by Gore et al.27Complete response was defined by the disappearance of the paraprotein (evaluated by immunoelectrophoresis) from the serum and the concentrated urine and less than 5% plasma cells in the bone marrow. Patients were considered to be in partial response when a decrease of more than 50% was observed in measurable paraprotein and bone marrow infiltration. All others were regarded as nonresponders.
Preparation of effector cells.
Peripheral blood or bone marrow samples were obtained from healthy donors or patients with multiple myeloma after informed consent. Peripheral blood mononuclear cells (PBMCs) or bone marrow mononuclear cells (BMMCs) were separated by Ficoll-Conray (density, 1.077) gradient centrifugation. Polymorphprep (density, 1.113) gradient (Nycomed Pharma AS, Oslo, Norway) was used to purify neutrophils. PBSCs were collected by a Fenwal CS-3000 Plus cell separator (Baxter, Deerfield, IL) during the phase of bone marrow recovery after chemotherapy as described above. PBSCs were separated by 40% and 60% Percoll gradients (Pharmacia, Uppsala, Sweden).
Antibodies.
Mouse anti-HM1.24 MoAb (IgG2aκ) was produced by the fusion of mouse myeloma cells SP2/0 with spleen cells from Balb/c mice immunized with the human myeloma cell line, KPC-32.21 This MoAb recognizes a 29- to 33-kD glycoprotein as shown by immunoprecipitation assay under reducing conditions. The anti-HM1.24 MoAb was purified from the ascites fluid by ammonium sulfate precipitation and a protein A-affinity chromatography kit (Ampure PA; Amersham Japan, Tokyo, Japan). The mouse-human chimeric anti-HM1.24 MoAb was constructed by linking the cDNA sequences encoding the heavy and light chain variable regions of mouse anti-HM1.24 with the cDNAs encoding the human γ1 and κ constant regions, respectively,28using human elongation factor (HEF) expression vectors.29 Humanized anti-HM1.24 MoAb (IgG1κ) was constructed by grafting the complementarity-determining regions from the mouse MoAb to a human MoAb.28 A more detailed description of this procedure has been provided previously.30 Mouse IgG2a (UPC-10; Cappel, Malvern, PA) and human IgG1κ (Serotec, Oxford, UK) proteins were used as control IgG. For inhibition studies, heat-aggregated human IgG was prepared by a 20-minute incubation at 63°C.
Myeloma cells.
Myeloma cells were obtained from the bone marrow of a patient (no. 1) and from the malignant pleural effusion of three patients (no. 2, no. 3, and no. 4). Mononuclear cells were isolated by Ficoll-Conray density gradient centrifugation, and adherent cells and T cells were depleted as described.31 The mononuclear cell fraction of these samples included more than 95% CD38+ myeloma cells and was used for the target of ADCC assay.
The human plasma cell lines, U266 and ARH-77, were obtained from the American Type Culture Collection (Rockville, MD). The following cell lines were obtained from the Japanese Cancer Research Resources Bank (Tokyo, Japan): RPMI 8226, IM-9, HS-Sultan, Ramos, Daudi, and HEL. Myeloid HEL cells which do not express the HM1.24 antigen were used as control cells. These cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin.
Construction of HM1.24 expressing CHO cells.
To establish stable Chinese hamster ovary (CHO) transformants expressing the HM1.24 antigen, cDNA was transfected into CHO cells and transformants were selected in the presence of 500 μg/mL G418. Transformants that expressed different amounts of HM1.24 were obtained and used for the targets of ADCC assay.
Flow cytometry.
Expression of HM1.24 antigen on myeloma cells was examined by flow cytometry. Cells were washed with cold phosphate-buffered saline (PBS) and stained on ice for 30 minutes with mouse anti-HM1.24 MoAb or control IgG. After incubation with primary MoAb, cells were washed three times with cold PBS containing 0.1% bovine serum albumin and 0.02% sodium azide and then incubated on ice for an additional 30 minutes with fluorescein isothiocyanate (FITC)-conjugated goat F(ab′)2 anti-mouse IgG antibody (Tago, Burlingame, CA). The cells were washed again and resuspended in 1% paraformaldehyde. In some experiments, cells were stained with biotin-labeled isotype control IgG or anti-HM1.24 MoAb, and phycoerythrin (PE)-conjugated anti-CD38 MoAb (Becton Dickinson, San Jose, CA), and then with streptavidin-RED 670 (GIBCO-BRL, Rockville, MD). The analysis was performed on a flow cytometer (EPICS XL; Coulter Electronics, Hialeah, FL). The mean specific fluorescence intensity (MSFI) was calculated as the ratio of mean fluorescence channel of anti-HM1.24 MoAb/control MoAb.
To analyze the HM1.24 expression on hematopoietic progenitor cells in PBSC collections, cells were stained with FITC-conjugated control IgG or mouse anti-HM1.24 MoAb, and PE-conjugated anti-CD34 MoAb (PharMingen, San Diego, CA). The CD34+ fraction was gated based on CD34 fluorescence intensity and side scatter profile. To examine the phenotype of effector cells from patients, cells were stained with FITC-labeled anti-CD3, anti-CD4, anti-CD8, anti-CD16, anti-CD20, or PE-labeled anti-CD56 MoAbs (Becton Dickinson) and were analyzed as above.
Complement-dependent cytotoxicity.
Cell lysis with complement was determined using a51Cr-release assay. Target cells were labeled with 0.1 mCi51Cr-sodium chromate (New England Nuclear, Boston, MA) at 37°C for 1 hour. The cells were then washed three times with RPMI 1640 medium. 51Cr-labeled cells (1 × 104cells) were incubated with various concentrations of anti-HM1.24 MoAb or control IgG on ice for 30 minutes. The unbound antibody was removed by washing the cells three times with medium. The cells were then distributed into 96-well plates and incubated with serial dilutions of baby rabbit complement (Cedarlane, Ontario, Canada) or human serum at 37°C for 2 hours. After incubation, supernatants from each well (50 μL) were harvested and 51Cr was measured using a gamma counter. Spontaneous release of 51Cr was measured after incubating 51Cr-labeled cells with medium alone. The maximum release of 51Cr was determined after incubation of51Cr-labeled cells with 1% NP-40. Percentage of cytotoxicity was calculated from the formula: specific cytotoxicity (%) = (A − C)/(B − C) × 100, where A = experimental51Cr release, B = maximum 51Cr release, and C = spontaneous 51Cr release.
ADCC assay.
ADCC activity was determined by standard 4-hour51Cr-release assay. In some experiments, effector cells were cultured in RPMI 1640 medium with or without recombinant human interleukin-2 (IL-2) (Genzyme, Cambridge, MA), IL-10 (Genzyme), IL-12 (R&D Systems, Minneapolis, MN), IL-15 (Genzyme) or macrophage colony-stimulating factor (M-CSF; Morinaga Milk Industry Co, Ltd, Tokyo, Japan) for 3 days, then washed and resuspended in medium before use. 51Cr-labeled target myeloma cells (1 × 104 cells) were placed in 96-well plates and various concentrations of anti-HM1.24 MoAb or control IgG were added to wells. Effector cells were then added to the plates at various effector to target (E/T) ratios. After 4-hour incubation, supernatants were removed and counted in a gamma counter. The percentage of cell lysis was determined as above.
Hematopoietic progenitor cell assay.
The effect of humanized anti-HM1.24 MoAb on the growth of granulocyte-macrophage colony-forming units (GM-CFU) and erythroid burst-forming units (E-BFU) was evaluated as described previously.31 Briefly, PBSC collections (1 × 105 cells) from myeloma patients were incubated with 10 μg/mL control human IgG or humanized anti-HM1.24 MoAb in Iscove’s modified Dulbecco’s medium (IMDM) for 30 minutes at 37°C. The cells were then plated in 35-mm tissue-culture dishes in 1 mL of IMDM containing 1% methylcellulose, 20% fetal calf serum, 1% bovine serum albumin, 450 ng/mL iron-saturated transferrin, 10 ng/mL IL-3, and 2 U/mL erythropoietin or 10 ng/mL G-CSF in triplicate. After 14 days of culture, the numbers of colonies were counted by an inverted microscope.
Statistical analysis.
The statistical significance of difference between groups was analyzed by unpaired t-test. The correlation between ADCC activity and HM1.24 expression on target cells or phenotypic data of PBMCs was evaluated by Pearson’s rank correlation analysis.
RESULTS
ADCC activity of humanized anti-HM1.24 MoAb against RPMI 8226 cells.
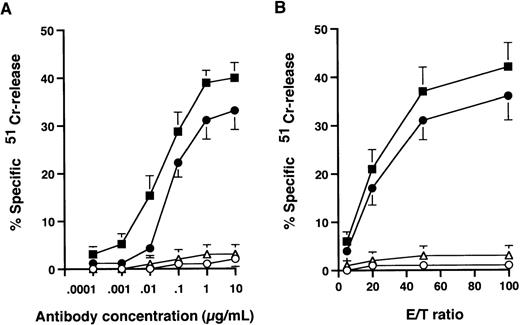
The ability of chimeric and humanized versions of anti-HM1.24 to mediate ADCC was determined in a 51Cr-release assay. As shown in Fig 1, mouse anti-HM1.24 MoAb did not induce ADCC activity against RPMI 8226 cells in the presence of PBMCs from healthy donors, suggesting that the mouse form of this MoAb is completely unrecognized by effector cells. In contrast, humanized as well as chimeric anti-HM1.24 MoAb induced ADCC activity in a dose-dependent manner (Fig1A) and the extent of cytotoxicity was dependent on the E/T ratio (Fig 1B). This cytotoxicity was mediated by humanized anti-HM1.24 MoAb even at a low concentration of 0.01 μg/mL. No additional killing was seen at concentrations above 10 μg/mL. In contrast, neutrophils isolated from healthy donors or myeloma patients did not exhibit ADCC activity in the presence of humanized anti-HM1.24 MoAb even after stimulation with G-CSF (data not shown).
ADCC activity against RPMI 8226 cells by normal human PBMCs. (A) 51Cr-labeled RPMI 8226 cells were incubated with PBMCs at an E/T ratio of 50 along with various concentrations of antibodies. (B) 51Cr-labeled RPMI 8226 cells were incubated with PBMCs in the presence of 1 μg/mL of antibodies. Symbols represent control human IgG (○), mouse anti-HM1.24 MoAb (▵), chimeric anti-HM1.24 MoAb (▪) and humanized anti-HM1.24 MoAb (•). Data represent the mean ± SD of triplicates.
ADCC activity against RPMI 8226 cells by normal human PBMCs. (A) 51Cr-labeled RPMI 8226 cells were incubated with PBMCs at an E/T ratio of 50 along with various concentrations of antibodies. (B) 51Cr-labeled RPMI 8226 cells were incubated with PBMCs in the presence of 1 μg/mL of antibodies. Symbols represent control human IgG (○), mouse anti-HM1.24 MoAb (▵), chimeric anti-HM1.24 MoAb (▪) and humanized anti-HM1.24 MoAb (•). Data represent the mean ± SD of triplicates.
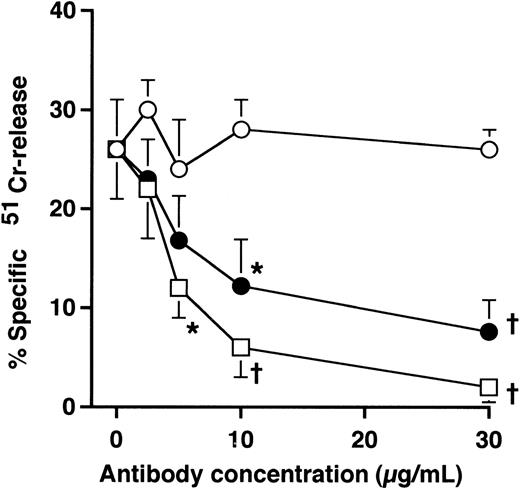
To further examine the role of the Fc region of the humanized anti-HM1.24 MoAb, competitive inhibition studies with mouse anti-HM1.24 MoAb were performed. The humanized anti-HM1.24 MoAb-mediated cytotoxicity was inhibited by mouse anti-HM1.24 MoAb in a dose-dependent manner, suggesting that cytotoxicity was mediated by the Fc region of the humanized MoAb (Fig 2).
Effects of mouse anti-HM1.24 MoAb and human IgG on ADCC activity mediated by humanized anti-HM1.24 MoAb.51Cr-labeled RPMI 8226 cells were incubated with PBMCs from healthy donors (E/T ratio, 50) and humanized anti-HM1.24 MoAb (1 μg/mL) in the presence of mouse anti-HM1.24 MoAb (□), heat-aggregated human IgG (•), or monomeric human IgG (○). Effector cells were preincubated for 15 minutes at room temperature with monomeric or heat-aggregated IgG. Data represent the mean ± SD of triplicates. *P < .05 or †P < .005, compared with the data in the absence of mouse anti-HM1.24 MoAb or human IgG preparations.
Effects of mouse anti-HM1.24 MoAb and human IgG on ADCC activity mediated by humanized anti-HM1.24 MoAb.51Cr-labeled RPMI 8226 cells were incubated with PBMCs from healthy donors (E/T ratio, 50) and humanized anti-HM1.24 MoAb (1 μg/mL) in the presence of mouse anti-HM1.24 MoAb (□), heat-aggregated human IgG (•), or monomeric human IgG (○). Effector cells were preincubated for 15 minutes at room temperature with monomeric or heat-aggregated IgG. Data represent the mean ± SD of triplicates. *P < .05 or †P < .005, compared with the data in the absence of mouse anti-HM1.24 MoAb or human IgG preparations.
We next determined whether human IgG or paraproteins from myeloma patients could inhibit ADCC activity by blocking the FcγR of effector cells. The preincubation of PBMCs with monomeric human IgG did not affect the cytotoxicity mediated by humanized anti-HM1.24 MoAb, whereas heat-aggregated human IgG inhibited the effect of humanized anti-HM1.24 MoAb in a dose-dependent manner (Fig 2). Similarly, serum from IgG or IgA myeloma patients (serum dilution, 1:4) did not abrogate this ADCC activity (data not shown), indicating that this humanized MoAb mediates ADCC through FcγR even in the presence of monomeric IgG or paraproteins. These findings also suggest that soluble HM1.24 antigen blockage of the binding of humanized anti-HM1.24 MoAb did not occur in the serum of myeloma patients.
Complement-dependent cytotoxicity.
The complement-dependent cytotoxicity of anti-HM1.24 MoAb was examined in the presence of rabbit or human complement. Both humanized and mouse anti-HM1.24 MoAb (10 μg/mL) mediated the complement-dependent cytotoxicity (24.2% ± 5.7% and 67.1% ± 2.5%, mean ± SD of triplicates, respectively) against RPMI 8226 cells with rabbit complement (complement dilution, 1:5). However, no cytotoxicity was observed when human serum from healthy donors or myeloma patients was used as the source of complement. There was no complement-dependent cytotoxicity elicited by humanized or mouse anti-HM1.24 MoAb against HEL cells.
Expression of HM1.24 and the sensitivity to humanized anti-HM1.24 MoAb of myeloma cells.
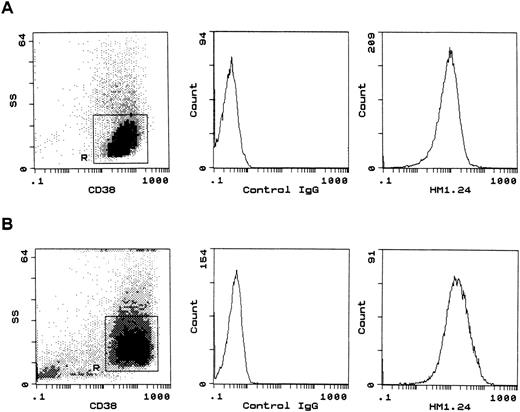
Next, additional myeloma cell lines as well as myeloma cells from patients were used as targets, and surface expression of HM1.24 on these cells was examined using indirect immunofluorescence techniques. HM1.24 antigen was strongly expressed on ARH-77, IM-9, RPMI 8226, Ramos, U266, HS-Sultan, Daudi, and CD38+ myeloma cells from patients (Fig 3). Mean specific fluorescence intensity (MSFI) was determined by immunofluorescence staining, and the results are shown in Table1. The MSFI ranged between 12 and 43 in these myeloma cells.
Flow cytometric analysis of HM1.24 expression on myeloma cells. RPMI 8226 (A) and BMMCs from patient no. 1 (B) were stained with biotin-labeled isotype control IgG or anti-HM1.24 MoAb, and PE-conjugated anti-CD38 MoAb, and then with streptavidin-RED 670. Myeloma cell regions (R) were gated for further analysis according to the side scatter (SS) profile and CD38 expression. BMMCs from patient no. 1 contained 95% of CD38+ myeloma cells (B). The MSFI was calculated as the ratio of mean fluorescence channel of anti-HM1.24 MoAb/control IgG.
Flow cytometric analysis of HM1.24 expression on myeloma cells. RPMI 8226 (A) and BMMCs from patient no. 1 (B) were stained with biotin-labeled isotype control IgG or anti-HM1.24 MoAb, and PE-conjugated anti-CD38 MoAb, and then with streptavidin-RED 670. Myeloma cell regions (R) were gated for further analysis according to the side scatter (SS) profile and CD38 expression. BMMCs from patient no. 1 contained 95% of CD38+ myeloma cells (B). The MSFI was calculated as the ratio of mean fluorescence channel of anti-HM1.24 MoAb/control IgG.
Expression of HM1.24 and the Sensitivity to Humanized Anti-HM1.24 MoAb of Neoplastic Myeloma Cells
| Target Cells . | HM1.24 Expression (MSFI) . | Cytotoxicity (%) . | |
|---|---|---|---|
| Control Human IgG . | Humanized Anti-HM1.24 . | ||
| ARH-77 | 43 | 2 | 44 |
| IM-9 | 38 | 6 | 61 |
| RPMI 8226 | 36 | 0 | 37 |
| Ramos | 33 | 0 | 33 |
| U 266 | 30 | 38* | 59 |
| HS-Sultan | 21 | 0 | 31 |
| Daudi | 12 | 0 | 11 |
| Patient no. 1 | 34 | 4 | 29 |
| 1† | 25† | ||
| Patient no. 2 | 33 | 0 | 21 |
| 0† | 27† | ||
| Patient no. 3 | 30 | 0 | 16 |
| Patient no. 4 | 30 | 7 | 32 |
| CHO-1 | 50‡ | 5 | 38 |
| CHO-2 | 20‡ | 11 | 28 |
| CHO-3 | 5‡ | 7 | 8 |
| CHO-4 | 1‡ | 0 | 1 |
| HEL | 11-153 | 6 | 6 |
| Target Cells . | HM1.24 Expression (MSFI) . | Cytotoxicity (%) . | |
|---|---|---|---|
| Control Human IgG . | Humanized Anti-HM1.24 . | ||
| ARH-77 | 43 | 2 | 44 |
| IM-9 | 38 | 6 | 61 |
| RPMI 8226 | 36 | 0 | 37 |
| Ramos | 33 | 0 | 33 |
| U 266 | 30 | 38* | 59 |
| HS-Sultan | 21 | 0 | 31 |
| Daudi | 12 | 0 | 11 |
| Patient no. 1 | 34 | 4 | 29 |
| 1† | 25† | ||
| Patient no. 2 | 33 | 0 | 21 |
| 0† | 27† | ||
| Patient no. 3 | 30 | 0 | 16 |
| Patient no. 4 | 30 | 7 | 32 |
| CHO-1 | 50‡ | 5 | 38 |
| CHO-2 | 20‡ | 11 | 28 |
| CHO-3 | 5‡ | 7 | 8 |
| CHO-4 | 1‡ | 0 | 1 |
| HEL | 11-153 | 6 | 6 |
HM1.24 expression was examined by flow cytometry. Cytotoxicity against tumor cells was determined by a 4-hour 51Cr-release assay using normal human PBMCs as effector cells at an E/T ratio of 50 in the presence of 1 μg/mL control human IgG or humanized anti-HM1.24 MoAb. Data represent the mean value of three independent experiments. The standard error of cytotoxicity was <10% in all target cells.
U266 is NK-sensitive.
Autologous PBMCs were used as effector cells.
CHO transformants expressing different levels of HM1.24 antigen were used.
HEL does not express HM1.24 antigen.
The ADCC activity of PBMCs from a healthy donor was tested against these myeloma cells. Both myeloma cell lines and myeloma cells from patients were killed with PBMCs in the presence of humanized anti-HM1.24 MoAb (Table 1). There was no significant correlation between HM1.24 expression levels and cytolysis of these myeloma cells. However, the degree of target cell cytolysis was related to the level of HM1.24 expression in CHO cells transfected with HM1.24 cDNA (r = .94, P = .08). PBMCs from myeloma patients also mediated ADCC activity against autologous myeloma cells (Table 1).
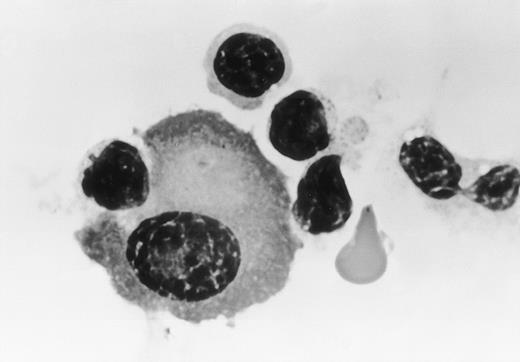
Morphological examination showed that these myeloma cells were attached to large granular lymphocytes, and cytolysis of myeloma cells was only observed in the presence of humanized anti-HM1.24 MoAb (Fig4). In contrast, no cytolysis of HEL cells was seen with humanized anti-HM1.24 MoAb.
Morphology of effector cells attacking myeloma cells. Myeloma cells were purified from the bone marrow (patient no. 1) and were cultured with PBMCs from a healthy donor in the presence of humanized anti-HM1.24 MoAb (1 μg/mL) for 30 minutes. Cytospin preparations were stained with Wright-Giemsa (original magnification × 330).
Morphology of effector cells attacking myeloma cells. Myeloma cells were purified from the bone marrow (patient no. 1) and were cultured with PBMCs from a healthy donor in the presence of humanized anti-HM1.24 MoAb (1 μg/mL) for 30 minutes. Cytospin preparations were stained with Wright-Giemsa (original magnification × 330).
ADCC activity of PBMCs from myeloma patients.
The ADCC activity of PBMCs from both treated and untreated myeloma patients was examined using RPMI 8226 cells as target cells. The mean ADCC activity of PBMCs from healthy donors was 31.2% ± 7.6% (mean ± SD, n = 12). As shown in Fig5, PBMCs of untreated myeloma patients had ADCC activity as efficient as that of healthy donors despite different clinical stages. No significant differences in ADCC activity were found between the various clinical stages. The mean values of ADCC activity were not significantly different in patients with different types of paraproteins (data not shown). ADCC activity was also observed in treated patients, including those after PBSCT (n = 3), while in three of the stage III patients decreased ADCC activity was exhibited (less than 2 SD of controls, <16%).
ADCC activity of PBMCs from myeloma patients according to clinical stages. PBMCs from healthy donors or untreated (○), treated (•), or post PBSCT (▪) myeloma patients were compared as effector cells against RPMI 8226 cells at an E/T ratio of 50 in the presence of 1 μg/mL humanized anti-HM1.24 MoAb. Horizontal bars represent the mean values of each group. The shaded area represents the mean ± 2 SD of ADCC activity of PBMCs from healthy donors.
ADCC activity of PBMCs from myeloma patients according to clinical stages. PBMCs from healthy donors or untreated (○), treated (•), or post PBSCT (▪) myeloma patients were compared as effector cells against RPMI 8226 cells at an E/T ratio of 50 in the presence of 1 μg/mL humanized anti-HM1.24 MoAb. Horizontal bars represent the mean values of each group. The shaded area represents the mean ± 2 SD of ADCC activity of PBMCs from healthy donors.
Clinical characteristics according to ADCC activity.
To assess different ADCC activities among the treated patients in stage III, clinical characteristics at the time of ADCC assay were evaluated. Three patients displaying low ADCC activity (<16%, <2 SD of controls) were nonresponders to chemotherapy with relatively severe anemia and high levels of paraprotein. In contrast, patients with high ADCC activity (>46%, >2 SD of controls) were all in complete response after treatment. Although there was no significant difference in white blood cell counts between the patients with low and high ADCC activity, the number of CD16+ cells in the peripheral blood was relatively lower in the group with low ADCC activity.
Correlation between the percentage of NK cells and ADCC activity in myeloma.
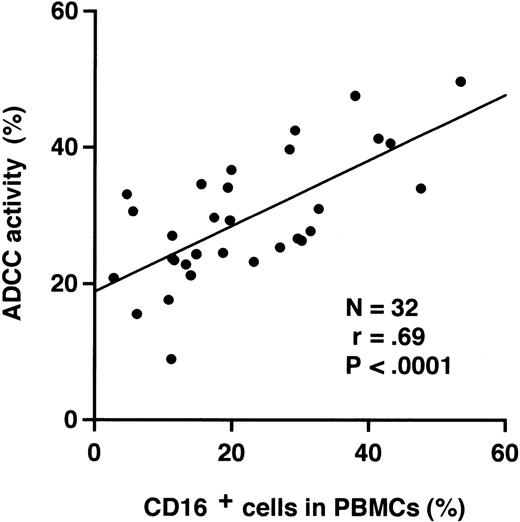
Because NK cells, which express CD16 and CD56, are known to be the major effector cells of ADCC,9 we analyzed the percentage of NK cells among the PBMCs by flow cytometry. ADCC activity of PBMCs correlated significantly with the percentages of CD16+cells (r = .69, P < .0001; Fig6) or CD56+ cells (r = .56,P = .0011) in the peripheral blood. In contrast, the percentages of CD3, CD4, CD8, CD20, or CD19 positive cells did not correlate with ADCC activity (data not shown).
Correlation between ADCC activity and the percentage of CD16+ cells in PBMCs from myeloma patients.51Cr-labeled RPMI 8226 cells were incubated with PBMCs from myeloma patients at an E/T ratio of 50 in the presence of 1 μg/mL humanized anti-HM1.24 MoAb. A regression line is shown (Y = 0.48X + 18.8).
Correlation between ADCC activity and the percentage of CD16+ cells in PBMCs from myeloma patients.51Cr-labeled RPMI 8226 cells were incubated with PBMCs from myeloma patients at an E/T ratio of 50 in the presence of 1 μg/mL humanized anti-HM1.24 MoAb. A regression line is shown (Y = 0.48X + 18.8).
Effect of cytokines on ADCC activity of effector cells.
To evaluate whether cytokines can enhance the diminished ADCC activity found in certain myeloma patients, we examined the effect of various cytokines (IL-2, IL-10, IL-12, IL-15, or M-CSF) on the ADCC activity of PBMCs. PBMCs were incubated for 3 days in culture medium with IL-2 (500 U/mL), IL-10 (20 ng/mL), IL-12 (20 ng/mL), IL-15 (20 ng/mL), or M-CSF (5,000 U/mL) and then added to cultures of 51Cr-labeled RPMI 8226 cells with humanized anti-HM1.24 MoAb (1 μg/mL). As shown in Fig 7, the cytolytic activity of PBMCs activated by IL-2, IL-12, or IL-15 without humanized anti-HM1.24 MoAb, ie, lymphokine-activated killer (LAK) cell activity, was found in both healthy donors and myeloma patients. ADCC activity with humanized anti-HM1.24 MoAb was not augmented by the stimulation with these cytokines in healthy donors. In contrast, reduced ADCC activity of PBMCs from certain myeloma patients was significantly enhanced by IL-2, IL-12, or IL-15. Cytolytic activity by ADCC was always higher than NK or LAK activity. Although IL-10 and M-CSF have been shown to stimulate the ADCC activity of monocytes,32 33 both cytokines failed to enhance the cytolytic activity of PBMCs from either healthy donors or myeloma patients.
Effects of cytokines on ADCC activity of PBMCs against RPMI 8226. PBMCs from healthy donors (A, n = 5) and myeloma patients (B, n = 5) were cultured with various cytokines for 3 days and were used as effector cells in the presence of 1 μg/mL control human IgG (□) or humanized anti-HM1.24 MoAb (▪). Cytotoxicity was determined by a 4-hour 51Cr-release assay at an E/T ratio of 50. Data represent the mean ± SD of triplicates. *P < .05 or †P < .001, compared with the data of nonactivated PBMCs in the presence of humanized anti-HM1.24 MoAb.
Effects of cytokines on ADCC activity of PBMCs against RPMI 8226. PBMCs from healthy donors (A, n = 5) and myeloma patients (B, n = 5) were cultured with various cytokines for 3 days and were used as effector cells in the presence of 1 μg/mL control human IgG (□) or humanized anti-HM1.24 MoAb (▪). Cytotoxicity was determined by a 4-hour 51Cr-release assay at an E/T ratio of 50. Data represent the mean ± SD of triplicates. *P < .05 or †P < .001, compared with the data of nonactivated PBMCs in the presence of humanized anti-HM1.24 MoAb.
Effector cell analysis of ADCC in myeloma patients.
Finally, the ADCC activity of PBMCs, BMMCs, and PBSC collections from myeloma patients was examined (Table 2). The proportion of CD16+ cells and ADCC activity was relatively higher in PBMCs than in BMMCs. However, despite a high percentage of CD16+ cells in PBSC collection, the magnitude of ADCC activity was relatively low as compared with that of PBMCs. Again, the ADCC activity of PBSC collections as well as in PBMCs was significantly enhanced by the stimulation with IL-2.
ADCC Activity of Various Effector Cells in Myeloma Patients
| Source of Effector Cells . | CD16+ Cells (%) . | ADCC Activity (%) . | |
|---|---|---|---|
| Medium . | IL-2– Stimulated . | ||
| PBMCs | 12 ± 6 | 30 ± 9 | 45 ± 9* |
| BMMCs | 7 ± 5 | 17 ± 20 | 31 ± 12 |
| PBSC collections | 29 ± 6† | 21 ± 9 | 36 ± 10* |
| Source of Effector Cells . | CD16+ Cells (%) . | ADCC Activity (%) . | |
|---|---|---|---|
| Medium . | IL-2– Stimulated . | ||
| PBMCs | 12 ± 6 | 30 ± 9 | 45 ± 9* |
| BMMCs | 7 ± 5 | 17 ± 20 | 31 ± 12 |
| PBSC collections | 29 ± 6† | 21 ± 9 | 36 ± 10* |
PBMCs and BMMCs were obtained from five myeloma patients before PBSC harvest. Effector cells were cultured alone or with IL-2 (500 U/mL) for 3 days, then tested for ADCC activity against RPMI 8226 cells at an E/T ratio of 50 in the presence of humanized anti-HM1.24 MoAb (1 μg/mL). Results are expressed as the mean ± SD.
P < .05, compared with the data of nonactivated effector cells.
P < .005, compared with the percentage of PBMCs.
Effect of humanized anti-HM1.24 MoAb on hematopoietic progenitor cells.
To determine the safety of humanized anti-HM1.24 MoAb for hematopoietic progenitor cells, the expression of HM1.24 and the effect of humanized anti-HM1.24 MoAb on these cells were investigated. CD34+progenitor cells in PBSC collections did not express HM1.24 antigen by two-color flow cytometry (data not shown). In addition, the humanized anti-HM1.24 MoAb did not significantly inhibit the growth of GM-CFU and E-BFU grown from PBSCs of myeloma patients (n = 5) when compared with control IgG.
DISCUSSION
In this report, we have shown that humanized anti-HM1.24 MoAb can mediate ADCC activity against myeloma cell lines as well as against neoplastic myeloma cells from patients in the presence of effector cells. These findings confirm our previous observation that HM1.24 serves as a target molecule for myeloma immunotherapy.23-25Moreover, most myeloma patient PBMCs showed ADCC activity comparable with that of healthy donors, although a small number of PBMCs from patients with refractory disease did show decreased ADCC activity. This decreased ADCC activity, however, could be significantly enhanced by treatment with IL-2, IL-12, or IL-15. Previous studies have suggested that the PBMCs of myeloma patients have NK and LAK activity,34 35 but little is known about ADCC specific for myeloma cells. We have shown that various effector cells, including PBMCs, BMMCs, and PBSC collections, have the ability to induce ADCC activity against myeloma cells together with humanized anti-HM1.24 MoAb. Indeed, this cytotoxicity was specific and more effective than NK or LAK activity against myeloma cells. Thus, humanized anti-HM1.24 MoAb has therapeutic potential for myeloma patients even with advanced disease.
Among antiplasma cell antibodies previously described, only chimeric and humanized forms of anti-CD38 have been reported to have cytotoxic potential against myeloma cells in the presence of effector cells. Stevenson et al18 and Ellis et al20 have shown that the maximum ADCC activity of these chimeric and humanized anti-CD38 MoAbs was 40% to 60% in the presence of normal PBMCs. Although the target cells were different in our cytotoxicity assay, we found that humanized anti-HM1.24 MoAb elicited ADCC activity as efficiently as anti-CD38 MoAbs in the presence of normal PBMCs. However, CD38 is also expressed on normal tissues such as hematopoietic cells.36 In contrast, HM1.24 antigen was not expressed on normal tissues including the peripheral blood, bone marrow, lymph node, liver, spleen, kidney, heart,21 and hematopoietic progenitor cells, suggesting that humanized anti-HM1.24 MoAb would not cause any adverse effects on these normal cells. Indeed, humanized anti-HM1.24 MoAb did not show toxicity for progenitor cells in PBSC collections, even though CD16+ effector cells were present in the assay.
The effector cells responsible for ADCC have not been fully identified, but several studies have indicated that human IgG1 MoAb is most effective in cell-mediated cytolysis, utilizing FcγRIII (CD16)-expressing NK cells as the effectors.37 We have shown that both humanized and mouse-human chimeric anti-HM1.24 MoAbs that have human Cγ1 can mediate ADCC against human myeloma cells, whereas the parent mouse MoAb has no effect. In addition, ADCC activity of PBMCs correlated significantly with the percentages of CD16+ cells. Moreover, heat-aggregated IgG blocked the binding site of FcγR on effector cells, but monomeric human IgG and patient serum containing paraprotein did not inhibit the ADCC activity by humanized anti-HM1.24 MoAb in accordance with the findings that FcγRIII was not blocked by monomeric IgG.38 In contrast, neutrophils that also express Fc receptors did not exhibit any cytotoxicity with humanized anti-HM1.24 MoAb. These results indicate that Fc-mediated effector functions, especially through FcγRIII, are responsible for the antitumor activity observed, and that an increase of NK cells that express CD16 and CD56 is a good indicator for high ADCC activity.
Because of the wide variation in the ADCC activity of PBMCs in healthy controls, there was no apparent suppression of ADCC activity in myeloma patients. However, three patients with refractory disease showed decreased ADCC activity of PBMCs (<2 SD of controls). In contrast, a high level of ADCC activity was observed in patients in which treatment proved successful. Similar observations have been reported that myeloma patients in stable remission phase showed a significant recovery of LAK activity.34 These findings suggest that cell-mediated immunity can recover following effective chemotherapy. Further studies will be necessary to determine whether ADCC activity is of prognostic value in patients with multiple myeloma.
The precise mechanism of reduced ADCC activity in patients with advanced disease remains unclear, but the presence of suppressor factors has been suggested that can alter NK cell function, regulated by several cytokines, such as IL-2, IL-12, and IL-15.39-41Abnormal cytokine production, ie, decreased serum levels of IL-2 and increased serum levels of IL-1β, IL-6, and prostaglandin E2, has been reported in patients with various types of cancer.42 Specifically, overproduction of IL-6 and transforming growth factor-β (TGF-β) was observed in myeloma patients in association with a poor prognosis.43-45 Because IL-6 and TGF-β are known to suppress the production of IL-2, interferon-γ, and tumor necrosis factor from T cells,46 47 they might negatively regulate the immune response of NK cells. In fact, we found that decreased ADCC activity of PBMCs can be restored by IL-2, IL-12, and IL-15 in myeloma patients, but that these cytokines cannot enhance ADCC activity of PBMCs from healthy donors. Although there is no direct evidence for an established role of these cytokines in cellular immunity, our data support the hypothesis that an abnormal cytokine network might contribute to reduced ADCC activity, at least in part, and suggest the potential benefits of ex vivo treatment with IL-2, IL-12, or IL-15 for immunotherapy, especially in patients with advanced disease.
PBSCs are increasingly used as an alternative to autologous bone marrow for hematological rescue after myeloablative chemotherapy for the treatment of malignancies including multiple myeloma.5Several investigators have reported that high numbers of NK and LAK cells are present in PBSC grafts, suggesting a potential role in tumor eradication after PBSCT.48,49 Although the number of samples is small, we also observed an expansion of the NK (CD16+) cell population and generation of ADCC activity in PBSC collections from myeloma patients. Because tumor cells have been detected in PBSC collections and may contribute to relapse,50 it might be possible to eliminate myeloma cells in harvested PBSC by incubation with humanized anti-HM1.24 MoAb. Furthermore, the combination of current PBSCT and immunotherapy could well result in enhanced tumor cytotoxicity and improve the prognosis of multiple myeloma.
In addition to the cytolytic activity of effector cells, there are certain other advantages to immunotherapy with humanized anti-HM1.24 MoAb in multiple myeloma. First, myeloma cells mainly localize in the bone marrow, where tumor cells are readily accessible to therapeutic MoAb.25 Second, there is no soluble HM1.24 antigen in the serum to block the binding of humanized anti-HM1.24 MoAb. Third, humanized anti-HM1.24 MoAb does not induce human complement activation, which could cause serious side effects in patients. Finally, the ability to generate antibodies against the therapeutic MoAb is likely to be suppressed because of compromised B-cell function in myeloma patients.51 These factors suggest that together, immunotherapy using humanized anti-HM1.24 MoAb may be beneficial in myeloma patients when significant tumor reduction has been achieved by conventional and/or high-dose chemotherapy. Moreover, our previous study has shown that anti-HM1.24 MoAb inhibits Ig secretion by human myeloma cells in SCID mouse models.25 Therefore, humanized anti-HM1.24 MoAb may also be useful to regulate humoral immunity in a variety of clinical situations.
In conclusion, the present study demonstrates that humanized anti-HM1.24 MoAb can mediate myeloma cell-specific cytolysis together with various effector cells from myeloma patients by an ADCC mechanism, and that the reduced ADCC activity of effector cells in certain myeloma patients can be restored by treatment with cytokines, such as IL-2, IL-12, or IL-15. These results encourage clinical trials with this humanized MoAb and warrant further investigation into the feasibility of ex vivo enhancement of ADCC activity by cytokines, especially in patients with cellular immunodeficiency.
ACKNOWLEDGMENT
We thank Dr Kevin Boru for review of the manuscript, Drs Shingo Wakatsuki, Toshiaki Takeichi, Yoshiyuki Miyamoto, Takashi Mizuguchi, and Yoshihito Okamura for their cooperation, and Tomoko Sei for excellent technical assistance.
Supported in part by a grant for Cancer-Induced Bone Diseases from the Ministry of Health and Welfare, Tokyo, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Masaaki Kosaka, MD, PhD, First Department of Internal Medicine, School of Medicine, University of Tokushima, 3-18-15 Kuramoto-cho, Tokushima 770-8503, Japan.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal