Intestinal mucosal mast cells (IMMC) express granule neutral proteases that are regulated by T-cell–derived cytokines, including interleukin-3 (IL-3) and IL-9, and by stem cell factor (SCF). The IMMC-specific chymase, mouse mast cell protease-1 (mMCP-1), is released in substantial quantities into the blood stream during gastrointestinal allergic responses. We used cultured bone marrow-derived mast cells (mBMMC) to identify cytokines that regulate the expression and extracellular release of mMCP-1. When grown in IL-3–rich WEHI (15% vol/vol) and 50 ng/mL recombinant rat SCF (rrSCF) bone marrow cells supplemented with IL-9 (5 ng/mL) differentiated into mBMMC that expressed a maximum of less than 250 ng mMCP-1/106 cells and 189 ng mMCP-1/mL of culture supernatant. Supplementation of the same three cytokines with transforming growth factor-β1(TGF-β1; 1 ng/mL) resulted in substantially enhanced expression (6 μg/106 mBMMC) and extracellular release (2 μg/mL of culture supernatant) of mMCP-1. The response to TGF-β1 was dose-dependent, with maximal effect at 1 ng/mL, and was associated with immunohistochemical and ultrastructural changes in the secretory granules. IL-9–induced expression of mMCP-1 may be due to endogenously expressed TGF-β1, because it was blocked by anti–TGF-β antibodies. In conclusion, the expression and extracellular release of the IMMC-specific chymase, mMCP-1, is strictly regulated by TGF-β1.
THE PROLIFERATION, differentiation, and survival of intestinal mucosal mast cells (IMMC) in murine rodents are regulated by several distinct mechanisms. The most critical of these involves the ligand for c-kit and its tyrosine kinase receptor encoded by the c-kit proto-oncogene.1 The crucial importance of this mechanism is demonstrated by the absence of IMMC in murine rodents with mutations affecting the c-kit gene or of its ligand,2,3 which is known as kit-ligand, Steel factor, and stem cell factor (SCF), the designation that will be used herein.1 Similarly, survival of IMMC undergoing hyperplasia in response to nematode infection is also substantially reduced in murine rodents treated with antibodies directed against SCF or against c-kit.4,5 These studies on mutant mice and rats, and using antibody treatments, show that the interaction of SCF and of c-kit is absolutely essential for the generation and survival of proliferating IMMC populations.2-5
The second mechanism that influences IMMC proliferation during intestinal nematode infection is mediated by T cells6,7 and both interleukin-3 (IL-3) and IL-4 are implicated in the proliferation of IMMC.8,9 Similarly, mice transgenic for increased expression of IL-9, a mast cell growth factor,10 have substantially increased numbers of IMMC in the absence of any additional immunological stimulus in the gut.11
The mechanisms regulating the differentiation of IMMC are not understood. In murine rodents, humans, and other species, IMMC are morphologically, histochemically, and functionally distinct from connective tissue mast cells (CTMC).12-14 Mucosal mast cells are significant because of their involvement in gut hypersensitivities,15 in gastric ulceration and inflammation,16,17 and in protective responses against gastrointestinal nematodes.18 Therefore, it is important to determine the regulatory mechanisms which promote the tissue-specific expression of genes unique to IMMC.
One of the key differences between IMMC and CTMC is in the expression of secretory granule neutral proteases.19-21 In the mouse, the β-chymase, mouse mast cell protease-1 (mMCP-1), is expressed only by mast cells at mucosal surfaces.22,23 Similarly, rat mast cell protease-II (rMCP-II), a β-chymase highly homologous to mMCP-1,19 and sheep mast cell protease-1 (sMCP-1) are abundantly expressed by mucosal mast cells (MMC) but not by CTMC.18,19 Each of these MMC-associated proteases is secreted systemically and into the gut lumen during gastrointestinal allergic responses.18 19
Attempts to generate an in vitro analog of IMMC in the mouse, using bone marrow-derived mast cells (mBMMC), have not been successful. Early studies in which mBMMC were grown in the presence of IL-3 alone suggested that the cultured cells shared many features in common with IMMC.13 However, it was subsequently shown that these cells expressed little or none of the IMMC-specific chymases and that, morphologically, they were distinct from IMMC.13,14,20 In contrast, mBMMC supported by T-cell–derived conditioned medium24 or by a combination of SCF/IL-9 or of SCF/IL-10 expressed the IMMC-specific chymase, mMCP-1.25 26
The initial aim of the present study was to determine whether the constitutive systemic secretion of mMCP-1 seen in normal mice,27 which is greatly enhanced in mice transgenic for the overexpression of IL-9,11 could be reproduced in vitro using mBMMC. The first series of experiments addresses this question. The results show that IL-9 stimulates levels of mMCP-1 expression and secretion by mBMMC in vitro that are much lower than might be expected from in vivo studies.19 27 This indicates that additional growth/differentiation factors are required.
Such factors could, like SCF,28 be associated with the mucosal epithelium, because greater than 95% of IMMC differentiate intraepithelially in mouse intestine.29-31 One possible candidate is transforming growth factor-β1(TGF-β1), which is expressed by enterocytes, T cells, eosinophils, and many other cells found in the intestinal microenvironment.32,33 This cytokine has multiple immunoregulatory roles, including that of augmenting the expression of the surface integrins αEβ7 on both T cells and mBMMC.34,35 TGF-β1 modulates expression of other genes in mast cells, including the FcεR1-induced members of the chemokine family.36 Therefore, we reasoned that the constitutive expression of SCF28 and of TGF-β132 by enterocytes could influence the differentiation of IMMC that are so intimately associated with gut epithelium.
The hypothesis that TGF-β1 is a key regulatory signal in the differentiation of IMMC was tested by growing mBMMC in the presence or absence of this cytokine. Our results show that the chymase mMCP-1 is expressed at very high levels and is released in a dose-dependant fashion into the supernatant when TGF-β1 is added to the cultures. They also show that the IL-9–induced expression of mMCP-1 is probably regulated through autostimulation by endogenously secreted TGF-β1. This novel role for TGF-β1 in the regulation of the IgE- independant extracellular release of mMCP-1 has substantial implications for our understanding of mast cell biology. Importantly, the profound immunoregulatory influence that TGF-β1 is known to have at mucosal surfaces may now also extend to the mucosal mast cell.
MATERIALS AND METHODS
Production of WEHI-3B IL-3–rich conditioned medium.
The mouse myelomonocyte cell line WEHI-3B37 (European Collection of Animal Cell Cultures, Porton Down, Salisbury, UK) was grown in RPMI 1640 (Life Technologies Ltd, Paisley, UK), 10% heat-inactivated fetal calf serum (FCS; Serotec, Kidlington, Oxford, UK), 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin to high cell density. The resultant maximum supernatant was tested for use as an IL-3–rich conditioned medium by its ability to sustain growth of the IL- 3–dependent cell line AC-2.
Bone marrow mast cell culture.
Groups of five 10- to 12-week-old male BALB/c mice were killed and their femurs were removed under sterile conditions. Bone marrow was washed from the femurs using a 23-gauge needle and 5-mL syringe filled with Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies Ltd) containing 10% FCS (Serotec), 100 U/mL penicillin, 100 μg/mL streptomycin, 2.5 μg/mL fungizone, 2 mmol/L L-glutamine, and 1 mmol/L sodium pyruvate (DMEM/FCS). Cells were suspended by passing them through a 19-gauge needle 3 times, followed by filtration through sterile lens tissue, and then were centrifuged at 230g for 7 minutes at room temperature. After resuspension in 50 mL DMEM/FCS, the cells were counted in an Improved Neubauer counting chamber (×10 magnification) using 0.2% Nigrosin exclusion to measure viability. Nucleated cells were counted after dilution in white blood cell counting fluid. Cell cultures were set up in a humid 5% CO2 incubator using 162 cm2 flat-bottomed flasks or 24-well microtiter plates at 5 × 105cells/mL in DMEM/FCS, containing various combinations of 15% WEHI-3B, conditioned medium (15% WEHI), 50 ng/mL rrSCF (Amgen, Thousand Oaks, CA), 5 ng/mL recombinant mouse IL-9 (rmIL-9; R&D Systems Ltd, Abingdon, UK), and 1 ng/mL recombinant human TGF-β1(rhTGF-β1; Sigma, Poole, UK). Where cultures were maintained for more than 4 days, they were fed at 3- to 4-day intervals. The concentrations of WEHI-3B and of SCF were determined in a series of pilot experiments quantifying maximum bone marrow cell growth with a yield of greater than 80% mBMMC within 7 days of initiating the culture. Optimal concentrations of IL-9 and TGF-β1 were determined experimentally as described in Results.
Treatment of mBMMC cultures with anti–TGF-β antibodies.
mBMMC grown in culture for 7 days in various combinations of cytokines, were incubated for 48 hours with chicken anti–TGF-β antibody (R&D Systems Ltd) or normal purified chicken IgG control (Sigma) at 1 μg/mL.
Preparation of cytosmears.
Cultured mBMMC (5 × 104 cells in 100 μL) were cytocentrifuged (Shandon, Runcorn, Cheshire, UK) onto clean glass slides for 5 minutes at 40g. The cytosmears were air-dried and fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS), pH 7.5, for 45 minutes at 45°C24 and stored in 70% ethanol at 4°C. Slides from each culture were also immediately stained with Leishmans24 for morphological analysis, and others were fixed in Carnoy’s fluid before staining with toluidine blue for mast cell counts.24
Immunohistochemistry and toluidine blue staining of cytosmears.
Endogenous peroxidase activity in paraformaldehyde-fixed cytosmears was eliminated by incubation for 30 minutes in methanol containing 1% H2O2.24 Cytosmears were then washed in H2O and nonspecific binding of antibodies was blocked with PBS/0.5 mol/L NaCl containing 0.5% Tween-80 (Serotec Ltd, Crawley, UK; PBS/T80). Cytosmears were probed with a rat IgG1 monoclonal antibody (MoAb) raised against mMCP-1 (MoAb RF6.1 maximum supernatant diluted 1:10 in PBS/T80)30 or rat IgG1 (10 μg/mL) in PBS/T80 as negative control. Detection was with biotinylated antirat IgG1 (Vector Laboratories Ltd, Peterborough, UK) followed by Vectastain standard ABC kit (Vector Laboratories Ltd) and diaminobenzidine (DAB) peroxidase substrate kit (Vector Laboratories Ltd), with hematoxylin as counterstain. Slides were also probed with a biotinylated sheep polyclonal antibody raised against mMCP-122 and detected with the ABC/DAB system described above. Cytosmears fixed in Carnoy’s were stained with 0.5% toluidine blue (pH 0.5) overnight,24 washed in distilled H2O, and counterstained for 30 seconds with 1% eosin in 70% ethanol. All slides were washed, dehydrated, and mounted in DPX (Fisher Scientific UK, Loughborough, UK). Median cell counts were compared using the nonparametric Mann-Whitney test (Minitab) with significance levels ofP < .05.
Cell pellets and supernatants for enzyme-linked immunosorbent assay (ELISA) and Western blotting.
Cells (2.5 × 106) were washed 3 times in PBS and centrifuged at 230g for 7 minutes. The supernatant was discarded and the cell pellet was frozen at −70°C for use in ELISA and Western blotting. Cell supernatants were obtained before feeding the culture. The cells were centrifuged at 230g for 7 minutes, and 1 mL of the supernatant was removed and frozen at −70°C.
ELISA to quantify mMCP-1.
Western blotting.
Western blotting of material from cell pellets lysed by repeated freeze-thawing and detection with MoAb RF 6.1 was performed as previously described.30 The detection system used was alkaline phosphatase-labeled monoclonal antirat IgG1(Sigma) followed by incubation with 5-bromo 4-chloro 3-indolyl phosphate/nitro blue tetrazolium (BCIP/NBT).
Flow cytometry to detect c-kit expression.
At various stages of bone marrow culture, 5 × 105cells from each culture were centrifuged at 230g for 7 minutes and incubated on ice with rat anti–c-kit MoAb (ACK-2)4 (a gift from Dr H. Faulkner, University of Manchester, Manchester, UK) at 3 μg IgG/mL for 30 minutes in PBS containing 1% bovine serum albumin (BSA) and normal rat IgG (Sigma) was used as a control at the same concentration. The cells were washed in PBS and incubated on ice for 30 minutes with biotinylated antirat IgG (Vector Laboratories Ltd). Cells expressing surface c-kit and binding MoAb ACK-2 and biotinylated mouse antirat IgG were labeled with streptavidin phycoerythrin (R&D Systems Ltd). Labeled cells were scanned on a Becton Dickinson fluorescence activated flow cytometer (FACScan).
Detection of transcripts by reverse transcription-polymerase chain reaction (RT-PCR).
Total RNA was extracted from cell pellets in 0.5 to 1 mL Tri-Reagent (Sigma) according to the manufacturer’s specifications. Semiquantitative RT-PCR was used to detect IMMC specific and nonspecific products essentially as described in our previous studies.38 Because mast cell-associated heparin can copurify with RNA and is known to inhibit the PCR reaction,39 aliquots of 4 μg RNA were incubated with 4 U heparinase I (Sigma), in addition to 115 U deoxyribonuclease I (Sigma) to remove any contaminating genomic DNA, in 5 mmol/L Tris-HCl, pH 7.5, 1 mmol/L CaCl2, and 40 U RNAase inhibitor (Promega, Southampton, UK) in a total volume of 40 μL for 2 hours at 25°C and then for 5 minutes at 95°C. Serial dilutions of the heparinase-treated samples were reverse transcribed in 20 μL volumes containing 1, 0.1, 0.01, or 0.001 μg RNA, 1 mmol/L dNTPs, 20 U RNAase inhibitor, 2.5 μmol/L oligo dT primers, 1× RT buffer, 2.5 mmol/L MgCl2, and 50 U of avian myeloblastosis virus (AMV) reverse transcriptase (Promega) at 42°C for 1 hour. After 5 minutes of incubation at 95°C, each RT reaction was diluted 10-fold to 200 μL and 10 μL was used for each PCR reaction. The cDNA was amplified for 1 minute at 94°C, 2 minutes at 63°C, and 3 minutes at 72°C for 30 thermocycles in 50 μL volumes containing 250 μmol/L dNTPs, 250 nmol/L of each primer, 1× Boehringer Mannheim PCR buffer [5 mmol/L Tris-HCl, pH 9.2, 16 mmol/L (NH4)2SO4, 1.5 mmol/L MgCl2], and 2.5 U Taq DNA polymerase (Boehringer Mannheim, Lewes, East Sussex, UK). Four pairs of oligonucleotide primers were used to identify transcription of chymase genes commonly expressed in mouse mast cells: mMCP-1 (460 bp)40 5′ primer, 5′-GGAAAACTGGAGAGAAAGAACCTAC, and 3′ primer, 5′-GACAGCTGGGGACAGAATGGGG; mMCP-2 (525 bp)41 5′ primer, 5′- ATTTCATTGCCTAGTTCCTCTGAC, and 3′ primer, 5′-CAGGATGAGAACAGGCTGGGAT; mMCP-4 (454 bp)42 5′ primer, 5′-GTAATTCCTCTGCCTCGTCCTTC, and 3′ primer, 5′-GGACAGGATGGACACATGCTTT; and mMCP-5 (418 bp)43 5′ primer, 5′-GGCAGAACAAACGTGAATGAGCC, and 3′ primer, 5′-AAGAACCTTCTGGAAGCTCAGGG. In addition, gene-specific oligonucleotide primers were used for mouse TGF-β1 (406 bp)44 5′ primer, 5′-CGGGGCGACCTGGGCACCATCCATGAC, and 3′ primer, 5′-CTGCTCCACCTTGGGCTTGCGACCCAC, and the mast cell nonspecific housekeeping genes mouse glyceraldehyde 3-phosphate dehydrogenase (G3PDH; 450 bp)45 5′ primer, 5′-GAAGGGCTCATGACCACAGTCCATG, and 3′ primer, 5′-TGTTGCTGTAGCCGTATTCATTGTC, or mouse β-actin (514 bp) 5′ primer, 5′-TGTGATGGTGGGAATGGGTCAG, and 3′ primer, 5′- TTTGATGTCACGCACGATTTCC (purchased from Stratagene, Cambridge, UK). Primers for housekeeping genes were included in each set of PCR reactions as a control to eliminate variations in the heparinase/reverse transcription reactions that could affect the efficiency of subsequent PCR reactions, in addition to controls containing heparinase–treated RNA only (no cDNA). PCR products were separated on 1.2% agarose gels stained with ethidium bromide and visualized and recorded under UV light with a CCD camera linked to an image processor (Oncor/Appligene, Watford, UK). The authenticity of the PCR products were confirmed by Southern hybridization using gene-specific oligonucleotide probes, as described previously.38
RESULTS
Expression of mMCP-1 in the presence of IL-9.
Analysis of the differentiation of bone marrow cells has shown that a combination of IL-3/SCF/IL-9 promotes the early differentiation of mBMMC.46 To determine whether the presence of IL-9 promoted significant expression and secretion of mMCP-1, bone marrow cells grown in IL-3–rich WEHI (15%), rrSCF (50 ng/mL), and rmIL-9 (5 ng/mL) and control cells grown in WEHI/rrSCF were harvested at intervals and the proportions of mMCP-1+ mBMMC, and the content of mMCP-1 in the cell pellets and culture supernatants was compared.
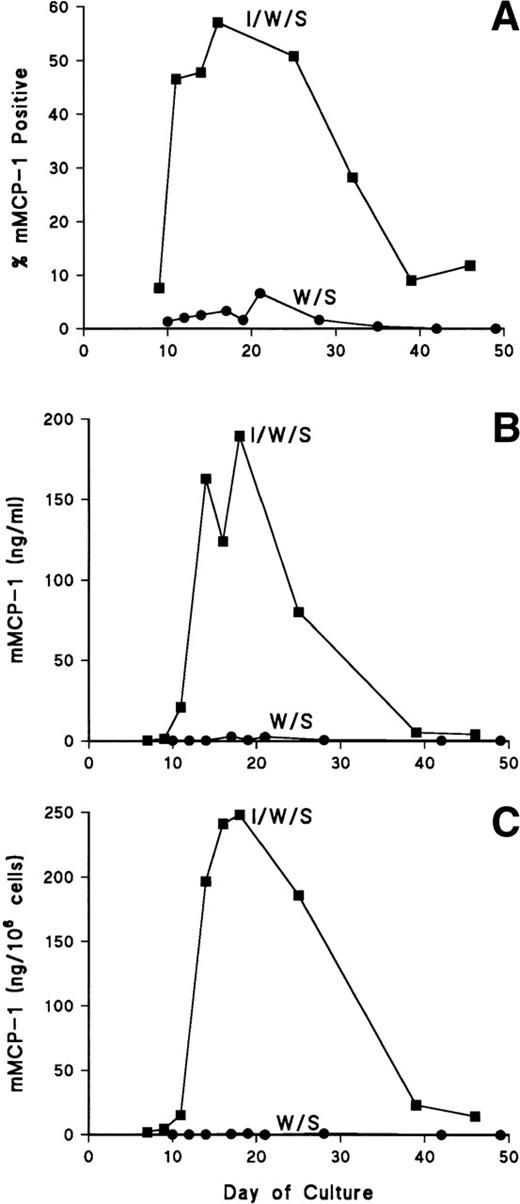
The results of one such experiment are shown in Fig 1. In both cultures, the percentage of mast cells as assessed from Leishman-stained cytosmears was greater than 80% by day 10 and remained at greater than 95% (>95% viable) from day 14 until completion of the study. Expression of mMCP-1 on day 10 was low in both cultures, but the proportion mMCP-1+cells increased rapidly, reaching a maximum of 57% in the presence of rmIL-9, on day 16, and 3% mMCP-1+ mBMMC in the absence of rmIL-9 (Fig 1A). The secretion of mMCP-1 into the culture supernatants reflected the proportions of positive cells in the two populations with a maximum concentration of 189 ng/mL in the rmIL-9 supplemented culture and less than 3 ng/mL in the controls (Fig 1B). Concentrations of mMCP-1 in the cell pellets, similarly, reflected the levels in the supernatants (Fig 1B and C). In four repeat experiments, results were similar, except that mMCP-1+mBMMC were often maximal (25% to 40%) at 11 to 14 days of culture and that levels of mMCP-1 rarely exceeded 50 ng/mL of supernatant in the rmIL- 9–supplemented cultures (see also below).
Expression of mMCP-1 in long-term bone marrow cultures. Cells were supplemented with rmIL- 9/WEHI/rrSCF (▪; I/W/S) or WEHI/rrSCF (•; W/S). In (A), the percentage of mMCP-1+mast cells was assessed by immunohistochemical staining of cytosmears with MoAb RF 6.1. (B) shows the concentrations of mMCP-1 in culture supernatants (in nanograms per milliliter) quantified by ELISA. (C) shows the concentrations of mMCP-1 in cell pellets (in nanograms per 106 cells).
Expression of mMCP-1 in long-term bone marrow cultures. Cells were supplemented with rmIL- 9/WEHI/rrSCF (▪; I/W/S) or WEHI/rrSCF (•; W/S). In (A), the percentage of mMCP-1+mast cells was assessed by immunohistochemical staining of cytosmears with MoAb RF 6.1. (B) shows the concentrations of mMCP-1 in culture supernatants (in nanograms per milliliter) quantified by ELISA. (C) shows the concentrations of mMCP-1 in cell pellets (in nanograms per 106 cells).
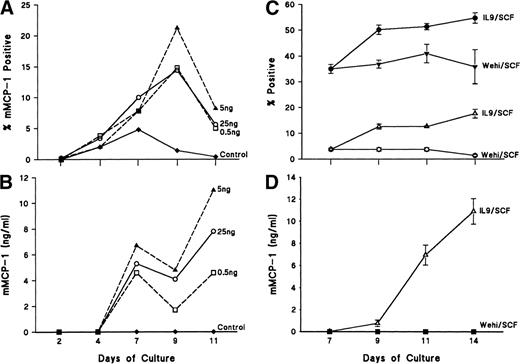
Because it was possible that the concentrations of rmIL-9 in the above-noted studies were not optimal, a dose-response experiment was set up in which bone marrow cells were cultured in 15% WEHI, 50 ng/mL rrSCF, and concentrations of 0.5, 5, and 25 ng rmIL-9/mL; cells and supernatants were harvested 2, 4, 7, 9, and 11 days later. With all three doses of rmIL-9, peak proportions of mMCP-1+ mBMMC were detected on day 9 (Fig 2A), with a slight trend towards a maximal response both for the percentage of positive cells and for the concentration of mMCP-1 in supernatants at a concentration of 5 ng rmIL-9/mL (Fig 2B). However, there was a greater accumulation of intracellular mMCP-1 on day 11 in the culture supplemented with 25 ng rmIL-9/mL (data not shown). Cell viability was greater than 90% throughout, and mast cell numbers increased twofold to threefold every 2 days between days 4 and 11.
(A) shows the dose response curves for the intracellular expression of mMCP-1 as assessed by immunohistochemical staining of cytosmears with MoAb RF 6.1 for mBMMC cultures set up in WEHI (15%)/rrSCF (50 ng/mL) alone (⧫) or in WEHI/rrSCF with 0.5 ng/mL (□), 5 ng/mL (▴), or 25 ng/mL (○) rmIL-9. (B) shows the concentrations of mMCP-1 in supernatants from the mBMMC cultures described in (A). (C) and (D) show expression of mMCP-1 in short-term bone marrow cultures in which cells were cultured for 7 days in the presence of WEHI/rrSCF (>90% mBMMC; >98% viable), before they were transferred to separate flasks and cultured for a further 7 days in quadruplicate in the presence of either WEHI/rrSCF or rmIL-9/rrSCF. In (C), percentages of mMCP-1+ (▵, ○) and chymase+ (•, ▾) mast cells were assessed by immunohistochemical staining of cytosmears using MoAb RF 6.1 or sheep polyclonal antibody that cross–reacts with other chymases. Results are shown from mBMMC cultured in WEHI/rrSCF (▾, ○) or rmIL-9/rrSCF (•, ▵). In (D), the concentration of mMCP-1 in supernatants was assessed in quadruplicate mBMMC cultures grown in WEHI/rrSCF (▪) or rmIL-9/rrSCF (▵). Data are expressed as the mean ± SE.
(A) shows the dose response curves for the intracellular expression of mMCP-1 as assessed by immunohistochemical staining of cytosmears with MoAb RF 6.1 for mBMMC cultures set up in WEHI (15%)/rrSCF (50 ng/mL) alone (⧫) or in WEHI/rrSCF with 0.5 ng/mL (□), 5 ng/mL (▴), or 25 ng/mL (○) rmIL-9. (B) shows the concentrations of mMCP-1 in supernatants from the mBMMC cultures described in (A). (C) and (D) show expression of mMCP-1 in short-term bone marrow cultures in which cells were cultured for 7 days in the presence of WEHI/rrSCF (>90% mBMMC; >98% viable), before they were transferred to separate flasks and cultured for a further 7 days in quadruplicate in the presence of either WEHI/rrSCF or rmIL-9/rrSCF. In (C), percentages of mMCP-1+ (▵, ○) and chymase+ (•, ▾) mast cells were assessed by immunohistochemical staining of cytosmears using MoAb RF 6.1 or sheep polyclonal antibody that cross–reacts with other chymases. Results are shown from mBMMC cultured in WEHI/rrSCF (▾, ○) or rmIL-9/rrSCF (•, ▵). In (D), the concentration of mMCP-1 in supernatants was assessed in quadruplicate mBMMC cultures grown in WEHI/rrSCF (▪) or rmIL-9/rrSCF (▵). Data are expressed as the mean ± SE.
It was reported that IL-3 suppressed the expression of mMCP-1 and mMCP-2, but that when the cells were transferred to a culture medium supplemented with rrSCF/rmIL-9, there was substantial expression of these two chymases.25 Therefore, bone marrow cells were cultured for 7 days in the presence of WEHI/rrSCF (>90% mBMMC; >98% viable), before they were transferred to separate flasks and cultured for a further 7 days in quadruplicate in the presence of either WEHI/rrSCF or rmIL-9/rrSCF. There was negligible expression of mMCP-1 in supernatants and cells in the absence of rmIL-9 (Fig 2C and D), but when mBMMC were transferred to rmIL- 9/rrSCF the culture supernatant and cells contained 11 ± 1 ng mMCP-1/mL (Fig 2D) and 24 ± 3 ng mMCP-1/106 cells, respectively, and 18% ± 2% of the mBMMC were positive for mMCP-1 by immunohistochemistry 7 days later (Fig 2C). Similarly, at all earlier time points, the percentage mMCP-1+ values for the rmIL-9/rrSCF–supplemented cultures were significantly increased (P < .03) over the values for the cultures maintained in WEHI/rrSCF (Fig 2C).
When the cytosmears were stained for the presence of other chymases, in addition to mMCP-1, using a polyclonal antibody against mMCP-1,22 the proportion of chymase+ mBMMCs remained constant in WEHI/rrSCfF, but increased significantly (P < .03) in the presence of rmIL-9 (Fig 2C).
The transfer of mBMMC to rmIL-9/rrSCF was associated with an initial 40% ± 13% decrease in cell numbers on day 2, followed by a 160% ± 38% increase on day 4 and steady state on day 7. In WEHI/rrSCF, the mBMMC were in steady state on day 2, increased 142% ± 34% on day 4, and had further increased by 83% ± 13% on day 7. However, there was a significant (P < .03) decrease in cell viability in the cells supplemented with rmIL-9/rrSCF to 56% ± 6% on day 7, whereas the viabilities of the WEHI/rrSCF cultures remained between 89% ± 4% and 93% ± 3% (n = 3).
TGF-β1 promotes the enhanced expression and secretion of mMCP-1.
Although the data given above were in agreement with previous studies showing that a combination of rmIL-9/rrSCF promoted the expression of mMCP-1, the proportions of mMCP-1+ cells rarely exceeded 50%, which contrasts with the in vivo picture, in which 100% of IMMC recruited during the early stages of nematode infection are mMCP-1+.29,30,47 Furthermore, the low nanogram levels of mMCP-1 present in culture supernatants and cell pellets are not commensurate with the microgram quantities noted in vivo.19 Therefore, we reasoned that an additional factor might be required to promote the expression and secretion of mMCP-1 and of another β-chymase associated with IMMC, mMCP-2.25 26
One potential candidate was TGF-β1, because several studies have shown that this cytokine is constitutively expressed by enterocytes32 and because mBMMC have been shown to be responsive to short-term (16 hours) exposure to this cytokine.36 To test the hypothesis that TGF-β1 regulates the expression of IMMC-specific β-chymases, 4 flasks of bone marrow cells were cultured in the presence of WEHI (15%), rrSCF (50 ng/mL), and rmIL-9 (5 ng/mL) for 7 days to produce greater than 95% mast cells that were greater than 90% c-kit+ by flow cytometry (data not shown) and 20% ± 4% mMCP-1+ by immunohistochemistry, with typically vacuolated granules and numerous pseudopod– like cytoplasmic extensions.13 The cells were split into 16 separate flasks at 5 × 105 mBMMC/mL and supplemented with WEHI (15%)/rrSCF (50 ng/mL)/rmIL-9 (5 ng/mL) to which was added either vehicle alone or rhTGF-β1 at a final concentration of 1 ng/mL of culture supernatant.
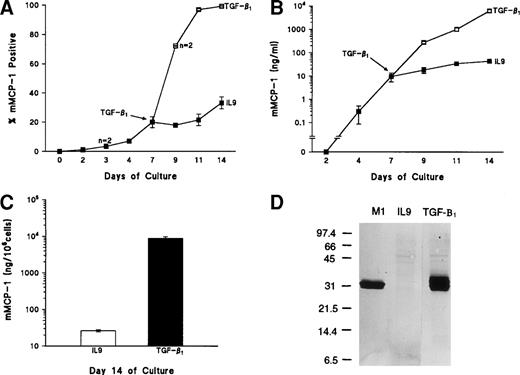
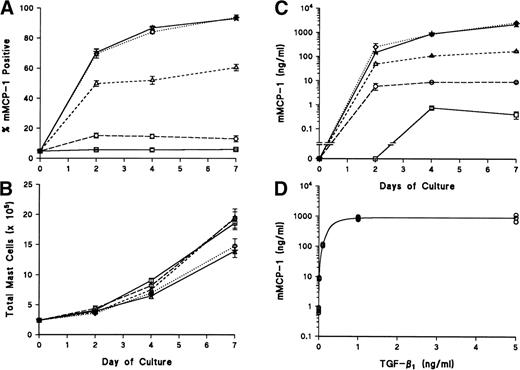
Within 48 hours of adding TGF-β1, the proportion of mMCP-1+ mBMMC increased from 20% ± 4% to 72% ± 0.3% (n = 2). At 4 days, the proportion had increased to 97% ± 1% (n = 4) and, at 7 days, 99% ± 0.5% (n = 4; Fig 3A). The proportions of mMCP-1+ mBMMC in the control flasks lacking TGF-β1 reached a maximum of 30% ± 5% (n = 3) at 7 days (Fig 3A). The differences between the values for control and TGF-β1–supplemented flasks were highly significant (P < .001) 4 and 7 days after adding TGF-β1(days 11 and 14, Fig 3A and 3B). The substantial change in the proportion of mMCP-1+ mBMMC, together with the greatly increased intensity of staining (Fig 4b and d) with time after supplementation with TGF-β1, was associated with a 500-fold increase in the level of mMCP-1 in the culture supernatants (Fig 3B) and, although there was a gradual increase in the controls, maximum values were 44 ± 5 ng/mL on day 7, as opposed to 6,000 ± 900 ng/mL in the TGF-β1–supplemented samples (P < .001; Fig 3B). Quantification of mMCP-1 in the cells recovered on day 7 after adding TGF-β1 (day 14), similarly, showed much higher concentrations of mMCP-1 in the TGF-β1–supplemented mBMMC than in controls (Fig 3C;P < .001), and this was confirmed using Western blotting (Fig 3D).
Upregulation of mMCP-1 expression in mBMMC cultures after the addition of TGF-β1. Four flasks of mBMMC were cultured in the presence of WEHI/rrSCF/rmIL-9 (5 ng/mL) for 7 days to produce greater than 95% mast cells which were 20% ± 4% mMCP-1+. They were split into 16 separate flasks at 5 × 105 mBMMC/mL, and supplemented with WEHI/rrSCF/rmIL-9 to which was added either vehicle alone or rhTGF-β1 (1 ng/mL of culture supernatant). (A) shows the percentage of mMCP-1+ mast cells from mBMMC cultures with (□) or without (▪) addition of TGF-β1 on day 7. Data are from quadruplicate cultures except where stated. (B) shows the concentrations of mMCP-1 in culture supernatants (in nanograms per milliliter). (C) shows the concentration of mMCP-1 in cell pellets (in nanograms per 106 cells) from these cultures 7 days after the addition of rhTGF-β1 (▪) or in rmIL-9/WEHI/rrSCF (□). (D) is a Western blot to show mMCP-1 expression in mBMMC grown in rhTGF-β1/rmIL-9/WEHI/rrSCF (TGF-β1) compared with an equivalent loading from mBMMC grown in rmIL-9/WEHI/rrSCF (IL-9) as detected by MoAb RF 6.1. A lane containing purified mMCP-1 (M1) was included as a control. Extracts from 2.5 × 104 mBMMC were loaded in the other lanes. Molecular weights in kilodaltons are shown. (E) shows the RT-PCR products of chymase genes from total RNA extracted from mBMMC cultures. RNA was from triplicate mBMMC cultures (A, B, and C) in rhTGF-β1/rmIL-9/WEHI/rrSCF (T/I/W/S) or rmIL-9/WEHI/rrSCF (I/W/S) (7 days after addition of TGF-β1). Initial dilutions of the RNA template before reverse transcription are indicated (1, 0.1, 0.01, and 0.001 μg/mL). Primer sets used for PCR were specific for the mMCP-1, mMCP-2, mMCP-4, mMCP-5, and β-actin genes as indicated.
Upregulation of mMCP-1 expression in mBMMC cultures after the addition of TGF-β1. Four flasks of mBMMC were cultured in the presence of WEHI/rrSCF/rmIL-9 (5 ng/mL) for 7 days to produce greater than 95% mast cells which were 20% ± 4% mMCP-1+. They were split into 16 separate flasks at 5 × 105 mBMMC/mL, and supplemented with WEHI/rrSCF/rmIL-9 to which was added either vehicle alone or rhTGF-β1 (1 ng/mL of culture supernatant). (A) shows the percentage of mMCP-1+ mast cells from mBMMC cultures with (□) or without (▪) addition of TGF-β1 on day 7. Data are from quadruplicate cultures except where stated. (B) shows the concentrations of mMCP-1 in culture supernatants (in nanograms per milliliter). (C) shows the concentration of mMCP-1 in cell pellets (in nanograms per 106 cells) from these cultures 7 days after the addition of rhTGF-β1 (▪) or in rmIL-9/WEHI/rrSCF (□). (D) is a Western blot to show mMCP-1 expression in mBMMC grown in rhTGF-β1/rmIL-9/WEHI/rrSCF (TGF-β1) compared with an equivalent loading from mBMMC grown in rmIL-9/WEHI/rrSCF (IL-9) as detected by MoAb RF 6.1. A lane containing purified mMCP-1 (M1) was included as a control. Extracts from 2.5 × 104 mBMMC were loaded in the other lanes. Molecular weights in kilodaltons are shown. (E) shows the RT-PCR products of chymase genes from total RNA extracted from mBMMC cultures. RNA was from triplicate mBMMC cultures (A, B, and C) in rhTGF-β1/rmIL-9/WEHI/rrSCF (T/I/W/S) or rmIL-9/WEHI/rrSCF (I/W/S) (7 days after addition of TGF-β1). Initial dilutions of the RNA template before reverse transcription are indicated (1, 0.1, 0.01, and 0.001 μg/mL). Primer sets used for PCR were specific for the mMCP-1, mMCP-2, mMCP-4, mMCP-5, and β-actin genes as indicated.
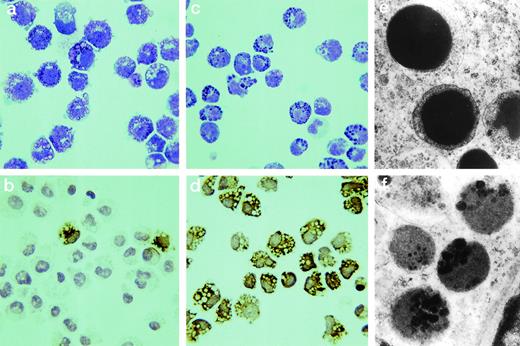
The morphology of mBMMC grown in the presence or absence of TGF-β1 is compared. Cytosmears are shown from mBMMC cultured in rmIL-9/WEHI/rrSCF stained with Leishman’s stain (a) or MoAb RF 6.1 for mMCP-1 (b) and from mBMMC cultured in rhTGF-β1/rmIL-9/WEHI/rrSCF stained with Leishman’s stain (c) or MoAb RF 6.1 for mMCP-1 (d). mBMMC cultured in the presence of TGF-β1 are more rounded, lack pseudopodia, and are highly positive for mMCP-1 compared with those cultured in IL-9 alone (original magnification ×800). The ultrastructure of granules from mBMMC cultured in the presence of TGF-β1 or in WEHI/rrSCF/rmIL-9 alone are shown in (e) and (f) (original magnification ×2,500). The granules of mBMMC supplemented TGF-β1 mBMMC are more electron-dense.
The morphology of mBMMC grown in the presence or absence of TGF-β1 is compared. Cytosmears are shown from mBMMC cultured in rmIL-9/WEHI/rrSCF stained with Leishman’s stain (a) or MoAb RF 6.1 for mMCP-1 (b) and from mBMMC cultured in rhTGF-β1/rmIL-9/WEHI/rrSCF stained with Leishman’s stain (c) or MoAb RF 6.1 for mMCP-1 (d). mBMMC cultured in the presence of TGF-β1 are more rounded, lack pseudopodia, and are highly positive for mMCP-1 compared with those cultured in IL-9 alone (original magnification ×800). The ultrastructure of granules from mBMMC cultured in the presence of TGF-β1 or in WEHI/rrSCF/rmIL-9 alone are shown in (e) and (f) (original magnification ×2,500). The granules of mBMMC supplemented TGF-β1 mBMMC are more electron-dense.
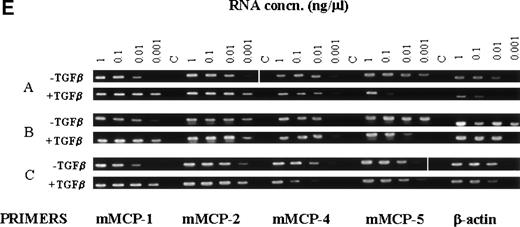
In previous studies of the expression of mast cell granule proteases by mBMMC, the levels of transcription of the IMMC-associated chymases mMCP-1 and mMCP-2 and of the CTMC-associated chymases mMCP-4 and mMCP-5 were compared after the addition of rmIL-9 or rmIL-10, together with SCF, to the cultured cells.25 Northern analysis showed upregulated transcription of the mMCP-1 and mMCP-2 genes.25Therefore, the transcription of these four chymases by the mBMMC cultured for 7 days in the presence or absence of TGF-β1was, similarly, analyzed using semiquantitative RT-PCR (Fig3E). In the culture supplemented with WEHI/rrSCF/rmIL-9, all four proteases were expressed to a similar extent (Fig 3E), which is largely in agreement with published data.25 When the culture was supplemented with WEHI/rrSCF/rmIL-9/rhTGF-β1, there was substantial upregulation of transcription of mMCP-1 and mMCP-2 (Fig3E), with no obvious variation in the transcription of mMCP-4 and mMCP-5 (Fig 3E). These data are consistent with the immunohistochemical and ELISA results described above and suggest that TGF-β1coregulates the increased expression of mMCP-1 and mMCP-2 and is without effect on mMCP-4 and mMCP-5.
At all stages of the culture, the cell viability remained at 95% to 98% and greater than 98% cells from both groups were strongly toluidine blue (pH 0.5) positive. However, there were substantial morphological differences between the two populations after Leishman’s staining and at the ultrastructural level. Using light microscopy, control mBMMC grown in the absence of TGF-β1 had pseudopodia and the granules were vacuolated and less distinct (Fig4a), as described previously by other workers.48 The addition of TGF-β1 was associated with a more compact mBMMC, lacking pseudopodia, and with densely stained granules of variable shape and size (Fig 4c).
Ultrastructurally, all of the cells (n = 54) exposed to TGF-β1 and harvested on day 7 contained homogeneous granules with, in some instances, a less dense rim between the matrix and granule membrane (Fig 4e) and, rarely, a crystalline core of the type extensively described for the granules of IMMC in parasitized gut.49 By contrast, the granules of the control cells (n = 40) were vacuolated and contained numerous small vesicles and clusters of more dense material; this vacuolated granule morphology (Fig 4f) has been described previously by others.49 The morphology of the cells supplemented with TGF-β1 is, thus, quite distinct from that described previously for mBMMC.13 49
Synergy between SCF and TGF-β1 in the expression and secretion of mMCP-1.
To identify the cytokines that best supported the expression and secretion of mMCP-1, bone marrow cells were grown in flasks for 1 week in WEHI/rrSCF (>80% BMMC, >90% viable) before they were transferred at 5 × 105 cells/mL into 48-well plates and cultured for a further 4 days in varying cytokine combinations as shown in Table 1. When compared with controls maintained in WEHI/rrSCF that supported the strongest growth of mBMMC, showing a fourfold increase over the 4 days (Table 1), the combination of rhTGF-β1/rmIL-9/WEHI/rrSCF resulted in a threefold increase in numbers of mBMMC (Table 1). In contrast, and as reported previously,50 rhTGF-β1/rrSCF was associated with very poor cell viability and a decrease in cell numbers.
Effect of Different Cytokines on Numbers and Viability of mBMMC and on the Expression and Release of mMCP-1
| . | Cells (×105/mL) . | % Viability . | % Mast Cells . | % mMCP-1+ . | mMCP-1 (ng/mL) . |
|---|---|---|---|---|---|
| T/I/W/S | 7.8 ± 2 | 88 ± 2.6 | 96 ± 0.8 | 90 ± 2.7 | 1,778 ± 337 |
| T/W/S | 5.3 ± 1.1 | 89 ± 2.2 | 88 ± 5.5 | 71 ± 6 | 745 ± 68 |
| T/I/W | 2.6 ± 0.4 | 75 ± 3.3 | 79 ± 2.2* | 34 ± 2.2* | 175 ± 26* |
| T/I/S | 2.7 ± 0.4 | 68 ± 3.3* | 90 ± 6† | 89 ± 1‡ | 1,866 ± 131 |
| T/W | 2.3 ± 0.4 | 72 ± 4.8 | 80 ± 2.3* | 18 ± 1.2* | 10 ± 2.4* |
| T/S | 1.2 ± 0.2* | 36 ± 4.7* | 92 ± 5.5† | 50 ± 4.1‡ | 201 ± 22* |
| I/W | 4.5 ± 0.2 | 74 ± 1.9 | 73 ± 4.5* | 1.6 ± 0.5* | 0 ± 0* |
| W/S | 10.6 ± 0.8 | 85 ± 2.8 | 94 ± 0.8 | 5 ± 1.3* | 1.3 ± 0.2* |
| . | Cells (×105/mL) . | % Viability . | % Mast Cells . | % mMCP-1+ . | mMCP-1 (ng/mL) . |
|---|---|---|---|---|---|
| T/I/W/S | 7.8 ± 2 | 88 ± 2.6 | 96 ± 0.8 | 90 ± 2.7 | 1,778 ± 337 |
| T/W/S | 5.3 ± 1.1 | 89 ± 2.2 | 88 ± 5.5 | 71 ± 6 | 745 ± 68 |
| T/I/W | 2.6 ± 0.4 | 75 ± 3.3 | 79 ± 2.2* | 34 ± 2.2* | 175 ± 26* |
| T/I/S | 2.7 ± 0.4 | 68 ± 3.3* | 90 ± 6† | 89 ± 1‡ | 1,866 ± 131 |
| T/W | 2.3 ± 0.4 | 72 ± 4.8 | 80 ± 2.3* | 18 ± 1.2* | 10 ± 2.4* |
| T/S | 1.2 ± 0.2* | 36 ± 4.7* | 92 ± 5.5† | 50 ± 4.1‡ | 201 ± 22* |
| I/W | 4.5 ± 0.2 | 74 ± 1.9 | 73 ± 4.5* | 1.6 ± 0.5* | 0 ± 0* |
| W/S | 10.6 ± 0.8 | 85 ± 2.8 | 94 ± 0.8 | 5 ± 1.3* | 1.3 ± 0.2* |
Data are expressed as the mean ± SE (n = 4 to 5, except where indicated). Bone marrow cells were grown in flasks for 1 week in WEHI (15%)/rrSCF (50 ng/mL) before they were transferred at 5 × 105/mL into 48-well plates and cultured for a further 4 days in the various cytokine combinations shown: T, rhTGF-β1 (1 ng/mL); I, rmIL-9 (5 ng/mL); W, WEHI-3B IL-3–rich supernatant (15%); S, rrSCF (50 ng/mL). The following parameters were measured 4 days after supplementation with different cytokine combinations: cell numbers and viability using nigrosin exclusion; percentage of mast cells in Leishman’s stained cytosmears; percentage of mMCP-1+ mast cells as assessed by immunohistochemical staining of cytosmears by MoAb RF 6.1; and concentrations of mMCP-1 in culture supernatants (in nanograms per milliliter) measured by ELISA using MoAb RF 6.1.
Data are significantly different (P < .03 Mann Whitney) from the control data for cells cultured in T/I/W/S.
n = 2.
n = 3.
In all of the cytokine combinations in which TGF-β1 was added, there was an increase in the proportion of mMCP- 1+mBMMC and in the concentrations of mMCP-1 found in the culture supernatants (Table 1). However, by day 4 of culture, it was clear that the maximal expression and secretion of mMCP-1 was associated with the presence of TGF-β1 and SCF and that either rmIL-9 or IL-3 (WEHI) synergized with these two cytokines to maintain cell viability. These data confirm that the addition of IL-9 and of TGF-β1 to mBMMC maintained in WEHI/rrSCF promotes the maximal cell growth in association with maximal expression and secretion of mMCP-1. However, it would appear that, on a per cell basis, maximum extracellular release of mMCP-1 occurred in the presence of TGF-β1/IL-9/SCF.
Expression and secretion of mMCP-1 is directly related to the concentration of TGF-β1.
In the preceding experiment, the cultures were supplemented with rhTGF-β1 at 1 ng/mL, and it was not clear whether this concentration was optimal for the induced expression of mMCP-1. It was also not clear whether the rate of mBMMC growth was affected by the presence of TGF-β1, because it is reported that TGF-β1 is inhibitory for mast cell growth.51Therefore, cells grown for 7 days in WEHI/rrSCF (>95% mBMMC, <1% mMCP-1+, >95% viable) were transferred into 48-well plates at 5 × 105 mBMMC/mL and supplemented with WEHI (15%)/rrSCF (50 ng/mL)/rmIL-9 (5 ng/mL) to which was added, in quadruplicate wells, vehicle (controls) or rhTGF-β1 at final concentrations of 0.01, 0.1, 1, and 5 ng/mL. Supernatants and cells for immunohistochemistry were harvested 2, 4, and 7 days later. Similarly, cells were harvested from additional quadruplicate wells at 2 and 7 days to measure the content of stored mMCP-1.
Over the 7 days of the experiment, concentrations of 1 and 5 ng TGF-β1/mL augmented the expression and secretion of mMCP-1 to the same extent (Fig 5A and C). The content of stored mMCP-1 was not significantly different for these two doses with, on day 7, concentrations of 4,708 ± 420 and 4,478 ± 448 ng mMCP-1/106 mBMMC. Whereas the addition of lower doses of TGF-β1 (10 and 100 pg/mL) was without effect on the growth of the mBMMC over the 7-day period, concentrations of 1 and 5 ng TGF-β1/mL did result in some growth inhibition on days 2 and 4 (P < .03), although significant growth of the cells in all the cultures occurred between days 4 and 7 (Fig 5B). The lower doses of 10 and 100 pg TGF-β1/mL induced the extracellular release of mMCP-1 (Fig 5C and D), and dose-response analysis on day 4 shows that the extracellular release of mMCP-1 is strictly dose-related, with a maximum release at 1 ng TGF-β1/mL (Fig 5D). Similarly, the intracellular storage of mMCP-1 was significantly greater than in control cultures (P < .03), with, for example, 100 pg/mL promoting expression in cell pellets of 239 ± 53 ng mMCP-1/106 mBMMC on day 7.
The upregulation of mMCP-1 supplemented with TGF-β1 is dose–dependant. mBMMC were grown for 7 days in WEHI/rrSCF, transferred into 48-well plates at 5 × 105mBMMC/mL, and supplemented with WEHI/rrSCF/rmIL-9 to which was added, in quadruplicate wells, WEHI/rrSCF/rmIL-9 alone (□) or 0.01 (○), 0.1 (▵), 1 (◊), or 5 (▴) ng/mL rhTGF-β1. (A) shows the percentage of mMCP-1+ mast cells. Counts from all the TGF-β1–supplemented cultures were significantly higher than the WEHI/rrSCF/rm IL-9 controls on days 4 and 7 (P < .03). (B) shows the total numbers of mast cells per culture. The counts from cultures supplemented with 1 or 5 ng/mL rhTGF-β1were significantly lower than in the controls on day 4 (P < .03). (C) shows the concentrations of mMCP-1 in culture supernatants (in nanograms per milliliter). The mMCP-1 concentrations from all the TGF-β1–supplemented cultures were significantly higher than the controls on days 4 and 7 (P < .03). (D) shows the concentration of mMCP-1 detected in culture supernatants on day 4 plotted against concentrations of TGF-β1.
The upregulation of mMCP-1 supplemented with TGF-β1 is dose–dependant. mBMMC were grown for 7 days in WEHI/rrSCF, transferred into 48-well plates at 5 × 105mBMMC/mL, and supplemented with WEHI/rrSCF/rmIL-9 to which was added, in quadruplicate wells, WEHI/rrSCF/rmIL-9 alone (□) or 0.01 (○), 0.1 (▵), 1 (◊), or 5 (▴) ng/mL rhTGF-β1. (A) shows the percentage of mMCP-1+ mast cells. Counts from all the TGF-β1–supplemented cultures were significantly higher than the WEHI/rrSCF/rm IL-9 controls on days 4 and 7 (P < .03). (B) shows the total numbers of mast cells per culture. The counts from cultures supplemented with 1 or 5 ng/mL rhTGF-β1were significantly lower than in the controls on day 4 (P < .03). (C) shows the concentrations of mMCP-1 in culture supernatants (in nanograms per milliliter). The mMCP-1 concentrations from all the TGF-β1–supplemented cultures were significantly higher than the controls on days 4 and 7 (P < .03). (D) shows the concentration of mMCP-1 detected in culture supernatants on day 4 plotted against concentrations of TGF-β1.
In this experiment, supplementation with IL-9 in the control cultures resulted in a minimal change in the number of mMCP-1+mBMMC, but the levels of this chymase in culture supernatants increased to 0.4 ± 0.1 ng/mL (Fig 5C), and in cell pellets reached 5.8 ± 1 ng/106 cells at day 7. These data show that the levels of expression and secretion of mMCP-1 by mBMMC are related to both the concentration of TGF-β1 and the length of time the cells are exposed to this cytokine. They also show that IL-9 stimulates a relatively low level of mMCP-1 expression and extracellular release that is significantly augmented by as little as 10 pg/mL of TGF-β1. Importantly, the release of mMCP-1 occurred in the absence of any exogenous stimulus with secretagogue.
Continued expression and extracellular release of mMCP-1 requires the continuous presence of TGF-β1.
The capacity of mouse mast cells to express mMCP-1 in vivo appears to be a consequence of their localization close to, or within, mucosal epithelia.29-31 Furthermore, the expression of mMCP-1 in, for example, the gastric mucosa varies according to exogenous stimuli.30 Therefore, the first question is whether mBMMC that have been induced to express and release mMCP-1 by addition of TGF-β1 continue to store and release this chymase when TGF-β1 is withdrawn from the culture. The second question was whether any other combination of cytokine supported mMCP-1 expression in the absence of TGF-β1.
For this experiment, bone marrow cells were grown in the presence of WEHI (15%)/rmIL-9 (5 ng/mL)/rrSCF (50 ng/mL) and rhTGF-β1 (1 ng/mL) for 7 days. These cultures yielded 15.2 × 105 mBMMC/mL, 88% viable, and 99.6% mMCP-1+, with 547 ng mMCP-1/mL of supernatant. Cells were transferred in quadruplicate at 5 × 105/mL to 48-well plates and cultured in varying combinations of cytokines as shown in Table 2. The withdrawal of TGF-β1 was associated with substantial decrease in the concentrations of mMCP-1 in the supernatants, and this was already apparent 2 days after withdrawing TGF-β1 (Table 2). By contrast, the levels of mMCP-1 in culture supernatants reached a maximum 1.9 μg/mL in the presence of rhTGF-β1/rmIL-9/WEHI/rrSCF and of 2.5 μg/mL at 4 days in the presence of rhTGF-β1/rmIL-9/rrSCF, with an intracellular accumulation of 10.3 μg mMCP-1/106 mBMMC (Table 2). However, the viability of the cells in the latter culture was only 66% when compared with 90% in cultures containing the same combination of cytokines, but including WEHI (Table 2).
Effect of Withdrawal of TGF-β1 on the Number and Viability mBMMC and on the Expression and Release of mMCP-1
| . | Cells (×105/mL) . | % Viability . | % Mast Cells . | % mMCP-1+ . | mMCP-1 (ng/mL) . | mMCP-1 (ng/106 cells) . |
|---|---|---|---|---|---|---|
| Day 2 | ||||||
| T/I/W/S | 16.5 ± 0.5 | 88 ± 0.9 | 97 ± 1.8 | 96 ± 4.0 | 1,849 ± 351* | 2,155 ± 305 |
| I/W/S | 18.1 ± 1.4 | 81 ± 1.7† | 99 ± 0.7 | 89 ± 3.0 | 59 ± 4.0‡ | 565 ± 65‡ |
| W/S | 22.8 ± 1.9† | 86 ± 1.0 | 99 ± 0.4 | 90 ± 2.6 | 100 ± 47‡ | 464 ± 65‡ |
| T/W/S | 17.8 ± 2.4 | 85 ± 2.7 | 98 ± 0.9 | 95 ± 0.5 | 977 ± 46‡ | 2,715 ± 725 |
| T/I/S | 13.9 ± 3.0 | 79 ± 1.1† | 98 ± 0.5 | 98 ± 0.8 | 1,476 ± 109 | 5,061 ± 660‡ |
| Day 4 | ||||||
| T/I/W/S | 8 ± 0.8 | 90 ± 1.9 | 99 ± 0.2 | 99 ± 0.5 | 1,935 ± 376* | 6,038 ± 466 |
| I/W/S | 6.5 ± 0.6 | 87 ± 3.4 | 99 ± 0.7 | 60 ± 1.3† | 75 ± 23† | 167 ± 24† |
| W/S | 6.7 ± 0.2 | 83 ± 2.0 | 99 ± 0.3 | 55 ± 28† | 90 ± 7† | 140 ± 11† |
| T/W/S | 5.3 ± 0.8 | 80 ± 4.1 | 98 ± 0.7 | 93 ± 1.4† | 908 ± 97† | 3,365 ± 330† |
| T/I/S | 4.1 ± 0.4† | 66 ± 3.3† | 99 ± 0.3 | 98 ± 0.2 | 2,460 ± 301 | 10,320 ± 1,216† |
| . | Cells (×105/mL) . | % Viability . | % Mast Cells . | % mMCP-1+ . | mMCP-1 (ng/mL) . | mMCP-1 (ng/106 cells) . |
|---|---|---|---|---|---|---|
| Day 2 | ||||||
| T/I/W/S | 16.5 ± 0.5 | 88 ± 0.9 | 97 ± 1.8 | 96 ± 4.0 | 1,849 ± 351* | 2,155 ± 305 |
| I/W/S | 18.1 ± 1.4 | 81 ± 1.7† | 99 ± 0.7 | 89 ± 3.0 | 59 ± 4.0‡ | 565 ± 65‡ |
| W/S | 22.8 ± 1.9† | 86 ± 1.0 | 99 ± 0.4 | 90 ± 2.6 | 100 ± 47‡ | 464 ± 65‡ |
| T/W/S | 17.8 ± 2.4 | 85 ± 2.7 | 98 ± 0.9 | 95 ± 0.5 | 977 ± 46‡ | 2,715 ± 725 |
| T/I/S | 13.9 ± 3.0 | 79 ± 1.1† | 98 ± 0.5 | 98 ± 0.8 | 1,476 ± 109 | 5,061 ± 660‡ |
| Day 4 | ||||||
| T/I/W/S | 8 ± 0.8 | 90 ± 1.9 | 99 ± 0.2 | 99 ± 0.5 | 1,935 ± 376* | 6,038 ± 466 |
| I/W/S | 6.5 ± 0.6 | 87 ± 3.4 | 99 ± 0.7 | 60 ± 1.3† | 75 ± 23† | 167 ± 24† |
| W/S | 6.7 ± 0.2 | 83 ± 2.0 | 99 ± 0.3 | 55 ± 28† | 90 ± 7† | 140 ± 11† |
| T/W/S | 5.3 ± 0.8 | 80 ± 4.1 | 98 ± 0.7 | 93 ± 1.4† | 908 ± 97† | 3,365 ± 330† |
| T/I/S | 4.1 ± 0.4† | 66 ± 3.3† | 99 ± 0.3 | 98 ± 0.2 | 2,460 ± 301 | 10,320 ± 1,216† |
Data are expressed as the mean ± SE (n = 4, unless otherwise stated). Data significantly different from the results for control cultures grown in the presence of T/I/W/S are indicated. mBMMC were grown in the presence of WEHI (15%)/rmIL-9 (5 ng/mL)/rrSCF (50 ng/mL) and rhTGF-β1 (1 ng/mL) for 7 days. These cultures yielded 15.2 × 105 mBMMC/mL, 88% viable, and 99.6% mMCP-1+, with 547 ng mMCP-1/mL of supernatant. Cells were transferred in quadruplicate at 5 × 105/mL to 48-well plates and cultured in varying combinations of cytokines as shown: T, rhTGF-β1 (1 ng/mL); I, rmIL-9 (5 ng/mL); W, WEHI-3B IL-3–rich supernatant (15%); S, rrSCF (50 ng/mL). The table shows the following parameters that were measured 2 and 4 days after transfer into cytokines: cell numbers and viability, percentage of mast cells in Leishman’s stained cytosmears, percentage of mMCP-1+ mast cells, and concentrations of mMCP-1 in culture supernatants (in nanograms per milliliter) and cell pellets (in nanograms per 106 cells) assessed by ELISA.
n = 3.
P < .03 (Mann Whitney).
P < .05 (Mann Whitney).
Cell growth was greatest in the presence of WEHI/rrSCF, but this combination was associated with a substantial decrease in the percentage of mMCP-1+ cells and, at 4 days, intracellular levels of 140 ng mMCP-1/106 mBMMC. These results confirm that the continued presence of TGF-β1 is required to maintain the expression and release of mMCP-1 and that the combination of rhTGF-β1/rmIL-9/rrSCF promotes the greatest intracellular storage of mMCP-1. However, the presence of IL-3–rich WEHI appears to be important for supporting mBMMC viability and growth.
Expression of mMCP-1 is autostimulated by endogenous TGF-β1 in the presence of IL-9.
Previous studies have shown that, in the presence of IL-9 or IL-10, there is increased expression of mMCP-1 and mMCP-2.25 26The current work supports these observations, but shows that TGF-β1 is a much more potent stimulus for the expression of mMCP-1 than IL-9. Therefore, it seemed possible that IL-9 was promoting the endogenous secretion of active TGF-β1and/or the processing of latent TGF-β1 and that low levels of this cytokine were responsible for the suboptimal expression and secretion of mMCP-1 in cultures containing IL-9.
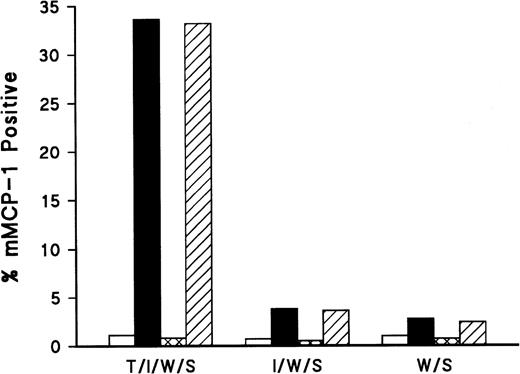
To test this hypothesis, a pilot study was performed in which mBMMC were grown in WEHI (15%)/rrSCF (50 ng/mL) for 7 days before transferring them in duplicate to 48-well plates supplemented with rhTGF-β1 (100 pg/mL)/rmIL- 9 (5 ng/mL)/WEHI (15%)/rrSCF (50 ng/mL) or with rmIL-9/WEHI/rrSCF or WEHI/rrSCF at the same concentrations. This concentration of TGF-β1 resulted in 33% mMCP-1+ mBMMC 48 hours later, whereas the addition of IL-9 resulted in 6% mMCP-1+ mBMMC. The addition of 1 μg/mL or 10 μg/mL chicken anti–TGF-β antibody to these cultures substantially suppressed the expression of mMCP-1 in both cultures after 48 hours (Fig 6) compared with controls to which 1 or 10 μg/mL of chicken IgG was added.
Addition of anti–TGF-β antibody results in reduced expression of mMCP-1. mBMMC were grown in WEHI/rrSCF for 7 days before transferring them in duplicate to 48-well plates supplemented with rhTGF-β1 (100 pg/mL)/rmIL-9 (5 ng/mL)/WEHI (15%)/rrSCF (50 ng/mL) (T/I/W/S ) or with rmIL-9/WEHI/rrSCF (I/W/S) or WEHI/rrSCF (W/S) at the same concentrations. To these cultures were also added chicken anti–TGF-β at 1 μg/mL (□) or 10 μg/mL (▩), or chicken IgG control at 1 μg/mL (▪) or 10 μg/mL (▨). The mean percentages of mMCP-1+ mast cells were assessed by immunohistochemical staining of cytosmears with MoAb RF 6.1 48 hours after addition of the cytokines and antibodies (n = 2).
Addition of anti–TGF-β antibody results in reduced expression of mMCP-1. mBMMC were grown in WEHI/rrSCF for 7 days before transferring them in duplicate to 48-well plates supplemented with rhTGF-β1 (100 pg/mL)/rmIL-9 (5 ng/mL)/WEHI (15%)/rrSCF (50 ng/mL) (T/I/W/S ) or with rmIL-9/WEHI/rrSCF (I/W/S) or WEHI/rrSCF (W/S) at the same concentrations. To these cultures were also added chicken anti–TGF-β at 1 μg/mL (□) or 10 μg/mL (▩), or chicken IgG control at 1 μg/mL (▪) or 10 μg/mL (▨). The mean percentages of mMCP-1+ mast cells were assessed by immunohistochemical staining of cytosmears with MoAb RF 6.1 48 hours after addition of the cytokines and antibodies (n = 2).
This experiment was repeated with minor modifications to measure the effect of anti–TGF-β on the extracellular release of mMCP-1. mBMMC were grown in WEHI (15%)/rrSCF (50 ng/mL) for 7 days before transferring them in quadruplicate to 48-well plates supplemented with rhTGF-β1/rmIL-9/WEHI/rrSCF, rmIL-9/rrSCF, or WEHI/rrSCF, as described above, before the addition of anti–TGF-β or chicken IgG at 1 μg/mL. At 48 hours, the results were very similar to those of the previous experiment; release of mMCP-1 was not detected in the cultures supplemented with IL-9, despite 5% of the cells being mMCP-1+ (Table 3). However, the addition of anti–TGF-β antibody significantly suppressed the intracellular staining for mMCP-1 in both the IL-9– and TGF-β1–supplemented cultures (P < .03; Table3). It also significantly suppressed the extracellular release of mMCP-1 in the cultures supplemented with TGF-β1(P < .03; Table 3).
Effect of TGF-β Antibody on Numbers and Viability of mBMMC and on the Expression and Release of mMCP-1
| Treatment . | Cells (×105/mL) . | % Viability . | % Mast Cells . | % mMCP-1+ . | mMCP-1 (ng/mL) . |
|---|---|---|---|---|---|
| W/S + αTGFβ Ab | 4.8 ± 0.5 | 92 ± 1 | 90 ± 1 | 0.5 ± 0.1 | 0 ± 0 |
| W/S + N. Chicken Ab | 5.4 ± 0.2 | 91 ± 1 | 88 ± 2 | 0.9 ± 0.4 | 0 ± 0 |
| W/S + no Ab | 6.0 ± 0.4 | 94 ± 1 | 92 ± 1 | 1.3 ± 0.3 | 0 ± 0 |
| I/S + αTGFβ Ab | 3.2 ± 0.3 | 87 ± 4 | 91 ± 0.5 | 1.1 ± 0.2a | 0 ± 0 |
| I/S + N. Chicken Ab | 4.5 ± 0.2 | 87 ± 1 | 95 ± 1 | 5.1 ± 0.3b | 0 ± 0 |
| I/S + no Ab | 4.3 ± 0.3 | 84 ± 2 | 91 ± 2 | 5.4 ± 1.3b | 0 ± 0 |
| T/I/W/S + αTGFβ Ab | 6.3 ± 0.1 | 90 ± 2 | 87 ± 1 | 0.6 ± 0.2c | 0 ± 0e |
| T/I/W/S + N. Chicken Ab | 7.0 ± 0.4 | 91 ± 2 | 88 ± 1 | 23 ± 0.5d | 15 ± 0.1f |
| T/I/W/S + no Ab | 5.7 ± 0.4 | 89 ± 3 | 89 ± 2 | 26 ± 1.9d | 14 ± 0.9f |
| Treatment . | Cells (×105/mL) . | % Viability . | % Mast Cells . | % mMCP-1+ . | mMCP-1 (ng/mL) . |
|---|---|---|---|---|---|
| W/S + αTGFβ Ab | 4.8 ± 0.5 | 92 ± 1 | 90 ± 1 | 0.5 ± 0.1 | 0 ± 0 |
| W/S + N. Chicken Ab | 5.4 ± 0.2 | 91 ± 1 | 88 ± 2 | 0.9 ± 0.4 | 0 ± 0 |
| W/S + no Ab | 6.0 ± 0.4 | 94 ± 1 | 92 ± 1 | 1.3 ± 0.3 | 0 ± 0 |
| I/S + αTGFβ Ab | 3.2 ± 0.3 | 87 ± 4 | 91 ± 0.5 | 1.1 ± 0.2a | 0 ± 0 |
| I/S + N. Chicken Ab | 4.5 ± 0.2 | 87 ± 1 | 95 ± 1 | 5.1 ± 0.3b | 0 ± 0 |
| I/S + no Ab | 4.3 ± 0.3 | 84 ± 2 | 91 ± 2 | 5.4 ± 1.3b | 0 ± 0 |
| T/I/W/S + αTGFβ Ab | 6.3 ± 0.1 | 90 ± 2 | 87 ± 1 | 0.6 ± 0.2c | 0 ± 0e |
| T/I/W/S + N. Chicken Ab | 7.0 ± 0.4 | 91 ± 2 | 88 ± 1 | 23 ± 0.5d | 15 ± 0.1f |
| T/I/W/S + no Ab | 5.7 ± 0.4 | 89 ± 3 | 89 ± 2 | 26 ± 1.9d | 14 ± 0.9f |
Data are expressed as the mean ± SE (n = 4). Statistical analysis shows that a versus b, c versus d, and e versus f are significantly different (P < .03 Mann Whitney). mBMMC were grown in WEHI (15%)/rrSCF (50 ng/mL) for 7 days before transferring them in quadruplicate to 48-well plates supplemented with rhTGF-β1 (100 pg/mL)/rmIL-9 (5 ng/mL)/WEHI (15%)/rrSCF (50 ng/mL) or with rmIL-9/rrSCF or WEHI/rrSCF at the same concentrations. These cytokines are denoted as follows: T, rhTGF-β1; I, rmIL-9; W, WEHI-3B IL-3–rich supernatant; S, rrSCF. Included in these cultures were chicken anti–TGF-β antibody (1 μg/mL), control chicken IgG antibody (1 μg/mL), or vehicle alone (no Ab) as indicated. The table shows the following parameters measured 2 days after transfer into cytokines: cell numbers and viability as assessed by nigrosin exclusion, percentage of mast cells in Leishman’s stained cytosmears, percentage of mMCP-1+ mast cells, and concentrations of mMCP-1 in culture supernatants (in nanograms per milliliter).
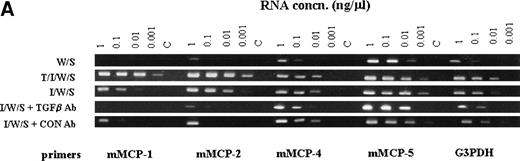
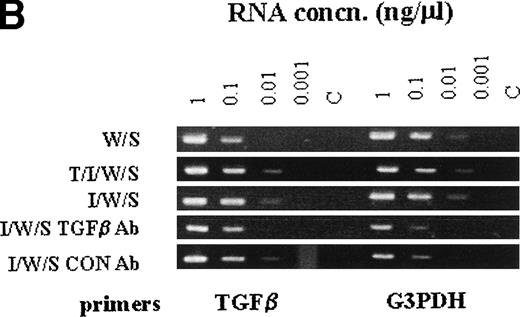
When the transcription of granule proteases was analyzed in cultures stimulated with WEHI/rrSCF, with rhTGF-β1 (100 pg/mL)/rmIL-9/WEHI/rrSCF, or with rmIL-9/WEHI/rrSCF in the presence of anti–TGF-β antibody or of control IgG, using semiquantitative RT-PCR, there was almost complete suppression of transcription of mMCP-1 and mMCP-2 by anti–TGF-β antibody, but transcripts for mMCP-4 and mMCP-5 were apparently unaffected (Fig7A). In contrast, abundant transcripts for TGF-β1 were detected in all cell pellets regardless of whether mBMMC were cultured in the presence or absence of IL-9, of anti–TGF-β antibody, or of control chicken IgG (Fig 7B). These results support the hypothesis that IL-9–induced mMCP-1 expression is mediated via autostimulation with TGF-β1 but suggest that the mechanism of IL-9 stimulation of TGF-β1 production is posttranscriptional.
The RT-PCR products of chymase genes from total RNA extracted from mBMMC cultures. Initial dilutions of the RNA template before reverse transcription are indicated (1, 0.1, 0.01, and 0.001 μg/mL). (A) shows the RT-PCR products using primer sets specific for the mMCP-1, mMCP-2, mMCP-4, mMCP-5, and G3PDH genes as indicated. Target RNA was from mBMMC cultured in WEHI/rrSCF alone (W/S), in rhTGF-β1 (1 ng/mL)/rmIL-9 (5 ng/mL)/WEHI (15%)/rrSCF (50 ng/mL) (T/I/W/S), in rmIL-9/WEHI/rrSCF (I/W/S) (day 14, as described in Fig 3), or in rmIL-9/WEHI/rrSCF (I/W/S) in the presence of anti–TGF-β antibody (TGFβ Ab) or a control antibody (Con Ab) as indicated (cultures described in Fig 6). Transcription of mMCP-1 and mMCP-2 is suppressed by the presence of anti–TGF-β antibodies, whereas transcripts for mMCP-4 and mMCP-5 are unaffected. Note the much higher level of transcription of mMCP-1 and mMCP-2 in the culture supplemented with TGF-β1. (B) shows the RT-PCR products using primer sets specific for the TGF-β1 and G3PDH genes as indicated. Target RNA was as described for (A). The transcripts for TGF-β1 appear to be unaffected by the culture conditions or the presence of anti–TGF-β antibodies.
The RT-PCR products of chymase genes from total RNA extracted from mBMMC cultures. Initial dilutions of the RNA template before reverse transcription are indicated (1, 0.1, 0.01, and 0.001 μg/mL). (A) shows the RT-PCR products using primer sets specific for the mMCP-1, mMCP-2, mMCP-4, mMCP-5, and G3PDH genes as indicated. Target RNA was from mBMMC cultured in WEHI/rrSCF alone (W/S), in rhTGF-β1 (1 ng/mL)/rmIL-9 (5 ng/mL)/WEHI (15%)/rrSCF (50 ng/mL) (T/I/W/S), in rmIL-9/WEHI/rrSCF (I/W/S) (day 14, as described in Fig 3), or in rmIL-9/WEHI/rrSCF (I/W/S) in the presence of anti–TGF-β antibody (TGFβ Ab) or a control antibody (Con Ab) as indicated (cultures described in Fig 6). Transcription of mMCP-1 and mMCP-2 is suppressed by the presence of anti–TGF-β antibodies, whereas transcripts for mMCP-4 and mMCP-5 are unaffected. Note the much higher level of transcription of mMCP-1 and mMCP-2 in the culture supplemented with TGF-β1. (B) shows the RT-PCR products using primer sets specific for the TGF-β1 and G3PDH genes as indicated. Target RNA was as described for (A). The transcripts for TGF-β1 appear to be unaffected by the culture conditions or the presence of anti–TGF-β antibodies.
DISCUSSION
Mast cell heterogeneity is recognized in many species, including humans, but it has been difficult to determine the regulatory mechanisms underlying this heterogeneity, particularly with regard to the apparent tissue specificity of granule chymase expression.13,14 Mouse BMMC have been widely used to explore the mechanisms of heterogeneity. They were originally considered to be IMMC-like,48 although it has subsequently been demonstrated that they lack the granule structure seen in vivo, and do not express the IMMC-specific chymase mMCP-1.13,14However, they do have the potential to develop into cells with the morphology of CTMC,14 expressing mMCP-4, mMCP-5, and CPA.14 It is also apparent that, under certain circumstances, they can be induced to express the β-chymases mMCP-140 and mMCP-2,25 although in our hands, using T-cell–conditioned medium,24 the level of expression of mMCP-1 was not commensurate with the level occurring in vivo in nematode-infected gut.19 27
The present study focussed on the expression of mMCP-1 in mBMMC, because this is the only chymase expressed uniquely at mucosal surfaces,27,30 and mechanisms that significantly upregulate expression in vitro are also likely to operate in vivo. The present data strongly suggest that TGF-β1 is a potent stimulus for the expression of mMCP-1 and that mBMMC cultured for several days in this cytokine not only express and release microgram quantities of this chymase, but that the cells themselves are more heavily granulated and the granules are more homogeneous than has been described previously for mBMMC.14 Further work will be required to show whether these cells are true homologs of mouse IMMC.
The culture system used was a modification of that described by Lantz and Huff46 in which greater than 35% mBMMC are generated within 7 days and involved the supplementation of bone marrow cells in either WEHI/rrSCF or WEHI/IL-9/rrSCF. In the present study, mast cell differentiation was usually greater than 80% with both combinations of cytokines by day 7, when the cells were exposed to other cytokine combinations. The results are in agreement with previous studies showing that WEHI/rrSCF was associated with barely detectable expression of mMCP-1, but that the presence of IL-9 was associated with upregulated expression of mMCP-1, albeit to low levels.25Importantly, there was mBMMC heterogeneity as described previously where a high proportion of cells did not express this chymase.24 However, the addition of TGF-β1resulted in a very rapid increase in the expression of mMCP-1 in greater than 95% of mBMMC, in increased transcription of mMCP-2, and in substantial release of mMCP-1 into the supernatant.
The mMCP-1 responses were strictly dependant on the concentration of TGF-β1 in the culture. The expression and release of this chymase fell substantially on withdrawal of TGF-β1 from the culture. Furthermore, using anti–TGF-β antibodies, it was evident that IL-9 probably induced mMCP-1 expression through a TGF-β–dependant pathway. Together, these data suggest that TGF-β1 drives not only the mucosal mast cell-specific expression, but also the IgE-independant release of mMCP-1. Such a mechanism could explain why, in normal mice, there is systemic secretion of mMCP-1 and why such high systemic levels of this protease are evident at very early stages of intestinal nematode infection, before specific IgE responses can be detected.18,27 47
It would appear that growth of 1-week-old, differentiated mBMMC is only slightly inhibited by TGF-β1, even at a maximal effective concentration of 1 ng/mL, provided that either IL-3 or IL-9 were also present in the culture medium. This probably reflects the fact that TGF-β1 is inhibitory to the growth of early hematopoietic progenitor cells, but is less inhibitory to differentiated hematopoietic cells.52 Nevertheless, the results in Table 1are in agreement with previous studies that showed that a combination of TGF-β1 and SCF did not support mast cell survival.50 However, other work suggested that TGF-β1 inhibited the growth of mBMMC without affecting differentiation or activation of the cells.51Interestingly, these studies were performed using recombinant IL- 3 or WEHI-3B conditioned medium and TGF-β151 and, again, are in agreement with the results shown in Table 1. The combination of WEHI and TGF-β1 supported cell survival without any significant growth when compared with WEHI/SCF– or WEHI/SCF/IL-9/TGF-β1–supplemented cultures. Similarly, WEHI/TGF-β1 supplementation induced only very low levels of expression of mMCP-1 when compared with combinations that included TGF-β1/IL-9/SCF or TGF-β1/WEHI/SCF. Therefore, it is reasonable to conclude that the differentiation process involving the expression of IMMC-specific secretory granule proteases as well as hyperplasia of the population in parasitised gut is optimal in the presence of all four cytokines. In vivo studies clearly demonstrate the importance of IL-3,8,9IL-9,11 and SCF4 5 in the induction of IMMC hyperplasia; comparable experiments have yet to show whether the TGF-β family of cytokines are also functioning in this response.
It was beyond the scope of the present study to investigate other mast cell parameters, apart from granule histochemistry and expression of c-kit. However, based on the current observations and on previous studies on short-term exposure to TGF-β1,35 36 it is probable that the biochemistry and functional properties of mBMMC exposed to this cytokine are substantially altered. It is also clear that the chymase expression induced by TGF-β1 is readily reversible and that cells exposed to this cytokine lose their ability to express mMCP-1 when it is withdrawn or its activity is blocked with anti–TGF-β antibodies.
The rapidity with which expression of mMCP-1 is induced and can be measured in culture supernatants using sensitive immunoassays provides a novel system in which to analyze TGF-β receptor expression and signal transduction pathways. For example, it should be possible to determine the relative contributions of TGF-β receptors I and II (TβI and TβII) to the expression of mMCP-1 and, importantly, explore whether the mechanism of mRNA stabilization that is reported to occur for mMCP-2 in the presence of exogenous IL-1026 also applies to the WEHI/SCF/IL-9/TGF-β1–induced expression and extracellular release of mMCP-1 described here. This is assuming that IL-9 and IL-10 both induce autostimulation via endogenously secreted TGF-β. However, it was clear that IL-9 has no obvious effect on TGF-β1 transcription (Fig 7B). Some posttranslational regulation, as has been well described for macrophages and monocytes,33 may therefore be involved. It is interesting to note, for example, that an IL-3-independent mast cell line as well as IL-3–dependent mBMMC secrete significant quantities of bioactive TGF-β into the culture supernatant after IgE-dependent activation.53 This suggests that mBMMC- derived latent TGF-β can be processed into an active form with an appropriate stimulus.
In the present study, IL-3 appears to have little or no inhibitory effect on the expression of mMCP-1, which is in contrast with previous data on the role of IL-3 in IL-9–induced mMCP-1 expression.25 The reasons for this are not clear but could be due to the rather lower concentrations of SCF used in the current study. In general, IL-3–rich WEHI maintained mBMMC viability and was a potent growth factor when in the presence of SCF alone (Table 1). There was no obvious downregulation of mMCP-1 expression and extracellular release when IL-9/SCF/TGFβ1 was supplemented with WEHI (Table 1). It was more difficult to assign a role for IL-9, although both in vivo11 and in vitro,10 IL-9 apparently stimulates mast cell proliferation. The growth and viability data (Table 1) show that mBMMC grown initially in IL-3–rich WEHI and SCF continued to proliferate and maintained viability in the presence of these two cytokines. Transfer of the cells to IL-9/SCF was, for example, associated with a decline in cell viability over a 7-day period. Similarly, transfer of cells grown in WEHI/SCF into cultures supplemented with TGF-β1/IL-9/SCF resulted in poor viability and poor proliferation, but a high proportion of mMCP-1+ mBMMC and abundant release of mMCP-1 into the culture supernatant (Table 1). Again, as mentioned above, the lower concentrations of SCF (50 ng/mL compared with 200 ng/mL25) may have contributed to the lower viability of mBMMC in the absence of IL-3. Because in mice transgenic for overexpression of IL-9 there is mucosal mast cell hyperplasia and substantial levels of mMCP-1 in the blood,11 it is reasonable to assume that IL-9 synergizes with TGF-β1 and SCF in promoting the maturation of IMMC and the expression and secretion of mMCP-1.
These new data, showing that the expression and release of mMCP-1 is highly TGF-β1 dependant, extend the range of functions for this important cytokine. It will be of great interest to determine whether it will promote expression of mMCP-1 during the early differentiation of mBMMC, because it has been argued, on the basis of previous studies in which mBMMC were exposed to IL-9 or IL-10, that mMCP-1 is a gene expressed late in the differentiation of mBMMC.25 Our preliminary results suggest that expression of mMCP-1 can be detected as early as 48 hours after initiating bone marrow cultures, which is consistent with in vivo data on the early expression of this protease in parasitised mouse gut.47 In the longer term, analysis of the localization of the site of expression of TGF-β1 in parasitised gut, its regulation during helminth infection and in other gut allergies in which mucosal mast cell hyperplasia has been reported, and the way in which it promotes the expression and release of mMCP-1 and its regulation of other IMMC-specific genes will throw new light on the mucosal role of the TGF-β family of cytokines.
ACKNOWLEDGMENT
The authors thank Dr H. Faulkner (University of Manchester) for rat anti–c-kit MoAb (ACK-2), Jean Vaagenes for technical assistance, and Cheryl Scudamore for helpful discussion. Thanks also to Bob Munro for photography and Eileen Duncan and Liz Moore for maintenance of Balb/C mice.
Supported by Grant No. 050065 from the Wellcome Trust.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Hugh R.P. Miller, PhD, Department of Veterinary Clinical Studies, Royal (Dick) School of Veterinary Studies, The University of Edinburgh, Easter Bush Veterinary Centre, Easter Bush, Roslin, Midlothian EH25 9RG, Scotland; e-mail:Hugh.Miller@ed.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal