Abstract
We have analyzed factors influencing the outcome of 102 children with acute leukemia given a cord blood transplantation (CBT) and reported to the Eurocord Registry. Seventy patients with acute lymphoblastic and 32 with acute myeloid leukemia were given either a related (n = 42) or an unrelated (n = 60) CBT. Children given CBT during first or second complete remission were considered as belonging to the good-risk group (n = 66), whereas those who received a transplant in a more advanced stage of disease were assigned to the poor-risk group (n = 36). In the related group (RCBT), 12 of 42 patients received transplantation from an HLA-disparate donor, whereas in the unrelated group (UCBT) 54 of 60 received an HLA mismatched CBT. Kaplan-Meier estimates for neutrophil recovery at day 60 were 84% ± 7% in RCBT and 79 ± 6% in UCBT (P = .16). In multivariate analysis, the most important factor influencing neutrophil engraftment in UCBT was a nucleated cell dose infused greater than 3.7 × 107/kg (P = .05, relative risk [RR] of 1.85, 95% confidence interval [CI]: 0.98-3.4). The incidence of grade II through IV acute graft-versus-host disease was 41% ± 8% in the RCBT group and 37% ± 6% in the UCBT group (P = .59). Kaplan-Meier estimates of 2-year event-free survival (EFS) after RCBT or UCBT were 39% ± 8% and 30% ± 7%, respectively (P = .19). In multivariate analysis, the most important factor influencing EFS was disease status at time of transplantation: good-risk patients had a 2-year EFS of 49% ± 7% as compared to 8% ± 5% in patients with more advanced disease (P = .0003, RR: 0.40, 95% CI: 0.24 to 0.65). This was a consequence of both an increased 1-year transplant related mortality and a higher 2-year relapse rate in the poor-risk group (65% ± 9% and 77% ± 14%, respectively), as compared with good risk patients (34% ± 6% and 31% ± 9%, respectively). These data confirm that allogeneic CBT from either a related or an unrelated donor is a feasible procedure able to cure a significant proportion of children with acute leukemia, especially if transplanted in a favorable phase of disease.
OVER THE PAST decade, allogeneic cord blood transplantation (CBT) from both related and unrelated donors has been used increasingly for the treatment of pediatric patients with malignant disorders.1-5 In particular, the creation of cord blood banks worldwide has greatly facilitated the possibility of identifying suitable unrelated donors.5 In children with acute leukemia, peculiar advantages of CBT from unrelated individuals are both a prompt availability of cryopreserved hematopoietic stem cells and less stringent requirements of HLA-identity between donor and recipient because of the low risk of graft-versus-host disease (GVHD) observed after CBT.2-5
However, the reported low incidence of GVHD after CBT1-5might also be a major drawback in leukemic patients. In fact, because the role of allogeneic lymphocytes in the control and/or eradication of malignancy is well established,6 the absence or reduction of the component of graft-versus-leukemia (GVL) activity associated with GVHD could represent a theoretical concern in leukemic subjects given CBT.
Previously published reports enrolling a limited number of pediatric leukemia patients given CBT,1-4 or not specifically analyzing the outcome of children with hematological malignancies,5 did not provide clinical data allowing to conclusively establish whether cord blood has a GVL effect comparable with that of other stem cell transplants. This Eurocord report describes the results obtained in 102 pediatric patients with acute leukemia who received either a related or an unrelated transplantation of cord blood hematopoietic stem cells. In particular, we have specifically analyzed disease-, patient-, and transplant-related factors that have affected relapse, nonleukemia death, and leukemia-free survival of related and unrelated CBT recipients.
PATIENTS AND METHODS
Between April 1990 and December 1997, 102 children (58 boys and 44 girls) with acute leukemia were given an allogeneic CBT from either a relative (42 patients) or an unrelated donor (60 patients), and were reported to the Eurocord Registry. Information on these patients was collected through a form recording data addressing questions about the disease, the origin of cord blood, the number of cells collected and infused, HLA typing, and transplant outcome. Transplants were performed in 41 different centers, which reported from 1 to 12 cases (see). Centers reported on the outcome of transplantation and any complication 3 months after CBT and then at periodic (ie, between 3 and 6 months) intervals during follow-up.
Seventy children had acute lymphoblastic leukemia (ALL) and 32 had acute myeloid leukemia (AML). Median time interval between diagnosis and CBT was 19 months (range, 2.5 to 106 months). Because in patients with acute leukemia, disease phase has been reported to be the main factor influencing transplant outcome,7-10 children receiving transplantation during a first or second complete remission (CR) were considered as belonging to the good risk group (n = 66). By contrast, patients in third or subsequent remission, relapse, or partial remission or with refractory leukemia at time of CBT, were considered in advanced phase and belonging to the poor risk group (n = 36). Further details on pretransplant characteristics of patients belonging to the good and poor risk groups are reported in Table 1.
Patients’ Characteristics According to Status at Transplant
| Characteristics . | Poor Risk (N = 36) (≥3 CR, relapse and refractory) . | Good Risk (N = 66) (1 CR and 2 CR) . | P* . |
|---|---|---|---|
| Median age (yr) (range) | 6.3 (0.3-14) | 5.0 (0.5-15) | .08 |
| Median weight (kg) (range) | 21 (4.4-83) | 19 (6.5-67) | .16 |
| <20/≥20 | 14/22 | 35/31 | |
| Gender (male/female) | 23/13 | 35/31 | .30 |
| Type of donor | |||
| Related | 18 | 24 | .21 |
| Unrelated | 18 | 42 | .38 |
| ALL/AML | 27/9† | 43/23‡ | |
| Disease status at transplant | |||
| First CR | — | 18 | <.001 |
| Second CR | — | 48 | |
| ≥Third CR | 15 | — | |
| First relapse | 4 | — | |
| Second relapse | 10 | — | |
| Partial response | 3 | — | |
| Refractory to chemotherapy | 4 | ||
| Cytogenetics | .65 | ||
| Unfavorable | 8 | 20 | |
| Intermediate1-153 | 23 | 39 | |
| Favorable | 5 | 7 | |
| First relapse | |||
| On therapy | 20 | 27 | .65 |
| Off therapy | 12 | 21 | |
| Median d from diagnosis to first relapse | 506 (105-1,631) | 472 (42-1,725) | .80 |
| Median d from last CR to transplant1-155 | 62 (0-366) | 93 (18-442) | .08 |
| Previous autologous/allogeneic transplants | 11/2 | 5 | <.001 |
| Characteristics . | Poor Risk (N = 36) (≥3 CR, relapse and refractory) . | Good Risk (N = 66) (1 CR and 2 CR) . | P* . |
|---|---|---|---|
| Median age (yr) (range) | 6.3 (0.3-14) | 5.0 (0.5-15) | .08 |
| Median weight (kg) (range) | 21 (4.4-83) | 19 (6.5-67) | .16 |
| <20/≥20 | 14/22 | 35/31 | |
| Gender (male/female) | 23/13 | 35/31 | .30 |
| Type of donor | |||
| Related | 18 | 24 | .21 |
| Unrelated | 18 | 42 | .38 |
| ALL/AML | 27/9† | 43/23‡ | |
| Disease status at transplant | |||
| First CR | — | 18 | <.001 |
| Second CR | — | 48 | |
| ≥Third CR | 15 | — | |
| First relapse | 4 | — | |
| Second relapse | 10 | — | |
| Partial response | 3 | — | |
| Refractory to chemotherapy | 4 | ||
| Cytogenetics | .65 | ||
| Unfavorable | 8 | 20 | |
| Intermediate1-153 | 23 | 39 | |
| Favorable | 5 | 7 | |
| First relapse | |||
| On therapy | 20 | 27 | .65 |
| Off therapy | 12 | 21 | |
| Median d from diagnosis to first relapse | 506 (105-1,631) | 472 (42-1,725) | .80 |
| Median d from last CR to transplant1-155 | 62 (0-366) | 93 (18-442) | .08 |
| Previous autologous/allogeneic transplants | 11/2 | 5 | <.001 |
Fisher’s test was used for categorical variables and Kruskal-Wallis Test for noncategorical variables.
One patient with AML associated to Down syndrome.
One patient with AML associated to Down Syndrome and another with congenital AML.
Including normal and not available cytogenetics.
Only for patients in CR at time of transplant.
Karyotype analysis at diagnosis was available in 84 of the 102 children. Thirty-two patients had a normal karyotype, whereas clonal abnor- malities were present in the remaining 52 patients. For the purpose of this study, ALL patients with hyperdiploid karyotype and AML patients with inversion of chromosome 16, translocation 8;21 and 15;17, were classified as having good prognosis (12 cases). Chromosomal abnormalities classified as poor prognosis features (28 cases) included ALL with translocation 9;22 and 4;11, as well as AML with monosomy of chromosomes 5, 7, anomalies of 11q, or translocation 6;9. Patients with normal karyotype or other cytogenetic abnormalities, as well as those with unavailable cytogenetic data, were assigned to an intermediate-risk category (62 cases, Table 1). Notably, eight patients given a related and eight patients given an unrelated CBT had previously received an autologous bone marrow transplantation (BMT). Moreover, two children who had been treated with an allogeneic BMT from an unrelated volunteer and had relapsed were given an unrelated CBT.
Table 2 shows characteristics of patients according to the type of donor (related or unrelated). Median age at transplantation of the overall cohort was 5.5 years (range, 0.3 to 15), whereas median body weight was 20 kg (range 4.4 to 83). Cord blood units for unrelated transplants were provided by the New York (23 cases), Milan (18 cases), Dusseldorf (15 cases), Paris (2 cases), and Brussels (2 cases) banks, whereas in the majority of related CBT, cord blood was collected by local banks.
Patients’ Characteristics According to the Cord Blood Donor
| Characteristics . | Related (N = 42) . | Unrelated (N = 60) . | P† . |
|---|---|---|---|
| Median age (yr) range | 5.5 (1.7-14) | 5.5 (0.2-15) | |
| <6/≥6 | 24/18 | 36/24 | .64 |
| Median weight (kg) range | 19.5 (10-46) | 20.0 (4.4-83) | |
| <20/≥20 | 21/21 | 28/32 | .63 |
| Gender | |||
| Male | 25 | 33 | .70 |
| Female | 17 | 27 | |
| Diagnosis and status | |||
| ALL | 30 | 40 | .67 |
| Poor risk | 15 | 12 | |
| Good risk | 15 | 28 | .14 |
| AML | 12 | 20 | |
| Poor risk | 3 | 6 | |
| Good risk | 9 | 14 | 1.0 |
| CMV serology | |||
| Negative | 20 | 25 | .69 |
| Positive | 22 | 34 | |
| ABO compatibility | |||
| Matched | 31 | 27 | .005 |
| Mismatched | 11 | 33 | |
| HLA disparities* | |||
| Identical | 30 | 6 | .0006 |
| 1 difference | 1 | 27 | |
| 2 differences | 3 | 22 | |
| 3 differences | 7 | 4 | |
| 4 differences | 1 | 1 | |
| Median no. of nucleated cells collected (107)/kg of recipient (range) | 4.0 (1.0-12.2) | 5.0 (1.5-46.5) | .04 |
| Median no. of nucleated cells infused (107)/kg of recipient (range) | 3.2 (0.7-10) | 4.4 (0.9-36) | .30 |
| Conditioning | |||
| TBI-containing regimens | 22 | 34 | .69 |
| Chemotherapy-based regimens | 20 | 26 | |
| ALG/ATG or monoclonal antibody | 10 | 50 | <.0001 |
| GVHD prophylaxis | |||
| CsA | 20 | 5 | <.0001 |
| CsA + steroid | 3 | 41 | |
| CsA + MTX | 13 | 5 | |
| CsA + MTX + steroid | 2 | 3 | |
| CsA ± steroid ± ATG/ALG or Mab | 4 | 4 | |
| FK506 + MTX | — | 2 |
| Characteristics . | Related (N = 42) . | Unrelated (N = 60) . | P† . |
|---|---|---|---|
| Median age (yr) range | 5.5 (1.7-14) | 5.5 (0.2-15) | |
| <6/≥6 | 24/18 | 36/24 | .64 |
| Median weight (kg) range | 19.5 (10-46) | 20.0 (4.4-83) | |
| <20/≥20 | 21/21 | 28/32 | .63 |
| Gender | |||
| Male | 25 | 33 | .70 |
| Female | 17 | 27 | |
| Diagnosis and status | |||
| ALL | 30 | 40 | .67 |
| Poor risk | 15 | 12 | |
| Good risk | 15 | 28 | .14 |
| AML | 12 | 20 | |
| Poor risk | 3 | 6 | |
| Good risk | 9 | 14 | 1.0 |
| CMV serology | |||
| Negative | 20 | 25 | .69 |
| Positive | 22 | 34 | |
| ABO compatibility | |||
| Matched | 31 | 27 | .005 |
| Mismatched | 11 | 33 | |
| HLA disparities* | |||
| Identical | 30 | 6 | .0006 |
| 1 difference | 1 | 27 | |
| 2 differences | 3 | 22 | |
| 3 differences | 7 | 4 | |
| 4 differences | 1 | 1 | |
| Median no. of nucleated cells collected (107)/kg of recipient (range) | 4.0 (1.0-12.2) | 5.0 (1.5-46.5) | .04 |
| Median no. of nucleated cells infused (107)/kg of recipient (range) | 3.2 (0.7-10) | 4.4 (0.9-36) | .30 |
| Conditioning | |||
| TBI-containing regimens | 22 | 34 | .69 |
| Chemotherapy-based regimens | 20 | 26 | |
| ALG/ATG or monoclonal antibody | 10 | 50 | <.0001 |
| GVHD prophylaxis | |||
| CsA | 20 | 5 | <.0001 |
| CsA + steroid | 3 | 41 | |
| CsA + MTX | 13 | 5 | |
| CsA + MTX + steroid | 2 | 3 | |
| CsA ± steroid ± ATG/ALG or Mab | 4 | 4 | |
| FK506 + MTX | — | 2 |
HLA disparities were defined by using serological and low resolution typing for A, B locus, and DRB1 in related CBT and high resolution DRB1 typing for unrelated CBT.
Fisher’s test was used for categorical variables and Kruskal-Wallis test for noncategorical variables.
The methods of collecting, cryopreserving, and storing cord blood varied among centers. Usually, cord blood progenitors were thawed and washed following the procedure described by Rubinstein et al.11 The median number of collected and infused nucleated cells is reported in Table 2. The median number of CD34+cells infused was 1.85 × 105/kg (range, 0.1 to 78 × 105/kg). However, because the number of CD34+ cells infused was available in only 52 cases and the method of counting these cells varied according to the centers, this variable was not included in the analysis.
HLA Compatibility and GVHD Prophylaxis
HLA class I antigen serological typing of recipients and cord blood units was performed by standard NIH microlymphocytotoxicity, whereas low-resolution generic oligotyping was used for the DRB1 antigens in all donor/recipient pairs. High-resolution molecular typing of HLA-class II DRB1 was available for all children given CBT from an unrelated donor. Accuracy of the data was confirmed for each sample by the HLA typing laboratories including the confirmatory typing of donor and recipient pairs before transplant. Thirty of the 42 patients given a related CBT were transplanted from an HLA-identical sibling, whereas in the remaining 12 cases, there was a disparity for one or more HLA-antigens (Table 2). In the 60 patients given an unrelated CBT, the donor was HLA-identical to the recipient in only 6 cases (see Table 2for further details).
In patients transplanted from a family donor, GVHD prophylaxis mainly consisted of cyclosporine (CsA) alone (20 cases) or of the combination of CsA and methotrexate (13 cases), this latter scheme being used especially when the donor was HLA-disparate. By contrast, in the 60 children given an unrelated CBT the most commonly used GVHD prophylaxis was the combination of CsA and steroids (41 cases). Further details on GVHD prophylaxis are reported in Table 2. Established GVHD was usually treated with steroids, according to each center policy.
Preparative Regimen, Cytomegalovirus Serology, and Post-Transplant Use of Hematopoietic Growth Factors
Conditioning regimen varied according to the center policy, type of leukemia, previous treatments, and disease status at time of transplantation. Details on the preparative regimens, as well as on the use of anti–T-lymphocytes polyclonal or monoclonal antibodies are reported in Table 2.
Cytomegalovirus serological status was studied before transplantation in all children but one. In detail, 20 of the 42 related CBT recipients studied were seronegative, as well as 25 of the 59 patients given an unrelated transplant.
Supportive therapy, as well as prophylaxis and treatment of infections, varied among centers.
Recombinant human granulocyte colony-stimulating factor or recombinant human granulocyte-macrophage colony-stimulating factor was given to 28 of the 42 patients receiving a related CBT and to 48 of the 60 children transplanted from an unrelated donor. In 50 of the 76 patients receiving hematopoietic growth factors, drug inception started in the first week after transplantation.
Endpoints
Engraftment.
Hematopoietic and lymphoid engraftment was documented by chromosome analysis of marrow cells and peripheral blood lymphocytes, red blood cell antigen phenotyping, and by study of genetic polymorphism of variable number of tandemly repeated short DNA sequences. White blood cell (WBC) engraftment was defined as the first of 3 consecutive days when the absolute neutrophil count was ≥0.5 × 109/L with evidence of donor hematopoiesis, and platelet engraftment as the time to reach a sustained platelet count ≥20 × 109/L in the absence of platelet transfusions for 7 consecutive days. Patients in whom no engraftment occurred were censored if the patient died before day 60. Patients receiving a second transplant for nonengraftment were censored at the time of the second transplant. Neutrophil recovery that occurred after day 60 was considered as nonengraftment.
Acute and chronic GVHD.
Children were considered at risk, starting on day 1, for the occurrence of acute GVHD, whereas only those with sustained engraftment of donor hematopoiesis and surviving for more than 100 days after transplant were evaluated for the development of chronic GVHD. Acute and chronic GVHD were evaluated according to previously described criteria.12 13 Grades ≥ II were counted as acute GVHD.
Relapse.
Relapse was indicated by morphological evidence of leukemia in bone marrow, cerebrospinal fluid or peripheral blood or by cytogenetic recurrence of a neoplastic clone and it was considered as time interval between CBT and relapse, with censoring at death in complete remission.
Transplant-related mortality (TRM).
TRM was defined as all causes of nonleukemic deaths before 1 year after transplant.
Overall survival.
Overall survival was the time between transplantation and death due to any cause.
Event-free survival (EFS).
EFS was defined as time interval from CBT to first event (either relapse or death in complete remission).
Data Analysis and Presentation
Results of the study were analyzed as of February 1, 1998. The median duration of follow-up was 34 months for children transplanted from a family-donor (range, 3 to 92) and 14 months for patients given an unrelated CBT (range, 2 to 43). No patients were lost to follow-up on February 1, 1998, the day at which all centers verified data on transplant characteristics and patient outcome.
Differences were compared using the Fisher test for categorical and Kruskal-Wallis test for continuous variables. Overall survival, relapse, EFS, and other related curves after CBT (starting point interval) were evaluated by Kaplan-Meier product limit method and compared using log-rank test. All variables found to have a Pvalue of less than .5 by log-rank test were included as binary covariates in a Cox proportional-hazards model, with the use of a backward procedure with a type I error of 0.05. Relative risks (RR) for the association between covariates and events were estimated from the Cox’s model. SAS software (SAS Institute, Cary NC) was used.
RESULTS
Neutrophil and Platelet Engraftment
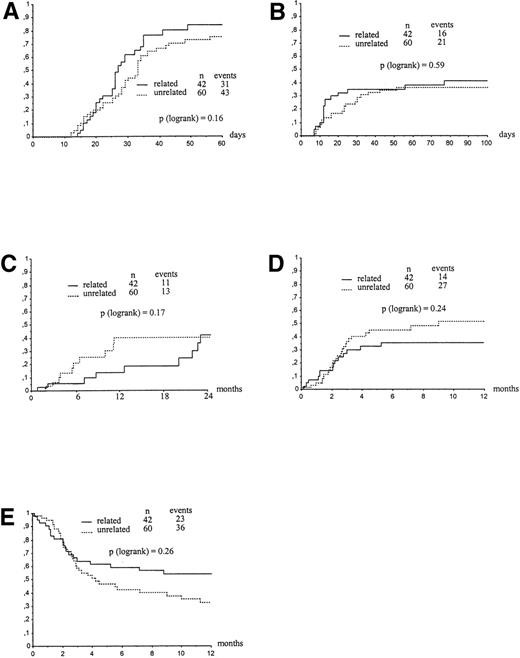
Graft failure occurred in 6 (16%) and 14 (22%) patients given related or unrelated CBT, respectively. In detail, 9 patients received a second transplant for persisting cytopenia, 5 did not have neutrophil recovery on day +60, 2 experienced autologous hematopoietic reconstitution, and 4 experienced regrowth of leukemia cells. Only two patients given an unrelated transplant had hematopoietic recovery of donor origin after day +60. Figure 1A shows the Kaplan-Meier estimates for neutrophil recovery of patients transplanted either from a relative or an unrelated donor, by day 60 after transplantation (84%v 79%, respectively, P = .16). The median time to neutrophil recovery for related and unrelated CBT recipients were 27 (range, 14 to 49) and 33 days (range, 12 to 56), respectively. In univariate analysis, the main factors favorably affecting myeloid recovery in children transplanted from a family donor were an age younger than 6 years (P = .04), a body weight lower than 20 kg (P = .006), a non-methotrexate containing GVHD prophylaxis (P = .03), and a favorable disease phase at time of CBT (P = .05). In patients transplanted from an unrelated donor, a number of cells infused greater than 3.7 × 107/kg was the only factor predicting for a higher probability of myeloid engraftment (P = .03). Hematopoietic growth factors had no observable effect on time to neutrophil recovery in both related and unrelated CBT recipients (data not shown). In the Cox analysis for the related group, a body weight lower than 20 kg was the only factor predicting for a higher likelihood of neutrophil recovery (P = .008, RR: 0.37, 95% confidence interval [CI]: 0.17 to 0.77), whereas in the unrelated cohort the only significant factor influencing neutrophil engraftment was a higher cell dose (≥3.7 × 107 nucleated cells per kg) (P = .05, RR: 1.85, 95% CI: 0.98 to 3.4).
Kaplan-Meier estimate of neutrophil engraftment (A), acute GVHD (B), relapse (C), TRM (D), and survival (E) of children given a CBT for acute leukemia according to donor type. (A) Neutrophil engraftment at day 60 in children who received transplant with a related and unrelated cord blood. (B) Acute GVHD in children who received transplant with a related and unrelated cord blood. (C) RR in children who received transplant with a related and unrelated cord blood. (D) TRM in children who received transplant with a related and unrelated cord blood. (E) Overall survival in children who received transplant with a related and unrelated cord blood.
Kaplan-Meier estimate of neutrophil engraftment (A), acute GVHD (B), relapse (C), TRM (D), and survival (E) of children given a CBT for acute leukemia according to donor type. (A) Neutrophil engraftment at day 60 in children who received transplant with a related and unrelated cord blood. (B) Acute GVHD in children who received transplant with a related and unrelated cord blood. (C) RR in children who received transplant with a related and unrelated cord blood. (D) TRM in children who received transplant with a related and unrelated cord blood. (E) Overall survival in children who received transplant with a related and unrelated cord blood.
In children given CBT from a related or an unrelated donor, the median time to reach a platelet count of at least 20 × 109/L was 56 (range, 17 to 180) and 85 days (range, 16 to 159), respectively. The probability of platelet recovery by day +180 after transplantation in patients given either a related or an unrelated CBT was 85% and 78%, respectively (P = .19). From the Cox model, we found that the most favorable factor for platelet recovery in children transplanted from a relative was age (<6 years) (P = .009, RR: 0.29, 95% CI: 0.11 to 0.73). In patients receiving an unrelated transplant, the most important factor was a higher number of cells infused (≥3.7 × 107 nucleated cells per kg) (P = .03, RR: 2.35, 95% CI: 1.07 to 5.14).
GVHD
Kaplan-Meier estimate of developing grade II-IV acute GVHD was comparable in patients given CBT from either a relative or an unrelated donor (41% v 37%, respectively, P = .59) (Fig 1B). There was also no difference in the probability of developing grade III-IV acute GVHD between the two groups (16 v 23%, respectively, P = .89). In the related group, 10 patients had grade II, 4 grade III, and 2 patients grade IV. In the unrelated group 8 patients had grade II, 7 patients grade III and six patients grade IV.
In univariate analysis, HLA disparity between donor and recipient was significantly associated with an increased probability of developing acute GVHD among patients given CBT from a family donor, the estimated incidence being 26% in recipients of HLA-identical cord blood and 75% in children given a mismatched transplant (P = .001). A number of cells infused lower than 3.7 × 107/kg was also associated with an increased risk of grade II-IV acute GVHD in unrelated CBT (P = .004). In unrelated CBT, the incidence of acute GVHD was not significantly affected by the number of HLA-mismatches: the incidence of acute GVHD ≥ grade II was 4 of 6 patients receiving an HLA identical (67%), 6 of 27 with one HLA disparity (24%), 10 of 22 (48%) with two HLA disparities and 1 of 4 (25%) patients with three HLA disparities (P = .08).
From the Cox model, in children given a related CBT, factors associated with acute GVHD were HLA disparity between donor and recipient (P = .0002, RR: 10.4, 95% CI: 3.02 to 36.1) and patient positive cytomegalovirus (CMV) serology before transplant (P =.01, RR: 0.21, 95% CI: 0.06-0.74). In the unrelated CBT group, we could not find any variable, including the number of HLA disparities, which was associated with grade II-IV acute GVHD.
Chronic GVHD affected 2 of 25 recipients of related CBT and 3 of 25 patients given transplantation from an unrelated donor. Two-year Kaplan-Meier estimate of developing chronic GVHD was comparable in patients given CBT from either a relative or an unrelated donor (13v 28%, respectively, P = .86).
As discussed in detail below, GVHD was the primary cause of death in seven children.
Relapse
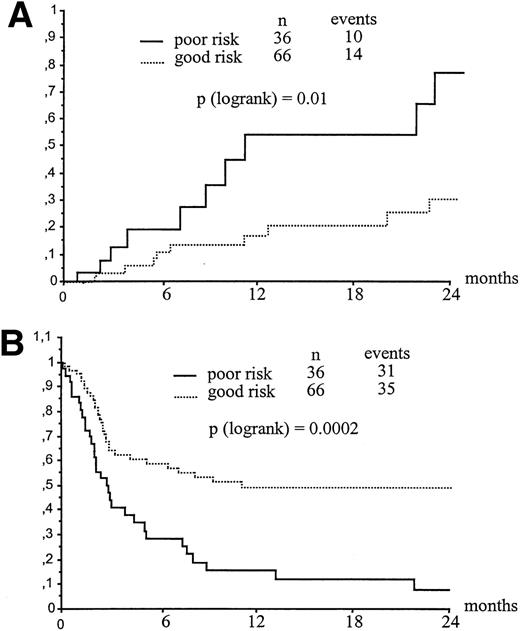
Clinical or cytogenetic relapse was detected after transplantation in 11 and 13 recipients of related or unrelated CBT, respectively. This yielded a 2-year relapse estimate of 42% and 40% for children transplanted from family or unrelated donors, respectively (P = .17) (Fig 1C). Table 3 shows a univariate analysis of factors influencing relapse either in related or in unrelated transplants. Not shown in Table 3, WBC count, blast percentage in blood, organs involved, morphology, immunophenotype at diagnosis, and interval between diagnosis and CBT did not influence 2-year relapse rate, as well as transplant-related mortality (TRM) and EFS. Relapse rate was higher when the transplant was performed in patients with more advanced disease. The 2-year probability of leukemia recurrence for children belonging to the poor- and good-risk group was 77 ± 14% and 31 + 9%, respectively (P = .01) (Fig2A). The impact on relapse rate of disease state at time of transplantation was observed both in patients transplanted from a relative and, even though less evident, in recipients of unrelated CBT (see Table 3). In the overall cohort of patients transplanted beyond first CR, irrespective of the donor type, those experiencing leukemia recurrence during chemotherapy had a higher probability of post-transplant relapse as compared with those who had off-therapy pretransplant relapse (73% v 28%, respectively,P = .03). Children younger than 6 years of age and with a body weight lower than 20 kg had a significantly increased risk of relapse (P = .03), this probably reflecting more aggressive biological characteristics of leukemia in young children. None of the factors usually associated with the prognosis of leukemia, including type of leukemia, karyotype abnormalities, WBC at diagnosis, blasts in blood, organs involved, phenotype. French-American-British morphology, and interval between CR and CBT were associated with an increasing risk of post-transplant relapse (Table 3). In a Cox model for related and unrelated transplants, the most important factors associated with increasing risk of relapse were, respectively, advanced disease at transplant (P = .03, RR: 0.25, 95% CI: 0.07 to 0.90) and weight less than 20 kg (P = .03, RR: 0.10, 95% CI: 0.012 to 0.77).
Univariate Analyses of Factors Associated to TRM, Relapse, EFS for Related and Unrelated CBT
| Factors . | Related n = 42 . | Unrelated n = 60 . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n . | 1 yr TRM . | 2 yr Relapse . | 2 yr EFS . | n . | 1 yr TRM . | 2 yr Relapse . | 2 yr EFS . | |||||||
| % . | P . | % . | P . | % . | P . | % . | P . | % . | P . | % . | P . | |||
| Age | ||||||||||||||
| <2 yr | 2 | 0 | .04 | 0 | .41 | 100 | .17 | 14 | 37 | .59 | 66 | .04 | 24 | .13 |
| 2 to 10 yr | 35 | 31 | 47 | 39 | 36 | 64 | 42 | 22 | ||||||
| ≥10 yr | 5 | 80 | 0 | 20 | 10 | 40 | 0 | 60 | ||||||
| Weight | ||||||||||||||
| <20 kg | 21 | 15 | .008 | 58 | .04 | 40 | .46 | 28 | 43 | .46 | 66 | .006 | 21 | .29 |
| ≥20 kg | 21 | 57 | 5 | 38 | 32 | 59 | 4 | 39 | ||||||
| CMV | ||||||||||||||
| Negative | 20 | 21 | .07 | 29 | .03 | 56 | .02 | 25 | 47 | .37 | 43 | .95 | 33 | .39 |
| Positive | 22 | 48 | 58 | 25 | 34 | 56 | 37 | 28 | ||||||
| ABO | ||||||||||||||
| Matched | 31 | 38 | .63 | 59 | .07 | 28 | .09 | 27 | 48 | .39 | 41 | .42 | 31 | .23 |
| Mismatched | 11 | 28 | 0 | 72 | 33 | 56 | 37 | 29 | ||||||
| Gender | ||||||||||||||
| Matched | 20 | 22 | .06 | 41 | .75 | 48 | .13 | 29 | 49 | .80 | 49 | .54 | 27 | .77 |
| Mismatched | 21 | 49 | 37 | 33 | 29 | 51 | 31 | 35 | ||||||
| Type | ||||||||||||||
| ALL | 30 | 43 | .14 | 42 | .54 | 33 | .34 | 40 | 57 | .29 | 38 | .66 | 28 | .68 |
| AML | 12 | 18 | 39 | 58 | 20 | 42 | 45 | 32 | ||||||
| Karyotype | ||||||||||||||
| Good | 7 | 57 | 33 | 29 | 5 | 47 | 25 | 40 | ||||||
| Intermediate | 24 | 37 | .33 | 44 | .90 | 35 | .62 | 38 | 49 | .79 | 37 | .39 | 34 | .41 |
| Poor | 11 | 18 | 35 | 55 | 17 | 59 | 56 | 18 | ||||||
| Relapse | ||||||||||||||
| On therapy | 17 | 27 | .16 | 67 | .10 | 24 | .72 | 30 | 55 | .23 | 57 | .16 | 22 | .07 |
| Off therapy | 16 | 50 | 17 | 44 | 17 | 44 | 16 | 48 | ||||||
| Time from diagnosis to relapse | ||||||||||||||
| <500 d | 12 | 25 | .28 | 54 | .94 | 36 | .40 | 29 | 54 | .99 | 36 | .91 | 30 | .98 |
| ≥500 d | 21 | 46 | 39 | 32 | 18 | 51 | 40 | 31 | ||||||
| Disease status | ||||||||||||||
| Poor risk | 18 | 58 | .04 | 71 | .02 | 11 | .001 | 18 | 71 | .07 | 75 | .22 | 7 | .02 |
| Good risk | 24 | 21 | 26 | 65 | 42 | 43 | 31 | 40 | ||||||
| Time from last CR to CBT | ||||||||||||||
| <92 d | 11 | 36 | .79 | 0 | .18 | 64 | .49 | 29 | 56 | .74 | 36 | .69 | 28 | .53 |
| ≥92 d | 12 | 32 | 49 | 53 | 19 | 47 | 17 | 44 | ||||||
| HLA3-150 | ||||||||||||||
| Identical | 30 | 21 | 45 | 48 | 6 | 50 | 50 | 25 | ||||||
| 1 difference | 1 | 100 | .01 | 0 | .43 | 0 | .005 | 27 | 34 | .07 | 29 | .81 | 49 | .19 |
| 2 differences | 3 | 33 | 0 | 67 | 22 | 76 | 44 | 14 | ||||||
| 3 differences | 7 | 82 | 100 | 0 | 4 | 25 | 100 | 25 | ||||||
| 4 differences | 1 | 100 | 0 | 0 | 1 | 0 | 0 | 100 | ||||||
| Cell dose | ||||||||||||||
| <3.7 × 107/kg | 23 | 51 | .03 | 15 | .04 | 39 | .60 | 25 | 62 | .21 | 14 | .24 | 33 | .78 |
| ≥3.7 × 107/kg | 19 | 16 | 72 | 39 | 35 | 44 | 54 | 28 | ||||||
| Factors . | Related n = 42 . | Unrelated n = 60 . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n . | 1 yr TRM . | 2 yr Relapse . | 2 yr EFS . | n . | 1 yr TRM . | 2 yr Relapse . | 2 yr EFS . | |||||||
| % . | P . | % . | P . | % . | P . | % . | P . | % . | P . | % . | P . | |||
| Age | ||||||||||||||
| <2 yr | 2 | 0 | .04 | 0 | .41 | 100 | .17 | 14 | 37 | .59 | 66 | .04 | 24 | .13 |
| 2 to 10 yr | 35 | 31 | 47 | 39 | 36 | 64 | 42 | 22 | ||||||
| ≥10 yr | 5 | 80 | 0 | 20 | 10 | 40 | 0 | 60 | ||||||
| Weight | ||||||||||||||
| <20 kg | 21 | 15 | .008 | 58 | .04 | 40 | .46 | 28 | 43 | .46 | 66 | .006 | 21 | .29 |
| ≥20 kg | 21 | 57 | 5 | 38 | 32 | 59 | 4 | 39 | ||||||
| CMV | ||||||||||||||
| Negative | 20 | 21 | .07 | 29 | .03 | 56 | .02 | 25 | 47 | .37 | 43 | .95 | 33 | .39 |
| Positive | 22 | 48 | 58 | 25 | 34 | 56 | 37 | 28 | ||||||
| ABO | ||||||||||||||
| Matched | 31 | 38 | .63 | 59 | .07 | 28 | .09 | 27 | 48 | .39 | 41 | .42 | 31 | .23 |
| Mismatched | 11 | 28 | 0 | 72 | 33 | 56 | 37 | 29 | ||||||
| Gender | ||||||||||||||
| Matched | 20 | 22 | .06 | 41 | .75 | 48 | .13 | 29 | 49 | .80 | 49 | .54 | 27 | .77 |
| Mismatched | 21 | 49 | 37 | 33 | 29 | 51 | 31 | 35 | ||||||
| Type | ||||||||||||||
| ALL | 30 | 43 | .14 | 42 | .54 | 33 | .34 | 40 | 57 | .29 | 38 | .66 | 28 | .68 |
| AML | 12 | 18 | 39 | 58 | 20 | 42 | 45 | 32 | ||||||
| Karyotype | ||||||||||||||
| Good | 7 | 57 | 33 | 29 | 5 | 47 | 25 | 40 | ||||||
| Intermediate | 24 | 37 | .33 | 44 | .90 | 35 | .62 | 38 | 49 | .79 | 37 | .39 | 34 | .41 |
| Poor | 11 | 18 | 35 | 55 | 17 | 59 | 56 | 18 | ||||||
| Relapse | ||||||||||||||
| On therapy | 17 | 27 | .16 | 67 | .10 | 24 | .72 | 30 | 55 | .23 | 57 | .16 | 22 | .07 |
| Off therapy | 16 | 50 | 17 | 44 | 17 | 44 | 16 | 48 | ||||||
| Time from diagnosis to relapse | ||||||||||||||
| <500 d | 12 | 25 | .28 | 54 | .94 | 36 | .40 | 29 | 54 | .99 | 36 | .91 | 30 | .98 |
| ≥500 d | 21 | 46 | 39 | 32 | 18 | 51 | 40 | 31 | ||||||
| Disease status | ||||||||||||||
| Poor risk | 18 | 58 | .04 | 71 | .02 | 11 | .001 | 18 | 71 | .07 | 75 | .22 | 7 | .02 |
| Good risk | 24 | 21 | 26 | 65 | 42 | 43 | 31 | 40 | ||||||
| Time from last CR to CBT | ||||||||||||||
| <92 d | 11 | 36 | .79 | 0 | .18 | 64 | .49 | 29 | 56 | .74 | 36 | .69 | 28 | .53 |
| ≥92 d | 12 | 32 | 49 | 53 | 19 | 47 | 17 | 44 | ||||||
| HLA3-150 | ||||||||||||||
| Identical | 30 | 21 | 45 | 48 | 6 | 50 | 50 | 25 | ||||||
| 1 difference | 1 | 100 | .01 | 0 | .43 | 0 | .005 | 27 | 34 | .07 | 29 | .81 | 49 | .19 |
| 2 differences | 3 | 33 | 0 | 67 | 22 | 76 | 44 | 14 | ||||||
| 3 differences | 7 | 82 | 100 | 0 | 4 | 25 | 100 | 25 | ||||||
| 4 differences | 1 | 100 | 0 | 0 | 1 | 0 | 0 | 100 | ||||||
| Cell dose | ||||||||||||||
| <3.7 × 107/kg | 23 | 51 | .03 | 15 | .04 | 39 | .60 | 25 | 62 | .21 | 14 | .24 | 33 | .78 |
| ≥3.7 × 107/kg | 19 | 16 | 72 | 39 | 35 | 44 | 54 | 28 | ||||||
HLA low resolution typing for related transplants and high resolution typing for unrelated transplants.
Influence of leukemia status at transplant on RR (A) and EFS (B). (A) Kaplan-Meyer estimate of leukemia relapse according to disease status at transplant. (B) Kaplan-Meyer estimate of EFS according to disease status at transplant.
Influence of leukemia status at transplant on RR (A) and EFS (B). (A) Kaplan-Meyer estimate of leukemia relapse according to disease status at transplant. (B) Kaplan-Meyer estimate of EFS according to disease status at transplant.
It is noteworthy that 2 of the 11 patients who relapsed after CBT from a relative were rescued with a second BMT or donor lymphocyte infusion from the same donor and are currently alive and in remission. Seventeen patients, 9 after a related CBT and 8 after transplantation from an unrelated donor, died from complications of recurrent leukemia.
Survival and EFS
The overall 1-year survival and 2-year EFS for all 102 patients was 42 and 34%, respectively (Kaplan-Meier estimate). Figure 1E, reporting the survival distribution of patients given a related and an unrelated transplant, shows that the outcome in recipients of CBT did not differ according to the type of donor (P = .26). Two-year EFS estimate of children given a related and an unrelated transplantation were 39 versus 30%, respectively (P = .19).
Univariate analysis of factors related to patients, leukemia and transplant, influencing EFS in both related and unrelated group is listed on Table 3. EFS was not significantly different for patients with ALL versus AML (P = .44), as well as for children with favorable, intermediate, or unfavorable cytogenetic characteristics (P = .93). Irrespective of whether the cord blood was from a relative or an unrelated donor, the most important factor influencing survival was disease state at time of transplantation. In fact, among all 102 children, those belonging to the good risk group had a significantly better post-transplant outcome than poor-risk patients, 2-year Kaplan-Meier estimate of EFS for these groups being 49% and 8%, respectively (P = .0002) (Fig 2B). In related CBT, HLA-disparity between donor and recipient was a predictor of poor outcome, since only 2 of 12 recipients of HLA-disparate cord blood were alive as compared with 17 of 30 patients given transplantation from an HLA-identical sibling (P = .01). In the 42 patients who received cord blood from a family donor, recipient negative CMV serology was associated with a better EFS probability (P = .02). In unrelated CBT neither number of HLA differences nor CMV were predictors of EFS (Table 3). From the Cox analysis, we found that the only factor predicting for a better EFS was a favorable disease phase at time of transplantation in related (P = .002, RR 0.28, 95% CI: 0.12 to 0.63) and unrelated groups (P = .02, RR: 0.47, 95% CI: 0.24 to 0.90).
TRM and Causes of Death
Forty-two patients died from transplant-related causes with a Kaplan-Meier estimate of 1-year TRM of 44% for the overall population. Univariate analysis of factors associated with 1-year TRM is listed in Table 3. TRM did not differ between children given a related and an unrelated transplant (35% v 52%, P = .24, Fig 1D). Causes of death according to disease status at transplant are reported in Table 4. The main causes of CBT-related deaths were acute GVHD, bacterial and viral infections, and interstitial pneumonia. In univariate analysis, the main factors unfavorably affecting TRM in the overall population were a number of cells infused lower than 3.7 × 107/kg (P = .02), an age older than 6 years (P = .02), a body weight greater than 20 kg (P = .02), HLA-disparity between donor and recipient (P = .01), and an advanced disease phase at time of CBT (P = .009). In the Cox analysis, patients transplanted in a favorable disease phase (P = .01, RR: 0.46, CI: 0.25 to 0.84) and receiving more than 3.7 × 107 nucleated cells/kg (P = .04, RR: 0.53, CI: 0.28 to 0.97) had a lower probability of 1-year TRM. However, in a Cox model for the related group, the most important factor adversely affecting TRM was HLA incompatibility (P = .01, RR: 3.67, CI: 1.31 to 10.27). For the unrelated group, the Cox model did not show any important factor affecting TRM (Table 3).
Causes of Death According to Status at Transplant
| Causes of Death . | Poor Risk (≥3 CR, relapse and refractory) N = 36 . | Good Risk (1 CR and 2 CR) N = 66 . |
|---|---|---|
| Relapse | 9 | 84-150 |
| Transplant-related causes | 21 | 21 |
| Acute GVHD | 2 | 5 |
| Bacterial infection | 4 | 2 |
| Fungal infection | ||
| Aspergillus | 2 | 1 |
| Viral infection | ||
| CMV | 2 | 3 |
| Adenovirus | — | 2 |
| EBV lymphoma | — | 1 |
| Toxoplasmosis | — | 1 |
| Interstitial pneumonia | 5 | 2 |
| VOD | 2 | — |
| Toxicity (cardiac and MOF) | 4 | 1 |
| Others | — | 34-151 |
| Causes of Death . | Poor Risk (≥3 CR, relapse and refractory) N = 36 . | Good Risk (1 CR and 2 CR) N = 66 . |
|---|---|---|
| Relapse | 9 | 84-150 |
| Transplant-related causes | 21 | 21 |
| Acute GVHD | 2 | 5 |
| Bacterial infection | 4 | 2 |
| Fungal infection | ||
| Aspergillus | 2 | 1 |
| Viral infection | ||
| CMV | 2 | 3 |
| Adenovirus | — | 2 |
| EBV lymphoma | — | 1 |
| Toxoplasmosis | — | 1 |
| Interstitial pneumonia | 5 | 2 |
| VOD | 2 | — |
| Toxicity (cardiac and MOF) | 4 | 1 |
| Others | — | 34-151 |
One patient died in complete remission (unknown origin of cause of death) 1 year after treatment for relapse.
Cerebral hemorrhage, gastro-intestinal hemorrhage after a biopsy, hemolytic uremic syndrome.
DISCUSSION
This study confirms that allogeneic CBT from either a related or an unrelated donor is a feasible procedure able to cure a significant proportion of children with acute leukemia, especially if transplanted in a favorable phase of the disease. In fact, as a result of lower risk of leukemia relapse and transplant-related mortality, children given CBT early during the course of their disease had a better probability of leukemia-free survival in comparison with those transplanted in more advanced disease (49% v 8%, respectively).
Concerns raised about the possibility of an increased risk of leukemia recurrence in CBT recipients derived from the following considerations: (1) there is a close association of GVL with GVHD in allograft recipients, such that patients developing either acute or chronic GVHD experience a much lower risk of relapse6,14; (2) the incidence and severity of both acute and chronic GVHD appeared to be less after transplantation of cord blood progenitors than after marrow transplantation1-4; and (3) immaturity of infused cord blood lymphocytes15-18 could further impair the immune-mediated antileukemia effect. Other theoretically opposing clinical considerations can be made on the risk of leukemia relapse after CBT. In fact, the prompt availability of placental blood may permit transplants to be given to children in a more favorable disease state, and the greater tolerable HLA-disparity between donor and recipient in unrelated CBT could be associated with a greater GVL effect. However, for patients given an unrelated CBT, the most widely used regimens of GVHD prophylaxis are those based on the combination of CsA with either low- or high-dose steroids, and this latter combination in particular can unfavorably affect lymphocyte function, thus impairing GVL effect in CBT recipients. Even though a formal, well-matched comparison between the relapse rate of our patients and that observed after marrow transplantation from related or unrelated donor was not performed, the outcome of our good-risk children is comparable with those reported in studies on allograft of bone marrow cells from either a family or an unrelated donor in pediatric leukemia patients.19-25 In particular, the 30% 2-year EFS of our overall cohort of unrelated CBT recipients was not remarkably different from that reported in the studies by Balduzzi et al22 and Davies et al,23 who recently analyzed the posttransplant outcome of children with acute leukemia given an unrelated, unmanipulated BMT. In the report of Balduzzi et al,22 the disease-free survival and relapse of patients with ALL in first or second CR were 47% and 20%, respectively, whereas the values for patients transplanted in more advanced disease were 10% and 60%, respectively. In the study of the Minneapolis group,23 the 2-year EFS was 30% and 33% for patients with ALL and AML, respectively. Notably, the incidence of chronic GVHD in our population was significantly lower than that (on the order of 50% to 60%) documented in the above mentioned studies on pediatric recipients of unrelated BMT22,23 and this is of particular relevance considering the detrimental impact that can have this complication in a growing organism. A recently published single center study comparing matched sibling and unrelated donor T-cell–depleted BMT in children with leukemia showed a remarkably better 2-year disease-free survival, which was 81% in HLA identical sibling and 73% in unrelated BMT for standard-risk patients and 31% and 32%, respectively, for high-risk patients.26 The risk of relapse at 2 years was 17% in HLA-matched and 29% in unrelated BMT. This improvement can be due to a center effect or to better patient selection, advances in HLA typing, T-cell depletion, and antiviral prophylaxis. It remains to be shown by a case control study that cord blood transplant gives similar results to matched related or unrelated BMT.
In contrast to other studies on outcome of HLA identical sibling BMT for ALL, we did not find any influence of leukemia risk factors, including tumor burden at diagnosis, karyotype abnormalities, and immunophenotype on relapse.12,27 The increased probability of leukemia recurrence that we observed in patients given CBT later in the course of the disease is not surprising given that disease status has been reported to be the most important factor influencing the risk of relapse, irrespective of the source of progenitors (bone marrow, peripheral blood, or cord blood) and the type of donor (HLA-compatible relative, partially matched family donor, or unrelated volunteer) used.7-10,22,25 The higher relapse rate of patients who received transplant in advanced disease may be the consequence of either a greater tumor burden or an intrinsic reduced susceptibility of leukemia cells to chemoradiotherapy used during the preparative regimen, and to the GVL effect displayed by donor cells. In this regard, it is likely that leukemia blasts, under strong negative selection pressure after an allograft, may downregulate critical surface molecules to escape immune control.28 Few data have been published on the biological mechanisms of cord blood–related GVL effect. In particular, while several authors have shown that cytotoxic T lymphocytes directed against allogeneic leukemia blasts can be detected in the peripheral blood of healthy donors,29-31 no study on a cord blood T-cell–specific HLA-restricted activity toward leukemia blasts has been reported. By contrast, available evidence indicates that cord blood LAK cells are able to lyse noncultured fresh leukemia blasts32 and that their activity towards cell lines such as Daudi and YAC-1 cells is greater than that of bone marrow cells.33 These findings, together with the prompt activation of aspecific immunity mediated by cord blood lymphocytes toward primary antigenic challenges,34 support the hypothesis that innate cell-mediated immunity probably is the most important mechanism for controlling the regrowth of leukemic blasts in CBT recipients and that, notwithstanding a lower incidence of GVHD, patients given CBT may benefit from efficacious donor-derived GVL effect. The possibility of obtaining a new remission after relapse in recipients of a related CBT by means of allogeneic BMT or leukocyte infusion from the same donor is of particular interest. In this regard, it must be emphasized that the GVL effect associated with donor leukocyte infusion has the greatest efficacy in conditions characterized by a limited tumor burden, as proved by studies on patients with chronic leukemia, where better responses are obtained for cytogenetic relapse or hematological relapse in chronic phase as compared with recurrence in accelerated phase or blastic crisis.35 36 Thus, it is rational to hypothesize that in patients given CBT from a relative, posttransplant infusion of donor cells, collected with only minor discomfort to the donor, should be considered when close monitoring with sensitive molecular tools documents regrowth of malignant cells or reappearance of recipient hematopoiesis.
HLA-disparity predicted for the occurrence of A-GVHD in our limited number of patients who received transplant from a relative, whereas this association was not observed when the donor was an unrelated individual. The lack of correlation between GVHD occurrence and donor/recipient HLA diversity for children given an unrelated CBT could be explained by the fact that some mismatch for HLA-class I antigens may have been undetected by conventional serological typing. Using molecular techniques for HLA-class I typing, it is possible that the same correlation found in children who received transplant from relatives could be observed. This hypothesis is supported by two reports recently published, documenting the relevance of HLA-class I disparity for acute GVHD occurrence in unrelated BMT recipients.37,38 Moreover, it should be considered that also in the New York Cord Blood Bank experience, the frequency of severe GVHD was lower in patients with six of six with HLA antigen matches than in other patients, but did not otherwise correlate with the number of mismatches.5 Finally, we cannot exclude that the use of steroids for GVHD prophylaxis in children given an unrelated CBT has blunted the impact of HLA disparity.
Mortality from causes other than leukemia relapse in our cohort was not negligible because more than one third of children given a related and half of those receiving an unrelated CBT died of nonrelapse causes. This finding could be explained by considering the increased risk of graft failure, the delay of hematological reconstitution (particularly evident for platelet recovery) observed in a number of patients, and particularly the organ toxicity associated with the intensive treatment administered to patients before CBT. This latter consideration is also supported by the increased risk of treatment failure observed in children who received transplant late during the course of their disease. Moreover, because infections accounted for one third of nonrelapse deaths, it can be hypothesized that the absence of adoptive transfer of specific immunity toward infectious agents due to fetal immune immaturity and lack of previous antigenic exposure could have also contributed to treatment failure. It is also noteworthy that seven patients died of GVHD suggesting that, although certainly reduced in incidence and severity, GVHD may have a detrimental effect on survival, especially when an unrelated donor is used. Finally, given the heterogeneity of the methods of collecting, cryopreserving, and storing of cord blood at the different centers, we did not have a reliable evaluation of the possible impact of these variables on hematopoietic engraftment and TRM.
In summary, this study supports the conclusion that CBT from either a relative or an unrelated donor is a feasible procedure, capable of curing a relevant number of children with acute leukemia who failed conventional chemotherapy or who are at risk of relapse. It is therefore evident from this analysis that if the aim of CBT is to achieve a definitive cure in leukemia patients, this transplant should not be considered as a treatment of last resort for patients with end-stage disease, but rather as a well-established and accepted therapy for patients who have achieved a second remission after relapse and for those children who are in first hematological remission with a high risk of recurrence. For the time being, concerns raised about the possibility of an increased risk of leukemia recurrence in patients given CBT are not supported by these as well as other available data.1-5 However, only analysis performed on a larger sample and including comparison with well-matched controls given transplantation of bone marrow or peripheral blood stem cells will be able to provide definitive conclusions on the relative risk of leukemia relapse and long-term outcome of CBT recipients.
Eurocord-Cord Blood Transplant Group Centers: Abecasis M, Machado A: BMT Unit, Inst. Portugues Oncologia, Lisboa, Portugal. Arcese W, Carmini D: Inst. of Hematology, Univ. degli Studi La Sapienza, Roma, Italy. Badell Serra I: Dept of Pediatrics, Hospital Santa Creu i Santa Pau, Barcelona, Spain. Bernaudin F, Service d’Hématologie, Hôpital Henri-Mondor, Créteil. France. Bosi A, Saccardi R: BMT Unit, Department of Hematology, Ospedale di Carregi, Firenze, Italy. Chapuis B: Hôpital Cantonal Universitaire, Geneva, Switzerland. Cornu G, Brichard B, Vermylen C: Dept of Pediatrics, University of Louvain, Brussels, Belgium. Favre C: BMT Unit, Pediatric Department, University of Pisa, Pisa, Italy. Fernandez MN: Servicio de Hematologia y Hemoterapia, Clinica Puerta de Hierro, Madrid, Spain. Ferreira E: Serviço de Transplante de Medula Ossea, Hospital Israelita Albert Einstein, Sao Paulo, Brazil. Fisher A, Haddad E: Unité d’Immunologie et d’Hématologie, Hôpital Necker, Paris, France. Gibson BES: Royal Hospital for Sick Children, Glasgow, UK. Gluckman E: Service d’Hématologie Greffe de Moelle, Hôpital Saint-Louis, Paris, France. Harris R: Stem Cell Transplant Program, Hospital Medical Center, Cincinatti, OH. Sievers E: Fred Hutchinson Cancer Research Center, Seattle, WA. Jouet JP: Service des maladies du Sang, Hôpital Claude Huriez, Lille, France. Laporte JP, Gorin N: Dept of Hematology, Hôpital Saint-Antoine, Paris, France. Locatelli F: Pediatric Clinic, University of Pavia, Pavia, Italy. Madero LM: Autonomous University of Madrid, Madrid, Spain. Michel G: Service d’Oncologie, Hopital d’Enfants de La Timone, Marseille, France. Miniero R: Dept of Pediatrics, Univ. of Torino, Ospedale Regina Margherita, Torino, Italy. Nagler A, Slavin S: Dept of Bone Marrow Transplantation, Hadassah University Hospital, Jerusalem, Israel. Nurnberger W, Burdach S: Medizinische Einrichtungen, Heinrich-Heine-Universitat Dusseldorf, Germany. Ortega J, Diaz de Heredia C: Hospital M. Infantil Vall d’Hebron, Barcelona, Spain. Pasquini R, Bittencourt M: Serviço de Transplante de Medula Ossea, Hospital de Clinicas, Curitiba, Brazil. Pession A: BMT Unit Clinica Pediatrica III, Policlinico S. Orsola Malphighi, Bologna, Italy. Pihkala U, Vetteranta K: Children’s Hospital, University of Helsinki, Finland. Plouvier E: Service d’Oncologie-Hématologie Pédiatrique, Hôpital Saint-Jacques, Besançon, France. Roittman S: Serviço de Hematologia e Transplante de Medula Ossea, Hospital de Clinicas, Porto Alegre, Brazil. Rowe J: Dept of Hematology and Bone Marrow transplantation, Rambam Medical Center, Haifa, Israel. Guillot F, Sadoun A: Bone Marrow Transplant Unit, Hôpital La Milétrie, Poitiers, France. Souillet G: Immuno-Hématologie Pédiatrique et Transplantation de moelle osseuse, Hôpital Debrousse, Lyon, France. Spruce W, Allen J: Children’s Hospital, San Diego, CA. Stary J: Pediatric Dept Oncology, Univ. Hospital Motol, Praha, Czech Republic. Stone S, Chan K: The University of Texas, MDACC, Houston, TX. Takaue Y: University of Tokushima, Japan. Vilmer E: Hôpital Robert Debré, Paris, France. Verdeguer A, Castel V: Hospital Infantil La Fe, Valencia, Spain. Vossen JM, Jacobs H: BMT Unit and Department of Hematology, University Hospital Leiden, The Netherlands. Zanesco L, Varotto S, Messina C: Clinica Oncoematologica Pediatrica e Centro Leucemie Infantil, Padova, Italy. Zintl F: Dept of Pediatrics, University of Jena, Jena, Germany.
NETCORD Banks: Benbunan M, Marolleau J-P; Paris Cord Blood Bank. Contreras M; London Cord Blood Bank. Garcia J, Querol S; Barcelona Cord Blood Bank. Rubinstein P, Stevens C; New York Cord Blood Bank. Sirchia G; Milano Cord Blood Bank. Wernet P, Kögler G; Dusseldorf Cord Blood Bank.
Deceased.
Submitted September 11, 1998; accepted January 26, 1999.
This paper is dedicated to the memory of Prof Claude Chastang.
Supported by the program Biomed II (Grant Eurocord BMH4CT96) from the European Union to E.G. and by grants from AIRC (Associazione Italiana Ricerca sul Cancro) and IRCCS Policlinico S. Matteo to F.L. Cooperative work of the European Blood and Marrow Transplantation Group. Members of Eurocord-Cord Blood Transplant group are listed in the .
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Eliane Gluckman, MD, Eurocord Pavilion Lallier, Unité de Recherche clinique, Hôpital Saint Louis, 1 Ave Glaude Vellefaux 75475, Paris Cedex 10, France; e-mail:eliane.gluckman@chu-stlouis.fr.