Abstract
The sialomucin CD34 is a useful marker for hematopoietic stem/progenitor cells. However, the role of CD34 remains poorly understood. Here we investigate the functions of CD34 and another sialomucin CD43 coexpressed on hematopoietic stem/progenitor cells. Stimulation of undifferentiated hematopoietic KG1a cells with anti-CD34 or anti-CD43 induced homotypic cytoadhesion, accompanied by formation of a long-lived cap of CD34 and CD43 respectively, which colocalized with F-actin. Stimulation with either antibody specifically increased tyrosine phosphorylation of the identical set of proteins of Lyn, Syk, pp60, pp69, and pp77 at the capping site. These events were similar to those observed in monocytic U937 cells ectopically expressing CD34. After stimulation of KG1a cells, coimmunoprecipitation of Lyn with pp69 and pp77 and of Syk with pp37 was detected in the membrane fraction. Blockade of antibody-induced cap formation by treatment with cytochalasin D leads to inhibition of tyrosine phosphorylation of Syk and pp77 and homotypic cytoadhesion. Moreover, normal human CD34+ bone marrow cells showed cap formation of CD34 or CD43 after stimulation. These results suggest that crosslinking of either CD34 or CD43 activates the same signaling pathway for cytoadhesion through Lyn, Syk, and the novel tyrosine-phosphorylated proteins within hematopoiesis.
PROTEIN-TYROSINE phosphorylation is critical in signal transduction pathways by which extracellular ligands induce proliferation, differentiation, and functional activation of multiple cell types, including hematopoietic cells.1 2Tyrosine phosphorylation is mediated by either receptor-type protein-tyrosine kinases, which possess extracellular domains that bind ligands, or by nonreceptor-type protein-tyrosine kinases, which appear to associate with receptor-type proteins directly or indirectly.
CD34 is a heavily O-glycosylated sialomucin-type transmembrane glycoprotein that is selectively expressed on hematopoietic stem/progenitor cells and vascular endothelial cells.3-5Cloning of human and murine CD34 has shown that the protein has no significant sequence homology to other proteins in the database. The cytoplasmic domain bears a consensus site for tyrosine phosphorylation and two potential targeting sites for protein kinase C, but lacks enzymatic domain motifs.6-8 A truncated form of the protein with a shorter cytoplasmic domain is produced by alternative splicing.9,10 The natural ligand or counter-receptor for CD34 has not yet been found in the hematopoietic milieu, whereas CD34 present on high endothelial venules in lymph nodes can function as a ligand for the lymphocyte-homing receptor L-selectin.11
Monoclonal antibodies (MoAbs) directed against CD34 are useful for enrichment of hematopoietic progenitor cells from bone marrow (BM) and umbilical cord blood and are useful in analysis of “mobilized” hematopoietic progenitor cells in leukemia, in BM transplantation, and in gene therapy.4,12 Despite the significance of CD34 as a marker for hematopoietic stem/progenitor cells, the function of CD34 during hematopoiesis is unclear. Recent studies on CD34 function thus far indicate that engagement of particular determinants of CD34 by MoAbs increases cell adhesion of CD34-expressing hematopoietic cells,13-15 and that expression of CD34 inhibits terminal differentiation of hematopoietic cells.16 It is hypothesized that CD34 is involved in maintenance of a primitive hematopoietic progenitor cell phenotype in BM stem cell niches.12-16
CD43 (leukosialin/sialophorin), whose glycosylation is aberrant in patients with Wiskott-Aldrich syndrome, is a major transmembrane cell-surface sialomucin-type glycoprotein expressed on a variety of hematopoietic cells.17,18 CD43 is a molecule mediating both adhesion and repulsion, the latter through its negative charge and extended structure.17,19 Not only does CD43 function in passive cell adhesion, but it actively participates in signal transduction of hematopoietic cells, such as T lymphocytes, monocytes, and natural killer cells.18 There is increasing evidence that signaling activated by stimulation with anti-CD43 MoAbs involves tyrosine phosphorylation.20,21 In addition, CD43 is reported to interact with actin filaments through ezrin/radixin/moesin (ERM) family members, suggesting that CD43 regulates cytoskeletal reorganization.22
Recent observations with specific inhibitors suggest that tyrosine phosphorylation is involved in homotypic cell aggregation induced by crosslinking of CD34 upon stimulation with anti-CD34 MoAbs.13,15 Both CD34 and CD43 are heavily O-glycosylated sialomucins with an extended rodlike conformation, and they are coexpressed on hematopoietic stem/progenitor cells.7 23-26These similarities suggest that CD34 and CD43 have overlapping or partially redundant functions as signal transducers in hematopoietic cell adhesion.
In this study, we show that crosslinking of either CD34 or CD43 with specific MoAbs induces an increase in tyrosine phosphorylation of the same set of proteins through nonreceptor-type tyrosine kinases Syk and Lyn at the capping sites. Thus, our results provide the first demonstration that both CD34 and CD43 are capable of generating equivalent signals through tyrosine phosphorylation in cytoadhesion.
MATERIALS AND METHODS
Antibodies and reagents.
Anti-CD34 (4A1, Nichirei, Tokyo, Japan; My10 and 8G12, Becton Dickinson, San Jose, CA; QBEND10, Immunotech S.A., Marseille, France), anti-CD43 (MEM59, Sanbio, Am Uden, The Netherlands), anti-phosphotyrosine (4G10 and biotinylated 4G10, UBI, Lake Placid, NY), anti-Syk (4D10) and anti-Lyn (Lyn44) (Santa Cruz Biotechnology, Santa Cruz, CA), anti-CD11a (G43-25B) and anti-CD49e (IIA1) (PharMingen, San Diego, CA), CD18 (MEM48, Southern Biotechnology Associates, Birmingham, AL), anti-CD49d (BU49) and anti-CD54 (15.2) (Ancell Immunology Research Products, Bayport, MN), and MOPC21 (control IgG1, Sigma, St Louis, MO) were used. Horseradish peroxidase-F(ab′)2 fragments of anti-mouse Ig and of anti-rabbit Ig (Amersham, Buckinghamshire, UK), goat anti-mouse IgG Fcγ fragment specific (Jackson Immunoresearch, West Grove, PA), fluorescein isothiocyanate (FITC)-F(ab′)2 fragment of anti-mouse IgG and IgM (BioSource International, Camarillo, CA), FITC-avidin (GIBCO-BRL, Grand Island, NY), and rhodamine-phalloidin (Molecular Probes, Eugene, OR) were used.
Cell culture and flow cytometry.
The human undifferentiated myeloblastic leukemia cell line KG1a27 was cultured in RPMI 1640 medium containing 10% fetal bovine serum (FBS). The human monocytic cell line U93728 was cultured in Iscove’s modified Dulbecco’s medium supplemented with 10% FBS. Cells were stained with My10, 4A1, QBEND10, 8G12, MEM59, or MOPC21 (control IgG1), followed by an FITC-F(ab′)2 fragment of anti-mouse Ig, and analyzed by a FACScan (Becton Dickinson).
Preparation of normal CD34+ BM cells.
Light-density mononuclear cells were separated from BM cells of a normal adult volunteer by Lymphoprep (Nycomed Pharmaas Diagnostics, Oslo, Norway). Cells were washed, resuspended at 1 × 108 cells/mL in phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA), and incubated with 20 μg/mL anti-CD34 (8G12, class III) at 4°C for 30 minutes. After washing, cells were resuspended at 1 × 108 cells/mL with MACS rat anti-mouse IgG1 microbeads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany), and incubated at 4°C for 15 minutes. CD34+ cells were separated in magnetic field of a MACS separator.
Plasmid transfection.
cDNAs for the full-length human CD34 (kindly provided by Drs D.L. Simmons and B. Seed, Massachusetts General Hospital, Boston, MA)7 and the truncated human CD34 (kindly provided by Drs Y. Nakamura and H. Nakauchi, Institute of Basic Medical Sciences, The University of Tsukuba, Tsukuba, Japan)10 were cloned into the pBCMGSNeo vector.29 U937 cells were transfected by electroporation with pBCMGSNeo vector alone or with pBCMGSNeo vector containing full-length or truncated CD34. Stably transfected clones were selected in 500 μg/mL G418.
Stimulation with MoAbs.
Cells were adjusted to 1.25 × 107 cells/mL in phosphorylation medium (RPMI 1640 medium supplemented with 25 mmol/L HEPES, pH 7.2, 0.1% BSA, and 0.2 mmol/L Na3VO4). Cells were incubated at 4°C with either anti-CD34, anti-CD43, anti-CD11a, anti-CD18, anti-CD49d, anti-CD49e, anti-CD54 or control IgG1 (MOPC21) (12.5 μg/mL) for 30 minutes. After incubation with 25 μg/mL goat anti-mouse Ig antibody for an additional 30 minutes, the cells were stimulated for the indicated times at 37°C. In blocking experiments, cells were preincubated in phosphorylation medium without Na3VO4 at 37°C for 30 minutes with 10 μg/mL cytochalasin D (Sigma) or 30 mmol/L NaF, and the cells were then stimulated in the presence of 0.2 mmol/L Na3VO4 plus each inhibitor as described above.
Immunofluorescence.
Cells (1.25 × 107 cells/mL) were stimulated with various antibodies as described above. The cells were fixed with 2.5% paraformaldehyde at room temperature for 30 minutes. After washing, the cells were suspended in PBS containing 5% BSA for 30 minutes, and subsequently double-stained with FITC-anti-mouse Ig for CD34, CD43, or other cell-surface molecules and rhodamine-phalloidin for F-actin staining in PBS containing 3% BSA for 30 minutes. For detection of tyrosine-phosphorylated proteins, cells were stimulated with either anti-CD34 or anti-CD43, and fixed as described above. After being permeabilized in PBS containing 0.1% saponin, 5% BSA, and 5% normal mouse serum as described previously,30 the cells were stained with biotinylated 4G10 for 40 minutes, followed by FITC-avidin for 40 minutes. To avoid dephosphorylation, 10 mmol/L Na3VO4 was included throughout the staining procedure. Confocal and Nomarski differential-interference-contrast images were obtained using a Fluoview laser scanning microscope (Olympus, Tokyo, Japan). Z-series sections were recorded at 0.5- to 1-μm intervals and all sections were merged.
Western blotting and immunoprecipitation.
Cells were washed with HEPES-buffered saline (50 mmol/L HEPES, pH 7.4, 150 mmol/L NaCl, and 1 mmol/L Na3VO4), and then lysed with Triton X-100 lysis buffer (50 mmol/L HEPES, pH 7.4, 1% Triton X-100 [Sigma, St Louis, MO], 4 mmol/L EDTA, 100 mmol/L NaF, 2 mmol/L Na3VO4, and protease inhibitors [50 μg/mL aprotinin, 200 μmol/L leupeptin, 50 μmol/L pepstatin A, and 2 mmol/L phenylmethylsulfonyl fluoride]). Lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred onto polyvinylidene difluoride membranes. Immunodetection was performed by enhanced chemiluminescence, as described previously.31 Precleared lysates with Pansorbin (Calbiochem, La Jolla, CA) were incubated at 4°C with protein G-Sepharose beads (Pharmacia) precoated with the appropriate MoAb. The immune complexes were analyzed, as described previously.31 32
Subcellular fractionation.
Cells were suspended in hypotonic buffer (10 mmol/L HEPES, pH 7.4, 10 mmol/L NaCl, 1 mmol/L KH2PO4, 5 mmol/L NaHCO3, 5 mmol/L EDTA, 5 mmol/L EGTA, 0.2 mmol/L Na3VO4, and protease inhibitors), freeze/thawed in liquid nitrogen, and sonicated (6.25 W, 10 pulses for 2 seconds). After addition of an equal volume of adjusting buffer (10 mmol/L HEPES, pH 7.4, 290 mmol/L NaCl, 1 mmol/L KH2PO4, 5 mmol/L NaHCO3, 5 mmol/L EDTA, 5 mmol/L EGTA, 0.2 mmol/L Na3VO4, and protease inhibitors), the lysate was centrifuged at 100,000g for 30 minutes, and the cytosol was collected. The pellets were extracted in SDS-PAGE sample buffer or 1% Triton X-100 lysis buffer. All steps were performed at 4°C.
Kinase assays.
After washing immune complexes with modified RIPA buffer (50 mmol/L HEPES, pH 7.4, 1% Triton X-100, 0.25% SDS, 4 mmol/L EDTA, 2 mmol/L Na3VO4, and 2 mmol/L phenylmethylsulfonyl fluoride) and Triton X-100 lysis buffer containing 2 mol/L NaCl, an aliquot of each immunoprecipitate was subjected to an in vitro kinase assay with 2.5 μmol/L [γ-32P]ATP and an equal aliquot was applied to a quantitative immunoblot, as described previously.31,32 Myelin basic protein (MBP) was used as an exogenous substrate for Syk.33 The samples were separated on SDS-PAGE gels. The gels were treated with 1N KOH at 56°C for 2 hours and subjected to a BAS 2000 BioImage Analyzer (Fujix, Tokyo, Japan).
RESULTS
Tyrosine phosphorylation upon stimulation with anti-CD34.
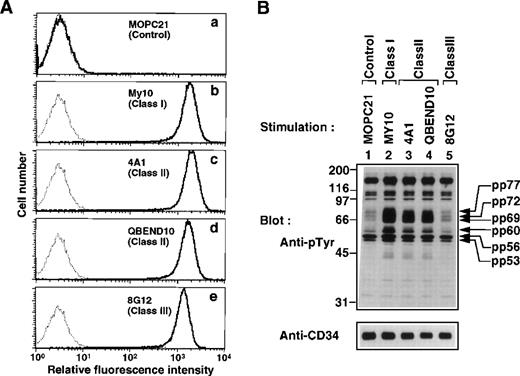
To investigate CD34 signaling, we used three classes of anti-CD34 MoAbs reacted to different epitopes on CD34: class I MoAb (My10) binds to an epitope on sialylated oligosaccharide chains extended from the CD34 core polypeptide; class II MoAb (4A1 and QBEND10) to an epitope on asialo-oligosaccharide chains also extended from the core polypeptide; and class III MoAb (8G12) to an epitope present in the core polypeptide.34-38 Fluorescence-activated cell sorter (FACS) analysis with the CD34+ hematopoietic cell line KG1a showed that there was no significant difference in fluorescence intensities of cells stained with respective MoAbs (Fig 1A, b through e).
Induction of tyrosine phosphorylation after stimulation with anti-CD34 MoAbs. (A) FACS analysis of KG1a cells stained with the anti-CD34 MoAbs used in this study. Cells were stained with MOPC21 (a, control IgG), My10 (b, class I MoAb), 4A1 (c, class II MoAb), QBEND10 (d, class II MoAb), and 8G12 (e, class III MoAb). Solid lines represent staining with specific antibodies, and dotted lines represent staining with the secondary reagent alone. (B) Western blot of equal amounts of Triton X-100 lysates from KG1a cells stimulated for 30 minutes with anti-CD34 MoAbs (lanes 2 through 5) or MOPC21 as a control (lane 1) as described in Materials and Methods, probed with anti-pTyr (4G10, top), and reprobed with anti-CD34 (4A1, bottom). Molecular size markers are indicated on the left (kD).
Induction of tyrosine phosphorylation after stimulation with anti-CD34 MoAbs. (A) FACS analysis of KG1a cells stained with the anti-CD34 MoAbs used in this study. Cells were stained with MOPC21 (a, control IgG), My10 (b, class I MoAb), 4A1 (c, class II MoAb), QBEND10 (d, class II MoAb), and 8G12 (e, class III MoAb). Solid lines represent staining with specific antibodies, and dotted lines represent staining with the secondary reagent alone. (B) Western blot of equal amounts of Triton X-100 lysates from KG1a cells stimulated for 30 minutes with anti-CD34 MoAbs (lanes 2 through 5) or MOPC21 as a control (lane 1) as described in Materials and Methods, probed with anti-pTyr (4G10, top), and reprobed with anti-CD34 (4A1, bottom). Molecular size markers are indicated on the left (kD).
KG1a cells were stimulated with anti-CD34 MoAbs and analyzed by Western blotting using the anti-phosphotyrosine MoAb 4G10 (anti-pTyr). We found that addition of either My10, 4A1, or QBEND10 increased tyrosine phosphorylation of several proteins (Fig 1B, lanes 2 through 4). Prominent tyrosine-phosphorylated proteins were detected at 60, 69, 72, and 77 kD, and a small increase in tyrosine phosphorylation was observed at 53 and 56 kD. However, addition of 8G12 did not increase tyrosine phosphorylation nor did the control MoAb MOPC21 (lanes 1 and 5). These results indicate that crosslinking of CD34 via the particular determinants recognized by class I or class II MoAbs increases tyrosine phosphorylation of cellular proteins, suggesting that the signaling via CD34 involves tyrosine phosphorylation.
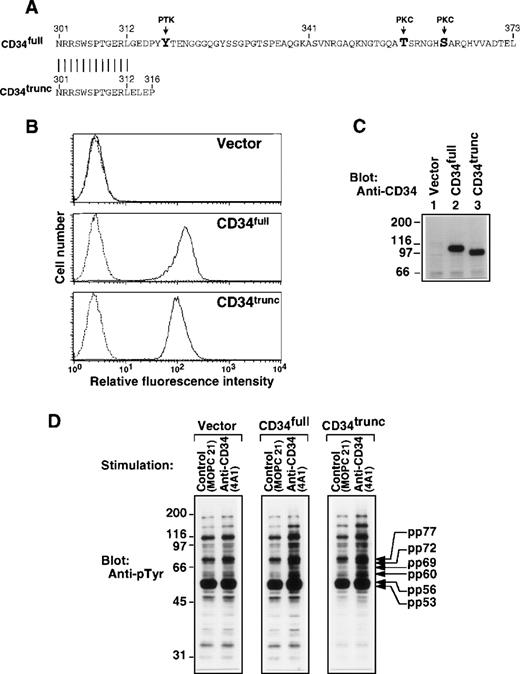
To examine the role of the cytoplasmic portion of CD34, we generated stable clones of the human monocytic cell line U937 transfected with vector alone, with full-length CD34 having a cytoplasmic domain containing possible sites of phosphorylation (Fig 2A; CD34full), or with the alternatively spliced form with a shorter cytoplasmic portion lacking these sites (CD34trunc). FACS analysis and Western blotting showed similar expression of CD34full and CD34trunc (Fig 2B and C). The level of their expression in established clones (Fig 2B) was less than one tenth that of endogenous CD34 in KG1a cells (Fig 1A). Crosslinking of CD34trunc and CD34full increased tyrosine phosphorylation of proteins of 53, 56, 60, 69, 72, and 77 kD (Fig 2D), as seen in KG1a cells (Fig 1B). These increases were moderate, probably due to lower expression of CD34full and CD34trunc. By contrast, addition of 4A1 did not increase tyrosine phosphorylation of cellular proteins in vector-transfected U937 cells (Fig 2D, left panel). These results suggest that the observed increase in tyrosine phosphorylation is independent of possible sites of phosphorylation in the cytoplasmic portion of CD34.
Role of the cytoplasmic portion of CD34 in tyrosine phosphorylation. (A) Amino acid sequence of the cytoplasmic portions of the full-length CD34 (CD34full; top) and the truncated CD34 (CD34trunc; bottom). Vertical bars indicate conserved residues. PTK, a candidate tyrosine phosphorylation site; PKC, a consensus phosphorylation site for protein kinase C. (B) FACS analysis of U937 cells stably transfected with vector alone, CD34full, or CD34trunc. Cells were stained with anti-CD34 (4A1, solid lines) or with the secondary reagent alone (dotted lines). (C) Western blot of Triton X-100 lysates from U937 cells stably transfected with Vector alone (lane 1), CD34full (lane 2), or CD34trunc (lane 3), probed with anti-CD34 (4A1). (D) Western blots of equal amounts of Triton X-100 lysates from U937 cells stably transfected with vector, CD34full, or CD34trunc, probed with anti-pTyr (4G10). Cells were stimulated for 30 minutes with anti-CD34 (4A1) or MOPC21 (control) as described in the legend to Fig 1. Molecular size markers are indicated on the left (kD).
Role of the cytoplasmic portion of CD34 in tyrosine phosphorylation. (A) Amino acid sequence of the cytoplasmic portions of the full-length CD34 (CD34full; top) and the truncated CD34 (CD34trunc; bottom). Vertical bars indicate conserved residues. PTK, a candidate tyrosine phosphorylation site; PKC, a consensus phosphorylation site for protein kinase C. (B) FACS analysis of U937 cells stably transfected with vector alone, CD34full, or CD34trunc. Cells were stained with anti-CD34 (4A1, solid lines) or with the secondary reagent alone (dotted lines). (C) Western blot of Triton X-100 lysates from U937 cells stably transfected with Vector alone (lane 1), CD34full (lane 2), or CD34trunc (lane 3), probed with anti-CD34 (4A1). (D) Western blots of equal amounts of Triton X-100 lysates from U937 cells stably transfected with vector, CD34full, or CD34trunc, probed with anti-pTyr (4G10). Cells were stimulated for 30 minutes with anti-CD34 (4A1) or MOPC21 (control) as described in the legend to Fig 1. Molecular size markers are indicated on the left (kD).
Tyrosine phosphorylation upon stimulation with anti-CD43.
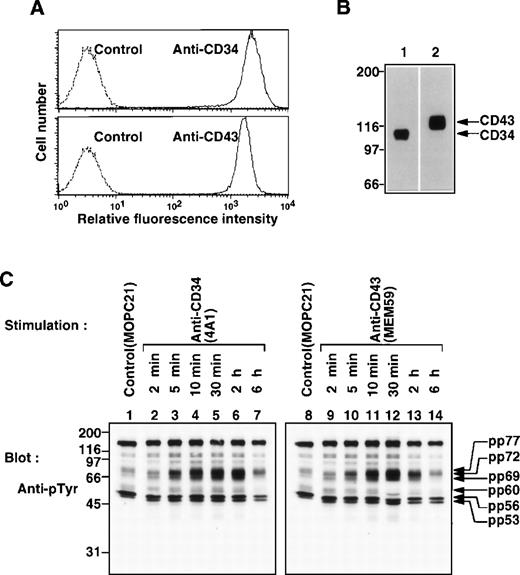
Both CD34 and CD43 are sialomucins expressed on hematopoietic stem cells.7,12,23,25 26 We found with FACS analysis and Western blotting that CD34 and CD43 were coexpressed on KG1a cells (Fig 3A) and detected at 110 kD and 120 kD, respectively (Fig 3B).
Coexpression of CD34 and CD43 on KG1a cells and stimulation with MoAbs to CD34 or CD43. (A) FACS analysis of KG1a cells stained with anti-CD34 (4A1) or anti-CD43 (MEM59). Solid lines represent staining with specific antibodies, and dotted lines represent staining with the secondary reagent alone. (B) Western blots of Triton X-100 lysates from KG1a cells, probed with anti-CD34 (4A1, lane 1) or anti-CD43 (MEM59, lane 2). (C) Western blots of equal amounts of Triton X-100 lysates from KG1a cells stimulated with anti-CD34 (4A1, lanes 2 through 7), anti-CD43 (MEM59, lanes 9 through 14) for the indicated times or incubated with MOPC21 (control IgG, lanes 1 and 8) for 30 minutes at 37°C, probed with anti-pTyr (4G10). Molecular size markers are indicated on the left (kD).
Coexpression of CD34 and CD43 on KG1a cells and stimulation with MoAbs to CD34 or CD43. (A) FACS analysis of KG1a cells stained with anti-CD34 (4A1) or anti-CD43 (MEM59). Solid lines represent staining with specific antibodies, and dotted lines represent staining with the secondary reagent alone. (B) Western blots of Triton X-100 lysates from KG1a cells, probed with anti-CD34 (4A1, lane 1) or anti-CD43 (MEM59, lane 2). (C) Western blots of equal amounts of Triton X-100 lysates from KG1a cells stimulated with anti-CD34 (4A1, lanes 2 through 7), anti-CD43 (MEM59, lanes 9 through 14) for the indicated times or incubated with MOPC21 (control IgG, lanes 1 and 8) for 30 minutes at 37°C, probed with anti-pTyr (4G10). Molecular size markers are indicated on the left (kD).
Because the anti-CD43 MoAb MEM59, which reacts to a CD43-specific epitope on sialylated oligosaccharide chains extended from the CD43 core polypeptide, corresponds to class I anti-CD34 MoAbs in the characteristics of the epitope,26 we investigated whether crosslinking of CD43 also induced tyrosine phosphorylation. KG1a cells were stimulated with either 4A1 or MEM59 and analyzed by Western blotting for phosphotyrosine. Intriguingly, stimulation with MEM59 was found to increase tyrosine phosphorylation of proteins at 53, 56, 60, 69, 72, and 77 kD, with a profile identical to that observed and kinetics similar to those seen upon stimulation with 4A1 (class II Ab to CD34) (Fig 3C). The increase was apparent 5 minutes after stimulation, peaked at ≈30 minutes after stimulation, and then gradually decreased until 6 hours after stimulation. In addition, similar kinetics were observed upon stimulation with My10 (class I MoAb to CD34) (data not shown). These results indicate that stimulation with either anti-CD34 or anti-CD43 increases tyrosine phosphorylation of the same set of cellular proteins.
Capping of CD34 or CD43 upon stimulation.
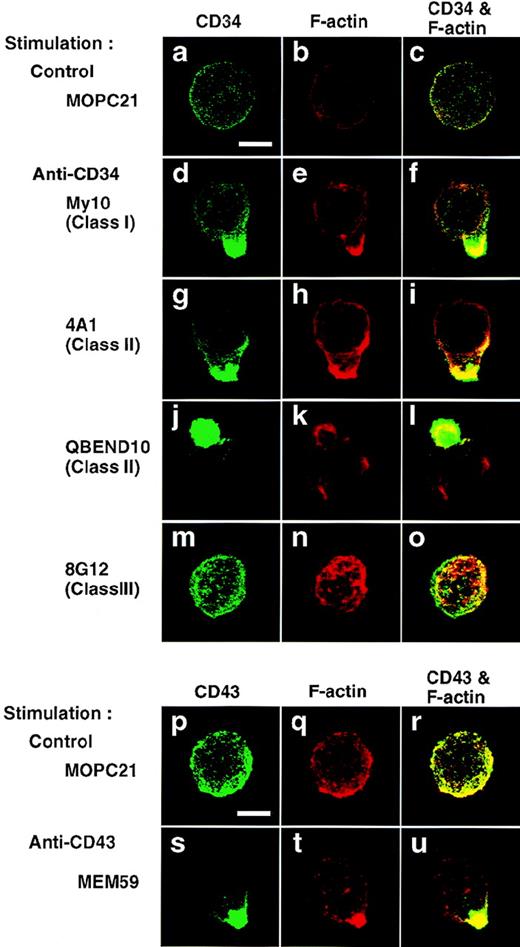
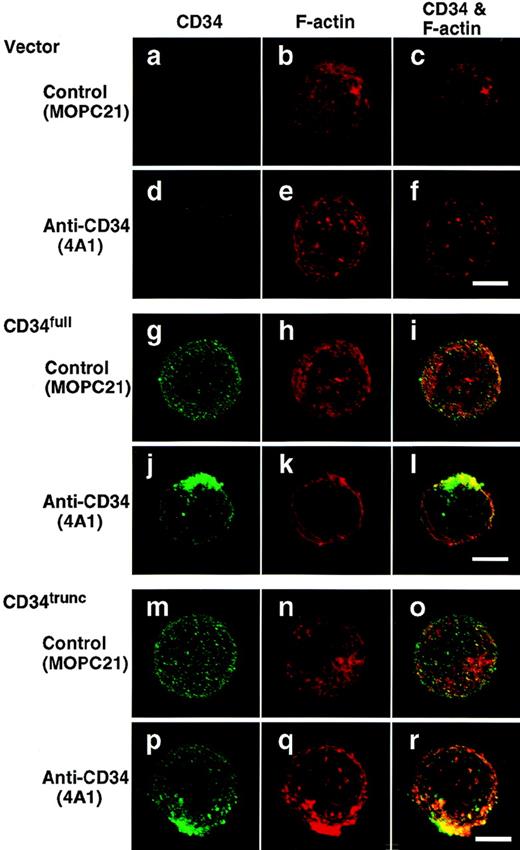
To examine the effect of stimulation with anti-CD34 on distribution of CD34, immunofluorescence microscopy studies were performed. After 5 minutes of stimulation with My10, 4A1, or QBEND10, KG1a cells became polarized with a spherical projection and displayed colocalization of CD34 with F-actin on the projection (Fig 4, d through l). Noticeably, only one large cap was formed on each cell and that cap resembled a cellular uropod, a kind of membrane protrusion. However, cells that reacted with 8G12 showed uniform distributions of CD34 and F-actin without the shape changes (Fig 4, m through o), as observed in control cells (a through c). Cap formation of CD34 was temperature-dependent and the CD34 caps were not internalized but stable on the cell surface at least 2 days after stimulation (data not shown). Furthermore, after stimulation with 4A1, CD34full- or CD34trunc-expressing U937 cells displayed caps of the respective CD34 species colocalized with F-actin (Fig 5, j through l, p through r), accompanied by equivalent increases in tyrosine phosphorylation in cells stably transfected with either construct (Fig 2D).
Capping of CD34 and CD43. KG1a cells were stimulated for 30 minutes with MOPC21 (control, a through c, p through r), anti-CD34 (My10, d through f; 4A1, g through i; QBEND10, j through l; 8G12, m through o), or anti-CD43 (MEM59, s through u) as described in the legend to Fig1. After fixation, cells were double-stained with anti-CD34 (green channel: a, d, g, j, and m) and phalloidin (red channel: b, e, h, k, and n) or with anti-CD43 (green channel: p and s) and phalloidin (red channel: q and t), and analyzed by confocal immunofluorescence microscopy. The right-hand panels show superimposed images of green and red channels. Bars, 5 μm.
Capping of CD34 and CD43. KG1a cells were stimulated for 30 minutes with MOPC21 (control, a through c, p through r), anti-CD34 (My10, d through f; 4A1, g through i; QBEND10, j through l; 8G12, m through o), or anti-CD43 (MEM59, s through u) as described in the legend to Fig1. After fixation, cells were double-stained with anti-CD34 (green channel: a, d, g, j, and m) and phalloidin (red channel: b, e, h, k, and n) or with anti-CD43 (green channel: p and s) and phalloidin (red channel: q and t), and analyzed by confocal immunofluorescence microscopy. The right-hand panels show superimposed images of green and red channels. Bars, 5 μm.
Capping of CD34full and CD34trunc. U937 cells stably transfected with vector (a through f), CD34full (g through l), or CD34trunc (m through r) were stimulated for 30 minutes with anti-CD34 (4A1) (d through f, j through l, p through r) or MOPC21 (control) (a through c, g through i, m through o). After fixation, cells were double-stained with anti-CD34 (green channel: a, d, g, j, m, and p) and phalloidin (red channel: b, e, h, k, n, and q), and analyzed by confocal immunofluorescence microscopy. The right-hand panels show superimposed images of green and red channels. Bars, 5 μm.
Capping of CD34full and CD34trunc. U937 cells stably transfected with vector (a through f), CD34full (g through l), or CD34trunc (m through r) were stimulated for 30 minutes with anti-CD34 (4A1) (d through f, j through l, p through r) or MOPC21 (control) (a through c, g through i, m through o). After fixation, cells were double-stained with anti-CD34 (green channel: a, d, g, j, m, and p) and phalloidin (red channel: b, e, h, k, n, and q), and analyzed by confocal immunofluorescence microscopy. The right-hand panels show superimposed images of green and red channels. Bars, 5 μm.
Next we examined whether capping was induced by stimulation with anti-CD43. Similarly, addition of MEM59 induced formation of uropod-like caps of CD43 on KG1a cells (Fig 4, s through u). Cap formation of CD43, as well as CD34, was almost complete 5 to 10 minutes after stimulation, and CD43 caps, like CD34 caps, were colocalized with F-actin (Fig 4, compare panel u with panels f, i, and l). However, CD43 cap formation did not affect the uniform distribution of CD34, and vice versa (data not shown).
Furthermore, to test the ability of normal cells to form caps of CD34 and CD43, we enriched CD34+ cells from normal human BM cells by MACS. As shown in Fig 6, stimulation with anti-CD34 (4A1) or anti-CD43 (MEM59) induced cap formation on normal CD34+ cells. However, cap formation of CD34 appeared incomplete, such as large clustering of CD34, probably because the level of CD34 expression in normal CD34+ cells was lower than that detected in KG1a cells and U937 cells ectopically expressing CD34. These results indicate that normal CD34+cells are able to become polarized after crosslinking of CD34 or CD43.
Capping/clustering of CD34 or CD43 on normal CD34+ cells. Normal CD34+ BM cells were stimulated with anti-CD34 (4A1) for 0 minutes (a through c) and 30 minutes (d through f), or with anti-CD43 (MEM59) for 0 minutes (g through i) and 30 minutes (j through l). After fixation, cells were double-stained with anti-CD34 (green channel: a and d) and phalloidin (red channel: b and e) or with anti-CD43 (green channel: g and j) and phalloidin (red channel: h and k), and analyzed by confocal immunofluorescence microscopy. The right-hand panels show superimposed images of green and red channels. Bar, 5 μm.
Capping/clustering of CD34 or CD43 on normal CD34+ cells. Normal CD34+ BM cells were stimulated with anti-CD34 (4A1) for 0 minutes (a through c) and 30 minutes (d through f), or with anti-CD43 (MEM59) for 0 minutes (g through i) and 30 minutes (j through l). After fixation, cells were double-stained with anti-CD34 (green channel: a and d) and phalloidin (red channel: b and e) or with anti-CD43 (green channel: g and j) and phalloidin (red channel: h and k), and analyzed by confocal immunofluorescence microscopy. The right-hand panels show superimposed images of green and red channels. Bar, 5 μm.
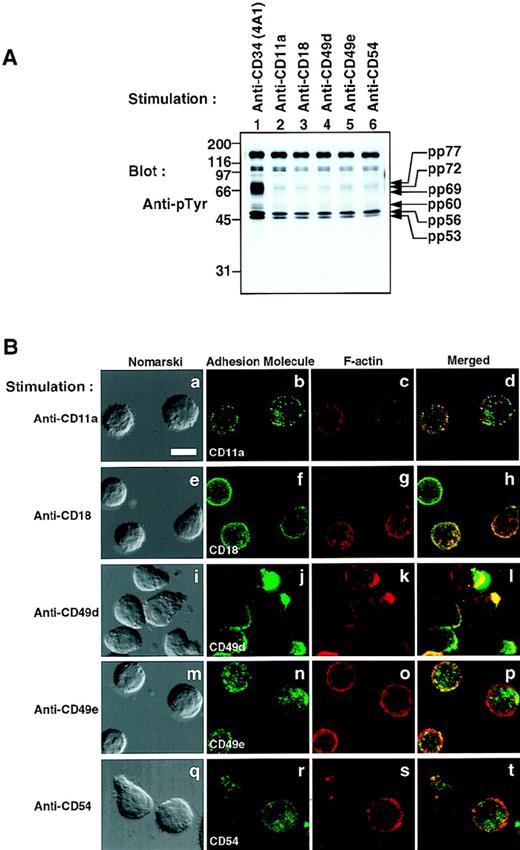
To exclude the possibility that the other adhesion molecules similarly induce an increase in tyrosine phosphorylation, we stimulated KG1a cells with a variety of MoAbs against the integrins α4 (CD49d), α5 (CD49e), αL (CD11a), and β2 (CD18), and the intercellular adhesion molecule ICAM-1 (CD54). No increase in tyrosine phosphorylation of proteins at 53, 56, 60, 69, 72, and 77 kD was induced by these stimulations (Fig 7A), although stimulation with anti-CD11a, anti-CD18, anti-CD49d, anti-CD49e, or anti-CD54 induced cap/patch formation of the respective molecules (Fig 7B). These results indicate that stimulation with either anti-CD34 or anti-CD43 specifically induces not only cell polarization (capping/uropod formation), but also an increase in tyrosine phosphorylation of the same set of cellular proteins.
Crosslinking of various cell-surface molecules by MoAbs. (A) Western blot of Triton X-100 lysates of KG1a cells stimulated for 30 minutes with anti-CD34 (4A1), anti-CD11a, anti-CD18, anti-CD49d, anti-CD49e, or anti-CD54 as described in the legend to Fig1. Molecular size markers are indicated on the left (kD). (B) Distribution of adhesion molecules on KG1a cells stimulated as described above. After fixation, cells were double-stained with FITC–anti-mouse Ig (green channel: b, f, j, n, and r) and phalloidin (red channel: c, g, k, o, and s), and analyzed by Nomarski differential-interference-contrast (left-hand panels) and confocal immunofluorescence microscopy. The right-hand panels show superimposed images of green and red channels. Bars, 10 μm.
Crosslinking of various cell-surface molecules by MoAbs. (A) Western blot of Triton X-100 lysates of KG1a cells stimulated for 30 minutes with anti-CD34 (4A1), anti-CD11a, anti-CD18, anti-CD49d, anti-CD49e, or anti-CD54 as described in the legend to Fig1. Molecular size markers are indicated on the left (kD). (B) Distribution of adhesion molecules on KG1a cells stimulated as described above. After fixation, cells were double-stained with FITC–anti-mouse Ig (green channel: b, f, j, n, and r) and phalloidin (red channel: c, g, k, o, and s), and analyzed by Nomarski differential-interference-contrast (left-hand panels) and confocal immunofluorescence microscopy. The right-hand panels show superimposed images of green and red channels. Bars, 10 μm.
A complex of tyrosine-phosphorylated proteins.
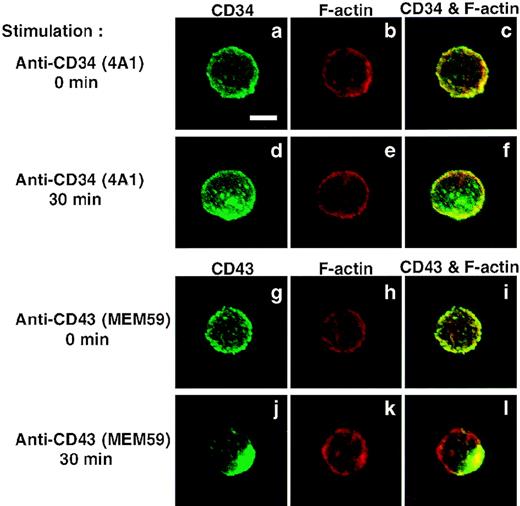
To examine the intracellular localization of tyrosine-phosphorylated proteins upon crosslinking of CD34 or CD43, KG1a cells were stimulated for 30 minutes, permeabilized, and stained for phosphotyrosine. Figure 8 shows that after stimulation with anti-CD34 (4A1, panels e through h) or anti-CD43 (MEM59, panels i through l), capping of tyrosine-phosphorylated proteins occurred in the projection colocalized with F-actin. These results indicate that both CD34 and CD43 are capable of forming caps accompanied by colocalization of both F-actin and tyrosine-phosphorylated proteins, suggesting that cap formation of CD34 or CD43 induces tyrosine phosphorylation at the capping sites.
Localization of phosphotyrosine in KG1a cells after stimulation. KG1a cells were stimulated for 30 minutes with anti-CD34 (4A1), anti-CD43 (MEM59), or MOPC21 (control) as described in the legend to Fig 1. After fixation and permeabilization, cells were double-stained with anti-pTyr (green channel: b, f, and j) and phalloidin (red channel: c, g, and k), and analyzed by Nomarski differential-interference-contrast (left-hand panels) and confocal immunofluorescence microscopy. The right-hand panels show superimposed images of green and red channels. Bars, 5 μm.
Localization of phosphotyrosine in KG1a cells after stimulation. KG1a cells were stimulated for 30 minutes with anti-CD34 (4A1), anti-CD43 (MEM59), or MOPC21 (control) as described in the legend to Fig 1. After fixation and permeabilization, cells were double-stained with anti-pTyr (green channel: b, f, and j) and phalloidin (red channel: c, g, and k), and analyzed by Nomarski differential-interference-contrast (left-hand panels) and confocal immunofluorescence microscopy. The right-hand panels show superimposed images of green and red channels. Bars, 5 μm.
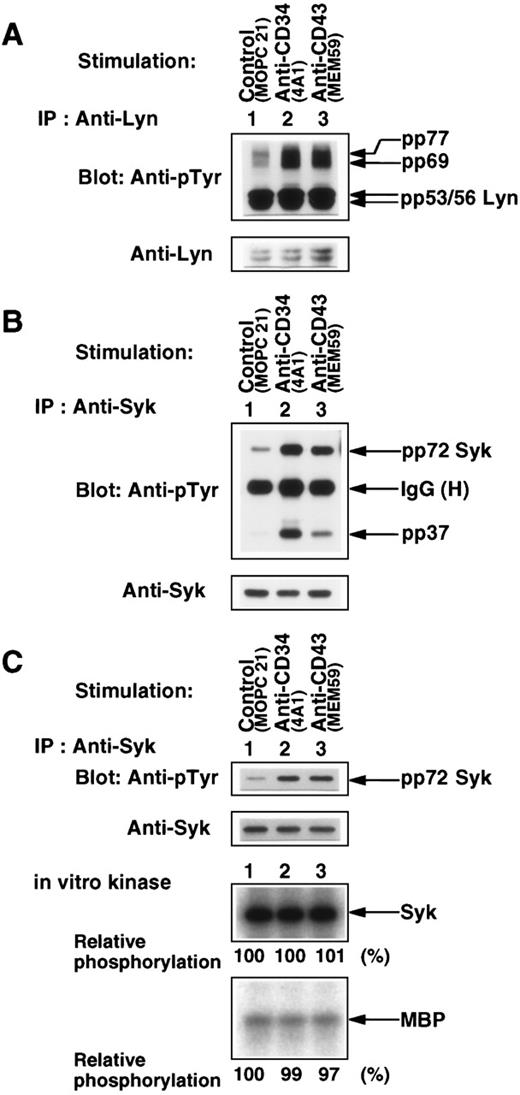
To further characterize the tyrosine-phosphorylated proteins pp53, pp56, pp60, pp69, pp72, and pp77 (Figs 1B, 2D, 3C, and 7A), we performed Western blotting and immunoprecipitation with anti-CD34, anti-CD43, anti-Lyn, anti-Syk, anti-Src, anti-paxillin, anti-HS1, or anti-phosphatidylinositol 3-kinase p85 subunit (PI3-kinase p85). CD34 and CD43 per se were not tyrosine-phosphorylated after any stimulation, and neither CD34 nor CD43 associated with pp53, pp56, pp60, pp69, pp72, and pp77 in Triton X-100 lysates or digitonin lysates (data not shown). Figure 9A shows that pp53/56 was identified as the nonreceptor-type Src family kinase Lyn. Intriguingly, pp69 and pp77 were coimmunoprecipitated with Lyn from Triton X-100 lysates. When KG1a cells were stimulated with anti-CD34 (4A1) or anti-CD43 (MEM59), tyrosine phosphorylation of pp69 and pp77 coimmunoprecipitated with Lyn was greatly enhanced, although an increase in tyrosine phosphorylation of Lyn was barely detected (Fig 9A, compare lanes 2 and 3 with lane 1). Immune complexes with Lyn did not contain CD34, CD43, Syk, c-Src, paxillin, HS1, and PI3-kinase p85 (data not shown). These results suggest that CD34 and CD43 are functionally linked to Lyn because CD34 and CD43 can enhance tyrosine phosphorylation of pp69 and pp77 associated with Lyn.
Characterization of tyrosine-phosphorylated proteins. (A and B) Western blots of Lyn and Syk immunoprecipitated with anti-Lyn (A) or anti-Syk (B) from Triton X-100 lysates of KG1a cells stimulated for 30 minutes with MOPC21 (control, lane 1), 4A1 (lane 2), or MEM59 (lane 3) as described in the legend to Fig 1, probed with anti-pTyr (top panels), and reprobed with anti-Lyn (A, bottom panel) or anti-Syk (B, bottom panel). IgG (H), heavy chain of immunoglobulin G. (C) (Top two panels) Western blots of modified RIPA-washed Syk immunoprecipitates from Triton X-100 lysates prepared as described above, probed with anti-pTyr, and reprobed with anti-Syk. (Bottom two panels) Autoradiograms of in vitro phosphorylation of Syk and MBP by aliquots of the modified RIPA-washed Syk immunoprecipitates. Syk kinase activities were determined by measuring the incorporation of32PO4 into Syk per se and MBP and expressed as values relative to the specific activity of Syk in the control sample (lane 1).
Characterization of tyrosine-phosphorylated proteins. (A and B) Western blots of Lyn and Syk immunoprecipitated with anti-Lyn (A) or anti-Syk (B) from Triton X-100 lysates of KG1a cells stimulated for 30 minutes with MOPC21 (control, lane 1), 4A1 (lane 2), or MEM59 (lane 3) as described in the legend to Fig 1, probed with anti-pTyr (top panels), and reprobed with anti-Lyn (A, bottom panel) or anti-Syk (B, bottom panel). IgG (H), heavy chain of immunoglobulin G. (C) (Top two panels) Western blots of modified RIPA-washed Syk immunoprecipitates from Triton X-100 lysates prepared as described above, probed with anti-pTyr, and reprobed with anti-Syk. (Bottom two panels) Autoradiograms of in vitro phosphorylation of Syk and MBP by aliquots of the modified RIPA-washed Syk immunoprecipitates. Syk kinase activities were determined by measuring the incorporation of32PO4 into Syk per se and MBP and expressed as values relative to the specific activity of Syk in the control sample (lane 1).
Furthermore, pp72 was identified as the nonreceptor-type tyrosine kinase Syk (Fig 9B). Stimulation of KG1a cells with anti-CD34 (4A1) or anti-CD43 (MEM59) induced an increase in tyrosine phosphorylation of Syk and the tyrosine-phosphorylated protein pp37 coimmunoprecipitated with Syk (lanes 2 and 3). The levels of tyrosine phosphorylation of Syk corresponded to those of Syk-associated pp37. Immune complexes with Syk did not contain CD34, CD43, Lyn, c-Src, paxillin, HS1, PI3-kinase p85, p44/p42 MAP kinases, and p38 MAP kinase (data not shown).
To determine whether different phosphorylation states of Syk affected its kinase activity, Syk was separated from pp37 under strong washing conditions and subjected to in vitro kinase assays with MBP as substrate. As shown in Fig 9C, purified Syk exhibited both high autophosphorylation and high tyrosine phosphorylation of MBP (lane 1). However, increases in tyrosine phosphorylation of Syk did not affect tyrosine kinase activity of Syk (lanes 1 through 3). These results suggest that CD34 and CD43 are capable of inducing an increase in tyrosine phosphorylation of Syk leading to an increase in tyrosine phosphorylation of pp37 associated with Syk.
Colocalization of CD34, CD43, Lyn, and Syk to membranes.
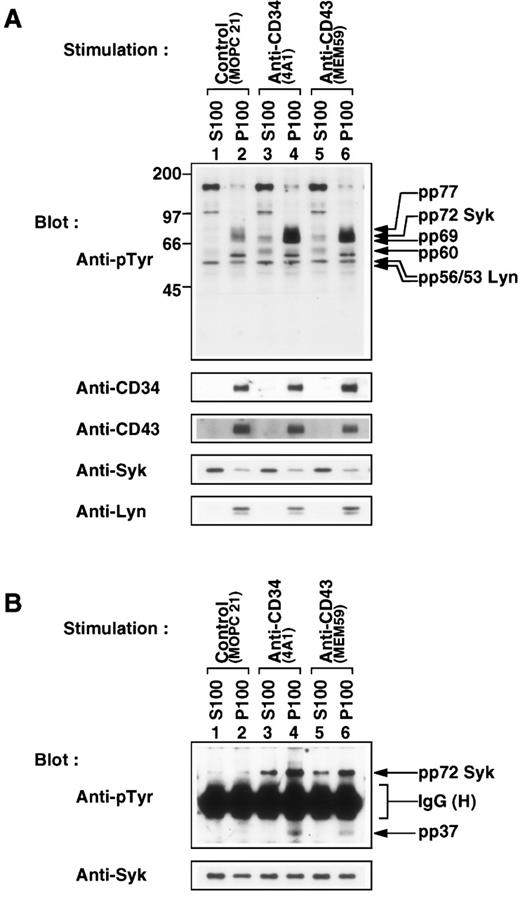
The cytoplasmic tyrosine kinase Lyn is anchored to the plasma membrane via lipid moieties attached to its N-terminal. To examine whether Syk, another cytoplasmic tyrosine kinase, localized to the membrane fraction, subcellular fractionation was performed by isolating the particulate fraction (P100), which contained cellular membranes, and the cytosolic fraction (S100). CD34, CD43, and Lyn were localized to the P100 fraction regardless of stimulation (Fig 10A, bottom panels, lanes 2, 4, and 6). pp69 and pp77, as well as Lyn, were clearly detected only in the P100 fraction (top panel, lanes 4 and 6), indicating that a complex of Lyn with pp69 and pp77 is formed at the plasma membrane after stimulation. A large fraction of Syk was present in the S100 fraction, and became tyrosine-phosphorylated after stimulation (top panels, lanes 1, 3, and 5). A small fraction of Syk was, in particular, constitutively localized to the P100 fraction (the second lowest panel, lanes 2, 4, and 6), and tyrosine phosphorylation of membrane-bound Syk was also induced (top panel). The proportion of membrane-bound Syk to cytosolic Syk was unchanged after stimulation, suggesting that cytosolic Syk is not translocated to membranes during stimulation. In addition, pp60 was distributed to cytosolic and membrane fractions, and stimulation with anti-CD34 (4A1) or anti-CD43 (MEM59) increased tyrosine phosphorylation of pp60 present in both fractions (top panel).
Subcellular localization of tyrosine-phosphorylated proteins. (A) Western blots of cytosolic (S100, lanes 1, 3, and 5) and particulate fractions (P100, lanes 2, 4, and 6) fractionated from KG1a cells stimulated for 30 minutes with MOPC21 (control, lanes 1 and 2), anti-CD34 (4A1, lanes 3 and 4) or anti-CD43 (MEM59, lanes 5 and 6) as described in the legend to Fig 1, probed with anti-pTyr (top), reprobed with anti-CD34 (4A1), anti-CD43 (MEM59), anti-Syk, and anti-Lyn. Molecular size markers are indicated on the left (kD). (B) Western blots of equally adjusted amounts of Syk immunoprecipitates from cytosolic (S100, lanes 1, 3, and 5) or Triton X-100–solubilized membrane fractions (P100, lanes 2, 4, and 6) of KG1a cells stimulated as above, probed with anti-pTyr (top), and reprobed with anti-Syk (bottom). IgG (H), heavy chain of immunoglobulin G.
Subcellular localization of tyrosine-phosphorylated proteins. (A) Western blots of cytosolic (S100, lanes 1, 3, and 5) and particulate fractions (P100, lanes 2, 4, and 6) fractionated from KG1a cells stimulated for 30 minutes with MOPC21 (control, lanes 1 and 2), anti-CD34 (4A1, lanes 3 and 4) or anti-CD43 (MEM59, lanes 5 and 6) as described in the legend to Fig 1, probed with anti-pTyr (top), reprobed with anti-CD34 (4A1), anti-CD43 (MEM59), anti-Syk, and anti-Lyn. Molecular size markers are indicated on the left (kD). (B) Western blots of equally adjusted amounts of Syk immunoprecipitates from cytosolic (S100, lanes 1, 3, and 5) or Triton X-100–solubilized membrane fractions (P100, lanes 2, 4, and 6) of KG1a cells stimulated as above, probed with anti-pTyr (top), and reprobed with anti-Syk (bottom). IgG (H), heavy chain of immunoglobulin G.
To examine the role of membrane-bound Syk, cytosolic and membrane-bound Syk were immunoprecipitated. Stimulation with anti-CD34 (4A1) or anti-CD43 (MEM59) greatly increased tyrosine phosphorylation of both cytosolic and membrane-bound Syk (Fig 10B, top panel, compare lanes 3 through 6 with lanes 1 and 2), whereas levels of Syk were generally comparable (bottom panel). The levels of tyrosine phosphorylation of membrane-bound Syk were more than threefold higher than those of cytosolic Syk (top panel, lanes 3 through 6). Only membrane-bound Syk was coimmunoprecipitated with pp37 (top panel, lanes 4 and 6). These results suggest that a complex of membrane-bound Syk with pp37 may localize with CD34 and CD43 at the plasma membrane.
Inhibition of anti-CD34– or anti-CD43–induced cap formation.
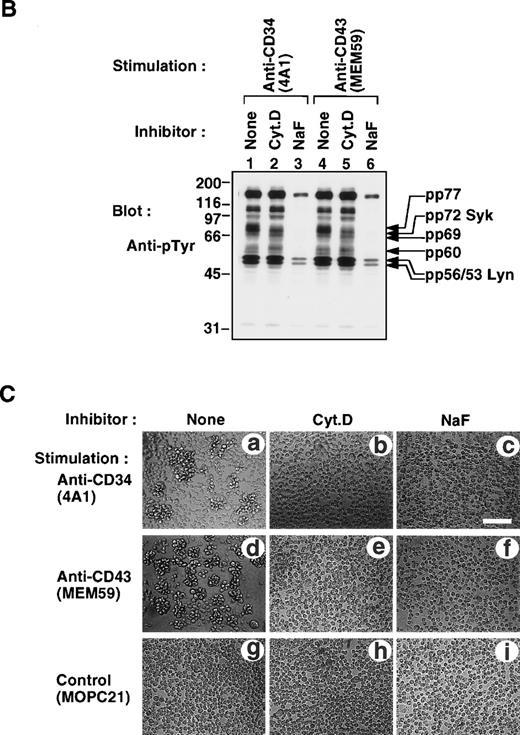
To further dissect the signaling pathway, we examined the effects of inhibitors on anti-CD34– or anti-CD43–induced cap formation and tyrosine phosphorylation. As shown in Fig 11A (see page 3730), treatment of KG1a cells with cytochalasin D (Cyt.D), an inhibitor of actin polymerization, or with NaF, an adenosine triphosphate (ATP)-depleting agent, blocked both anti-CD34– and anti-CD43–induced cap formation. We found that treatment with Cyt.D specifically inhibited an increase in tyrosine phosphorylation of Syk and pp77 upon stimulation with anti-CD34 or anti-CD43 (Fig 11B [see page 3731], compare lanes 2 and 5 with lanes 1 and 4). Incubation with NaF blocked an increase in tyrosine phosphorylation of cellular proteins including Lyn, pp60, pp69, Syk, and pp77 (lanes 3 and 6). Moreover, we observed that not only anti-CD34 but also anti-CD43 induced homotypic cell aggregation of KG1a cells (Fig 11C [see page 3731], a and d), and that treatment with either Cyt.D or NaF completely inhibited antibody-induced homotypic cell aggregation (b, c, e, and f). These data are consistent with recent observations that homotypic cell aggregation induced by anti-CD34 requires cellular ATP, a functional cytoskeleton, and protein-tyrosine kinase activity.13 15 Thus, these results suggest that cap formation of CD34 or CD43 increases tyrosine phosphorylation of Syk and pp77 through Lyn, pp60, and pp69 during induction of cytoadhesion.
(A) Inhibition of antibody-induced cap formation. (A) Distribution of CD34 or CD43 on KG1a cells stimulated for 30 minutes with anti-CD34 (4A1, a through l) or anti-CD43 (MEM59, m through x) in the presence of medium alone (a through d, m through p), Cyt.D (e through h, q through t), or NaF (i through l, u through x) as described in Materials and Methods. After fixation, cells were double-stained either with anti-CD34 (green channel: b, f, and j) and phalloidin (red channel: c, g, and k) or with anti-CD43 (green channel: n, r, and v) and phalloidin (red channel: o, s, and w), and then analyzed by Nomarski differential-interference-contrast (left-hand panels) and confocal immunofluorescence microscopy. The right-hand panels show superimposed images of green and red channels. Bars, 10 μm. (B) Anti-pTyr Western blot of equal amounts of Triton X-100 lysates from KG1a cells stimulated with anti-CD34 (lanes 1 through 3) or anti-CD43 (lanes 4 through 6) in the presence of medium alone (lanes 1 and 4), 10 μg/mL cytochalasin D (Cyt.D) (lanes 2 and 5), or 30 mmol/L NaF (lanes 3 and 6) as described above. Molecular size markers are indicated on the left (kD). (C) Homotypic cytoadhesion of KG1a cells stimulated for 90 minutes with anti-CD34 (a through c), anti-CD43 (d through f), or control antibody (g through i) in the presence of medium alone (a, d, and g), 10 μg/mL Cyt.D (b, e, and h), or 30 mmol/L NaF (c, f, and i). Bars, 80 μm.
(A) Inhibition of antibody-induced cap formation. (A) Distribution of CD34 or CD43 on KG1a cells stimulated for 30 minutes with anti-CD34 (4A1, a through l) or anti-CD43 (MEM59, m through x) in the presence of medium alone (a through d, m through p), Cyt.D (e through h, q through t), or NaF (i through l, u through x) as described in Materials and Methods. After fixation, cells were double-stained either with anti-CD34 (green channel: b, f, and j) and phalloidin (red channel: c, g, and k) or with anti-CD43 (green channel: n, r, and v) and phalloidin (red channel: o, s, and w), and then analyzed by Nomarski differential-interference-contrast (left-hand panels) and confocal immunofluorescence microscopy. The right-hand panels show superimposed images of green and red channels. Bars, 10 μm. (B) Anti-pTyr Western blot of equal amounts of Triton X-100 lysates from KG1a cells stimulated with anti-CD34 (lanes 1 through 3) or anti-CD43 (lanes 4 through 6) in the presence of medium alone (lanes 1 and 4), 10 μg/mL cytochalasin D (Cyt.D) (lanes 2 and 5), or 30 mmol/L NaF (lanes 3 and 6) as described above. Molecular size markers are indicated on the left (kD). (C) Homotypic cytoadhesion of KG1a cells stimulated for 90 minutes with anti-CD34 (a through c), anti-CD43 (d through f), or control antibody (g through i) in the presence of medium alone (a, d, and g), 10 μg/mL Cyt.D (b, e, and h), or 30 mmol/L NaF (c, f, and i). Bars, 80 μm.
DISCUSSION
In this study, we provide evidence that the sialomucins CD34 and CD43 can activate a common signaling pathway through tyrosine phosphorylation in cytoadhesion. First, a stable cap/cluster of CD34 or CD43, which colocalizes with reorganized F-actin, is rapidly formed after antibody-mediated crosslinking of the respective molecules. Second, formation of caps triggers tyrosine phosphorylation of cellular proteins at each capping site. Third, tyrosine phosphorylation of the same set of proteins, such as Lyn, Syk, and the novel proteins pp60, pp69, and pp77, is specifically induced by crosslinking of CD34 or CD43. Fourth, blockade of cap formation by treatment with Cyt.D leads to inhibition of tyrosine phosphorylation of Syk and pp77 and homotypic cytoadhesion.
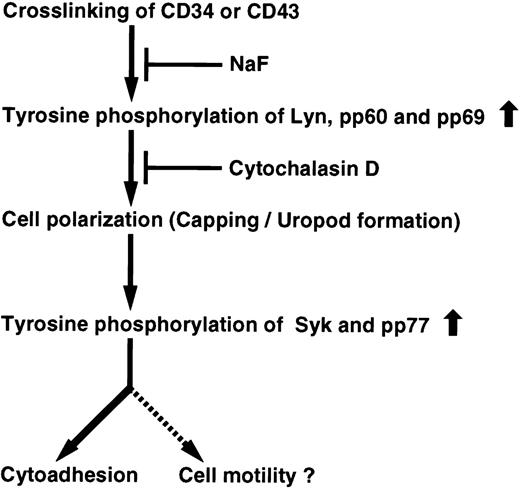
In Fig 12, we present a model to account for these findings. Crosslinking of CD34 or CD43 with the appropriate antibody triggers an increase in tyrosine phosphorylation of Lyn, pp60, and pp69, thereby leading to cell polarization, such as capping, clustering, and uropod formation. Subsequently, cell polarization initiates another increase in tyrosine phosphorylation of Syk and pp77 for homotypic cell aggregation. Although NaF inhibits the signaling pathway from the initial phase, Cyt.D blocks an increase in tyrosine phosphorylation induced by cell polarization, resulting in inhibition of homotypic cell aggregation. Thus, we propose that cell polarization characterized as cap formation is crucial for the signal transduction pathway in CD34- or CD43-mediated cytoadhesion.
Proposed pathway generated from CD34 and CD43. Crosslinking of either CD34 or CD43 leads to tyrosine phosphorylation and cell polarization (capping/uropod formation) for cytoadhesion and perhaps cell motility. NaF affects tyrosine phosphorylation of Lyn, pp60, pp69, Syk, and pp77 from the initial phase of stimulation, resulting in inhibition of cell polarization and cytoadhesion. Cyt.D suppresses not only tyrosine phosphorylation of Syk and pp77, but also cytoadhesion by inhibiting cell polarization through disruption of the actin cytoskeleton. Both CD34 and CD43 trigger activation of a common cascade of tyrosine phosphorylation signaling.
Proposed pathway generated from CD34 and CD43. Crosslinking of either CD34 or CD43 leads to tyrosine phosphorylation and cell polarization (capping/uropod formation) for cytoadhesion and perhaps cell motility. NaF affects tyrosine phosphorylation of Lyn, pp60, pp69, Syk, and pp77 from the initial phase of stimulation, resulting in inhibition of cell polarization and cytoadhesion. Cyt.D suppresses not only tyrosine phosphorylation of Syk and pp77, but also cytoadhesion by inhibiting cell polarization through disruption of the actin cytoskeleton. Both CD34 and CD43 trigger activation of a common cascade of tyrosine phosphorylation signaling.
In accordance with the previous findings,13 we showed that only class I and class II MoAbs directed against CD34, but not class III MoAbs, induce homotypic cytoadhesion of KG1a cells (Fig 11C, data not shown), and we found that those antibodies also induce homotypic cytoadhesion of U937 cells ectopically expressing CD34fullor CD34trunc (data not shown). Similarly, crosslinking of CD43 on KG1a cells with anti-CD43, which corresponds to class I anti-CD34 MoAbs, induces homotypic cytoadhesion (Fig 11C). The stimulatory MoAbs may act as surrogate ligands mimicking the binding of natural ligands or counter-receptors by interacting with glycosylated regions of CD34 or CD43, as suggested.13 15
It is worth noting that addition of greater than 0.1 mmol/L Na3VO4, an inhibitor of tyrosine phosphatases, to cell suspensions throughout stimulation periods is required to detect an increase in tyrosine phosphorylation of cellular proteins (Figs 1 through 3, 7, and 9 through 11), suggesting that the increase in tyrosine phosphorylation seen following crosslinking may be rapidly downregulated by dephosphorylation. The level of increase in tyrosine phosphorylation follows the extent of cap formation, indicating that capping triggers tyrosine phosphorylation.
Like CD34full, the alternatively spliced CD34trunc having 16 amino acid residues within the cytoplasmic region is capable of increasing tyrosine phosphorylation of the same set of proteins (Fig 2). Delay in myeloid differentiation of embryonic stem cells with CD34 null mutation was efficiently rescued by overexpression of either CD34trunc or CD34full.39 CD34 has one potential phosphorylation site for tyrosine kinases in the cytoplasmic region (Fig 2A). However, crosslinking of CD34 did not induce tyrosine phosphorylation of CD34 per se (data not shown). In addition, because CD34trunc lacks consensus phosphorylation sites for protein kinase C, phosphorylation by protein kinase C40 is unlikely to be involved in the CD34-mediated signaling. Furthermore, the truncated CD34 mutant having only one amino acid residue within the cytoplasmic region did not trigger CD34 MoAb-induced homotypic cell aggregation.15 These results indicate that the short cytoplasmic tail of CD34trunc, but not possible phosphorylation sites found in the cytoplasmic region of CD34full, is required for crosslinking-dependent signal transduction.
Among various MoAbs against cell-surface antigens that we tested, only anti-CD34 and anti-CD43 can induce an increase in tyrosine phosphorylation of the same set of proteins (Figs 3 and 7). These observations indicate that CD34 and CD43 share some overlapping functions. Although CD34, a member of the sialomucin family, has no significant sequence homology to known proteins, the heavily O-glycosylated extracellular region of CD34 is similar to that of CD43, which has an extended rodlike structure.7,12,19,24Stimulation with anti-CD34 MoAb triggers cell adhesion of CD34-expressing hematopoietic cells.13-15 In addition, stimulation with anti-CD43 MoAb is reported to induce capping of CD43 and activation of cell-type specific functions in different hematopoietic cells, such as T lymphocytes, neutrophils, and monocytes. These functions include cell polarization, cell adhesion, proliferation, costimulation, Ca2+ mobilization, factor production, and apoptosis.20,41-48 Furthermore, there is increasing evidence that CD43 regulates tyrosine phosphorylation of cellular proteins in T-lymphocyte activation, although neither CD43 nor CD34 possesses any tyrosine kinase structures.20,21,49 50
Given that CD34 and CD43 share some amino acid sequence identity clusters in the transmembrane domain and the cytoplasmic region near the transmembrane domain, it is possible that a common signal transducer could associate or interact with both CD34 and CD43 and initiate F-actin reorganization, thereby activating tyrosine phosphorylation. Crosslinking of CD34 or CD43 slightly increased autophosphorylation of Lyn (Figs 1 through 3, 7, and 9 through 11) and led to localization of Lyn to the capping site (data not shown). However, an increase in Lyn kinase activity was barely detected in in vitro kinase assays, and basal activity of Lyn kinase was detected at high levels (data not shown). We hypothesize that activation of a tyrosine kinase(s) takes place through a common signal transducer at the capping site after F-actin reorganization, and the resulting tyrosine-phosphorylated protein pp77 together with pp69 are recruited to Lyn to transmit the signals downstream. Alternatively, high levels of basal Lyn kinase activity might be involved in complex formation of pp69 and pp77 with Lyn after stimulation. In addition, another possibility is that activation of a tyrosine kinase(s) may lead to tyrosine phosphorylation of unphosphorylated pp69 and pp77 that already appear to be associated with Lyn.
Moreover, while crosslinking of CD34 or CD43 increased tyrosine phosphorylation of Syk, an increase in Syk kinase activity was not detected (Fig 9B and C). Rather, we found an increase in tyrosine phosphorylation of pp37 associated with tyrosine-phosphorylated Syk localized to the membrane (Figs 9B and 10B). These data suggest that tyrosine phosphorylation of Syk creates docking sites for proteins that possess the Src homologous 2 (SH2) domains, as suggested recently.51 52 Alternatively, it is also possible that cytoplasmic Syk is associated with unphosphorylated pp37. After stimulation, complexes with tyrosine-phosphorylated Syk and pp37 may be anchored to membranes. However, the latter possibility is unfavorable because cytoplasmic Syk is not translocated to the membrane (Fig 10A). Therefore, it is intriguing to assume that crosslinking either CD34 or CD43 promotes cooperation between Syk and Lyn, through an as yet unknown mechanism. Identification of pp37, pp69, and pp77 will help us more fully understand signaling initiated by activation of CD34 and CD43.
For several years there has been high interest in capping of cell-surface receptors.53,54 It should be emphasized that caps of CD34 or CD43 are quite stable and continue to be present on the cell surface for at least 2 days after stimulation, whereas other cell-surface molecules such as B-cell antigen receptors, T-cell antigen receptors, low-density lipoprotein receptors, transferrin receptors, and integrins are rapidly internalized/endocytosed from the cell surface for degradation or recycling upon stimulation. Thus, CD34 and CD43 caps are envisaged as unique features toward signal transduction. Recently, cell polarization and clustering of CD43 to a cellular uropod have been seen in T lymphocytes stimulated with anti-CD43.47 Similar clustering of CD43 on neutrophils was observed during neutrophil chemotaxis after stimulation with either chemokine peptides alone or with anti-CD43 antibodies.48CD43 expressed on T lymphocytes was associated with the ERM actin-binding proteins ezrin and moesin at the capping site after stimulation for cell aggregation and migration.55 These observations suggest that cell polarization leads to cytoadhesion and cell motility.
Although the physiological function of CD34 is still unclear, perhaps cell polarization via clustering of CD34, like CD43, plays a role in cell motility. One could therefore add a pathway for cell motility in Fig 12. Considering our findings that CD34 and CD43 can trigger tyrosine phosphorylation through cap formation, we imagine that cell polarization induced by capping of CD34 or CD43 leads to close localization of tyrosine-phosphorylated proteins including Lyn and Syk with CD34 or CD43, and actively participates in signal transduction generated from the capping sites for cytoadhesion and cell motility.
Finally, CD34 and CD43 are expressed on hematopoietic stem cells.23,25,26 KG1a cells, which express both CD34 and CD43, have many characteristics of immature hematopoietic cells.3 27 Like KG1a cells, CD13+CD34+ CD43+ peripheral mononuclear cells from a myelogenous leukemic patient exhibited cap formation of CD34 or CD43 after stimulation with respective antibodies (data not shown). Intriguingly, normal CD34+ CD43+ cells from normal human BM cells became polarized after crosslinking of CD34 or CD43 (Fig 6). Therefore, it will be necessary to investigate the physiological roles of CD34 and CD43 on immature hematopoietic cells in BM stem cell niches.
ACKNOWLEDGMENT
We are grateful to Drs B. Seed and D.L. Simmons (Massachusetts General Hospital, Boston, MA) for generously providing the human intact CD34 cDNA, Drs H. Nakauchi and Y. Nakamura (Institute of Basic Medical Sciences, The University of Tsukuba, Tsukuba, Japan) for the human truncated CD34 cDNA, Dr M. Nakamura (Nichirei, Tokyo, Japan) for 4A1 MoAb, and Dr T. Sudo (Basic Research Laboratories, Toray Industries, Kamakura, Japan) and Dr A. Kume (Jichi Medical School, Tochigi, Japan) for help in constructing the plasmids. We also thank Dr Y. Sakai (Showa University College of Medical Sciences, Yokohama, Japan) for the use of his confocal microscope in several experiments.
Supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Naoto Yamaguchi, PhD, Department of Cell Differentiation, Institute of Molecular Embryology and Genetics, Kumamoto University School of Medicine, Honjo 2-2-1, Kumamoto 860-0811, Japan; e-mail: bunseini@kaiju.medic.kumamoto-u.ac.jp.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal