Abstract
CrkL is a member of the Crk family of adapter proteins consisting mostly of SH2 and SH3 domains. CrkL is most abundantly expressed in hematopoietic cells and has been implicated in pathogenesis of chronic myelogenous leukemia. However, its function has not been precisely defined. Here, we show that overexpression of CrkL enhances the adhesion of hematopoietic 32D cells to fibronectin. The CrkL-induced increase in cell adhesion was blocked by antibodies against VLA-4 (4β1) and VLA-5 (5β1) but was observed without changes in surface expression levels of these integrins. Studies using CrkL mutants demonstrated that the SH2 domain is partially required for enhancing cell adhesion, whereas the C-terminal SH3 domain as well as the tyrosine phosphorylation site (Y207) is dispensable. In contrast, the N-terminal SH3 domain, involved in binding C3G and other signaling molecules, was showed to play a crucial role, because a mutant defective of this domain showed an inhibitory effect on the cell adhesion to fibronectin. Furthermore, overexpression of C3G also increased the adhesion of hematopoietic cells to fibronectin, whereas a C3G mutant lacking the guanine nucleotide exchange domain abrogated the CrkL-induced increase in cell adhesion. On the other hand, a dominant negative mutant of H-Ras or that of Raf-1 enhanced the basal and CrkL-induced cell adhesion and that of R-Ras modestly decreased the adhesion. Taken together, these results indicate that the CrkL-C3G complex activates VLA-4 and VLA-5 in hematopoietic cells, possibly by activating the small GTP binding proteins, including R-Ras, through the guanine nucleotide exchange activity of C3G.
HEMATOPOIESIS takes place in close contact with the bone marrow microenvironment, which is composed of stromal cells and extracellular matrix components, including fibronectin. Members of the integrin superfamily of adhesion molecules mainly mediate adherence of hematopoietic cells to both the extracellular matrix and stromal cells. Integrins are heterodimers of α and β subunits that can pair to form more than 20 receptors.1,2 Integrins of the β1 subfamily, mostly VLA-4 (α4β1) and VLA-5 (α5β1), have been identified on most of the hematopoietic progenitor cells as well as on various hematopoietic cell lines3-5 and shown to bind ligands especially when these cells are stimulated with growth-stimulating cytokines such as interleukin-3 (IL-3), granulocyte-monocyte colony-stimulating factor, erythropoietin (Epo), and stem cell factor.6-9 One of the ligands involved in the adhesion of hematopoietic cells through VLA-4 and VLA-5 is fibronectin, which preferentially mediates adhesion of primitive progenitor cells to the bone marrow microenvironment.10 Recently, accumulating evidence has suggested that adhesive interaction mediated by integrins of β1 subfamily and fibronectin plays a critical role in controling proliferation, apoptosis, migration, and mobilization of hematopoietic cells.4,8 11-18 Thus, knowledge of the mechanisms by which the functional states of these integrins are regulated is critical to our understanding of the physiologic mechanisms responsible for the regulation of normal hematopoiesis.
The Crk proteins, originally identified as homologues of the product of the v-crk oncogene,19 are adapter proteins composed of SH2 and SH3 domains with very short intervening sequences. Three forms of cellular Crk proteins have been found; both Crk II and CrkL (for Crk-like) have the domain structure SH2-SH3-SH3, although Crk I, the alternatively spliced form of Crk II, lacks the C-terminal SH3 domain.20,21 The N-terminal SH3 domain of CrkL has been shown to bind Sos1 and C3G, two guanine nucleotide exchange proteins for the Ras family of small GTPases.22,23 Interestingly, recent studies have established that CrkL, which is most abundantly expressed in hematopoietic cells,24 also binds through its N-terminal SH3 domain to the BCR-ABL fusion protein expressed in chronic myelogenous leukemia cells and becomes phosphorylated at Y-207.25-28 CrkL also becomes tyrosine phosphorylated in hematopoietic cells stimulated with stem cell factor,29thrombopoietin,30 Epo,31 IL-3,31and IL-2.32 In addition, CrkL, through its SH2 domain, forms complexes with tyrosine-phosphorylated signaling molecules, including c-Cbl,29,31-33 Shc,31 and SHP-231 in hematopoietic cells stimulated with cytokines. Thus, it is implied that CrkL may play a role in growth control and leukemic transformation of hematopoietic cells. However, the function of CrkL in hematopoietic cells has not been precisely defined. In the present study, we show that overexpression of CrkL activates adhesion of hematopoietic cells to fibronectin through VLA-4 and VLA-5. CrkL was further shown to transduce the signal to activate these integrins through the guanine nucleotide exchange activity of C3G.
MATERIALS AND METHODS
Cells and reagents.
A clone of IL-3–dependent 32D cells expressing the wild-type murine Epo receptor (32D/EpoR-Wt) was previously described34 and maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and 1 U/mL human recombinant Epo. COS7 cells were cultured in Dulbecco’s modified Eagle medium (Nissui Seiyaku, Tokyo, Japan) supplemented with 10% FCS. Recombinant human Epo was kindly provided by Chugai Pharmaceutical Co Ltd (Tokyo, Japan). Recombinant murine IL-3 was purchased from PeproTech Inc (Rocky Hill, NJ).
Antibodies against CrkL, C3G, and R-Ras were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal antibodies (MoAbs) against murine α4 (428) and α5 (5H10-27) integrin subunits were from Seikagaku Corp. (Tokyo, Japan) and from PharMingen (San Diego, CA), respectively. Fluorescein (DTAF)-conjugated goat anti-rat IgG secondary antibody was obtained from Immunotech (Marseille, France). Human plasma fibronectin was purchased from GIBCO-BRL (Grand Island, NY).
Expression plasmids.
An expression plasmid for human CrkL, pSG-CrkL,35 was kindly provided by Dr John Groffen (Childrens Hospital Los Angeles, Los Angeles, CA). Expression plasmids for various CrkL mutants were constructed by deletion of the following fragments from the CrkL cDNA: dSH2, a Cfr101 fragment (nucleotides 560 to 752); dSH3N, aDraI-AluI fragment (nucleotides 941 to 1049); dY, anAvaIII-PstI fragment (nucleotides 1127 to 1163); dSH3C, an AvaIII-BalI fragment (nucleotides 1127 to 1327).
For construction of pTet-CrkL, in which the CrkL cDNA is placed downstream of a tetracycline operator (tetO)-controlled promoter, a 5′ portion of the CrkL cDNA (nucleotides 514 to 844) was amplified by the polymerase chain reaction (PCR) with 5′ and 3′ primers of 5′-CCGGATCCTCCGCCAGGTTCGACTC-3′ and 5′-CCGAATTCATCCCATTGGTGGGCTTGGAT-3′, respectively. The primer sequences were designed to add the BamHI and EcoRI recognition sequences at the 5′ and 3′ ends, respectively, of the amplified fragment. These sites were then used for subcloning of the amplified fragment into the multiple-cloning site of an expression plasmid, pJ3H,36 obtained from American Type Culture Collection (Rockville, MD). The SalI-ClaI fragment, encompassing the amplified region, was then excised from this plasmid and subcloned between the SalI and ClaI sites of pTet-Splice (GIBCO-BRL). This plasmid was then digested withCpoI and EcoRV to subclone theCpoI-BglII fragment, containing nucleotides 536 to the 3′ end of the CrkL cDNA, from pSG-CrkL to replace the PCR-amplified region, thus giving pTet-CrkL.
An expression plasmid for C3G, pcDNA-C3G, was constructed by subcloning the HindIII-BamHI fragment (nucleotides 66 to 3377) of C3G cDNA,22 obtained through the Riken Gene Bank (Ibaraki, Japan) with the permission from Dr Michiyuki Matsuda (National Institute of Health, Tokyo, Japan), into pcDNA3 (Invitrogen, San Diego, CA). An expression plasmid for mutant C3G, pcDNA-C3G-dSS, was created by deletion of the SmaI-ScaI fragment of C3G cDNA (nucleotides 2609 to 2999) from pcDNA-C3G. Tetracycline responsive expression plasmid for C3G and the C3G mutant, pTet-C3G and pTet-C3G-dSS, were constructed by subcloning theHindIII-AvrII fragments (nucleotides 66 to 3360) from pcDNA-C3G and pcDNA-C3G-dSS, respectively, between the HindIII and SpeI sites of pTet-Splice.
For construction of an expression plasmid for mutant Raf-1, pcDNA-Raf-dSE, the Raf-1 cDNA was excised from p627,37obtained from the Riken Gene Bank, by digestion with EcoRI andXbaI and subcloned into pcDNA3 to give pcDNA-Raf-1. TheStuI-EcoRV fragment (nucleotides 1122 to 2028) was then deleted to give pcDNA-Raf-dSE. An expression plasmid for a dominant negative mutant of R-Ras, pcDNA-R-Ras43N,38 was kindly provided by Dr Erkki Ruoslahti (La Jolla Cancer Research Center, La Jolla, CA). An expression plasmid for dominant negative H-Ras, pcDNA-H-Ras17N, was constructed by subcloning cDNA coding for H-Ras17N (Upstate Biotechnology, Lake Placid, NY) into the pcDNA3 vector.
Transfection.
Transfection for stable expression was performed essentially as described previously.34 In brief, 32D/EpoR-Wt cells were transfected with or without 5 μg of pTet-CrkL along with 5 μg of pTet-tTAk (GIBCO-BRL), which is an expression plasmid for the tetracycline transactivator (tTA),39 and 1 μg of pSV-Zeo (Invitrogen) by electroporation at 960 μF and 300 V, followed by selection in medium containing Zeocine (Invitrogen) and 500 ng/mL tetracycline. Six clones transfected with pTet-CrkL were isolated by limiting dilution and examined for the induction of CrkL expression by anti-CrkL immunoblotting of cell lysates prepared after withdrawal from tetracycline for 24 hours. The clone inducibly expressing the highest level of CrkL, 32DE/Tet-CrkL, was selected for the subsequent studies. Clones transfected with pTet-tTAk and pSV-Zeo alone were similarly selected and examined for the expression of tTA by the luciferase assay by using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI), the pUHC13-3 (GIBCO-BRL) and a control Renilla luciferase plasmid, pRL-SV (Promega), a reporter plasmid, as described previously.40 The clone inducibly expressing the highest level of tTA, which was comparable with that expressed by 32DE/Tet-CrkL, was designated as 32DE/TA and used for the subsequent studies. 32DE/Tet-C3G and 32DE/Tet-C3G-dSS clones were similarly obtained by transfecting pTet-C3G and pTet-C3G-dSS, respectively, into 32DE/TA cells along with pMAM2-BSD41 (Funakoshi, Tokyo, Japan) followed by selection in medium containing blasticidin-S (Funakoshi).
Transfection of expression plasmids into COS7 cells was performed with the Lipofectamin reagent (GIBCO-BRL), as described previously.42 Cells were harvested for analysis with immunoprecipitation and immunoblotting 2 days after transfection.
Immunoprecipitation and immunoblotting.
Cells were solubilized with a lysis buffer composed of 1% Triton X-100, 20 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L sodium orthovanadate, 1 mmol/L phenylmethylsulfonyl fluoride, 10 μg/mL aprotinin, and 10 μg/mL leupeptin. Cell lysates were subjected to immunoprecipitation and immunoblotting as described previously.34
Cell adhesion assays.
For stable transfectants, cell adhesion assay was performed essentially as described with some modifications.38 In brief, 96-well, flat-bottom tissue culture plates were coated with indicated concentrations of fibronectin overnight at 4°C. Plates were then blocked with 1% bovine serum albumin (BSA) at 37°C for 1 hour followed by washing three times with RPMI 1640 containing 0.2% BSA, referred to as cell adhesion medium. Cells were washed twice and resuspended in cell adhesion medium supplemented with 5 ng/mL IL-3, unless indicated otherwise. Cells (5 to 10 × 104/well) were added to each well in triplicate and incubated for 30 minutes at 37°C. In some experiments, cells were incubated with indicated concentrations of anti-integrin antibodies or irrelevant MoAb for 15 minutes at room temperature before plating on fibronectin coated wells. Plates were then washed three times with cell adhesion medium to remove unbound cells. Cells remaining attached to the plates were measured by the sodium 3′-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro) benzene sulfonic acid hydrate (XTT) colorimetric assay (Boehringer-Mannheim, Indianapolis, IN) according to the manufacturer’s recommendation. After subtraction of background cell binding to BSA-coated wells, the percentage of adherent cells was determined by dividing the optical density of the adherent cells by that of the initial cell input.
For adhesion assay of transiently transfected cells, 32D/EpoR-Wt cells were electroporated at 960 μF and 300 V with indicated amounts of relevant plasmids and 1 μg of a control Renilla luciferase plasmid, pRL-SV. After a recovery period of 1 day, cells were subjected to the cell adhesion assay described above except that 4 × 105cells were plated on fibronectin-coated 24-well plates in duplicate, and the adhesion was assayed by the luciferase activity of cell lysates.
All the cell adhesion assays in Results were repeated at least three times, and the results were reproducible.
Flow cytometry.
To analyze the surface expression of VLA-4 and VLA-5, 32DE/Tet-CrkL cells were cultured in the presence or absence of tetracycline for 24 hours and stained with anti-α4 or anti-α5 antibody or left unstained as control. Cells were further stained with fluorescein-labeled secondary antibody and analyzed with an Epics Elite flow cytometer (Coulter Electronics, Miami, FL).
RESULTS
Overexpression of CrkL increases adhesion of cells to fibronectin.
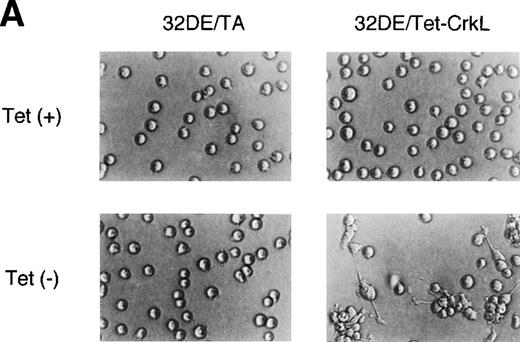
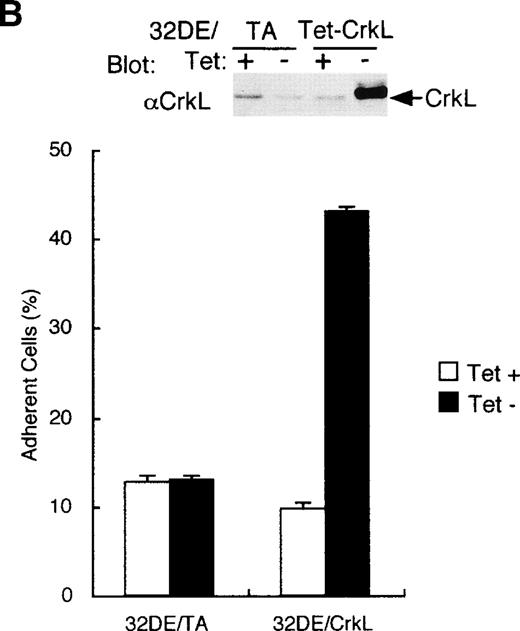
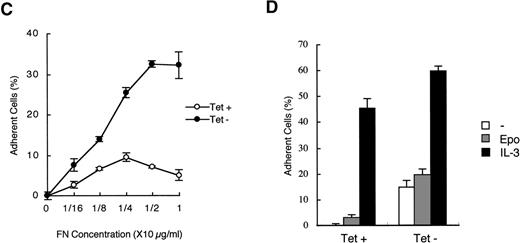
To explore the functions of CrkL in hematopoietic cells, we established a clone of 32D/EpoR-Wt cells, 32DE/Tet-CrkL, which overexpresses CrkL when withdrawn from tetracycline, as described in Materials and Methods. As shown in Fig 1A, 32DE/Tet-CrkL cells, which grow in suspension, became highly adherent when cultured in the absence of tetracycline and extended long protrusions. 32DE/TA cells, which express only tTA at a comparable level with 32DE/Tet-CrkL when withdrawn from tetracycline, did not show this change. To confirm that overexpression of CrkL activates cell adhesion, 32DE/Tet-CrkL cells as well as 32DE/TA cells were cultured with or without tetracycline for 24 hours and allowed to attach to wells coated with a defined substrate, fibronectin, for 30 minutes in the presence of IL-3. As shown in Fig 1B, 32DE/Tet-CrkL cells attached dramatically better to fibronectin when tetracycline was removed from culture medium, whereas 32DE/TA cells, cultured with or without tetracycline, attached poorly to this substrate. Ant-CrkL immunoblotting of lysates obtained from cells cultured under the same conditions confirmed that CrkL was overexpressed in 32DE/Tet-CrkL cells in the absence of tetracycline (Fig 1B, upper panel). When removed from tetracycline to overexpress CrkL, 32DE/Tet-CrkL cells were also shown to attach better to wells coated with various concentrations of fibronectin (Fig 1C). In accordance with previous reports,6 9 32DE/Tet-CrkL cells starved from Epo and IL-3 for 16 hours barely attached to fibronectin, whereas the adhesion was remarkably activated when cultured in the presence of IL-3 (Fig 1D). Although Epo also activated the adhesion of 32DE/Tet-CrkL or 32D/EpoR-Wt cells in repeated experiments, the Epo-induced increase in cell adhesion was always only moderate and much less than that induced by IL-3 (Fig 1D; data not shown). As shown in Fig 1D, the overexpression of CrkL induced by withdrawal from tetracycline dramatically increased the low adhesion levels of 32DE/Tet-CrkL cells starved from cytokines or cultured in Epo, while the IL-3–induced, high level of adhesion was only moderately increased by the CrkL overexpression.
Overexpression of CrkL increases adhesion of 32D cells. (A) Morphology of 32D cells overexpressing CrkL. A clone of 32D/EpoR-Wt cells stably transfected with the expression plasmid for tetracycline transactivator alone (32DE/TA) or a clone also transfected with pTet-CrkL (32DE/Tet-CrkL) was cultured in the presence (+) or absence (−) of 100 ng/mL of tetracycline (Tet), as indicated, for 24 hours and photographed under an inverted microscope (Olympus, Tokyo, Japan). (B) Adhesion of CrkL-overexpressing 32D cells to fibronectin. 32DE/TA and 32DE/Tet-CrkL cells were cultured in the presence (+) or absence (−) of Tet, as indicated, for 24 hours and allowed to attach to wells coated with 10 μg/mL fibronectin for 30 minutes at 37°C in the presence of IL-3. The extent of cell adhesion was quantitated as described in Materials and Methods. The data represent averages ± SD of triplicate determinations. Anti-CrkL immunoblotting of cell lysates obtained under the same conditions is also shown. (C) Effect of fibronectin concentration on adhesion of CrkL-overexpressing 32D cells. 32DE/Tet-CrkL cells, cultured with or without tetracycline, as indicated, were allowed to attach to wells coated with indicated concentrations of fibronectin for the cell adhesion assay. (D) Effect of cytokines on adhesion of CrkL-overexpressing 32D cells. 32DE/Tet-CrkL cells were cultured with or without tetracycline, as indicated, for 24 hours. During the last 16 hours, cells were cultured with 1 U/mL Epo (Epo), 5 ng/mL IL-3 (IL-3), or without any cytokine (−), as indicated. Cells were allowed to attach to wells coated with 10 μg/mL fibronectin for the cell adhesion assay in the presence or absence of cytokine, as indicated.
Overexpression of CrkL increases adhesion of 32D cells. (A) Morphology of 32D cells overexpressing CrkL. A clone of 32D/EpoR-Wt cells stably transfected with the expression plasmid for tetracycline transactivator alone (32DE/TA) or a clone also transfected with pTet-CrkL (32DE/Tet-CrkL) was cultured in the presence (+) or absence (−) of 100 ng/mL of tetracycline (Tet), as indicated, for 24 hours and photographed under an inverted microscope (Olympus, Tokyo, Japan). (B) Adhesion of CrkL-overexpressing 32D cells to fibronectin. 32DE/TA and 32DE/Tet-CrkL cells were cultured in the presence (+) or absence (−) of Tet, as indicated, for 24 hours and allowed to attach to wells coated with 10 μg/mL fibronectin for 30 minutes at 37°C in the presence of IL-3. The extent of cell adhesion was quantitated as described in Materials and Methods. The data represent averages ± SD of triplicate determinations. Anti-CrkL immunoblotting of cell lysates obtained under the same conditions is also shown. (C) Effect of fibronectin concentration on adhesion of CrkL-overexpressing 32D cells. 32DE/Tet-CrkL cells, cultured with or without tetracycline, as indicated, were allowed to attach to wells coated with indicated concentrations of fibronectin for the cell adhesion assay. (D) Effect of cytokines on adhesion of CrkL-overexpressing 32D cells. 32DE/Tet-CrkL cells were cultured with or without tetracycline, as indicated, for 24 hours. During the last 16 hours, cells were cultured with 1 U/mL Epo (Epo), 5 ng/mL IL-3 (IL-3), or without any cytokine (−), as indicated. Cells were allowed to attach to wells coated with 10 μg/mL fibronectin for the cell adhesion assay in the presence or absence of cytokine, as indicated.
CrkL increases cell adhesion to fibronectin by activating VLA-4 and VLA-5.
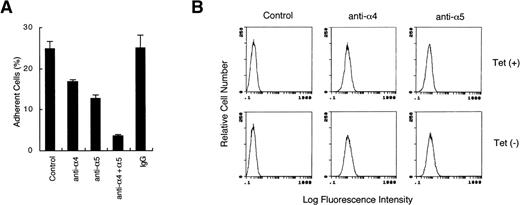
Integrins of the β1 family, mostly VLA-4 (α4β1) and VLA-5 (α5β1), have been shown to mediate adhesion of hematopoietic cells, including 32D cells38 to fibronectin. Therefore, to identify the receptors involved in the CrkL-induced increase of cell adhesion to fibronectin, we examined the effects of antibodies against VLA-4 and VLA-5 on adhesion of CrkL-overexpressing cells to fibronectin. As shown in Fig 2A, a function-blocking anti-α4 or anti-α5 integrin antibody, when added alone, partially inhibited the adhesion of 32DE/Tet-CrkL cells cultured without tetracycline to fibronectin. Notably, when the two antibodies were added in combination, adherent cells were reduced to less than 5% of the total cells added to fibronectin-coated wells. These results agree with the previous report38 that 32D cell attachment to fibronectin is mediated by VLA-4 and VLA-5 integrins and further indicate that overexpression of CrkL enhanced 32D cell attachment to fibronectin through these integrins. As shown in Fig 2B, the flow cytometry analysis using anti-α4 and anti-α5 antibodies confirmed the presence of these integrins on the cell surface of 32DE/Tet-CrkL cells and further showed that the expression levels of these integrins were not significantly altered by withdrawal from tetracycline. Thus, these results suggest that CrkL enhances cell adhesion by increasing the activities of VLA-4 and VLA-5.
CrkL increases adhesion of 32D cells to fibronectin by activating VLA-4 and VLA-5. (A) Antibodies against VLA-4 and VLA-5 inhibit adhesion of CrkL-overexpressing 32D cells to fibronectin. 32DE/Tet-CrkL cells were cultured for 24 hours in the absence of tetracycline and allowed to attach to wells coated with 10 μg/mL fibronectin in the absence (Control) or in the presence of indicated anti-integrin MoAbs or irrelevant MoAb (IgG), as indicated. The extent of cell adhesion was quantitated as described in Materials and Methods. (B) Analysis of VLA-4 and VLA-5 expression in 32DE/Tet-CrkL cells by flow cytometry. 32DE/Tet-CrkL cells were cultured in the presence (upper panels) or absence (lower panels) of tetracycline for 24 hours and stained with indicated anti-integrin MoAbs or left unstained as control (Control), as indicated. Cells were further stained with fluorescein-labeled secondary antibody and subjected to flow cytometry.
CrkL increases adhesion of 32D cells to fibronectin by activating VLA-4 and VLA-5. (A) Antibodies against VLA-4 and VLA-5 inhibit adhesion of CrkL-overexpressing 32D cells to fibronectin. 32DE/Tet-CrkL cells were cultured for 24 hours in the absence of tetracycline and allowed to attach to wells coated with 10 μg/mL fibronectin in the absence (Control) or in the presence of indicated anti-integrin MoAbs or irrelevant MoAb (IgG), as indicated. The extent of cell adhesion was quantitated as described in Materials and Methods. (B) Analysis of VLA-4 and VLA-5 expression in 32DE/Tet-CrkL cells by flow cytometry. 32DE/Tet-CrkL cells were cultured in the presence (upper panels) or absence (lower panels) of tetracycline for 24 hours and stained with indicated anti-integrin MoAbs or left unstained as control (Control), as indicated. Cells were further stained with fluorescein-labeled secondary antibody and subjected to flow cytometry.
The N-terminal SH3 domain of CrkL plays a critical role in enhancement of cell adhesion.
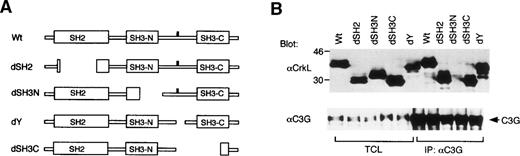
To explore the mechanisms by which CrkL increases the activity of VLA-4 and VLA-5, we examined the functional significance of each CrkL domain for the enhancement of cell adhesion. For this purpose, we constructed expression plasmids for CrkL mutants shown in Fig3A. These mutants were first expressed along with C3G in COS7 cells. As shown in Fig 3B, anti-CrkL immunoblotting of transfected COS7 cell lysates showed that the CrkL mutants with expected sizes were expressed at comparable levels. Furthermore, anti-CrkL immunoblotting of anti-C3G immunoprecipitates confirmed that C3G bound all the CrkL mutants except the dSH3N mutant, which has a deletion in the N-terminal SH3 domain involved in binding C3G (Fig 3B). These results suggest that the deletions introduced into CrkL did not significantly affect the expression level or the overall structure of mutant CrkL.
The N-terminal SH3 domain of CrkL plays a critical role in enhancement of cell adhesion. (A) Schematic representation of CrkL and its mutants. (B) Transient expression of CrkL and its mutants with C3G in COS7 cells. Expression plasmids for wild type and various mutants of CrkL, as indicated, were transfected with that of C3G into COS7 cells. Cells were harvested 2 days after transfection, and total cell lysates (TCL) and anti-C3G immunoprecipitates were subjected to anti-CrkL immunoblotting followed by reprobing with anti-C3G. (C) Effects of transiently expressed CrkL mutants on adhesion of 32D/EpoR-Wt cells to fibronectin. The expression plasmids for wild type and various mutants of CrkL, as indicated, were transfected into 32D/EpoR-Wt cells along with pRL-SV. Transiently transfected cells were subjected to the cell adhesion assay as described in Materials and Methods. (D) Dose-dependent effects of the CrkL dSH3N and dSH2 mutants on 32D/EpoR-Wt cell adhesion to fibronectin. 32D/EpoR-Wt cells were transfected with indicated amounts (microgram) of the expression plasmids for dSH3N and dSH2 mutants of CrkL or the pSG5 vector plasmid and subjected to the cell adhesion assay.
The N-terminal SH3 domain of CrkL plays a critical role in enhancement of cell adhesion. (A) Schematic representation of CrkL and its mutants. (B) Transient expression of CrkL and its mutants with C3G in COS7 cells. Expression plasmids for wild type and various mutants of CrkL, as indicated, were transfected with that of C3G into COS7 cells. Cells were harvested 2 days after transfection, and total cell lysates (TCL) and anti-C3G immunoprecipitates were subjected to anti-CrkL immunoblotting followed by reprobing with anti-C3G. (C) Effects of transiently expressed CrkL mutants on adhesion of 32D/EpoR-Wt cells to fibronectin. The expression plasmids for wild type and various mutants of CrkL, as indicated, were transfected into 32D/EpoR-Wt cells along with pRL-SV. Transiently transfected cells were subjected to the cell adhesion assay as described in Materials and Methods. (D) Dose-dependent effects of the CrkL dSH3N and dSH2 mutants on 32D/EpoR-Wt cell adhesion to fibronectin. 32D/EpoR-Wt cells were transfected with indicated amounts (microgram) of the expression plasmids for dSH3N and dSH2 mutants of CrkL or the pSG5 vector plasmid and subjected to the cell adhesion assay.
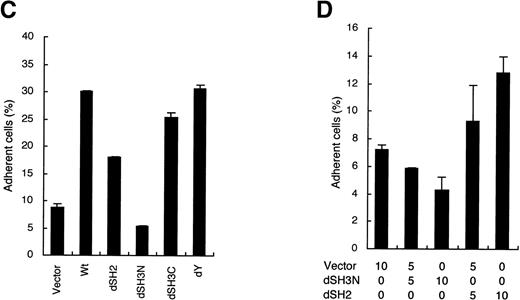
To examine the abilities of CrkL mutants to enhance the cell adhesion, each mutant was then transiently expressed in 32D/EpoR-Wt cells for the cell adhesion assay, as described in Materials and Methods. As shown in Fig 3C, the dY mutant, which lacks a 12-amino-acid region containing the site of tyrosine phosphorylation,28 did not show any impairment in the ability to enhance cell adhesion. The dSH3C mutant, which lacks most of the C-terminal SH3 domain in addition to the tyrosine phosphorylation site, also showed the adhesion-enhancing ability comparable with that of wild-type CrkL. On the other hand, the dSH2 mutant, in which most of the SH2 domain is lost by deletion, showed a significantly impaired ability to enhance the 32D cell adhesion to fibronectin. However, repeated experiments (data not shown) as well as a dose-dependent experiment shown in Fig 3D confirmed that the dSH2 mutant has retained the ability, although impaired, to enhance cell adhesion. In contrast, the dSH3N mutant, lacking the significant portion of the N-terminal SH3 domain, not only failed to enhance but also significantly inhibited the attachment of 32D/EpoR-Wt cells to fibronectin in repeated experiments (Fig 3C; data not shown). The inhibitory effect of the dSH3N mutant was also demonstrated to be dose dependent (Fig 3D). These results indicate that the N-terminal SH3 domain of CrkL, through which CrkL binds C3G and other signaling molecules, plays a crucial role in integrin activation. Although the SH2 domain may also play a role in integrin activation, this domain is not crucial for this function. On the other hand, neither the tyrosine phosphorylation site nor the C-terminal SH3 domain was shown to be involved in integrin activation.
The guanine nucleotide exchange activity of C3G is involved in CrkL-induced integrin activation.
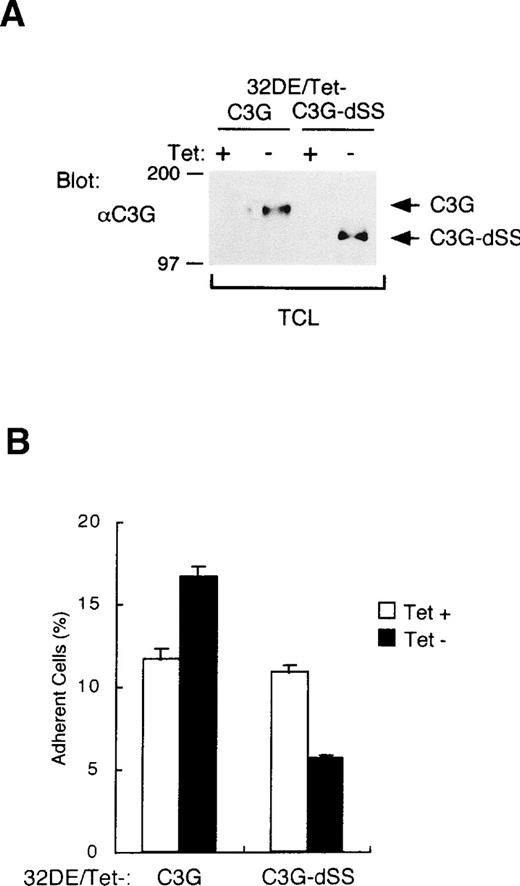
Although the N-terminal SH3 domain has been shown to bind both C3G and Grb2, we previously showed that CrkL predominantly binds C3G in 32D cells.31 In addition, it was confirmed that an increased amount of C3G was associated with CrkL in 32DE/Tet-CrkL cells when CrkL was overexpressed (data not shown). Thus, we examined whether C3G is also involved in integrin activation. As described in Materials and Methods, we established 32D/EpoR-Wt clones, 32DE/Tet-C3G and 32DE/Tet-C3G-dSS, which overexpress wild-type C3G and the C3G-dSS mutant, respectively, when cultured without tetracycline. When the expression level of C3G was increased by withdrawal from tetracycline (Fig 4A), 32DE/Tet-C3G cells showed a moderately increased adhesion to fibronectin, as shown in Fig 4B. In contrast, when the expression of C3G-dSS mutant, lacking the guanine nucleotide exchange domain, was induced by withdrawal from tetracycline (Fig 4A), the adhesion of 32DE/Tet-C3G-dSS cells was significantly inhibited, as shown in Fig 4B. These results indicate that the guanine nucleotide exchange activity of C3G should play a role in integrin activation.
The guanine nucleotide exchange domain of C3G is involved in enhancement of cell adhesion. (A) Inducible expression of C3G or its mutant in 32D cells. A clone of 32D/EpoR-Wt cells transfected with pTet-C3G (32DE/Tet-C3G) or pTet-C3G-dSS (32DE/Tet-C3G-dSS), coding for C3G or its mutant lacking the guanine nucleotide exchange domain, respectively, was cultured for 24 hours with (+) or without (−) Tet, as indicated. TCL were extracted and subjected to anti-C3G immunoblotting. Positions of C3G and its mutant, C3G-dSS, are indicated. (B) 32DE/Tet-C3G and 32DE/Tet-C3G-dSS cells were cultured for 24 hours with (+) or without (−) Tet, as indicated, and subjected to the cell adhesion assay as described in Materials and Methods.
The guanine nucleotide exchange domain of C3G is involved in enhancement of cell adhesion. (A) Inducible expression of C3G or its mutant in 32D cells. A clone of 32D/EpoR-Wt cells transfected with pTet-C3G (32DE/Tet-C3G) or pTet-C3G-dSS (32DE/Tet-C3G-dSS), coding for C3G or its mutant lacking the guanine nucleotide exchange domain, respectively, was cultured for 24 hours with (+) or without (−) Tet, as indicated. TCL were extracted and subjected to anti-C3G immunoblotting. Positions of C3G and its mutant, C3G-dSS, are indicated. (B) 32DE/Tet-C3G and 32DE/Tet-C3G-dSS cells were cultured for 24 hours with (+) or without (−) Tet, as indicated, and subjected to the cell adhesion assay as described in Materials and Methods.
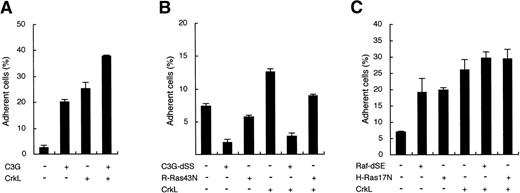
To further confirm the involvement of C3G in CrkL-induced integrin activation, we transiently expressed wild-type C3G or the C3G-dSS mutant in 32D/EpoR-Wt cells and examined the effect on cell adhesion. As shown in Fig 5A, the transient overexpression of C3G drastically enhanced the adhesion of 32D/EpoR-Wt cells to fibronectin, thus confirming the observation in 32DE/Tet-C3G cells. When overexpressed along with CrkL, C3G further increased the cell adhesion enhanced by CrkL (Fig 5A). On the other hand, the C3G-dSS mutant drastically inhibited the adhesion of transfected cells, in accordance with the result in 32DE/Tet-C3G-dSS (Fig 5B). Importantly, when coexpressed with CrkL, the adhesion-enhancing effect of CrkL was also significantly inhibited by this mutant (Fig 5B). Taken together with the result that the C3G-binding domain of CrkL is crucial for the enhancement of cell adhesion, these data indicate that the CrkL-induced integrin activation is mediated through the guanine nucleotide exchange activity of C3G.
Effects of C3G and various mutant signaling molecules on adhesion of 32D cells. (A) Effects of overexpression of C3G on adhesion of 32D cells to fibronectin. 32D/EpoR-Wt cells were transfected with 30 μg of pcDNA-C3G (C3G) and 10 μg of pSG-CrkL (CrkL), as indicated, along with 1 μg of pRL-SV. The total amount of expression plasmids for each transfection was adjusted to become equal by addition of pcDNA3. Transiently transfected cells were subjected to the cell adhesion assay as described in Materials and Methods. (B) Effects of dominant negative mutants of C3G and R-Ras on adhesion of 32D cells to fibronectin. 32D/EpoR-Wt cells were transfected with 40 μg of pcDNA-C3G-dSS (C3G-dSS) or pcDNA-R-Ras43N (R-Ras43N) and 5 μg of pSG-CrkL (CrkL), as indicated, along with 1 μg of pRL-SV. Transiently transfected cells were subjected to the cell adhesion assay. (C) Effects of dominant negative mutants of Raf-1 and H-Ras on adhesion of 32D cells to fibronectin. 32D/EpoR-Wt cells were transfected with 35 μg of pcDNA-Raf-dSE (Raf-dSE) or pcDNA-H-Ras17N (H-Ras17N) and 5 μg of pSG-CrkL (CrkL), as indicated, along with 1 μg of pRL-SV. Transiently transfected cells were subjected to the cell adhesion assay.
Effects of C3G and various mutant signaling molecules on adhesion of 32D cells. (A) Effects of overexpression of C3G on adhesion of 32D cells to fibronectin. 32D/EpoR-Wt cells were transfected with 30 μg of pcDNA-C3G (C3G) and 10 μg of pSG-CrkL (CrkL), as indicated, along with 1 μg of pRL-SV. The total amount of expression plasmids for each transfection was adjusted to become equal by addition of pcDNA3. Transiently transfected cells were subjected to the cell adhesion assay as described in Materials and Methods. (B) Effects of dominant negative mutants of C3G and R-Ras on adhesion of 32D cells to fibronectin. 32D/EpoR-Wt cells were transfected with 40 μg of pcDNA-C3G-dSS (C3G-dSS) or pcDNA-R-Ras43N (R-Ras43N) and 5 μg of pSG-CrkL (CrkL), as indicated, along with 1 μg of pRL-SV. Transiently transfected cells were subjected to the cell adhesion assay. (C) Effects of dominant negative mutants of Raf-1 and H-Ras on adhesion of 32D cells to fibronectin. 32D/EpoR-Wt cells were transfected with 35 μg of pcDNA-Raf-dSE (Raf-dSE) or pcDNA-H-Ras17N (H-Ras17N) and 5 μg of pSG-CrkL (CrkL), as indicated, along with 1 μg of pRL-SV. Transiently transfected cells were subjected to the cell adhesion assay.
Because C3G activates members of the Ras subfamily of small GTP binding proteins through its guanine nucleotide exchange activity, we next examined whether these molecules are involved in the downstream signaling pathway from the CrkL-C3G complex leading to integrin activation. First, the possible involvement of R-Ras, which activates integrin in 32D cells,38 was examined. Thus, a dominant negative mutant of R-Ras, R-Ras43N,38 was transiently expressed alone or along with CrkL in 32D/EpoR-Wt cells and its effect on cell adhesion was examined. In accordance with the previous report,38 R-Ras43N inhibited, although modestly, the basal level of 32D/EpoR-Wt cell adhesion to fibronectin (Fig 5B). This mutant also inhibited the cell adhesion enhanced by overexpression of CrkL to a similar extent. However, the extent of inhibition induced by R-Ras43N was much less than that induced by C3G-dSS, although R-Ras43N was expressed, under the same condition, at a much higher level than that of endogenous R-Ras (data not shown). Next, the possible involvement of H-Ras was examined by using dominant negative mutants of H-Ras and Raf-1, an effector molecule of H-Ras. As shown in Fig 5C, these mutants, H-Ras17N and Raf-dSE, significantly enhanced the adhesion of 32D/EpoR-Wt cells, which is in accordance with a previous report that H-Ras and Raf-1 inhibited integrin activity.43 The enhanced adhesion of cells overexpressing CrkL was also increased slightly by coexpression of these mutants (Fig 5C). These results indicate that R-Ras and H-Ras modulate the integrin activity positively and negatively, respectively, in 32D cells and raise a possibility that the CrkL-C3G complex may transduce the integrin activation signal, although partly, through activation of R-Ras. However, because the inhibitory effect of R-Ras43N on the CrkL-enhanced cell adhesion was only modest, it is speculated that other signaling molecules, most likely other small GTPases, may play more significant roles in integrin activation by CrkL and C3G.
DISCUSSION
In this study, we have showed that overexpression of CrkL activates the adhesion of hematopoietic cells to fibronectin. The enhancement of cell adhesion was observed without changes in expression levels of VLA-4 and VLA-5 but was specifically blocked by antibodies against these integrins, thus indicating that overexpression of CrkL activates VLA-4 and VLA-5 to increase the cell adhesion to fibronectin. Studies using CrkL mutants have showed that the N-terminal SH3 domain of CrkL, required for binding C3G, plays a crucial role for integrin activation, because a mutant defective in this domain decreased the cell adhesion to fibronectin. In accordance with this, overexpression of C3G also increased the cell adhesion to fibronectin, whereas a C3G mutant defective in the guanine nucleotide exchange domain significantly inhibited the basal and CrkL-enhanced adhesion. These data indicate that the CrkL-C3G complex activates VLA-4 and VLA-5 in hematopoietic cells through the guanine nucleotide exchange activity of C3G.
During the preparation of this report, Senechal et al44reported that overexpression of CrkL in hematopoietic cells increased adhesion to fibronectin. Senechal et al44 further showed that individual mutations or deletions of each SH2 and SH3 domain of CrkL abrogated the increase in adhesion. This is at variance with our structure function studies, which showed that the SH2 or C-terminal SH3 domain of CrkL is partially or totally, respectively, dispensable for the increase in cell adhesion and that the N-terminal SH3 domain-defective mutant exerted a dominant negative effect on adhesion. The basis for these discrepancies are unknown but may reflect differences in the structures of CrkL mutants or other experimental conditions. Although Senechal et al44 suggested the involvement of integrins in CrkL-induced cell adhesion by showing an inhibitory effect of RGD peptides, the integrins involved in adhesion were not identified. The present study, thus, complements and extends that of Senechal et al44 by showing that VLA-4 and VLA-5, without changes in their expression levels, mediate the CrkL-induced cell adhesion to fibronectin and that CrkL transduces the signal leading to integrin activation through the guanine nucleotide exchange activity of C3G, which is complexed through the N-terminal SH3 domain of CrkL.
Although it has remained to be known how the CrkL-C3G complex activates the signaling pathway leading to integrin activation through the guanine nucleotide exchange activity of C3G, it is reasonable to speculate that CrkL functions as an adapter protein to recruit C3G to its substrate involved in regulation of the integrin function. In this regard, it is noteworthy that the SH2 domain of CrkL was also required, although partially, for the enhancement of cell adhesion, because previous studies have shown that CrkL binds through its SH2 domain to tyrosine-phosphorylated adhesion-associated proteins, such as paxillin,45 CAS,46 HEF-1,47,48 and Cbl29,31-33,49,50 in hematopoietic cells expressing BCR/Abl or stimulated with cytokine. In addition, overexpression of CrkL in fibroblasts has been shown to activate many of the same signal transduction pathways as BCR/Abl51 and to induce tyrosine phosphorylation of paxillin and its association with CrkL.44 In accordance with these observations, Cbl was constitutively tyrosine phosphorylated and associated with CrkL in 32DE/Tet-CrkL cells withdrawn from tetracycline and starved from cytokine (Y.N., A.A., O.M., unpublished observation, July 1998). Thus, it is possible that overexpression of CrkL induces recruitment of C3G to the vicinity of its substrates involved in regulation of integrin function through interaction between the CrkL SH2 domain and these tyrosine-phosphorylated proteins.
Previously, R-Ras was shown to upregulate the binding affinity of integrins, including VLA-4 and VLA-5 in 32D cells.38 In contrast, H-Ras or its downstream kinase Raf-1, inhibited the activation of chimeric integrins with multiple α and β subunit cytoplasmic domains expressed in CHO cells.43 Although C3G most efficiently activates Rap1/K-Rev1, it also activates R-Ras moderately and H-Ras rather weakly.52 Therefore, the possible involvement of R-Ras or H-Ras in CrkL-induced activation of cell adhesion was examined in the present study. In accordance with the report by Hughes et al,43 a dominant negative mutant of H-Ras or that of Raf-1 expressed alone or in combination with CrkL significantly increased the 32D cell adhesion to fibronectin (Fig 5C). Because Rap1/K-Rev1, which C3G activates most efficiently, antagonizes the function of Ras in certain cell lines,53-55 it is formally possible that the CrkL-C3G complex activates integrins by downregulating the Ras/Raf-1 signaling pathway through activation of Rap1/K-Rev1. However, this possibility is unlikely because overexpression of the CrkL-C3G complex in 32D cells activates the Raf/MAP kinase pathway in 32D cells (A.A., Y.N., O.M., unpublished observation, July 1998). A dominant negative mutant of R-Ras, on the other hand, downregulated the basal as well as CrkL-induced adhesion of 32D/EpoR-Wt cells to fibronectin (Fig 5B), which is in agreement with the previous report.38 So, it is possible that CrkL may activate VLA-4 and VLA-5 partly through activation of R-Ras. However, the reduction in cell adhesion induced by the dominant negative R-Ras, which was expressed at high levels, was only moderate, thus suggesting that biochemical events involving signaling molecules other than R-Ras should play more important roles in activation of hematopoietic cell adhesion induced by CrkL and C3G. Therefore, to elucidate the signaling pathways mediating the CrkL-induced activation of cell adhesion, further studies are required to analyze the possible modulation of the activities of these Ras family GTPases and to examine the possible involvement of other signaling molecules. The Rho family GTPases, which have also been implicated in enhancing cell adhesion,56,57 are of particular interest because v-Crk has recently been reported to activate the Rho-signaling pathway in PC12 cells,58although C3G may not directly activate Rho.
CrkL has been implicated in hematopoietic cell signaling from the receptors for Epo, IL-3, thrombopoietin, and stem cell factor, because these factors induce the tyrosine phosphorylation of CrkL and its binding with tyrosine-phosphorylated signaling molecules, including Cbl.29-33 Intriguingly, these factors also activate the hematopoietic cell adhesion to fibronectin through VLA-4 and VLA-5.6-9 It is tempting to speculate that CrkL may mediate the signal from these receptors to activate integrins (“inside out” signaling), possibly by recruiting C3G to the vicinity of its effector molecule at the plasma membrane through the binding of CrkL SH2 domain with tyrosine-phosphorylated signaling molecules, such as Cbl. Consistent with this hypothesis, overexpression of CrkL enhanced the Epo- or IL-3-induced cell adhesion as shown in Fig 1D. In addition, it should be noted that, except for the results shown in Fig 1D, all the other cell adhesion assays in the present study were performed under the condition in which cells had been cultured in Epo-containing medium and subsequently allowed to adhere to fibronectin-coated wells for 30 minutes in the presence of IL-3 as described in Materials and Methods. Therefore, the effects of mutants of CrkL or other signaling molecules examined in this study should represent their effects on cytokine-stimulated adhesion of hematopoietic cells. As shown in Fig1D, overexpression of CrkL also activated the adhesion of cytokine-starved cells. However, this could be explained by the observation that overexpression of CrkL per se induces tyrosine phosphorylation of adhesion-associated proteins and their association with CrkL44 (Y.N., A.A., O.M., unpublished observation, July 1998). In addition to playing a possible role in the “inside out” signaling, CrkL may also play a role in the “outside in” signaling, because binding of ligands with integrins or cross linking of integrins also induces tyrosine phosphorylation of signaling molecules, including Hefl48,59 and Cbl48 and their association with CrkL. Noteworthy in this regard is the observation that the CrkL-overexpressing cells not only showed increased adhesion but also exhibited morphologic changes when attached to culture plate (Fig 1A). Further studies are in progress in our laboratory to examine the effects of CrkL overexpression on the “outside in” signaling pathways as well as on the control of growth, differentiation, apoptosis, motility, and morphologic changes of various hematopoietic cell lines, including those derived from chronic myelogenous leukemia.
ACKNOWLEDGMENT
We are grateful to Drs John Groffen, Erkki Rouslahti, and Michiyuki Matsuda for the generous gifts of expression plasmids for CrkL, R-Ras43N, and C3G, respectively. We also thank Dr Shuji Tohda for assistance in photography as well as Eiko Nishimura, Kiyomi Kaneki, Mihoko Suzuki, and Kaori Okada for excellent technical assistance.
Supported in part by grants from the Ministry of Education, Science and Culture of Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Osamu Miura, MD, First Department of Internal Medicine, Tokyo Medical and Dental University, 1-5-45 Yushima, Bunkyoku, Tokyo 113, Japan.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal