Abstract
Current methods for direct gene transfer into hematopoietic cells are inefficient. Here we show that functional complementation of Fanconi anemia (FA) group C cells by protein replacement can be as efficacious as by transfection with wild-type FAC cDNA. We expressed a chimeric protein (called His-ILFAC) consisting of the mature coding portion of gibbon interleukin-3 (IL-3) and full-length FAC inEscherichia coli. The purified bacterial protein is internalized by hematopoietic cells via IL-3 receptors. The intracellular half-life of His-ILFAC is approximately 60 minutes, which is comparable to that of the transgene-encoded FAC protein. In this cell-culture model His-ILFAC completely corrects the sensitivity of FA group C cells to mitomycin C, but it has no effect on FA cells that belong to complementation groups A and B. We suggest that receptor-mediated endocytosis of cytokine-fusion proteins may be of general use to deliver macromolecules into hematopoietic progenitor cells.
FANCONI ANEMIA (FA) is widely regarded as a prototype hematopoietic disorder that is amenable to gene therapy.1,2 FA is genetically heterogeneous and manifested clinically by birth defects, progressive failure of hematopoiesis, and transformation to acute leukemia.3 Over the past several years, great strides have been made in the genetic classification of FA into distinct complementation groups, in the assignment of chromosomal loci for three groups, and in cloning of the disease genes for complementation groups A and C, called FAA and FAC, respectively.4,5 The latter two groups account for the majority of FA patients. Although the FAA and FAC genes encode unique proteins with no homology to each other,6-8all FA cells are classically distinguished by having an enhanced sensitivity to bifunctional cross-linking agents, such as mitomycin C (MMC) and diepoxybutane (DEB). In addition, FA cells have a propensity to apoptosis and manifest variable cell cycle defects.9,10Progress has also been made toward an understanding of the function of proteins encoded by these genes. The FAC protein has been shown to localize predominantly to the cytoplasmic compartment11-16and function in a prerepair pathway.13 Indeed, cytoplasmic localization of FAC is essential for cross-linker (MMC and DEB) complementation of FA group C cells, whereas forced entry into nuclei completely abolishes this function.13 FAC also binds to NADPH cytochrome P450 reductase and attenuates its activity, which suggests that it plays a role in cellular detoxification.17By contrast, FAA localizes to both the cytoplasm and nucleus,15,18,19 and forced exit from the nucleus abolishes its cross-linker complementation function.19
Death in FA usually results from complications of hematopoietic failure.2 Although many questions still remain about the molecular pathogenesis of FA, parallel efforts to improve the therapy of this devastating disorder by bone marrow and cord blood transplantation are yielding encouraging results. Gene therapy is also being explored, although to date the success of this approach has been limited.20 The central premise of gene therapy is to introduce genes directly into target cells of interest. The paucity of hematopoietic stem or progenitor cells and other unknown barriers for successful transduction and expression may be difficult to overcome with current methods. Here we show an alternative method for functional complementation of hematopoietic cells using cell culture models of FA group C. This method exploits the ability of a cytokine ligand-receptor complex to undergo endocytosis. We show that a cytokine fusion protein can be internalized by a similar mechanism and correct a deficient function of FA cells.
MATERIALS AND METHODS
Cell culture and transfection.
Lymphoblasts and WEHI cells were maintained in RPMI 1640 medium containing 10% fetal calf serum (FCS). Two different human lymphoblastoid cell lines were used in these studies: HSC536 (compound heterozygous for a missense mutation in FAC on one allele and an unknown mutation on the second allele) and GM4510 (homozygous for a splicing mutation in FAC). HSC72 (FA group A) and HSC230 (FA group B) cells have been described previously.6 Stably transfected lymphoblasts generated by electroporation were grown in RPMI 1640–10% FCS supplemented with 200 μg/mL hygromycin B.
Constructs.
A 1.66-kb BamHI-Xba I restriction fragment containing full-length human FAC was obtained from pGEX2TK-FAC, as described previously,21 and cloned into the corresponding restriction sites of pQE9 (QIAGEN, Valencia, CA) to obtain pQE9-FAC. This construct would be expected to encode a His-tagged full-length FAC protein. To derive pQE9-ILFAC, a 0.4-kb interleukin-3 (IL-3) cassette was obtained by polymerase chain reaction (PCR) using the primers 5′-CGGGATCCGCTCCCATGACCCAGACAA-3′ and 5′-CGGGATCCAGAGATCTCAAGGCTCAAAGT-3′, which contain artificial BamHI sites. After amplification of the mature coding region of gibbon IL-3 from pXM-IL-3 (gift of Dr Steve Clark, Genetics Institute),22 the BamHI-digested PCR fragment was inserted into the unique BamHI site of pQE9-FAC. The sequence and reading frame of the junctions were confirmed by DNA sequencing. This arrangement added two amino acids, Gly-Ser, between the carboxy terminus of IL-3 and amino terminus of FAC. The His-IL-3–FAC cDNA insert was also subcloned into the BamHI andXba I sites of the mammalian episomal expression vector pDR2 downstream of an ATG in the context of a Kozak consensus sequence (details of this construction are available upon request).18
Expression and purification of His-tagged proteins.
The plasmids pQE9-FAC and pQE9-ILFAC were used to transformEscherichia coli strain M15 (pREP4). A 50-mL culture of LB containing ampicillin and kanamycin bacteria grown overnight at 37°C was expanded to 1-L culture medium, grown to A595 = 0.7, induced with 1 mmol/L isopropyl-β-D-thiogalactopyranoside and grown for an additional 4 hours at 37°C. Bacteria were pelleted by centrifugation (5,000g, 10 minutes) and resuspended (5 g wet weight into 35 mL) in Buffer I (0.1 mol/L NaH2PO4, pH 8.0, and 10 mmol/L Tris-HCl, pH 8.0, and protease inhibitors). Using a French Press, the resuspended bacteria were lysed twice and crude inclusion bodies pelleted by centrifugation (10,000g, 15 minutes). Inclusion bodies were resuspended in Buffer I supplemented with 1 mol/L guanidine-HCl and 0.2% NP40, and then repelleted by centrifugation. Washed inclusion bodies were dissolved at room temperature in Buffer A (Buffer I containing 6 mol/L guanidine-HCl, 0.2% NP40, and 10 mmol/L 2-mercaptoethanol). The supernatant was clarified twice by centrifugation (10,000g, 15 minutes) and incubated with Ni2+-agarose (0.5 mL of 50% suspension in Buffer A per 15 mL lysate) for 1 hour at room temperature. The slurry was loaded onto PolyPrep columns (Bio-Rad Laboratories, Richmond, CA) and washed successively with 50 bead volumes of Buffer B (0.1 mol/L NaH2PO4, 10 mmol/L Tris-HCl, 8 mol/L urea [pH 8.0], 0.2% NP40, and protease inhibitors), Buffer C (same as Buffer B, except for pH 6.3), and Buffer D (same as Buffer B, except for pH 5.9 and absence of NP40). Soluble fusion protein was obtained by elution with 10 mL of Buffer E (0.1 mol/L NaH2PO4, 10 mmol/L Tris-HCl, 8 mol/L urea [pH 4.5]). After stepwise dialysis against phosphate-buffered saline (PBS; pH 7.5) containing 4 mol/L urea, 2 mol/L urea, and no urea. The His-ILFAC protein was purified further by fast protein liquid chromatography on a Superdex-75 column (Pharmacia Biotech Inc, Piscataway, NJ). Purified recombinant protein was concentrated by centrifugation through a CentriPrep-10 column (Amicon, Beverly, MA).
Binding and internalization studies.
Purified His-ILFAC or His-FAC were added to HSC536 cells at 4°C in RPMI 1640–10% FCS. After 10 minutes, cells were centrifuged, fresh medium was added in the absence of His-tagged proteins, and cells were rapidly warmed to 37°C. Internalization and intracellular turnover of the proteins were assessed by washing the cells rapidly with cold PBS, lysis in 1× Laemmli buffer, and analysis for the presence of FAC-related proteins by Western blotting.
Western blotting.
The affinity-purified anti-FAC antibody and the Western blotting procedure have been described previously.10,12 14 Briefly, cells were lysed in a buffer containing 20 mmol/L Tris-HCl, pH 8.0, 50 mmol/L NaCl, 2 mmol/L EDTA, 0.1% NP40 and supplemented with 0.5 mmol/L phenylmethylsulfonyl fluoride (PMSF). Aliquots representing 1 × 105 HSC536 cells or column fractions from the gel chromatography step were subjected to electrophoresis on a 10% polyacrylamide gel (SDS-PAGE) and transferred to Polyscreen membrane (NEN Life Science Products, Boston, MA). Blots were incubated with affinity-purified FAC antibody in 10 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 0.05% Tween 20, and 5% nonfat dry milk, followed by incubation in the same buffer with peroxidase-conjugated goat-anti-rabbit IgG (GIBCO-BRL, Grand Island, NY). Bands were detected by using enhanced chemiluminescence (Amersham Life Sciences, Arlington Heights, IL). Either densitometry or PhosphorImaging was used to quantify the intensity of bands on autoradiograms.
MMC sensitivity assay.
The MMC growth inhibition assay was performed by exposing lymphoblast cultures to a range of MMC concentrations in the presence or absence of His-tagged recombinant proteins. Cell numbers were obtained by either total cell counts using a Coulter (Hialeah, FL) counter or Trypan blue exclusion and hemocytometer as described.14
RESULTS
Construction and in vivo function of chimeric protein.
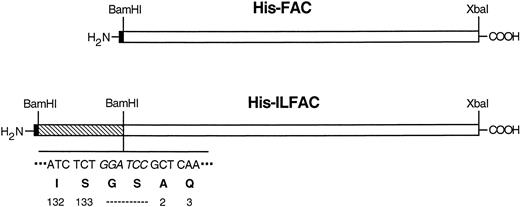
IL-3 is a 133–amino acid glycoprotein that is produced mainly by activated T cells. Its predicted tertiary structure consists of 4 α-helices flanked by exposed ends. Sites on helix A and helix D constitute the binding domain to high-affinity receptors.23We constructed a fusion cDNA consisting of a histidine tag (His-tag) at the amino-terminus, gibbon IL-3 (minus the signal peptide) at the center, and full-length human FAC (minus the initiation codon) at the carboxy terminus (called His-ILFAC; Fig 1). We reasoned that placement of a fusion partner at the exposed carboxy terminus of IL-3 would not result in steric hindrance. Despite these considerations, it is possible that the resulting protein may be intrinsically deficient, but the lack of an in vitro assay for FAC function would not allow us to test this possibility before protein replacement studies. We therefore asked whether or not the chimeric cDNA can encode a functional polypeptide in vivo. We placed an initiation codon with a Kozak consensus sequence at the 5′ end of the tripartite insert and subcloned it into the mammalian episomal expression vector pDR2. Stable expression of this construct in HSC536 cells completely corrected the sensitivity of these cells to MMC. Therefore, this construct is comparable in its in vivo activity to wild-type FAC (Fig 2).
Schematic structures of His-ILFAC and His-FAC. The structures are drawn approximately to scale. The restriction sites and sequence (BamHI site in italics) of the IL-3–FAC junction are indicated. The last two codons for IL-3 at the carboxy terminus, a two-residue linker, and the first two mature codons of FAC are also shown. Hexahistidine tag (filled block), mature gibbon IL-3 (striped block), FAC (open block).
Schematic structures of His-ILFAC and His-FAC. The structures are drawn approximately to scale. The restriction sites and sequence (BamHI site in italics) of the IL-3–FAC junction are indicated. The last two codons for IL-3 at the carboxy terminus, a two-residue linker, and the first two mature codons of FAC are also shown. Hexahistidine tag (filled block), mature gibbon IL-3 (striped block), FAC (open block).
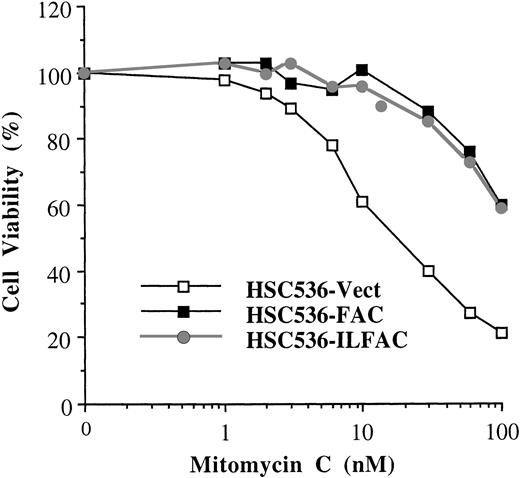
Activity of His-ILFAC transgene in vivo. HSC536 lymphoblasts transfected with pDR2–His-ILFAC or pDR2-FAC were selected for stable expression of the episomal vectors by Hygromycin B, followed by analysis of their growth in the presence of MMC. Growth inhibition was measured by cell counting after 3 days (approximately 3 cell divisions) of continuous exposure to MMC and compared with untreated cells. The final cell count of untreated cultures was set at 100%. The mean values of duplicate sets of experiments are shown.
Activity of His-ILFAC transgene in vivo. HSC536 lymphoblasts transfected with pDR2–His-ILFAC or pDR2-FAC were selected for stable expression of the episomal vectors by Hygromycin B, followed by analysis of their growth in the presence of MMC. Growth inhibition was measured by cell counting after 3 days (approximately 3 cell divisions) of continuous exposure to MMC and compared with untreated cells. The final cell count of untreated cultures was set at 100%. The mean values of duplicate sets of experiments are shown.
Expression and purification of His-ILFAC.
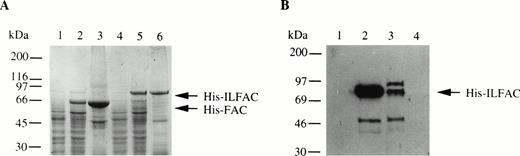
We also generated His-tagged FAC and cloned both His-FAC and His-ILFAC into the prokaryotic expression vector pQE9. Expression of His-FAC and His-ILFAC in E coli generated the expected 63-kD and 75-kD fusion proteins, respectively (Fig 3A). These fusion proteins were immobilized on nickel-agarose, washed, and eluted. Additional purification of His-ILFAC included size exclusion chromatography over Superdex-75 (Fig 3B). Western analysis confirmed that the 75-kD protein is immunoreactive with affinity-purified polyclonal antibodies directed against FAC. The final yield of partially pure His-ILFAC protein was approximately 0.25 mg from 4 L of induced bacterial culture.
Expression and purification of His-FAC and His-ILFAC. (A) Lysates from uninduced (lanes 1 and 4) or induced (lanes 2 and 5) E coli or solubilized proteins (lanes 3 and 6) that were immobilized on Ni2+-agarose, eluted with acid buffer, and analyzed by 10% SDS-PAGE and Coomassie blue staining. (B) Purification of His-ILFAC by size exclusion chromatography. Soluble His-ILFAC obtained after Ni2+-agarose chromatography was resolved further over Superdex-75. Fraction 1, wash buffer; fractions 2 and 3, void volume containing protein peaks as assessed by chromatography tracing (data not shown); fraction 4, void volume containing no protein as predicted by the flat chromatography tracing (data not shown). A 50-kD protein copurifying with His-ILFAC, His-IL▵FAC, most likely results from premature termination of translation as it is immuoreactive with an anti-His antibody. However, carboxy-terminal degradation cannot be excluded.
Expression and purification of His-FAC and His-ILFAC. (A) Lysates from uninduced (lanes 1 and 4) or induced (lanes 2 and 5) E coli or solubilized proteins (lanes 3 and 6) that were immobilized on Ni2+-agarose, eluted with acid buffer, and analyzed by 10% SDS-PAGE and Coomassie blue staining. (B) Purification of His-ILFAC by size exclusion chromatography. Soluble His-ILFAC obtained after Ni2+-agarose chromatography was resolved further over Superdex-75. Fraction 1, wash buffer; fractions 2 and 3, void volume containing protein peaks as assessed by chromatography tracing (data not shown); fraction 4, void volume containing no protein as predicted by the flat chromatography tracing (data not shown). A 50-kD protein copurifying with His-ILFAC, His-IL▵FAC, most likely results from premature termination of translation as it is immuoreactive with an anti-His antibody. However, carboxy-terminal degradation cannot be excluded.
Uptake and intracellular turnover of His-ILFAC by cells expressing IL-3 receptors.
High-affinity binding sites for IL-3 are found on immature CD34+ multipotential hematopoietic cells, myeloid cells, as well as some B cells.23-25 We tested FA group C lymphoblastoid cells (HSC536 and GM4510) as well as HSC72 and HSC230 cells of other FA subtypes for the expression of the IL-3 receptors α and β by reverse-transcription (RT)-PCR. Both cell lines tested showed expression of the α chain but not the β chain (data not shown). To determine whether these cells are capable of binding and internalizing His-ILFAC, we incubated HSC536 lymphoblasts with 10 μg/mL His-ILFAC and His-FAC simultaneously for 10 minutes at 4°C, washed in cold PBS, and shifted the temperature to 37°C to allow internalization. After 5 minutes, cells were washed and lysed, and the lysate was analyzed by immunoblotting with anti-FAC antibody. Immunoreactive His-ILFAC of ∼75-kD size was clearly detected under these conditions (Fig 4). An apparently prematurely truncated His-ILFAC, His-ILΔFAC, also internalized. By contrast, very little if any cell-associated His-FAC appeared to internalize. After the initial 5-minute pulse, the half-life of His-ILFAC was estimated by chase of the internalized protein for various time intervals and found to be approximately 60 minutes, similar to that of transfected FAC (40 to 45 minutes).21
Uptake and turnover of His-ILFAC. His ILFAC and His-FAC (10 μg/mL of cells; lane 1) were bound to HSC536 lymphoblasts at 4°C, internalized by warming to 37°C, and the intracellular fate of the fusion proteins was assessed by Western analysis with anti-FAC antibody after 5 minutes (lane 3), 15 minutes (lane 4), 30 minutes (lane 5), 60 minutes (lane 6), 120 minutes (lane 7), and 240 minutes (lane 8) postbinding times. Lane 2, no recombinant protein added.
Uptake and turnover of His-ILFAC. His ILFAC and His-FAC (10 μg/mL of cells; lane 1) were bound to HSC536 lymphoblasts at 4°C, internalized by warming to 37°C, and the intracellular fate of the fusion proteins was assessed by Western analysis with anti-FAC antibody after 5 minutes (lane 3), 15 minutes (lane 4), 30 minutes (lane 5), 60 minutes (lane 6), 120 minutes (lane 7), and 240 minutes (lane 8) postbinding times. Lane 2, no recombinant protein added.
Receptor-mediated ligand endocytosis.
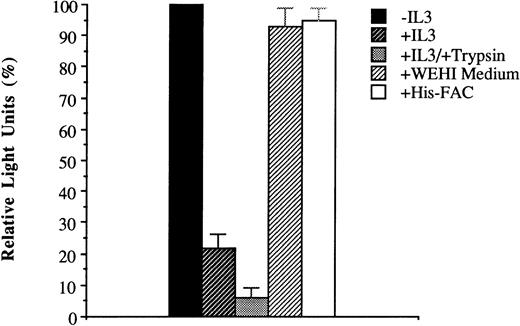
Internalization of His-ILFAC was inhibited when the incubated cells were kept at 4°C (Fig 5). Internalization was also inhibited by addition of recombinant human IL-3 (10 μg/mL; gift of Dr GD Longmore, Washington University, St Louis, MO) to the medium (Fig 6). The lack of complete inhibition may relate to inefficient stripping of surface IL-3 receptors with trypsin, as recognized previously.26 By contrast, no inhibition of His-ILFAC uptake was seen when His-FAC or murine IL-3 (WEHI conditioned medium) were used as competitors. Taken together, these data strongly support the notion that His-ILFAC is internalized by human lymphoblastoid cells by receptor-mediated endocytosis. It is conceivable, however, that IL-3 receptors bound to ligands could recycle back to the cell surface and release IL-3 into the medium, which could create a futile cycle and result in a lower effective intracellular concentration of His-ILFAC. To exclude this possibility, 2 × 106 cells were incubated with His-ILFAC for 10 minutes at 37°C, washed with PBS, and then allowed to incubate in RPMI–10% FCS for up to 1 hour. The medium was then clarified of cell debris and analyzed by immunoblotting. There was no detectable His-ILFAC in the medium (data not shown). The lack of extrusion into the medium suggests that, once inside the cell, His-ILFAC remains confined to the intracellular compartment.
Temperature-dependent uptake of His-ILFAC. HSC536 lymphoblasts were incubated with His-ILFAC for the indicated times at either 37°C or 4°C were lysed and analyzed by immunoblotting with anti-FAC antibody. Light units obtained by PhosphorImaging of the immunoblot are given as percentages of the value at 10 minutes (100%).
Temperature-dependent uptake of His-ILFAC. HSC536 lymphoblasts were incubated with His-ILFAC for the indicated times at either 37°C or 4°C were lysed and analyzed by immunoblotting with anti-FAC antibody. Light units obtained by PhosphorImaging of the immunoblot are given as percentages of the value at 10 minutes (100%).
Inhibition of His-ILFAC uptake. His-ILFAC internalization by HSC536 lymphoblasts was inhibited by addition of recombinant human IL-3 (10 μg/mL) to the medium, and inhibited further by pretreatment of intact cells with 0.25% Trypsin for 10 minutes before incubation with His-ILFAC (10 μg/mL). No inhibition was seen with His-FAC at 50 μg/mL (ie, 5-fold higher concentration than His-ILFAC) or with murine IL-3 (WEHI conditioned medium at a concentration 10-fold higher than that required for growth of the IL-3–dependent cell line HCD57). The mean of three independent measurements and standard error of the mean are shown.
Inhibition of His-ILFAC uptake. His-ILFAC internalization by HSC536 lymphoblasts was inhibited by addition of recombinant human IL-3 (10 μg/mL) to the medium, and inhibited further by pretreatment of intact cells with 0.25% Trypsin for 10 minutes before incubation with His-ILFAC (10 μg/mL). No inhibition was seen with His-FAC at 50 μg/mL (ie, 5-fold higher concentration than His-ILFAC) or with murine IL-3 (WEHI conditioned medium at a concentration 10-fold higher than that required for growth of the IL-3–dependent cell line HCD57). The mean of three independent measurements and standard error of the mean are shown.
Correction of MMC sensitivity in FA group C cells.
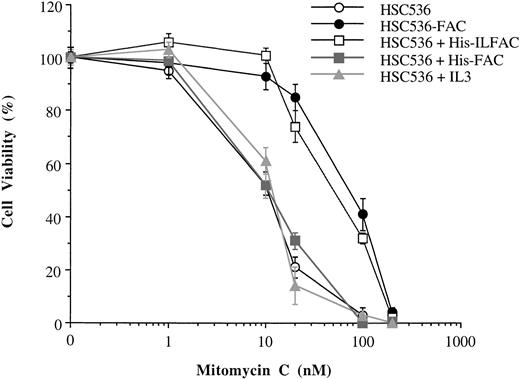
The effect of His-ILFAC on cell survival was evaluated in HSC536 cells exposed to MMC. Approximately 1 × 104 cells were incubated with different concentrations of MMC for 5 days in the presence or absence of His-ILFAC, and viable cells were quantified by Trypan blue exclusion. Cells supplemented with His-ILFAC at a concentration of 1 μg/mL, added at the beginning of the assay and supplemented daily, were significantly more resistant to MMC than control cells (Fig 7) and comparable to HSC536 cells stably expressing wild-type FAC. His-ILFAC also protected GM4510 cells from MMC cytotoxicity (data not shown). By contrast, under those conditions His-FAC at 1 μg/mL or recombinant human IL-3 at 1 μg/mL had no appreciable effect. Neither His-ILFAC nor His-FAC were able to protect HSC72 and HSC230 lymphoblasts from the cytotoxicity of MMC (data not shown). These data show that exogenous supplementation with His-ILFAC can protect FA group C cells from the toxicity of MMC to an extent similar to that of transfected FAC.
Complementation of FA group C cells with FAC molecules. Trypan blue exclusion was used to assess cellular viability of HSC536 lymphoblasts incubated in the presence of MMC continuously for 5 days. HSC536-FAC, HSC536 lymphoblasts stably transfected with wild-type FAC. Treatments with exogenous protein included His-ILFAC (1 μg/mL), His-FAC (1 μg/mL), and recombinant human IL-3 (1 μg/mL) added to the culture medium of HSC536 cells daily for 5 days. Each experiment was performed in triplicate. The mean and standard error of the mean are shown.
Complementation of FA group C cells with FAC molecules. Trypan blue exclusion was used to assess cellular viability of HSC536 lymphoblasts incubated in the presence of MMC continuously for 5 days. HSC536-FAC, HSC536 lymphoblasts stably transfected with wild-type FAC. Treatments with exogenous protein included His-ILFAC (1 μg/mL), His-FAC (1 μg/mL), and recombinant human IL-3 (1 μg/mL) added to the culture medium of HSC536 cells daily for 5 days. Each experiment was performed in triplicate. The mean and standard error of the mean are shown.
DISCUSSION
As an alternative to gene transfer, we have attempted to introduce the FAC protein into hematopoietic cells by exploiting the interaction of IL-3 with its receptor. In view of the difficulties associated with conventional methods of gene transfer (eg, by transduction with viral vectors) into hematopoietic cells, it seems reasonable to explore alternative methods with the eventual goal of generating clinically useful reagents.
Both murine and human IL-3 receptors are internalized upon binding IL-3, followed by digestion of IL-3 within endosomes.26,27The property of IL-3 receptors to undergo endocytosis upon ligand binding appears to be a general property as it is also observed in other members of the cytokine receptor superfamily. Although the IL-3 ligand–receptor pair was used in this study primarily because of the availability of mutant lymphoblasts that expressed the receptor, it may be possible to select other ligand-receptor pairs and target additional subsets of hematopoietic cells. Indeed, recent studies using analogous strategies have included the targeting of cytokine receptor components as fusion proteins with the constant region of IgG1 to Fc receptors on CD34+ cells and surface-modified retroviruses to cytokine receptors.28-31 The restricted expression of IL-3 receptors on immature hematopoietic cells primarily, specificity of binding to IL-3, and the ability of IL-3 receptors to internalize bound ligands led us to use this ligand-receptor pair for the delivery of FAC into hematopoietic cells. The His-ILFAC fusion protein was expressed in E coli and purified to greater than 80% homogeneity by two simple chromatographic techniques. Competition experiments with exogenous IL-3 and stripping of IL-3 receptors from the cell surface showed a significant inhibition in the uptake of the His-ILFAC protein. As expected, human IL-3 but not murine IL-3 was able to act as a competitor of His-ILFAC. Along with the observation that this internalization is temperature-dependent, our results show that His-ILFAC is internalized by receptor-mediated endocytosis.
We believe that several factors were responsible for the success of the experiments described herein, which may not necessarily be extrapolated to other systems. First, we reasoned that the inclusion of a tag at the termini of the IL-3 and FAC proteins would lead to correct folding and function of both domains of the fusion protein. The predicted tertiary structure of IL-3 suggests that modifications of the amino or carboxy termini may be tolerated well and would not interfere with the binding domain established by the α-helical structures.23 With regard to FAC, we knew that attachment of a tag at the amino terminus would not interfere with its function.12,14 By these rudimentary considerations we predicted that a fusion protein constructed in the His-ILFAC arrangement may fold correctly and preserve the function of both IL-3 and FAC. Other attempts to generate functional fusion proteins (eg, to target tumor cells with antibody-toxin or antibody-reporter conjugates) have relied on the placement of long linkers between fusion partners. Here, only two additional amino acids were introduced between IL-3 and FAC, and even those residues may be dispensible. Second, the availability of a simple functional readout made it possible to test both the activity of the transgene and the fusion protein in parallel. This was an important attempt to distinguish partially functional from fully functional fusion proteins. However, we cannot exclude the possibility that the FAC domain of His-ILFAC folds correctly only after entry into cells. Third, FAC is normally expressed at very low levels in most mammalian cells.12 For functional complementation, a relatively small amount of protein targeted to the appropriate cellular compartment may be sufficient to give phenotypic correction. Conversely, the demonstration that FAC overexpression causes no obvious toxicity in transgenic mice indicates that the therapeutic-to-toxic ratio of FAC is highly favorable.32 Fourth, the hydrophobic nature of FAC may be an important determinant which facilitates its exit from endosomes after endocytosis. In this way, a prolonged exposure to the acidic milieu of endosomes and excessive proteolysis may be circumvented. Finally, the size of a fusion protein may be a significant impediment to its expression in various host cells and penetration into cells.33 The relatively small size of the His-ILFAC fusion protein allows for efficient expression in bacteria and potentially good access to hematopoietic progenitor cells.
There are also a number of potential shortcomings with the projected use of this or similar reagents for protein replacement therapy. Chief among them is the paucity of cytokine receptors on hematopoietic progenitor cells. A higher receptor density may be more favorable. We have designed our expression cassette to enable the rapid exchange of IL-3 with other ligands. The short half-life of FAC also may be a limiting factor. Although it is reassuring that the intracellular half-life of His-ILFAC is at least comparable to that of transfected wild-type FAC cDNA,20 longer-acting forms of FAC may function in a more efficient manner. Finally, we do not know whether or not FAC is required during particular stages of the cell cycle. If FAC acts predominantly at a particular stage, pulse delivery of the fusion protein may be sufficient to achieve complementation. Alternatively, there may be a constant requirement for FAC if it is needed throughout the cell cycle. Hence, the precise time of FAC supplementation in the context of cell turnover is unclear. In our cytotoxicity assay (Fig 7), complementation was achieved by daily addition of His-ILFAC to cell cultures. In preliminary experiments, His-ILFAC at 100 μg/mL (10-fold higher concentration than that used in the previous regimen) added once at the beginning of the 5-day exposure period to MMC was also able to confer resistance to MMC (data not shown). Clearly, the optimum delivery schedule needs to be established. In future experiments we hope to define the temporal need for FAC activity, compare other receptor-ligand pairs, and identify FAC residues that, through in vitro mutagenesis, may lead to longer active forms of this molecule.
To our knowledge, this is the first report of an attempted restoration of function in a mendelian disorder by a protein engineered to undergo receptor-mediated endocytosis. It is functionally equivalent to enzyme replacement therapy for several inborn errors of metabolism using mannose-6-phosphate–dependent uptake of proteins into cells.34 In the absence of natural receptors for many intracellular proteins, the engraftment of IL-3 or possibly other cytokines may facilitate the delivery of therapeutic proteins into hematopoietic cells.
ACKNOWLEDGMENT
We thank Drs Steve Clark (Genetics Institute, Cambridge, MA) and Greg Longmore (Washington Universdity, St Louis, MO) for reagents. The early part of this work was performed at the Brigham and Women’s Hospital. The physical and intellectual support provided by that institution is gratefully acknowledged.
Supported by a Translational Research Award from the Leukemia Society of America.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Hagop Youssoufian, MD, Department of Molecular and Human Genetics, Baylor College of Medicine, One Baylor Plaza, S840, Houston, TX 77030; e-mail: hagopy@bcm.tmc.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal