Abstract
The routine use of bone marrow transplantation is limited by the occurrence of acute and chronic graft-versus-host disease (GVHD). Current approaches to decreasing the occurrence of GVHD after allogeneic transplantation use T-cell depletion, use immunosuppressive agents, or block costimulatory molecule function. The role of proteins in the recruitment of alloreactive lymphocytes has not been well characterized. Chemokines are a large family of proteins that mediate recruitment of mononuclear cells in vitro and in vivo. To investigate the role of T-cell production of the chemokine macrophage inhibitory protein-1 (MIP-1) in the occurrence of GVHD, splenocytes either from wild-type or from MIP-1−/− mice were administered to class I (B6.C-H2bm1) and class II disparate mice (B6-C-H2bm12). The incidence and severity of GVHD was markedly reduced in bm1 mice receiving splenocytes from MIP-1−/− mice as compared with mice receiving wild-type splenocytes. Bm1 mice receiving MIP-1−/− splenocytes had significantly less weight loss and markedly reduced inflammatory responses in the lung and liver than mice receiving C57BL/6 splenocytes. Bm1 mice receiving MIP-1−/− splenocytes had a markedly decreased production of antichromatin autoantibodies and impaired generation of bm1-specific T lymphocytes versus wild-type mice. However, MIP-1−/− splenocytes easily induced GVHD when administered to bm12 mice. This data show that blockade of chemokine production or function may provide a new approach to the prevention or treatment of GVHD but that chemokines that recruit both CD4+ and CD8+ lymphocytes may need to be targeted.
ALLOGENEIC BONE marrow transplantation (ABMT) has been increasingly used in the treatment of individuals with acute and chronic leukemia,1-3 lymphoma,4-10and congenital blood disorders.11 However, the widespread application of this therapy is currently limited by the occurrence of acute graft-versus-host disease (aGVHD) and chronic GVHD (cGVHD).12-16 Because of this, patients more than 55 years of age and individuals that do not have a family or unrelated six-antigen–matched donor are infrequently offered this treatment. As a result, only 25% to 35% of patients that could benefit from this therapy are eligible for ABMT.
GVHD is due to the recognition by alloreactive T lymphocytes of minor or major major histocompatibility complex (MHC) antigens.12In the context of an MHC-identical six-antigen sibling transplant, the antigens recognized are peptides from polymorphic proteins complexed to class I or II MHC molecules.17 T lymphocytes interact with MHC proteins on antigen-presenting cells through the T-cell receptor complex. This interaction can lead to the death of the antigen-presenting cell by the elaboration of proteins such as perforin and granzymes or to the production of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α).18 In animal models, both of these effector mechanisms are important in the pathology of GVHD.19-21 Current methods to decrease the occurrence of GVHD after allogeneic BMT include the use cyclosporine or tacrolimus, which inhibit the transcription of genes that occur after T-cell signaling. Alternatively, cytotoxic compounds such as methotrexate have been used to kill cells undergoing DNA synthesis.
Newer approaches to the prevention of GVHD have included the blockade of the costimulatory molecules CD80 and CD8612,22 and the blockade of interaction of CD4 with the α2 domain of the class II MHC protein.12 However, none of these methods has been validated in a large clinical trial, and additional approaches are needed to mitigate both the occurrence of GVHD as well as treatment of established GVHD. One perplexing aspect regarding the occurrence of GVHD is that, despite the presence of class I MHC molecules on all nonneuronal cells, GVHD typically involves specific target organs. In the setting of an MHC-matched sibling allograft, these differences may represent differences in the tissue distribution of minor MHC antigens. However, in the setting of an MHC-disparate transplant, other mechanisms must be involved in the occurrence of GVHD in specific target organs. Thus, there may be specific mechanisms involved in either the presentation of peptides by MHC molecules in certain tissue sites or in the recruitment of alloreactive T lymphocytes to the skin, liver, and gastrointestinal tract.
Chemokines are a group of predominantly small molecular weight proteins that are involved in the recruitment of multiple effector cells including mononuclear leukocytes and neutrophils.23-29 The C-C chemokine, macrophage inhibitory protein-1α (MIP-1α), has been shown to recruit multiple different cell types, including activated CD8+ lymphocytes in vitro.30 If GVHD involves the specific recruitment of activated lymphocytes to target organs, chemokines may play a role in this process. Additionally, previous investigators have shown that chemokines effect proliferation and cytotoxicity in vitro of T lymphocytes and natural killer (NK) cells. Therefore, we were interested in studying the role of specific chemokines in the pathophysiology of GVHD.
Using gene targeting, we produced mice with a disrupted gene encoding MIP-1α.31 These mice have a decreased inflammatory response after infection with influenza and Coxsackie viruses. More importantly, we have shown that CD8+ lymphocytes from these mice are not effective in the adoptive transfer of protection againstListeria monocytogenes because of both impaired killing and recruitment by these lymphocytes to the site of infection.31a
In the present studies, we have explored the role of MIP-1α production by T lymphocytes in the occurrence of GVHD across both a class I and class II MHC barrier. Splenocytes from MIP-1α−/− were impaired in the ability to cause GVHD across a class I but not class II MHC barrier. Blockade of T-lymphocyte production of specific different chemokines may provide a new approach to the prevention or treatment of GVHD.
MATERIALS AND METHODS
Mice.
B6.C-H2bm1/By (bm1), B6.C.H-2bm12/KhEg (bm12), DBA/2J (H-2d), and C57BL/6 (H-2b) female mice were purchased from Jackson Laboratories (Bar Harbor, ME). B6-129-Scya3tm1unc(MIP-1α−/−) mice were backcrossed to the eighth generation onto a C57BL/6 background (MIP-1α−/− B6). All mice were maintained specific-pathogen free in our colony under microisolator cage tops. Mice received food ad libitum. After irradiation, mice received neomycin-treated, acidified water. Female mice between 7 and 12 weeks of age were used.
Splenocyte transfer.
Recipient bm1 or bm12 mice received either 500 or 600 cGy of irradiation from a 137cesium source (Atomic Energy of Canada, Ottawa, Ontario, Canada) in the evening before the splenocyte transfer. The following morning, donor mice were killed by cervical transection and single-cell suspensions of splenocytes were prepared. Splenocytes were depleted of red blood cells using ACK lysis buffer and resuspended in nonbacteriostatic saline. Recipient mice received either 1 × 107 splenocytes or 5 × 106 CD8+ lymphocytes that had been selected using anti-CD8α monoclonal antibody (Ly-2; Miltenyi Biotec Bergishch, Gladbach, Germany) coupled to magnetic beads using the manufacturer’s protocol from either C57BL/6 or MIP-1α−/− B6 mice. After immunomagnetic separation, 98% of the cells infused expressed CD8 as shown by flow cytometry (data not shown). All mice were observed either until death or day 250. Moribund mice were killed and the data were censored from that point.
GVHD grading.
Mice were graded for the presence of skin and hair changes, hunched posture, diarrhea, and weight loss. Mice had to have skin lesions and hair loss consistent with GVHD and then either diarrhea or weight loss to be considered to have GVHD. Weight loss greater than 10% of initial weight was considered significant. Mice were weighed weekly for 50 days and then every 28 days until day 250.
Histology.
Mice were killed at day 150 after splenocyte transfer and tissues were placed in 10% neutral buffered formalin. Tissues were sectioned with a microtome and stained with hematoxylin and eosin. Tissues were evaluated by one of us (S.L.K.) blinded to the treatment administered.
Autoantibody production.
Surviving mice were bled at day 100 from the lateral tail vein. Blood was diluted 1:20 in 4 mol/L citric acid and tested for IgG to chromatin as previously reported.32 Control animals were irradiated C57BL/6 mice that received splenocytes from C57BL/6 mice.
Detection of antihost cytotoxic T-cell activity in vitro.
At day 35 after transfer, spleen cells were isolated from bm1 recipient mice and incubated with irradiated (2,500 cGy) splenocytes isolated from either bm1, DBA/2J, or C57BL/6 mice in the presence of 25% concavalin A supernatant. Four days later, effector cells were harvested and tested for the ability to lyse con A blasts from bm1, C57BL/6 (H-2b), or DBA/2J (H-2d) mice in a standard 4-hour 51Chromium release assay. Target to effector ratios of 2.5:1 and 25:1 were used. The percentage of lysis was calculated using the following formula: ([cpm sample − cpm spontaneous]/[cpm total – cpm spontaneous]) × 100. Spontaneous release was measured in wells that contained only target cells. Total release was measured by the addition of Triton X-100 to a final concentration of 5% to target cells. All samples were run in triplicate. Results are shown for an E:T ratio of 25:1.
Engraftment in an MHC-mismatched setting.
To demonstrate that splenocytes from MIP-1α−/− B6 (H-2b) mice were capable of engrafting in completely mismatched DBA/2J mice (H-2d), splenocytes were isolated from MIP-1α−/− B6 mice and 1 × 107 were administered intravenously to DBA/2J mice that had received 500 cGy of irradiation the previous evening. Splenocytes from DBA/2J mice isolated at day 7 were analyzed by flow cytometry for the expression of H-2Kb (transferred population) and H-2Kd(endogenous population). H-2Kb and H-2Kdantibodies were purchased from Pharmingen (LaJolla, CA). Mice were killed at day 14 and splenocytes were isolated and stimulated for 3 days with irradiated splenocytes from either C57BL/6 or DBA/2J mice. After this, T cells were tested for the ability to lyse con A blasts from either DBA/2J or MIP-1α−/− B6 mice in a standard 51Chromium-release assay.
Statistical analysis.
Groups were compared for the occurrence of GVHD using Fisher’s exact test. Groups were compared for differences in the production of autoantibodies and weight loss using the Student’s t-test. Estimates of the probability of survival for all groups were determined using the method of Kaplan and Meier.33P values less than .05 were considered significant.
RESULTS
Incidence of GVHD.
To examine the role of MIP-1α produced by T lymphocytes in the occurrence of GVHD caused by a single class I MHC difference, we transferred spleen cells from either B6 or MIP-1α−/− B6 mice to bm1 recipients. Previously, we have shown that MIP-1α−/− mice have similar numbers of total white blood cells and red blood cells in the bloodstream and similar numbers of CD4 and CD8 cells in lymph nodes and spleen as compared with control animals.31 Therefore, the splenocyte transfers should contain similar number of CD4+ and CD8+lymphocytes regardless of donor used. The results are shown in Table 1. For recipients receiving 500 cGy of irradiation before the splenocyte transfer, there was a significant difference in the incidence of GVHD at days 150 (P= .04) and a trend toward a decreased incidence in GVHD at day 100 (P = .054) in mice that received MIP-1α−/− B6 splenocytes (incidence of GVHD, 40%) as compared with those that received C57BL/6 splenocytes (incidence of GVHD, 75%).
Outcome of 20 bm1 Mice That Received Splenocytes From Either MIP-1α −/− or C57BL/6 Mice
| Day After Transfer . | MIP-1α−/− Splenocytes . | C57BL/6 Splenocytes . | . |
|---|---|---|---|
| 100 | 8 (40%) | 15 (75%) | GVHD |
| 12 (60%) | 5 (25%) | No GVHD | |
| 150 | 10 (50%) | 17 (85%) | GVHD |
| 10 (50%) | 3 (15%) | No GVHD |
| Day After Transfer . | MIP-1α−/− Splenocytes . | C57BL/6 Splenocytes . | . |
|---|---|---|---|
| 100 | 8 (40%) | 15 (75%) | GVHD |
| 12 (60%) | 5 (25%) | No GVHD | |
| 150 | 10 (50%) | 17 (85%) | GVHD |
| 10 (50%) | 3 (15%) | No GVHD |
Outcome of 20 bm1 mice that received splenocytes from either MIP-1α1 −/− or C57BL/6 mice was graded as indicated in the text.
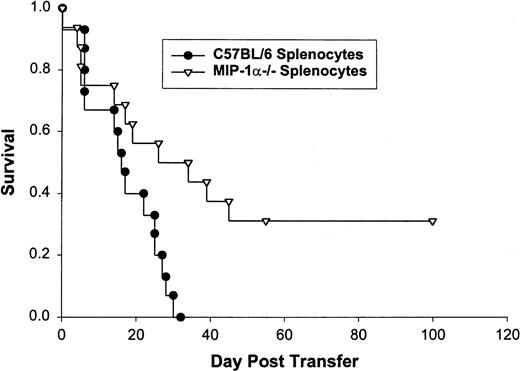
We confirmed these findings using survival as the end-point for analysis. bm1 mice receiving splenocytes from MIP-1α−/− B6 mice had a statistically increased survival (median survival, >250 v 105 days) over the 250 days after the transfer compared with those that received splenocytes from C57BL/6 mice (P = .02; Fig 1). We performed this same experiment using CD8+-selected splenocytes transferred into bm1 recipients who had received 600 cGy of irradiation. bm1 mice in this model die early of aGVHD when CD8+ lymphocytes from C57BL/6 animals were administered.34 As shown in Fig2, we saw a significant difference in mortality due to aGVHD in favor of bm1 recipients of MIP-1α−/− B6 splenocytes (median survival, 36 days) as compared with C57BL/6 splenocytes (median survival, 16.5 days; P = .04).
Survival of 10 bm1 mice that received splenocytes from MIP-1−/− or C57BL/6 mice. Bm1 mice were irradiated the day before transfer of 1 × 107 splenocytes depleted of red blood cells. Mice were observed until day 250 or death. This is one of three experiments (the pooled number of mice used was 30 for each condition).
Survival of 10 bm1 mice that received splenocytes from MIP-1−/− or C57BL/6 mice. Bm1 mice were irradiated the day before transfer of 1 × 107 splenocytes depleted of red blood cells. Mice were observed until day 250 or death. This is one of three experiments (the pooled number of mice used was 30 for each condition).
Survival of bm1 mice that received CD8+lymphocytes from MIP-1−/− or C57BL/6 mice. Bm1 mice received 600 cGy irradiation on the evening before splenocyte transfer. Splenocytes were prepared as indicated in the text. All mice were observed until day 100 or death. Sixteen mice were treated in each group.
Survival of bm1 mice that received CD8+lymphocytes from MIP-1−/− or C57BL/6 mice. Bm1 mice received 600 cGy irradiation on the evening before splenocyte transfer. Splenocytes were prepared as indicated in the text. All mice were observed until day 100 or death. Sixteen mice were treated in each group.
When similar experiments were performed using bm12 mice as the recipient, no difference in outcome was observed using splenocytes from either MIP-1α−/− mice or C57BL/6 mice (Fig 3; median survival, 125 v 145 days). Interestingly, the shape of the curves were different, with most of the deaths occurring early in bm12 mice receiving MIP-1α−/− B6 splenocytes, suggesting an enhanced effect on aGVHD in the setting of a class II MHC mismatch. Therefore, splenocytes from MIP-1α−/− B6 mice were impaired in the ability to cause GVHD across a class I but not class II MHC barrier.
Survival of 10 bm12 mice that received splenocytes from MIP-1−/− or C57BL/6 mice. Bm12 mice were irradiated as described in Fig 1. Splenocytes were prepared as indicated in the text and mice were observed until day 250 or death.
Survival of 10 bm12 mice that received splenocytes from MIP-1−/− or C57BL/6 mice. Bm12 mice were irradiated as described in Fig 1. Splenocytes were prepared as indicated in the text and mice were observed until day 250 or death.
Histology and weight loss.
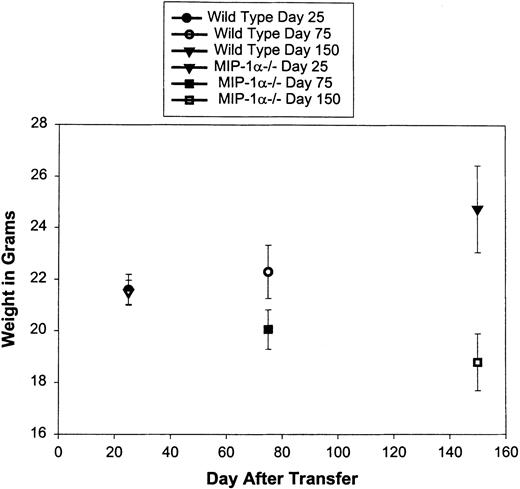
Tissue sections from bm1 mice that received either splenocytes from C57BL/6 or MIP-1α−/− B6 mice were evaluated for the presence of inflammatory foci in the lungs, liver, spleen, and gastrointestinal tract. We found a decreased inflammatory response in the liver and lungs of bm1 mice that received MIP-1α−/− B6 splenocytes as compared with C57BL/6 splenocytes (Fig 4a, A and B v D and E, and Fig 4b, B). Less striking was the decrease in the inflammatory response in the gastrointestinal tract of bm1 mice receiving MIP-1α−/− B6 splenocytes compared with C57BL/6 splenocytes (Fig 4b, A). The inflammatory infiltrate was made up primarily of mononuclear leukocytes (Fig 4b, C). Furthermore, bm1 mice that received MIP-1α−/− B6 splenocytes had significantly less weight loss at day 75 (P = .04) and at day 150 (P= .007) compared with mice that received splenocytes from C57BL/6 mice (Fig 5).
(a and b) Histopathology from bm1 mice after transfer of C57BL/6 or MIP-1−/− B6 splenocytes. Bm1 mice that received splenocytes from C57BL/6 or MIP-1−/− B6 were killed 150 days after transfer. Tissues were prepared as indicated in the text. (a) shows (A and B) sections of liver from bm1 mouse that received MIP-1−/− B6 splenocytes compared with sections from an animal that received C57BL/6 splenocytes (D and E). Sections (A and B) and (D and E) represent two different magnifications of the same liver zone. (a, C) is a section of the lung of a bm1 mouse that received MIP-1−/− B6 splenocytes compared with (F) and (b, B) sections of lung from a bm1 mouse that received C57BL/6 splenocytes (figures are of the same section at different magnifications). Foci of inflammation are indicated by the arrows. The arrowheads indicate extravasation of material into the lungs of bm1 mice that received C57BL/6 splenocytes. (b, A) shows a crypt abscess in the gastrointestinal tract of a bm1 animal that received C57BL/6 splenocytes; these were not seen in animals that received MIP-1−/− splenocytes. The character of the mononuclear cell infiltrate in the liver is shown in (b, C).
(a and b) Histopathology from bm1 mice after transfer of C57BL/6 or MIP-1−/− B6 splenocytes. Bm1 mice that received splenocytes from C57BL/6 or MIP-1−/− B6 were killed 150 days after transfer. Tissues were prepared as indicated in the text. (a) shows (A and B) sections of liver from bm1 mouse that received MIP-1−/− B6 splenocytes compared with sections from an animal that received C57BL/6 splenocytes (D and E). Sections (A and B) and (D and E) represent two different magnifications of the same liver zone. (a, C) is a section of the lung of a bm1 mouse that received MIP-1−/− B6 splenocytes compared with (F) and (b, B) sections of lung from a bm1 mouse that received C57BL/6 splenocytes (figures are of the same section at different magnifications). Foci of inflammation are indicated by the arrows. The arrowheads indicate extravasation of material into the lungs of bm1 mice that received C57BL/6 splenocytes. (b, A) shows a crypt abscess in the gastrointestinal tract of a bm1 animal that received C57BL/6 splenocytes; these were not seen in animals that received MIP-1−/− splenocytes. The character of the mononuclear cell infiltrate in the liver is shown in (b, C).
Weight loss in bm1 recipients of C57BL/6 or MIP-1−/− B6 splenocytes. After splenocyte transfer, bm1 mice were weighed weekly for the first 7 weeks and then monthly until day 250. After death, the weight just before death of each mouse was carried until day 250. N = 12 mice in each group.
Weight loss in bm1 recipients of C57BL/6 or MIP-1−/− B6 splenocytes. After splenocyte transfer, bm1 mice were weighed weekly for the first 7 weeks and then monthly until day 250. After death, the weight just before death of each mouse was carried until day 250. N = 12 mice in each group.
Autoantibody production.
cGVHD in mice is characterized by the Th2 cytokine-driven production of autoantibodies.22 Because our previous experiments had suggested greater differences later after the transfer of splenocytes, we wanted to test for differences in the production of autoantibodies in this model. As demonstrated in Fig 6, there was a striking decrease in the production of serum antichromatin antibodies in mice that received MIP-1α−/− B6 splenocytes as compared with C57BL/6 splenocytes (P < .001). Mice that received splenocytes from MIP-1α−/− mice had similar production of autoantibodies compared with control mice in which C57BL/6 splenocytes were administered to C57BL/6 mice. Thus, autoantibody production was increased even in the setting of only a class I MHC difference.
Autoantibody production after splenocyte transfer. Bm1 mice were transferred splenocytes from either C57BL/6 or MIP-1−/− B6 mice. Mice were bled from the lateral tail vein and 50 μL of blood was diluted in 1 mL of 4 mol/L citric acid. The serum was collected by centrifugation of the sample at 13,000g and frozen at −70°C. The serum was tested for the presence of IgG antichromatin autoantibodies using an enzyme-linked immunosorbent assay (ELISA). Control mice were C57BL/6 mice that received splenocytes from C57BL/6 mice.
Autoantibody production after splenocyte transfer. Bm1 mice were transferred splenocytes from either C57BL/6 or MIP-1−/− B6 mice. Mice were bled from the lateral tail vein and 50 μL of blood was diluted in 1 mL of 4 mol/L citric acid. The serum was collected by centrifugation of the sample at 13,000g and frozen at −70°C. The serum was tested for the presence of IgG antichromatin autoantibodies using an enzyme-linked immunosorbent assay (ELISA). Control mice were C57BL/6 mice that received splenocytes from C57BL/6 mice.
Generation of host-specific cytotoxic T lymphocytes (CTL).
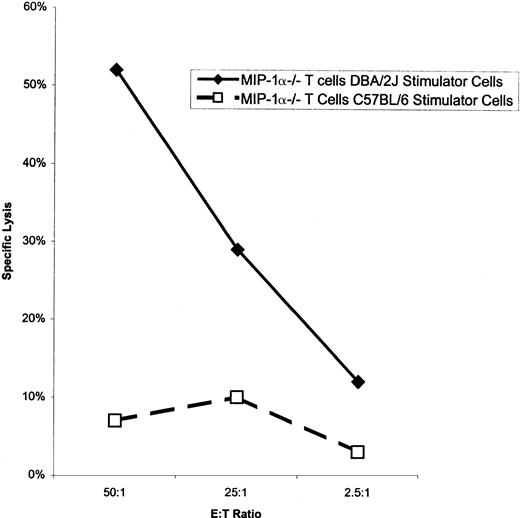
We have previously shown that pathogen-specific T lymphocytes are not recruited to the site of infection if they do not produce MIP-1α after adoptive transfer.31a We tested for the presence of donor-specific T lymphocytes in the spleen of bm1 mice after transfer of either wild-type or MIP-1α−/− B6 splenocytes. After the transfer of wild-type splenocytes, anti-host CTL activity was readily apparent (Fig 7). In contrast, there was no activity against host after the transfer of MIP-1α−/− B6 splenocytes. To evaluate if donor splenocytes were still present in the spleen, we tested splenocytes from bm1 recipients of either MIP-1α−/− or C57BL/6 for the ability to recognize third-party target cells. Bm1 recipients of both C57BL/6 and MIP-1α−/− splenocytes were able to recognize and kill third-party con A blasts from DBA/2J mice. There was no lytic activity directed against C57BL/6 con A blasts, showing that the lytic activity was not due to expansion of endogenous bm1 lymphocytes reacting with donor splenocytes. Thus, MIP-1α−/− splenocytes are capable of initiating a response against host in a completely mismatched setting. However, in the setting of differences in the Kb allele, splenocytes from MIP-1α−/− B6 mice do not cause a detectable antihost response.
Lytic activity of splenocytes isolated from bm1 mice against blast cells from bm1, B6, or DBA/2J mice. Bm1 mice were killed 35 days after splenocyte transfer of either C57BL/6 (▪) or MIP-1−/− (▦) mice. Splenocytes were stimulated in vitro as indicated and tested for lytic activity in a conventional51Cr-release assay. This is one of three representative experiments.
Lytic activity of splenocytes isolated from bm1 mice against blast cells from bm1, B6, or DBA/2J mice. Bm1 mice were killed 35 days after splenocyte transfer of either C57BL/6 (▪) or MIP-1−/− (▦) mice. Splenocytes were stimulated in vitro as indicated and tested for lytic activity in a conventional51Cr-release assay. This is one of three representative experiments.
Engraftment studies.
We evaluated the ability of MIP-1α−/− T cells to engraft in a completely MHC-mismatched setting. We transferred 1 × 107 MIP-1α−/− B6 splenocytes into completely mismatched irradiated (500 cGy) DBA/2J mice. We performed flow cytometry on splenocytes from DBA/2J mice 7 days after splenocyte transfer. Two percent to 4% of the splenocytes expressed the transferred Kb protein. Thus, of 1 × 107splenocytes transferred, 20% to 30% of these cells were either still present or had expanded to this number at day 7 after transfer in the spleen alone (data not shown). We also evaluated whether DBA/2J-specific lymphocytes were present in the spleen at day 14 after transfer of splenocytes from C57BL/6 or MIP-1α−/− B6 mice. As shown in Fig 8, we were able to show DBA/2J-specific T-cell activity after transfer of MIP-1α−/− B6 splenocytes. Thus, because con A blasts do not express detectable class II MHC complexes, we have shown that class I-reactive MIP-1α−/− B6 lymphocytes can engraft using sublethal irradiation in a completely MHC-mismatched setting.
Lytic activity from splenocytes of DBA/2J mice after transfer of MIP-1−/− B6 splenocytes. DBA/2J mice were irradiated as indicated in the text and the following day received 1 × 107 splenocytes from MIP-1−/− B6 or C57BL/6 mice. Fourteen days later, mice were killed, and their splenocytes were isolated and stimulated for 48 hours with irradiated (2,500 cGy) C57BL/6 or DBA/2J splenocytes. Lymphocytes were tested for lytic activity on con A blasts from C57BL/6 and DBA/2J mice as indicated in the text. Spontaneous release for each target cell was less than 20% of maximal. This is one of two experiments.
Lytic activity from splenocytes of DBA/2J mice after transfer of MIP-1−/− B6 splenocytes. DBA/2J mice were irradiated as indicated in the text and the following day received 1 × 107 splenocytes from MIP-1−/− B6 or C57BL/6 mice. Fourteen days later, mice were killed, and their splenocytes were isolated and stimulated for 48 hours with irradiated (2,500 cGy) C57BL/6 or DBA/2J splenocytes. Lymphocytes were tested for lytic activity on con A blasts from C57BL/6 and DBA/2J mice as indicated in the text. Spontaneous release for each target cell was less than 20% of maximal. This is one of two experiments.
DISCUSSION
GVHD is the limiting factor in the applicability of mismatched and unrelated allogeneic BMT for the treatment of tumors and congenital diseases. Current prevention and treatment of GVHD is not adequate to allow the majority of patients to receive this form of therapy. In the present study, we have investigated the role of T-cell production of the chemokine MIP-1α in the occurrence of GVHD. In our model system, MIP-1α−/− B6 splenocytes were markedly impaired in the ability to cause aGVHD and cGVHD across a class I but not class II MHC barrier. This was confirmed by demonstrating differences in overall survival, histopathological changes in the liver and lungs, weight loss, production of autoantibodies, and antihost CTL reactivity when comparing bm1 recipients receiving wild-type or MIP-1α−/− splenocytes.
The present work was undertaken to investigate if alloreactive lymphocytes are actively recruited out of the vasculature in GVHD and if the production of chemokines impacted this recruitment. Because of our previous finding that T-cell production of MIP-1α was important in the recruitment of listeria-specific T lymphocytes, we focused on the role of MIP-1α produced by T cells in GVHD. Our current data suggest that T-cell production of MIP-1α is important in the recruitment of alloreactive T lymphocytes. This was demonstrated by showing the lack of bm1-specific T lymphocytes in the spleens of bm1 mice that received splenocytes from MIP-1α−/− mice. We believe that the best explanation for the data presented is the lack of recruitment of alloreactive T lymphocytes to the spleen. However, although we have found that T lymphocytes that do not produce MIP-1α can function as allospecific cytolytic lymphocytes, these cells do not kill as well as wild-type cells. This would be consistent with data from Taub et al35 that suggest enhanced killing by T lymphocytes in the presence of β chemokines. The decreased ability to kill target cells may also play a role in the decreased incidence of GVHD in this setting.
We do not believe that the decreased incidence of GVHD after the transfer of MIP-1α−/− splenocytes is due to the lack of engraftment of the transferred splenocytes. This is based on the following observations. (1) The model that we have used has been previously shown to enable engraftment using sublethal irradiation.34 (2) We have shown that MIP-1α−/− B6 splenocytes engraft in the more stringent MHC-mismatched transfer and that CD8+ lymphocytes mediate lysis of host splenocytes after this transfer. (3) We have shown that third-party–specific lymphocytes are present in bm1 recipients after transfer of MIP-1α−/− B6 splenocytes. Because these lymphocytes do not lyse splenocytes from C57BL/6 mice, they cannot be from expanded endogenous cells but must come from the transferred population of splenocytes. Finally, (4) the incidence of GVHD is always greater than the severity of GVHD using splenocytes from MIP-1α−/− B6 mice. This finding is not consistent with enhanced survival solely being due to lack of engraftment and therefore occurrence of GVHD.
The finding that T lymphocytes’ production of MIP-1α is important in the setting of a class I but not class II MHC mismatch was not entirely unexpected. Previous investigators have also shown that CD8+ lymphocytes are the predominant source of C-C chemokines that block infectivity with human immunodeficiency virus.36-39 MIP-1α has been shown to have less of a role in the recruitment of activated CD4+lymphocytes.40-42
Our data also show that the blockade of T-cell production of only MIP-1α will have little effect on the occurrence of GVHD in the setting of an MHC class II mismatch. Interestingly, the incidence of lethal early GVHD was greater in bm12 mice that received splenocytes from MIP-1α−/− mice as compared with B6 mice. The reason(s) for the enhanced effects on GVHD of splenocytes from MIP-1α−/− mice has not been addressed in the current work. Quite possibly, splenocytes from MIP-1α−/− mice overexpress other chemokines that may be more important in the recruitment of activated CD4+ T lymphocytes. Alternatively, the loss of MIP-1α may have less of an effect on the production of cytokines or the lytic activity of activated CD4+lymphocytes. We are currently investigating these possibilities.
Our finding of enhanced autoantibody production after transfer of C57BL/6 splenocytes into irradiated bm1 recipients is intriguing. Previous investigators have shown that in the class II mismatched setting autoantibodies are produced by host B cells.43-45If production of autoantibodies is similar in this model, the data suggest that donor CD8+ lymphocytes either directly or indirectly are capable of stimulating host B cells to produce autoantibodies. We are currently performing work to examine this possibility.
We have focused on the production of chemokines by lymphocytes because of our data in the adoptive transfer model using Listeria monocytogenes. Current models suggest that local production of chemokines in the lung by alveolar macrophages may be quite important in the pathophysiology of asthma and infectious pneumonitis.46-48 Our work does not eliminate the possibility that local production of chemokines by macrophages, endothelial cells, fibroblasts, and other cell types contribute to the pathology in GVHD. Our findings of a marked decrease in the inflammatory infiltrate in the lungs and liver of recipients of MIP-1α−/− B6 splenocytes show that lymphocyte production of MIP-1α may be more important in the incidence and severity of GVHD in specific target organs than previously suspected.
We chose to use a nonlethal irradiation model for induction of GVHD for two reasons. MIP-1α is expressed in the bone marrow microenvironment and plays a critical role in stem cell kinetics.30Therefore, bone marrow transplanted from MIP-1α−/− mice is qualitatively different than bone marrow transplanted from C57BL/6 mice. This variable would be difficult to control and could lead to differences in outcome that are independent of the occurrence of GVHD. In addition, the system that we have used allows for differences in the timing of GVHD, which is dependent on the number of lymphocytes infused and the amount of irradiation administered to the recipients. This allowed us to study the role of MIP-1α in the early and late events associated with GVHD.
In summary, we have shown that blockade of T-cell production of a chemokine may offer a new avenue for the prevention of GVHD. We have shown that recipients that receive T cells that do not produce the C-C chemokine, MIP-1α, have a statistically improved survival and decreased incidence of GVHD across a class I MHC barrier. However, preventing T-cell production of MIP-1α had little effect on the occurrence of GVHD when splenocytes were administered to class II-mismatched recipients. Thus, an approach that blocks the function of multiple different chemokines may provide a new avenue of prevention in allogeneic BMT.
Supported by Public Health Service Grants No. CA66715 (J.S.S.) and AI20288 (J.A.F.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Jonathan S. Serody, MD, Division of Medical Oncology, Campus Box #7305, University of North Carolina School of Medicine, Chapel Hill, NC 27599-7305; e-mail: serody@med.unc.edu.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal