Interleukin-15 (IL-15) is a potent regulator of T-, B-, and natural killer cell proliferation and displays unusually tight controls of secretion. Even though IL-15 mRNA is constitutively expressed in monocytes/macrophages and is upregulated by a variety of stimuli, evidence for IL-15 cytokine secretion is only found exceptionally, eg, conditions of pathological, chronic inflammation. This raises the possibility that monocytes express membrane-bound IL-15 rather than secrete it. The current study explores this hypothesis. We demonstrate here that biologically active IL-15 is indeed detectable in a constitutively expressed, membrane-bound form on normal human monocytes, as well as on monocytic cell lines (MONO-MAC-6, THP-1, and U937), but not on human T or B cells (MT4, M9, C5966, JURKAT, DAUDI, RAJI, and Epstein-Barr virus-immortalized B-cell clones). Furthermore, cell surface-bound IL-15 is upregulated upon interferon-γ stimulation. Interestingly, monocyte/macrophage inhibitory cytokines such as IL-4 and IL-13 fail to downregulate both constitutive and induced cell-surface expression of IL-15. Membrane-bound IL-15 does not elute with acetate buffer or trypsin treatment, suggesting that it is an integral membrane protein and that it is not associated with the IL-15 receptor complex. Finally, membrane-bound IL-15 stimulates T lymphocytes to proliferate in vitro, indicating that it is biologically active. These findings enlist IL-15 in the fairly small family of cytokines for which the presence of a biologically active membrane-bound form has been demonstrated (eg, IL-1, tumor necrosis factor-, and IL-10) and invites the speculation that most of the biological effects of IL-15 under physiological conditions are exerted by the cell surface-bound form.
INTERLEUKIN-15 (IL-15) was originally discovered as a T-cell stimulatory activity that mimics several biological effects of IL-2.1,2 For example, like IL-2, IL-15 induces T-cell proliferation and chemotaxis, stimulates natural killer (NK) cell growth and interferon-γ (IFN-γ) production, generates cytotoxic effector cells, and costimulates B-cell growth and Ig production.2,3-6 In addition, IL-15 induces cytokine production by monocytes and polymorphonuclear cells and stimulates the antimicrobial activity of phagocytes.7-11 For binding and signaling, IL-15 uses the IL-2Rβ and γ chain12-14 as well as the specific IL-15Rα chain.15 16
Although IL-2 is mainly produced by T cells, IL-15 mRNA is constitutively expressed by a large variety of cell types and tissues, including monocytes/macrophages, fibroblasts, keratinocytes, and skeletal muscle.1,17 In monocytes/macrophages, IL-15 mRNA expression is upregulated by exogenous stimuli such as IFN-γ and lipopolysaccharide (LPS) or by bacteria, protozoa, and virus infection.5,17 18-21 However, despite the widespread expression of IL-15 mRNA, it has been very difficult to show IL-15 activity in cell culture supernatants using either bioassay or enzyme-linked immunosorbent assay (ELISA).
Two IL-15 isoforms have been identified that differ in the length of the signal peptide.22-25 IL-15 associated with the short signal peptide (SSP–IL-15) is not secreted, but rather stored intracellularly, where it appears in the nucleus and cytoplasm. The alternative isoform, characterized by the longer signal peptide (LSP–IL-15), is located in the endoplasmic reticulum and is supposed to follow a pathway ending in its secretion.22 23
This discrepancy between abundant IL-15 message, intracellularly detectable IL-15 protein, yet inefficient or absent cytokine secretion prompted us to speculate that, under physiological conditions, IL-15 may mainly be present in a membrane-bound, nonsecreted form. In this study, we have explored this hypothesis, studying normal human monocytes and monocytic cell lines as well as T and B cells. Biological activity was tested by a T-cell proliferation assay, and association of surface-bound IL-15 with plasma membrane or IL-15 receptor was investigated using acetate buffer and trypsin treatment. Collectively, our experiments suggest that human monocytes constitutively express membrane-bound, biologically active, and IFN-γ–inducible IL-15.
MATERIALS AND METHODS
Monocytes preparation and cell lines.
Peripheral blood mononuclear cells (PBMC) were obtained by Ficoll-Hypaque gradient centrifugation from normal volunteers after informed consent was received. Monocytes were purified by Percoll gradient. The purity of the monocytes preparations used in this study was 90% ± 4%, as assessed by morphology on Giemsa-stained cytocentrifuge preparations and by flow cytometry, using the monocyte-specific monoclonal antibody (MoAb) LeuM3.7 Cells were cultured in RPMI 1640 (GIBCO, Grand Island, NY) containing 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mmol L-glutamine, 20 mmol HEPES (GIBCO), and 10% heat-inactivated fetal bovine serum (HyClone Lab, Logan, UT). This will be referred to as complete medium. Human THP-1, U937, RAJI, DAUDI, JURKAT, SUPT-1, MT4, M9, and C5966 (American Type Culture Collection, Manassas, VA) were cultured in complete medium. The MONO-MAC-6 cell line, an acute leukemia monocytic cell line originally established by H.W. Ziegler-Heitbrok, was obtained from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany).26 These cells were maintained in complete medium supplemented with 1 mmol/L sodium pyruvate and 9 μg bovine insulin (Sigma Chemical Co, St Louis, MO) per milliliter.
Cytokines, antibodies, and reagents.
Human recombinant IFN-γ, IL-4, IL-13, and IL-10 were purchased from Peprotech (Rocky Hill, NJ). Recombinant IL-15 was a kind gift of Dr A. Troutt (Immunex, Seattle, WA). Escherichia coli 0111:B4 LPS was purchased from Sigma. Anti–IL-15 M111 and anti–IL-15 M112 (IgG2b) MoAbs were purchased from Genzyme (Boston, MA). Anti–IL-2 was purchased from Pharma Biotecnologie (Hannover, Germany). Anti–IL-2Rβ A41 was prepared as described.27 Control IgG2b was purchased from Becton Dickinson (San Jose, CA). Fluorescein isothiocyanate (FITC)-labeled goat antimouse Ab used for fluorescent staining was purchased from Southern Biotechnology Associates, Inc (Birmingham, AL).
To obtain human IL-15 mouse Ig G2b (IgG2b) fusion protein, cDNA encoding IL-15 was fused to genomic DNA encoding for the Fc portion of mouse IgG2b as described.28 Biotinylation of human IL-15 mouse IgG2b fusion protein resulted in high stability of the cytokine without reduction of its biological activity, as determined by CTLL proliferation assay.29
Flow cytometry analysis.
For indirect immunofluorescence analysis, cells were preincubated for 30 minutes at 4°C in phosphate-buffered saline (PBS) containing 2% goat serum plus 0.2% sodium azide (NaN3), washed with 1% bovine serum albumine (BSA), and incubated with mouse MoAbs for 30 minutes at 4°C or with an isotype-matched MoAb. The cells were then washed twice with 1% BSA in PBS and incubated with goat antimouse Ig-FITC for a further 30 minutes at 4°C. Cytoplasmic IL-15 was evaluated after permeabilization of the cell membranes using PermeaFix (1:2 dilution; Ortho, Raritan, NJ). To determine the binding of IL-15–IgG2b fusion protein, cells were incubated for 1 hour at 4°C with the biotinylated IL-15–IgG2b fusion protein or with the isotype-matched biotin-conjugated IgG (Pharmingen, San Diego, CA). The cells were then washed twice with 1% BSA in PBS and further incubated with FITC-conjugated avidin (Sigma) for 20 minutes at 4°C. IL-15 binding was assessed by flow cytometry. Labeled cells were analyzed by flow cytometry analysis with a FACSort (Becton Dickinson Immunocytometry Systems, San Jose, CA). Lysis II software was used to analyze FACS data and significance was analyzed by comparison between treated and untreated cells with z-test (*P < .001).
Reverse transcription-polymerase chain reaction (RT-PCR) analysis.
RNA extraction and RT-PCR analysis were performed as described.10 Briefly, total RNA was purified with TRIzol (Life Technologies, Gaithersburg, MD) as specified by the manufacturer’s instructions. cDNA synthesis was performed with 2 μg of RNA in a total volume of 20 μL, containing 50 mmol/L Tris-HCl, pH 8.3, 75 mmol/L KCl, 3 mmol/L MgCl2, 0.1 mol/L dithiothreitol, 40 U of RNase inhibitor (RNase OUT; Life Technologies), 0.5 μg oligo (dT), and 200 U Superscript II reverse transcriptase (Life Technologies). The reaction mixture was incubated at 42°C for 50 minutes and stopped at 90°C for 5 minutes. A 2-μL aliquot of the obtained cDNA was amplified in a 50 μL reaction containing 500 mmol/L KCl, 100 mmol/L Tris-HCl, pH 8.8, 25 mmol/L MgCl2, 2 mmol/L of each dNTP, 2 mg/mL BSA (Pharmacia, Uppsala, Sweden), 200 nmol/L of each primer, and 5 U Taq DNA polymerase (Life Technologies). The mixture was capped with 50 μL of sterile mineral oil. To ensure that equivalent amounts of cDNA were used in each reaction, PCR was also performed for β-actin from each sample, and the cDNA was adjusted to equivalent levels. The following oligonucleotides were used in the PCR reactions: IL-15 sense, 5′-GGATTTACCGTGGCTTTGAGTAATGAG-3′, and IL-15 antisense, 5′-GAATCAATTGCAATCAAGAAGTG-3′ (cycling conditions: 1 minute at 94°C, 1 minute at 80°C, and 2 minutes at 72°C, for 35 cycles25); and β-actin sense, 5′-GAGCGGGAAATCGTGCGTGACATT-3′, and β-actin antisense, 5′-GAAGGTAGTTTCGTGGATGCC-3′ (cycling conditions: 1 minute at 94°C, 1 minute and 30 seconds at 62°C, and 2 minutes at 72°C for 27 cycles).30 A sample (15 μL) of each PCR reaction was electrophoresed through a 2% agarose gel and visualized with ethidium bromide.
Acidic elution.
Acidic elution was performed as described.31 The cells were pelleted by centrifugation and resuspended in acetate buffer, pH 4.4, containing 0.05 mol/L sodium acetate, 0.09 mol/L NaCl, 0.005 mol/L KCl, and 0.05% fetal calf serum (FCS). The suspension was neutralized after 1 to 15 minutes by adding PBS containing 0.05% FCS. The suspended cells were immediately underlayed with 1 mL FCS and centrifuged to pellet the cells. The cells were then washed three times and stained as indicated above. Up to 15 minutes of acid treatment did not significantly change the cell viability as determined by forward and right angle scatter and trypan blue exclusion.
Trypsin treatment.
Cells were washed with PBS and treated with 10 μg/mL L-p-tosylamino-2-phenylethyl chloromethyl ketone (TPCK)-trypsin (Sigma) for 10 minutes at 37°C. The trypsin activity was neutralized by adding 100 μg/mL soybean trypsin inhibitor (Sigma).32
CD3+ T-cell proliferation assays.
CD3+ T cells were purified from peripheral blood lymphocytes (PBL) cell suspensions by using CD3+ T-cell subset enrichment columns (R&D Systems, Minneapolis, MN). For mitogen assays, CD3+ were cultured alone or with 12.5 × 103 monocytes in the presence of 10 μg/mL ConA (Sigma). Before the proliferation assay, monocytes were treated with IFN-γ for 72 hours and with mitomycin-C (Sigma) at 25 μg/107 cells/mL for 30 minutes at 37°C. Each experiment was performed in triplicate. Cultures were incubated for 72 hours at 37°C. Sixteen hours before the termination of the cultures, the plates were pulsed with 0.5 μCi/well 3H thymidine (Dupont, NEN, Boston, MA). The cells were harvested on paper filters and 3H thymidine uptake was measured in a liquid scintillation counter and expressed as total cpm.
Statistical analysis.
All in vitro experiments were performed independently at least three times. Comparison among treatments was performed by analysis of variance. When a difference among multiple treatments was found, the Fisher’s least significant difference test was used to identify which of the means were significantly different from the others at the .05 significance level.
RESULTS
Monocytes display membrane-bound IL-15.
Constitutive expression of IL-15 mRNA has previously been demonstrated in monocytes/macrophages.1 17 However, the IL-15 cytokine could be hardly detected in the supernatant by standard ELISA and CTL proliferation assays. Because of this apparent lack in cytokine secretion, we have tested the hypothesis that IL-15, rather than being released, is constitutively retained in functional form on the cell surface of monocytes.
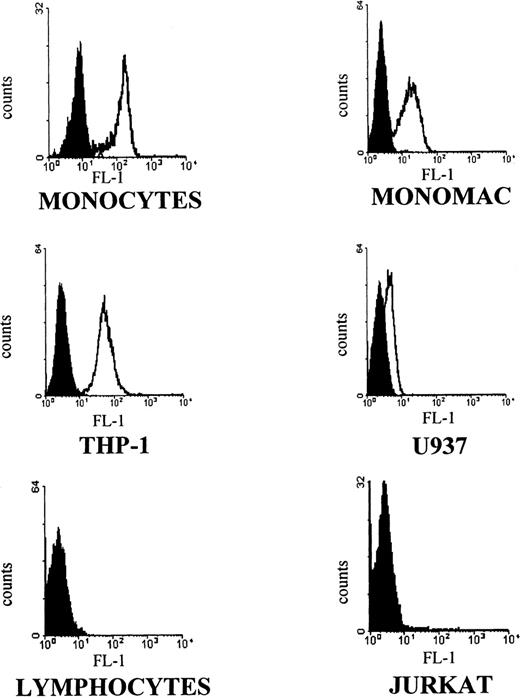
Using the anti–IL-15 antibody M112 in an indirect fluorescence assay, purified peripheral blood monocytes as well as the monocytic cell lines MONO-MAC-6, THP-1, and U937 demonstrated specific immunoreactivity on the surface of tested cells. Immunoreactivity was not seen with purified T lymphocytes (neither resting nor phytohemagglutinin [PHA]-activated T cells) or the JURKAT T-cell line (Fig 1). In addition, the screened panel of T- and B-cell lines (MT4, M9, C5966, SUPT-1; RAJI; DAUDI; and Epstein-Barr virus [EBV]-immortalized B-cell clones) gave negative immunostaining results. These data were fully reproduced using a different MoAb against IL-15 (M111; data not shown).
Flow cytometric analysis with anti–IL-15 M112 antibody. Human peripheral blood purified monocytes (MONOCYTES) and T lymphocytes (LYMPHOCYTES), human monocytic cell lines (MONO-MAC-6, THP-1, U937), and a human T-cell leukemia line (JURKAT) were stained with M112 MoAb followed by goat antimouse FITC-conjugated antibody. Fluorescence intensity is represented by white histograms; black histograms refer to the background staining of isotype-matched control MoAb. Data show one representative of five independent experiments.
Flow cytometric analysis with anti–IL-15 M112 antibody. Human peripheral blood purified monocytes (MONOCYTES) and T lymphocytes (LYMPHOCYTES), human monocytic cell lines (MONO-MAC-6, THP-1, U937), and a human T-cell leukemia line (JURKAT) were stained with M112 MoAb followed by goat antimouse FITC-conjugated antibody. Fluorescence intensity is represented by white histograms; black histograms refer to the background staining of isotype-matched control MoAb. Data show one representative of five independent experiments.
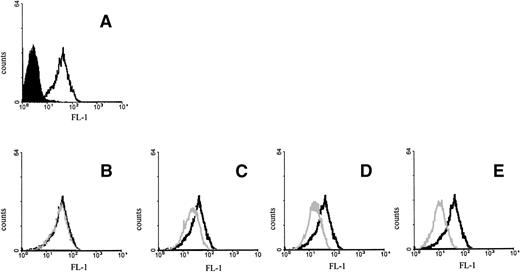
To show the specificity of the binding, we tested whether the addition of exogenous IL-15 competed for the binding of the M112 antibody to MONO-MAC-6 cells. This was the case, because a dose-dependent decrease in antibody staining was observed by preincubating the cells with IL-15 in a dosage range from 10 μg to ng dilution (Fig 2).
Inhibition of M112 MoAb binding by IL-15. MONO-MAC-6 cells were preincubated for 30 minutes at 4°C with medium (A), 0.01 μg (B), 0.1 μg (C), 1 μg (D), or 10 μg (E) of human recombinant IL-15 and then stained with M112 MoAb as described in Fig 1. Open histograms with a bold line represent M112 staining; open histograms with a gray line represent the residual staining after preincubation with IL-15; and solid histograms refer to the background fluorescence.
Inhibition of M112 MoAb binding by IL-15. MONO-MAC-6 cells were preincubated for 30 minutes at 4°C with medium (A), 0.01 μg (B), 0.1 μg (C), 1 μg (D), or 10 μg (E) of human recombinant IL-15 and then stained with M112 MoAb as described in Fig 1. Open histograms with a bold line represent M112 staining; open histograms with a gray line represent the residual staining after preincubation with IL-15; and solid histograms refer to the background fluorescence.
Thus, IL-15 is constitutively present in membrane-bound form on peripheral blood monocytes as well as on the tested monocytic cell lines and can be specifically recognized by antibodies normally used against the soluble cytokine.
IFN-γ significantly upregulates expression of the membrane-associated form of IL-15.
Various monocyte activators have been shown to upregulate IL-15 mRNA expression and to augment the levels of cytoplasmic IL-15.5,17,18 Monocyte activators may also give rise to detectable IL-15 activity in the supernatant of treated cells, although this remains controversial.17 Therefore, we next determined if IFN-γ and/or LPS, two standard monocyte/macrophage activating agents, are able to modulate IL-15 membrane expression as well as the cytoplasmic and mRNA levels of the cytokine.
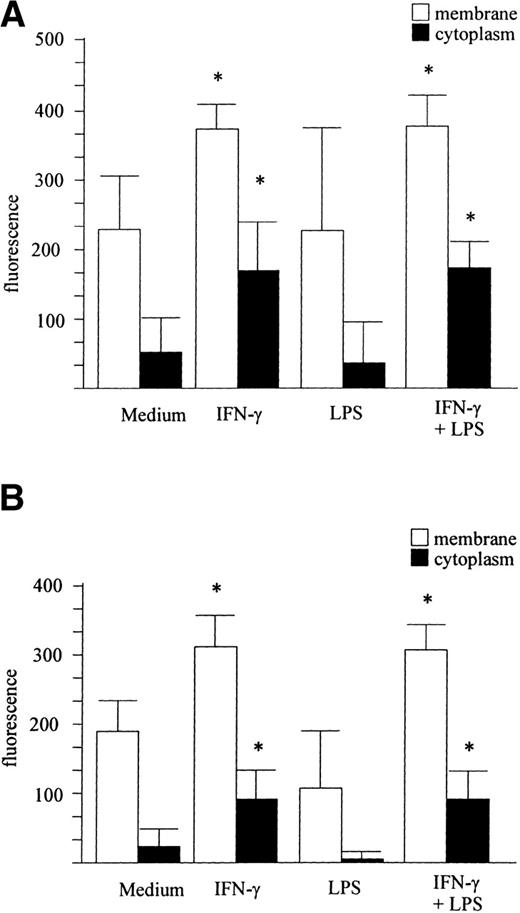
Purified peripheral blood monocytes (Fig 3A) and MONO-MAC-6 cells (Fig 3B) were stimulated for 24 hours with IFN-γ (500 U/mL), LPS (5 μg/mL), or LPS plus IFN-γ or left untreated and stained with M112 MoAb. As shown in Fig 3A and B, IFN-γ significantly increased IL-15 membrane levels, whereas LPS did not affect membrane IL-15 expression and did not potentiate the effect induced by IFN-γ.
IFN-γ upregulates IL-15 expression. The membrane and cytoplasmic forms of IL-15 were evaluated by FACS analysis. Human peripheral blood monocytes (A) and MONO-MAC-6 cells (B) were treated with IFN-γ, LPS, or LPS + IFN-γ or left untreated and were stained with the M112 MoAb as described in Materials and Methods. The mean ± SD from three independent experiments is shown. Significance was analyzed by comparison between treated and untreated cells with z-test (*P < .001). (C) cDNA derived from MONO-MAC-6 cells incubated for 6 hours with medium alone or supplemented with IFN-γ, LPS, or LPS plus IFN-γ were amplified with primers specific for IL-15. β-actin served as the control.
IFN-γ upregulates IL-15 expression. The membrane and cytoplasmic forms of IL-15 were evaluated by FACS analysis. Human peripheral blood monocytes (A) and MONO-MAC-6 cells (B) were treated with IFN-γ, LPS, or LPS + IFN-γ or left untreated and were stained with the M112 MoAb as described in Materials and Methods. The mean ± SD from three independent experiments is shown. Significance was analyzed by comparison between treated and untreated cells with z-test (*P < .001). (C) cDNA derived from MONO-MAC-6 cells incubated for 6 hours with medium alone or supplemented with IFN-γ, LPS, or LPS plus IFN-γ were amplified with primers specific for IL-15. β-actin served as the control.
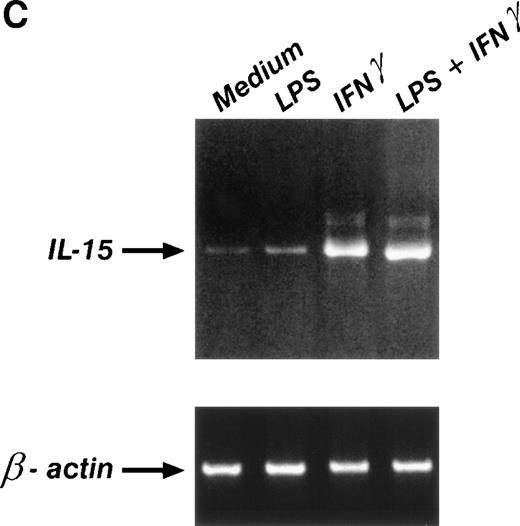
The expression of cytoplasmic IL-15 was evaluated by permeabilizing cell membranes before M112 staining. Untreated monocytes and MONO-MAC-6 cells both showed a basal level of intracellular staining that significantly increased after treatment with IFN-γ. However, LPS did not significantly affect intracytoplasmic IL-15 (Fig 3A and B). This pattern of response was confirmed at the gene level by RT-PCR analysis on MONO-MAC-6 cells. As shown in Fig 3C by a representative example, a low constitutive level of IL-15 mRNA expression was detectable in untreated MONO-MAC-6 but was strongly upregulated by incubation with IFN-γ for 6 hours. Stimulation with LPS alone did not significantly affect IL-15 mRNA and costimulation with LPS plus IFN-γ did not further upregulate the IL-15 mRNA level over the expression level stimulated by IFN-γ alone. The same pattern of response at the mRNA level was observed with peripheral blood monocytes (not shown).
When supernatants of LPS-treated, IFN-γ–treated, or LPS plus IFN-γ–treated monocytes and MONO-MAC-6 cells were tested for the presence of secreted, soluble IL-15 protein by ELISA and for IL-15 activity by CTLL proliferation assay, no detectable IL-15 could be demonstrated (not shown).
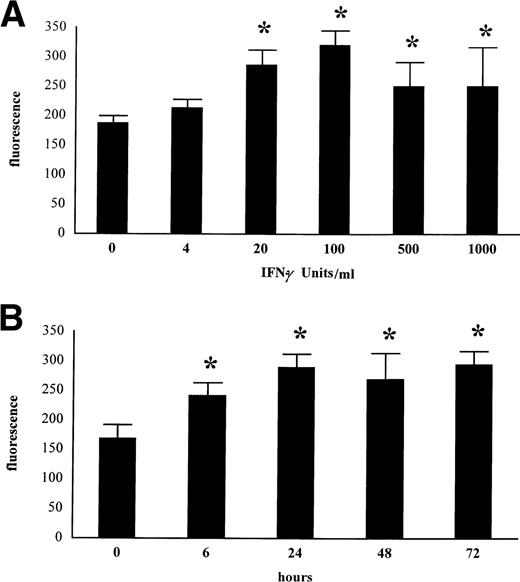
To determine the effect of the IFN-γ concentration on IL-15 membrane expression, MONO-MAC-6 were cultured for 24 hours with increasing concentrations of IFN-γ and were subsequently stained with M112 MoAb as described above. IFN-γ at 20 U/mL significantly increased IL-15 membrane expression, which reached a maximum at 100 U/mL (Fig 4A).
Dose- and time-dependent upregulation of membrane IL-15 after IFN-γ treatment. MONO-MAC-6 were treated with the indicated amounts of IFN-γ for 24 hours (A). In (B), MONO-MAC-6 were treated with medium alone or with IFN-γ (500 U/mL) for the indicated time. Cells were then stained with M112 MoAb as described in Materials and Methods and were analyzed by FACS. Results represent mean ± SD of three independent experiments. Significance was analyzed by comparison between treated and untreated cells with the z-test (*P< .001).
Dose- and time-dependent upregulation of membrane IL-15 after IFN-γ treatment. MONO-MAC-6 were treated with the indicated amounts of IFN-γ for 24 hours (A). In (B), MONO-MAC-6 were treated with medium alone or with IFN-γ (500 U/mL) for the indicated time. Cells were then stained with M112 MoAb as described in Materials and Methods and were analyzed by FACS. Results represent mean ± SD of three independent experiments. Significance was analyzed by comparison between treated and untreated cells with the z-test (*P< .001).
To determine the kinetic of the induction, membrane IL-15 was assessed at different time points after exposure to IFN-γ. As shown in Fig 4B, an increase in the IL-15–associated fluorescence intensity was observed 6 hours after IFN-γ stimulation and reached a plateau at 24 hours. Similar kinetics and dose-response were obtained with purified peripheral blood monocytes (not shown).
Thus, the constitutive expression of membrane-bound IL-15 is strongly and significantly upregulated upon monocyte activation in a dose-dependent manner by IFN-γ treatment. In our experimental setting, LPS failed to significantly induce IL-15 expression.
The membrane form of IL-15 is not associated with the IL-15R complex.
From the data presented above, it cannot be excluded that IFN-γ induces IL-15 to be secreted and that, after release, the cytokine binds to its own receptor complex. In fact, monocytes constitutively express the β33 and γ34 chains of the IL-2R, which are shared by the IL-15R complex. In addition, IFN-γ treatment is known to upregulate IL-2Rγ and to induce IL-15Rα expression.16,34 To investigate this possibility, IFN-γ–treated MONO-MAC-6 were incubated with acetate buffer, pH 4.4, which solubilizes cytokines associated with their cognate receptors.31 35
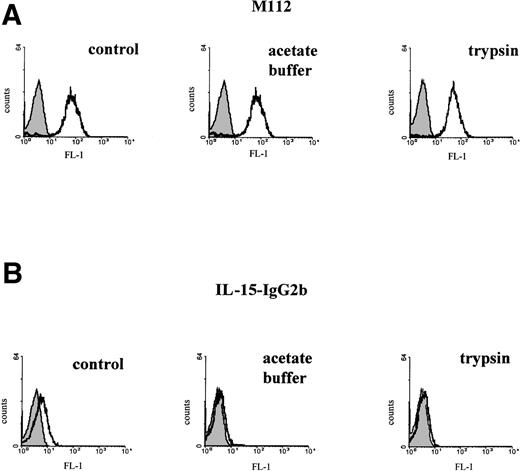
Figure 5A shows that there was no change in the mean fluorescence intensity of MONO-MAC-6 stained with anti–IL-15 MoAb after 10 minutes of treatment with acid buffer compared with cells incubated in normal medium. This suggests that membrane-bound IL-15 is not attached to its receptor but is an integral protein. As a control, we tested the effect of the acetate buffer treatment on the binding of an IL-15–IgG2b fusion protein to MONO-MAC-6 cells. As shown in Fig 5B (center panel), the binding of the IL-15 component to the IL-15R was totally displaced by such treatment.
Cell surface IL-15 is not associated with its own receptor. (A) MONO-MAC-6 were treated with IFN-γ (500 U/mL) for 24 hours. Cells were then washed and incubated in PBS, acetate buffer (pH 4.4), or trypsin as described in Materials and Methods. All groups were stained with M112 MoAb and analyzed on FACSort. Fluorescence intensity is represented by open histograms; solid histograms refer to the background staining of isotype-matched control MoAb. (B) MONO-MAC-6 treated with IFN-γ as described above were incubated with PBS (left panel) or trypsin (right panel). Cells were then incubated with biotin-conjugated IL-15 IgG2b fusion protein (open histogram) or with equal amount of isotype-matched biotinylated IgG (solid histogram). In the central panel, cells were incubated with the IL-15 IgG2b fusion protein and then with acetate buffer, pH 4.4. The x axis represents the intensity of green fluorescence expressed in a log scale as mean channel, and the y axis represents the number of cells per channel.
Cell surface IL-15 is not associated with its own receptor. (A) MONO-MAC-6 were treated with IFN-γ (500 U/mL) for 24 hours. Cells were then washed and incubated in PBS, acetate buffer (pH 4.4), or trypsin as described in Materials and Methods. All groups were stained with M112 MoAb and analyzed on FACSort. Fluorescence intensity is represented by open histograms; solid histograms refer to the background staining of isotype-matched control MoAb. (B) MONO-MAC-6 treated with IFN-γ as described above were incubated with PBS (left panel) or trypsin (right panel). Cells were then incubated with biotin-conjugated IL-15 IgG2b fusion protein (open histogram) or with equal amount of isotype-matched biotinylated IgG (solid histogram). In the central panel, cells were incubated with the IL-15 IgG2b fusion protein and then with acetate buffer, pH 4.4. The x axis represents the intensity of green fluorescence expressed in a log scale as mean channel, and the y axis represents the number of cells per channel.
This concept was further probed by an additional experimental approach. We have observed that IL-15R can be cleaved by trypsin treatment so that the cells lose their ability to bind the IL-15–IgG2b fusion protein used as receptor detector. This made it reasonable to expect that, if IL-15 detected on the membrane was indeed bound to its receptor, the whole ligand-receptor complex should be cleaved by trypsin, and trypsinized cells should not bind either M112 or IL-15–IgG2b. Therefore, IFN-γ–induced MONO-MAC-6 were treated with 10 μg/mL trypsin for 10 minutes at 37 °C and stained with M112 antibody or IL-15–IgG2b. As shown in Fig 5A, the fluorescence intensity due to M112 staining was unchanged, whereas IL-15–IgG2b did not bind any longer to the cell surface, indicating that IL-15R had successfully been cleaved from the cell membrane (Fig 5B).
These results indicate that the cell surface IL-15 immunoreactivity is not due to IL-15 bound to its cognate receptor.
Monocyte-inhibitory cytokines do not affect IL-15 membrane expression.
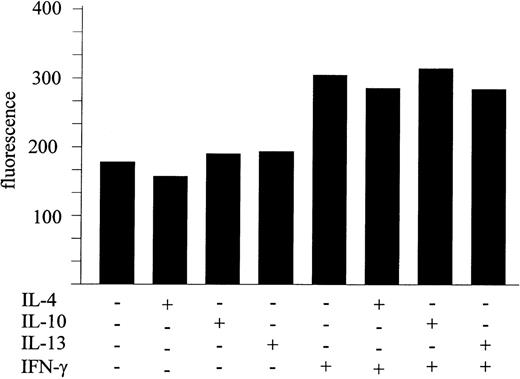
Many of the functionally important monocyte/macrophage activities induced by IFN-γ and other monocyte activators can be downregulated by IL-4, IL-13, and/or IL-10.36-41 However, it has recently been reported that IL-4 and IL-13 fail to significantly inhibit IL-15 gene expression, whereas IL-10 was even able to upregulate the message for IL-15 in murine macrophages.17 Therefore, it was of interest to establish whether these cytokines could modulate the expression of membrane-bound IL-15. To test this, anti–IL-15 staining of MONO-MAC-6 treated for 24 hours with medium alone, IFN-γ (500 U/mL), IL-4 (100 U/mL), IL-13 (50 ng/mL), IL-10 (100 U/mL), or IFN-γ plus IL-4, IL-13, or IL-10 was performed. Figure 6 shows that, among the tested cytokines, only IFN-γ significantly upregulated membrane IL-15, whereas IL-4, IL-13, and IL-10 had no modulatory effects when added to the cultures singly. Furthermore, these cytokines were unable to counteract the stimulatory effect of IFN-γ. Conversely, IL-4, IL-13, and IL-10 treatment of MONO-MAC-6 inhibited monocyte chemotactic protein-1 (MCP-1) production induced by IFN-γ (not shown)
Inhibitory cytokines do not affect IL-15 membrane expression. MONO-MAC-6 were treated as indicated for 24 hours and stained with M112 MoAb as described in Materials and Methods. One representative experiment of three independent experiments, which gave similar results, is shown.
Inhibitory cytokines do not affect IL-15 membrane expression. MONO-MAC-6 were treated as indicated for 24 hours and stained with M112 MoAb as described in Materials and Methods. One representative experiment of three independent experiments, which gave similar results, is shown.
Membrane-bound IL-15 is biologically active.
IL-15 is a potent growth factor for T-cell proliferation.1 42 To investigate the ability of membrane-bound IL-15 to support T-cell proliferation, ConA-stimulated human purified CD3+ T cells were cocultured with mitomycin-treated monocytes that had previously been stimulated with IFN-γ for 72 hours to assure an optimal membrane level of IL-15. T-cell proliferation was assessed by a [3H] thymidine uptake test.
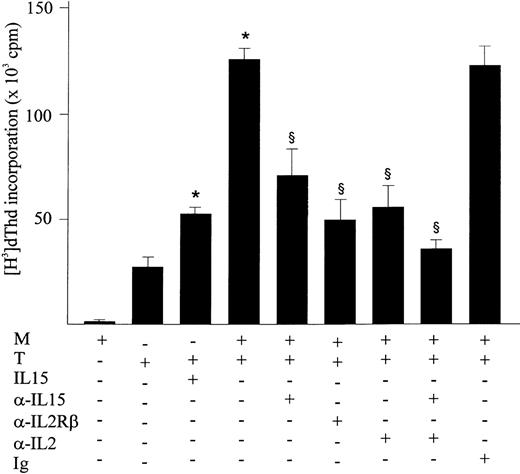
This assay showed that proliferation of CD3+ T cells was significantly enhanced by the presence of IFN-γ–treated monocytes as compared with T cells alone (Fig 7). Monocytes from 8 subjects were treated with IFN-γ, which increased their ability to stimulate T-cell proliferation up to 33% as compared with untreated monocytes (181.140 ± 22.700 v 136.630 ± 33.370, P < .01). The addition of neutralizing anti–IL-1543 and blocking anti–IL-2Rβ12 13antibodies to the culture significantly inhibited the CD3+T-cell proliferation induced by coincubation with IFN-γ–activated, mitomycin-treated monocytes (Fig 7). Furthermore, using ELISA it was determined that the anti–IL-15 treatment of the coculture inhibited the IFN-γ release from activated T cells in the supernatants by 50% (not shown). The addition of anti–IL-2 antibodies also had an inhibitory effect on proliferation by blocking the IL-2 released by stimulated T cells. The combination of neutralizing anti–IL-15 and anti–IL-2 antibodies showed an additive inhibitory effect on proliferation. The isotype-matched antibody showed no effect.
Membrane-bound IL-15 is biologically active. Human purified CD3+ T cells were incubated with medium, IL-15 (10 ng/mL), IFN-γ–treated and mitomycin C-treated monocytes, or the latter plus anti–IL-15, anti–IL-2, or anti–IL-2Rβ antibodies (10 μg/mL), all in presence of ConA (10 μg/mL). Proliferative activity was assessed by a (3H) thymidine incorporation assay. One representative experiment of three that produced similar results is shown. Significance was analyzed by comparison between antibody-treated and untreated cells with Fisher’s Least-test. *Significant induction by treatment (P < .05). §Significant inhibition by treatment (P < .05).
Membrane-bound IL-15 is biologically active. Human purified CD3+ T cells were incubated with medium, IL-15 (10 ng/mL), IFN-γ–treated and mitomycin C-treated monocytes, or the latter plus anti–IL-15, anti–IL-2, or anti–IL-2Rβ antibodies (10 μg/mL), all in presence of ConA (10 μg/mL). Proliferative activity was assessed by a (3H) thymidine incorporation assay. One representative experiment of three that produced similar results is shown. Significance was analyzed by comparison between antibody-treated and untreated cells with Fisher’s Least-test. *Significant induction by treatment (P < .05). §Significant inhibition by treatment (P < .05).
To exclude the possibility that the functional effects described above simply resulted from the shedding of previously membrane-bound IL-15 into the medium, the supernatants were also tested by ELISA for the presence of soluble IL-15. However, no IL-15 protein could be detected (data not shown), suggesting that the CD3+ proliferative effect observed after coculture with monocytes reflects genuine bioactivity of membrane-bound IL-15.
Taken together, these experiments suggest that IL-15 expressed on the cell surface of IFN-γ–stimulated monocytes is functionally active.
DISCUSSION
In this study, we show that IL-15 exists as a biologically active, cell surface-bound molecule on human monocytes and monocytic cell lines. Its expression is upregulated by IFN-γ treatment, but unaffected by IL-4, IL-13, and IL-10, and it is not associated with the IL-15R complex.
We have shown that, although IFN-γ is a potent inducer of IL-15 mRNA and of intracytoplasmic and membrane-bound IL-15 in both monocytes and MONO-MAC-6 cells, LPS does not significantly affect IL-15 expression at any level, although, in other respects, MONO-MAC are responsive to LPS.44 These results differ from those of Carson et al,5 who observed induction of intracytoplasmatic IL-15 in monocytes treated with LPS. However, these investigators used monocytes purified by adherence, a procedure known to provide additional stimuli for cytokine induction.45 Bamford et al19 have shown that high levels of IL-15 mRNA are expressed in monocytes treated with LPS plus IFN-γ. However, the effect of LPS or IFN-γ alone is not mentioned in that study,19 so the reported effect might well reflect the same IL-15 upregulation by IFN-γ reported here.
In murine systems, IL-15 mRNA is upregulated by several microbiological agents, including mycobacteria, Toxoplasma gondii, and LPS.17,19 Pretreatment of mouse macrophages with IFN-γ synergizes with BCG to increase IL-15 mRNA expression, but IFN-γ has little effect if added alone or after microbial stimulation. Our current data suggest that, in human monocytes, IFN-γ alone is a potent stimulus for IL-15 mRNA expression. This discrepancy could be explained by the differences between the two species in question as well as by the sources of cells (bone marrow or peritoneal macrophages v monocytes from peripheral blood). During the activation of murine macrophages, IFN-γ usually acts as a priming stimulus, and to achieve activation a second signal either physiologic (eg, IL-2) or environmental (eg, LPS) is required. In contrast, human monocytes can be activated by IFN-γ alone without the need for costimulatory signals.46
IL-4, IL-13, and IL-10 downregulate the activation of mononuclear phagocytes and antagonize IFN-γ–induced responses, including the production of several cytokines. Whereas IL-4, IL-13, and IL-10 inhibited IFN-γ–induced expression of MCP-1 (data not shown), the same cytokines failed to inhibit the constitutive expression of membrane IL-15 as well as the upregulation induced by IFN-γ treatment. Our results are in agreement with the study by Doherty et al17 in which the same cytokines did not influence IL-15 message expression in the murine system. Thus, IL-15 cytokine expression levels appear to be much more stable and less easily influenced than those of other proinflammatory cytokines.
Despite the very widespread distribution of IL-15 mRNA, it has been difficult to demonstrate IL-15 in the supernatants of many cells that express its message. In fact, only a single report demonstrates that murine macrophages activated in vitro with live mycobacteria produce detectable levels of IL-15 bioactivity as assessed with the CTLL-2 bioassay.17 We were unable to detect any IL-15 activity or IL-15 protein in the culture of monocytic cells, even upon a strong upregulation of IL-15 at the level of mRNA, membrane, and cytoplasmic form after IFN-γ stimulation. However, addition of neutralizing anti–IL-15 antibody to different in vitro models specifically abrogated IL-15 biological functions.5,18 43 In the current study, we show that, in the absence of detectable IL-15 in the supernatants and in the presence of a membrane-bound IL-15 form, IFN-γ–treated and mitomycin-treated monocytes can stimulate T-cell proliferation as well as IFN-γ release, which can be blocked by IL-15 neutralizing antibodies.
Thus, we can postulate that the membrane-bound form of IL-15 is likely to be the biologically active cytokine that directs the activities mentioned above. We are currently investigating if the IL-15 membrane-associated form also exerts other biological activities attributed to IL-15 such as preventing apoptosis in multiple systems.28 47
IL-15 production seems to be regulated at multiple levels. It has been proposed that the control is mainly posttranscriptional, ie, at the level of protein translation and intracellular trafficking rather than transcription.19,22,23 Two isoforms differing in the leader peptide sequences have been identified. Transfection of COS cells with the different isoforms showed that IL-15 associated with the short signal peptide is not secreted but is stored intracellularly, appearing in the nucleus and cytoplasmic components; on the contrary, the long signal peptide isoform is observed in the ER but its secretion is inefficient.22 23 Preliminary transfection experiments, with constructs carrying the two different isoforms, are being performed to clarify if the long signal peptide isoform is the form presented on the membrane. The results so far suggest that the expression of the membrane form of IL-15 does not depend on the signal peptide sequence.
The concept of the existence of a membrane-bound form of IL-15 is intriguing, because it enlists IL-15 into a small family of cytokines for which such cell surface-associated isoforms have also been detected. Like IL-15, other cytokines playing a pivotal role in the modulation of immune responses such as tumor necrosis factor-α (TNF-α), IL-1, IL-10, and transforming growth factor α (TGFα) have been reported to be secreted and to have a membrane form that is biologically active.31,35,48-51 It now needs to be clarified whether the secreted and membrane-bound IL-15 isoforms act differently and whether the membrane-bound IL-15 can be cleaved and released. We are currently testing if the activity of membrane bound IL-15 in juxtacrine signaling may be controlled by protease/antiprotease systems.52 It is tempting to speculate that membrane-associated IL-15 modulates constitutive, physiologic immune response, whereas the intracytoplasmic IL-15 pool is kept in store to be secreted only upon special demands and under pathological conditions.
ACKNOWLEDGMENT
The authors thank Dr Raffaele Badolato and Dr Thomas Pohl for helpful discussions.
Supported in part by grants from the 2nd National Project on Tuberculosis (ISS Rome, Italy), from AIRC (Milan, Italy), and from the Deutsch Forschungsgemainschaft (SFB 506/C5).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Tiziana Musso, PhD, Institute of Microbiology, Via Santena 9, 10126 Torino, Italy; e-mail:musso@molinette.unito.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal