Abstract
The natural history of chronic hepatitis C (HCV) infections in long-term leukemia survivors has not been well characterized. We studied the prevalence of HCV infections, measured HCV RNA levels, and evaluated the severity of liver disease in patients with leukemia who achieved long-term remissions after intensive chemotherapy or bone marrow transplantation (BMT). HCV antibody tests were performed by the enzyme-linked immunosorbent assay (ELISA) and positive tests confirmed by the recombinant immunoblot assay (RIBA). HCV RNA levels were measured by the branched DNA (bDNA) assay. Seventy-five leukemia survivors with 25 or more blood donor exposures were identified. Nine (12%) were anti-HCV positive. All were infected before 1992 when second generation HCV screening tests were implemented. Mean HCV RNA levels were 10.3 ×106 eq/mL versus 3.2 × 106 eq/mL (P = .056) in a control group of 20 anti-HCV positive immunocompetent individuals of comparable age who were infected twice as long (17.8 ± 6.5 years v 9.0 ± 4.4 years in leukemia survivors, P = .001). Liver biopsies were performed on six of the nine anti-HCV positive leukemia survivors. All showed at least moderate portal inflammation and half had evidence of bridging fibrosis. We conclude that viral loads in anti-HCV positive leukemia survivors are markedly higher than in immunocompetent controls. Our results suggest that long-term leukemia survivors with chronic HCV may have more rapidly progressive liver disease than has been previously recognized.
INDIVIDUALS WITH leukemia have an increased risk of acquiring transfusion-associated hepatitis C virus (HCV) infections.1,2 Adults with acute leukemia who have achieved remission after chemotherapy have an anti-HCV prevalence between 4.3% and 70%.3-7 Similarly, bone marrow transplant (BMT) recipients have an anti-HCV prevalence between 3.3% and 70%.8-14 A somewhat lower prevalence of 1% to 49% has been found in studies restricted to children with leukemia.15-20 The rate of infection has decreased since implementation of blood donor screening for anti-HCV in 1990 and second generation testing in 1992.3,13,14 18
Data are limited regarding the effects of immunosuppression on viral load and severity of liver disease in long-term anti-HCV positive leukemia survivors. However, numerous investigators have reported that anti-HCV positive individuals who are coinfected with human immunodeficiency virus (HIV) have significantly higher viral loads than those who are anti-HIV negative.21-26Some,21,22,27 but not others,23-26 have shown that HCV RNA levels increase as the immune deficiency progresses. Coinfection with HIV has also been shown to be associated with an increased risk of cirrhosis and liver failure.28-34 Similar findings have been reported in patients with primary hypogammaglobulinemia.35,36 Conversely, long-term follow-up of immunocompetent individuals has shown only a small increase in deaths related to liver disease.37
The objectives of this study were (1) to determine the prevalence of HCV infection and severity of liver disease in anti-HCV positive adults and children with leukemia who achieved a complete remission after intensive chemotherapy or allogeneic BMT and (2) to determine whether HCV load differed in the study group compared with a control group of immunocompetent anti-HCV positive hemophiliacs.
MATERIALS AND METHODS
Subjects.
The records of all patients with leukemia who were seen between 1973 and 1995 at the adult and pediatric hematology clinics at the Hershey Medical Center and in the practices of five physician groups in the Central Pennsylvania area were reviewed to identify those who had achieved a complete remission after intensive chemotherapy or BMT. Patients less than age 21 at the time of diagnosis were classified as children. Seventy-five patients in active follow-up who met study criteria were identified. They consisted of 46 adults and 29 children with acute leukemia or chronic myelogenous leukemia (CML) who had received 25 or more donor exposures to blood products. Four patients were known to be anti-HCV positive at the initiation of the study. After obtaining informed consent, medical records were reviewed and histories were obtained to exclude other causes for hepatitis. Blood was then drawn for HCV antibody testing and HCV RNA levels.
The control group consisted of 20 anti-HCV positive, anti-HIV negative, otherwise healthy, randomly selected hemophilia patients with known transfusion dates followed regularly at the hemophilia clinic at the Hershey Medical Center and enrolled in a prospective cohort study on HIV initiated in 1982. For these individuals, sera routinely collected during the previous year and stored frozen at −70°C was tested for HCV RNA.
Detection of HCV infection and quantitation of HCV RNA levels.
Sera were tested with the enzyme-linked immunosorbent assay (ELISA) (Ortho Diagnostic System, Raritan, NJ) and positive tests were confirmed by a second generation recombinant immunoblot assay test (RIBA-2) (Chiron Corp, Emeryville, CA). Samples were considered positive if they reacted with two or more of the four test antigens. Serum HCV RNA levels were measured by the Quantiplex 1.0 bDNA assay (Chiron Corp) on all who were anti-HCV positive except for one patient from a community practice who had the quantitative polymerase chain reaction (PCR)-based Monitor test (Roche Molecular Systems, Nutley, NJ) performed by an outside laboratory. All who were below the cut off (<3.5 × 105 eq/mL) were subsequently tested by the 2.0 assay (cut off 0.2 × 106 eq/mL). Those who were negative by the bDNA assay as well as a subset of leukemia patients who were anti-HCV negative were tested for HCV RNA by the more sensitive, qualitative PCR assay as previously described.26 To obtain a mean value of HCV RNA load for the control group, PCR positive samples below the detectable limits of the bDNA assay of 0.2 × 106 eq/mL were assigned a value of 0.1 × 106 eq/mL (half the detectable limit) and PCR negative samples were assigned a value of .001 × 106 eq/mL (approaching the lowest level of 1,000 viral copies/mL detectable by PCR).
Estimation of duration of infection.
For the patients with acute leukemia, the date of infection was estimated to be the initial exposure to blood products during the induction phase of chemotherapy. For patients with CML undergoing allogeneic BMT, the date of transplant was assumed to be the date of infection. For hemophilia patients, the midpoint between the maximum duration of infection (the number of years since first transfusion) and the minimum duration of infection (the number of years since first exposure to unsterilized clotting factor concentrates) was used. The minimum duration of infection for the hemophilia patients can be accurately estimated because studies have shown a near 100% incidence of infection with HCV after the first infusion of unsterilized clotting factor concentrates obtained from large donor pools.38 39For the five subjects who had received only single donor blood products or only virally attenuated concentrates, the minimum duration of infection was estimated from known duration of transaminase elevations.
Evaluation of liver histology.
Percutaneous liver biopsy specimens were evaluated blindly by a single pathologist and scored using the Knodell total histology activity index (HAl), which is a grading system for chronic hepatitis frequently used in clinical studies.40
Statistical analysis.
Exact P values were calculated using the Pearson’s χ2 test to determine the statistical significance of the difference between the prevalence of detectable HCV RNA in the study and control groups and prevalence of anti-HCV in (1) acute lymphoblastic leukemia (ALL) versus acute myelogenous leukemia (AML); (2) the two donor exposure groups; and (3) the three time periods examined. To compare the HCV RNA values between the two groups, a Wilcoxon rank sum test was used to determine the exact P value, and a two sample t-test was used to compare the duration of infection data.
RESULTS
Prevalence of HCV infection in the study cohort.
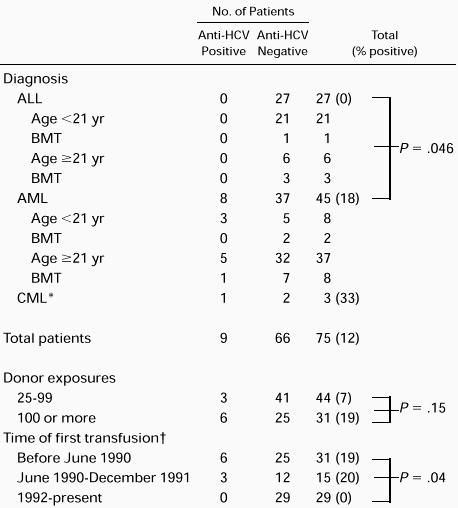
Nine (12.0%) of the 75 patients with leukemia who met the study criteria were found to be anti-HCV positive by ELISA and confirmed with the RIBA-2 assay (Table 1). Six were males and three were females. Eight were treated for AML. One of the eight received an allogeneic BMT. The ninth received an allogeneic BMT for CML. None of the 27 subjects with ALL including four allogeneic BMT recipients were anti-HCV positive compared with 8 of 37 (17.8%) with AML (P = .046). Of the 17 allogeneic BMT recipients, 2 (11.8%) were anti-HCV positive. Three (10.3%) of 29 children were anti-HCV positive. All 3 were among the 8 children treated for AML. None of 3 children treated with BMT were anti-HCV positive. The rate of HCV infection was 3 of 44 (6.8%) among those with 25 to 99 donor exposures compared with 6 of 31 (19.4%) among those with 100 or more donor exposures (P = .15). Six (19.4%) of 31 patients who were anti-HCV positive were transfused before June 1990, when first-generation anti-HCV testing was implemented. Three (20.0%) of 15 patients transfused while first generation tests were in use tested positive. None of the 29 subjects studied after second generation testing was implemented in 1992 were positive for anti-HCV (P = .04).
Characteristics of the anti-HCV positive leukemia patients.
The nine anti-HCV positive patients had a mean age of 36.2 ± 15.4 (standard deviation [SD]) years with a range of 7 to 50 years at the time of evaluation and were estimated to be infected for a mean of 9.0 ± 4.4 years with a range of 4 to 16 years (Table 2). Seven (78%) had mild to moderately elevated serum alanine transferases. All nine had normal bilirubin determinations and normal serum albumin levels (data not shown). None showed clinical evidence of liver disease. All had viral loads above the level of detection by quantitative HCV RNA assays. The mean HCV RNA level for the eight individuals tested by the bDNA assay was 9.6 × 106 eq/mL (range, 0.28 to 40.5 × 106 eq/mL). The individual tested by quantitative PCR had a level of 3.9 × 106 eq/mL, which is roughly equivalent to a fourfold higher value of 15.6 × 106 eq/mL by the bDNA test.41 When the adjusted value was included, the overall mean for the nine patients was 10.3 × 106eq/mL. None of the nine patients were receiving interferon-α when the HCV RNA tests were performed. Four were treated before the testing, but all had been off therapy for a minimum of 2 years. Six anti-HCV negative leukemia patients from the study group were randomly selected to have HCV testing using PCR. All six were HCV negative by PCR.
Characteristics of the Anti-HCV Positive Leukemia Patients
| Patient . | Sex/Age (yr) . | Estimated Duration of Infection (yr) . | ALT* (U/L) . | HCV RNA (×106 eq/mL) . | Piecemeal Necrosis (0-10) . | Biopsy Data† . | Portal Inflammation (0-4) . | Fibrosis (0-4) . | Knodell Score (0-22) . |
|---|---|---|---|---|---|---|---|---|---|
| Intralobular Inflammation Necrosis (0-4) . | |||||||||
| 1. TC | M/50 | 10 | 290 | .28 | |||||
| 2. EK | M/50 | 16 | 52 | 24.4 | 1 | 1 | 3 | 1 | 6 |
| 3. SS | F/24 | 4 | 43 | 3.6 | 1 | 1 | 3 | 0 | 5 |
| 4. WF‡ | M/47 | 4 | 119 | 1.04 | 1 | 1 | 3 | 1 | 6 |
| 5. JS | M/50 | 11 | 234 | 4.55 | 3 | 3 | 3 | 3 | 12 |
| 6. DP‡ | F/37 | 14 | 131 | 40.5 | 6 | 1 | 3 | 3 | 13 |
| 7. AS | F/22 | 9 | 75 | 1.08 | — | — | — | — | — |
| 8. NH | M/7 | 4 | 44 | 1.47 | — | — | — | — | — |
| 9. DM | M/39 | 9 | 78 | 15.62-153 | 1 | 1 | 4 | 4 | 10 |
| Mean ± SD | 36.2 ± 15.4 | 9.0 ± 4.4 | 118.4 | 10.3 | 2.2 | 1.3 | 3.2 | 2.0 | 8.7 |
| Range | 7-50 | 4-16 | 43-290 | .28-40.5 | 1-6 | 1-3 | 3-4 | 0-4 | 5-13 |
| Patient . | Sex/Age (yr) . | Estimated Duration of Infection (yr) . | ALT* (U/L) . | HCV RNA (×106 eq/mL) . | Piecemeal Necrosis (0-10) . | Biopsy Data† . | Portal Inflammation (0-4) . | Fibrosis (0-4) . | Knodell Score (0-22) . |
|---|---|---|---|---|---|---|---|---|---|
| Intralobular Inflammation Necrosis (0-4) . | |||||||||
| 1. TC | M/50 | 10 | 290 | .28 | |||||
| 2. EK | M/50 | 16 | 52 | 24.4 | 1 | 1 | 3 | 1 | 6 |
| 3. SS | F/24 | 4 | 43 | 3.6 | 1 | 1 | 3 | 0 | 5 |
| 4. WF‡ | M/47 | 4 | 119 | 1.04 | 1 | 1 | 3 | 1 | 6 |
| 5. JS | M/50 | 11 | 234 | 4.55 | 3 | 3 | 3 | 3 | 12 |
| 6. DP‡ | F/37 | 14 | 131 | 40.5 | 6 | 1 | 3 | 3 | 13 |
| 7. AS | F/22 | 9 | 75 | 1.08 | — | — | — | — | — |
| 8. NH | M/7 | 4 | 44 | 1.47 | — | — | — | — | — |
| 9. DM | M/39 | 9 | 78 | 15.62-153 | 1 | 1 | 4 | 4 | 10 |
| Mean ± SD | 36.2 ± 15.4 | 9.0 ± 4.4 | 118.4 | 10.3 | 2.2 | 1.3 | 3.2 | 2.0 | 8.7 |
| Range | 7-50 | 4-16 | 43-290 | .28-40.5 | 1-6 | 1-3 | 3-4 | 0-4 | 5-13 |
Average of the two earliest available values from 1995 to 1997.
All biopsies were performed within 1 year of HCV RNA assay with the exception of WF whose was done 2 years before HCV RNA testing.
BMT recipient.
Value determined by quantitative PCR-based monitor test (Roche Molecular Systems, Nutley, NJ) and multiplied by 4 (see text).
Liver histology in anti-HCV positive leukemia patients.
Six (67%) of nine anti-HCV positive patients had liver biopsies performed after a mean of 9 years (range, 4 to 16 years) from the estimated date of infection (Table 2). The mean Knodell score was 8.7 (range, 5 to 13). Two (33%) of the six showed moderate to marked piecemeal necrosis. One (17%) showed moderate intralobular inflammation/necrosis. All six showed at least a moderate degree of portal inflammation and half showed evidence of bridging fibrosis. One showed severe fibrosis consistent with cirrhosis. The patient with the highest Knodell score had the highest viral load, and two of the three patients with scores of 10 or greater had viral loads greater than 10.0 × 106 eq/mL.
Characteristics of the anti-HCV positive patients with hemophilia.
The 20 hemophiliacs chosen at random for the control group had a mean age of 30.7 ± 18.2 years with a range of 10 to 65 years. These individuals had an estimated mean duration of infection of 17.8 ± 6.5 years with a range of 9 to 28 years. Twelve (60%) had mild to moderately elevated transaminases. All had normal bilirubin and albumin levels, and none showed clinical evidence of liver disease at the time of study. One subsequently developed signs of portal hypertension with splenomegaly and mild thrombocytopenia. Twelve (60%) including 10 with elevated transaminases had detectable HCV RNA by the bDNA assay. The mean level of viremia for those 12 patients was 5.3 × 106 eq/mL (range, 0.5 to 15.5 × 106eq/mL). Eight hemophiliacs were HCV RNA negative by the bDNA assay. Six of the eight were also negative when tested by the more sensitive qualitative PCR assay. Overall, the mean HCV RNA level for the 20 controls was 3.2 × 106 eq/mL.
Comparison of HCV RNA levels in leukemia patients and hemophilia controls.
Mean HCV RNA levels were 10.3 × 106 eq/mL in the leukemia patients compared with 3.2 × 106 eq/mL in the hemophilia controls (P = .056). HCV RNA levels were above the detectable limit of 0.2 × 106 eq/mL in all nine leukemia patients compared with only 12 of 20 hemophilia controls (P = .067). Furthermore, values above 20.0 × 106 eq/mL were found only in the leukemia group. The estimated duration of infection was 9.0 ± 4.4 years in the leukemia group compared with 17.8 ± 6.5 years in the control group (P = .001). The mean age of the two groups was comparable, although the range was broader for the controls.
DISCUSSION
The 12% prevalence of anti-HCV seropositivity in this group of leukemia patients who achieved a complete remission after chemotherapy or BMT is in the range reported by others.3-7,15-20 The 10.3% prevalence in children and the 11.8% seropositivity rate among allogeneic BMT recipients are also within the reported ranges, which vary widely depending on the year of exposure, the number of transfusions, and geographic considerations.8-20 No further cases of anti-HCV positivity were found after second generation testing of blood donors was used in 1992. These results are similar to previous reports3,14 and are consistent with the current estimated risk of HCV infection via transfusion of 1 in 103,000 donor exposures reported by Schreiber et al42 using the second generation ELISA test to screen for anti-HCV.
Although 78% of our anti-HCV positive patients had elevated serum alanine transferases, none showed clinical evidence of hepatic decompensation after a mean of 9 years from the estimated date of infection. This relatively benign course in HCV positive leukemia survivors through the first decade of chronic infection is in agreement with previous reports.16,20 Cesaro et al16found little evidence of progression to liver failure in survivors of pediatric malignancies, of whom 63% had leukemia or lymphoma, after a mean of 14 years. They observed HAI scores between 2 and 7, and inactive cirrhosis and severe fibrosis in only 1 of 37 (2.7%) anti-HCV positive patients. Locasciulli et al20 reported similar findings in survivors of childhood leukemia 13 to 27 years (mean, 17 years) after chemotherapy withdrawal. The latter study did not include any histologic data.
Two large studies by Kiyosawa et al43 and Tong et al44 have focused on the pathologic evolution of hepatitis C-related liver disease in transfusion recipients. These studies showed progression to chronic hepatitis to cirrhosis to hepatocellular carcinoma after a mean duration of HCV infection of approximately 1, 2, and 3 decades, respectively. In another study, Yano et al45 showed that those with severe inflammation or fibrosis usually developed cirrhosis within 10 years. Our data show that all six patients who were biopsied showed moderate to severe hepatic inflammation and half had either severe fibrosis or cirrhosis after a mean of less than one decade of infection. Although these results must be interpreted with caution because of the small sample size, this finding suggests that leukemia survivors with chronic HCV may have more rapidly progressive liver disease than has been previously recognized.
The pathogenesis of liver cell damage by HCV is not well understood. However, evidence is emerging that both host cytotoxic T-lymphocyte responses and direct viral injury play a role. Reports that patients with high HCV RNA viral loads have more severe liver disease46-48 and that HCV runs a more aggressive course in patients with immune deficiency lend support to the direct hepatocellular toxic effect of the virus. However, HCV-specific CD8 and CD4 lymphocyte responses have been shown in the liver of individuals with HCV infection.49-53 Furthermore, in one study, those with intrahepatic HCV-specific CD8 activity had lower levels of viremia and more active liver disease than those without CD8 activity.52 This suggests that the level of CD8 response might be responsible for much of the ongoing hepatocellular injury, and that the balance between viral and host factors may be an important determinant of hepatocellular injury. In patients with leukemia, the disease process, chemotherapy, and BMT all affect cell-mediated and humoral immunity, which may contribute to the pathogenesis of liver disease. The immunocompromised status at the time of infection may also play a role, while chemotherapy-induced hepatotoxicity may accelerate the inflammatory and fibrotic hepatic changes.
Previous reports have paid much attention to the natural history of hepatitis C, as well as the many factors associated with disease progression including alcohol, male sex, older age at the time of infection, and longer duration of infection. However, little attention has been given to immunosuppression and its role in progression of HCV-related liver disease. After a mean follow-up of 18 years after transfusion-associated HCV infection, Seeff et al37 found that there was no increase in overall mortality and only modest increases in morbidity and deaths related to liver disease in the infected group when compared with noninfected controls. After 20 years of follow-up, approximately 10% of the infected group showed evidence of cirrhosis.54 Conversely, numerous studies involving HIV and HCV coinfected patients and two studies regarding patients with primary hypogammaglobulinemia and HCV35,36 have clearly shown more rapidly progressive HCV-related liver disease among those with immunodeficiency.29-36 In addition, several studies have demonstrated increased HCV RNA levels among HIV positive patients when compared with their immunocompetent, HIV negative controls.21-26 In one study, which examined serial samples, HCV RNA levels increased as immune deficiency progressed over time.21
We found that the HCV viral loads of leukemia survivors were markedly higher than a control group of HIV negative, immunocompetent, HCV positive patients of approximately the same age. This finding is more significant when one considers that the control group was infected for twice as long as the study group. These results suggest that the previously immunocompromised status of the leukemia survivors may have promoted more rapid viral replication or impaired host viral clearance. While none of our leukemia survivors have shown evidence of hepatic decompensation at a mean of 9 years after HCV infection, our results suggest that they may be at increased risk for more rapid disease progression. If so, a more aggressive approach regarding the use of antiviral therapy in the population of leukemia survivors with chronic HCV may be warranted.
ACKNOWLEDGMENT
We thank Mary Catherine Hess, RN, Drs James O. Ballard, Hamid Al-Mondhiry, W. Christopher Ehmann, Barbara Miller, L. Eamonn Boyle, Richard Dixon, David Prager, Michael Marrone, and Terrance Cescom for assistance with patient enrollment and data collection; Sara Neagley, RN, Elisabeth Crago, RN, and Linda Landis for assistance with data collection; Allen Kunselman, MA, for help with biostatistics; and Linda Nelson for assistance with preparing the manuscript.
Supported in part by a medical student scholarship grant to I.P. from the American Society of Hematology and by the Alice Livingston Trout and Dorothy Rider Pool Trust Funds.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to M. Elaine Eyster, MD, Division of Hematology/Oncology HO46, Department of Medicine, Pennsylvania State University College of Medicine, PO Box 850, Hershey, PA 17033.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal