Abstract
To clarify the differences of the signaling pathways used by granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), and tumor necrosis factor- (TNF), we investigated activation of mitogen-activated protein kinase (MAPK) subtype cascades in human neutrophils stimulated by these cytokines. G-CSF exclusively tyrosine-phosphorylated extracellular signal-regulated kinase (ERK). GM-CSF tyrosine-phosphorylated ERK strongly and p38 MAPK weakly, whereas TNF tyrosine-phosphorylated p38 MAPK strongly and ERK weakly. Consistent with these findings, MEK, an upstream kinase of ERK, was phosphorylated by G-CSF, GM-CSF, and TNF, whereas MKK3/MKK6, an upstream kinase of p38 MAPK, was phosphorylated by GM-CSF and TNF, but not by G-CSF. The potency of these cytokines to phosphorylate ERK and MEK was GM-CSF > G-CSF > TNF, whereas that to phosphorylate p38 MAPK and MKK3/MKK6 was TNF > GM-CSF. C-Jun amino-terminal kinase (JNK) was not tyrosine-phosphorylated by any cytokine despite the existence of JNK proteins in human neutrophils, whereas it was tyrosine-phosphorylated by TNF in undifferentiated and all-trans retinoic acid-differentiated HL-60 cells. Increased phosphorylation of ERK or p38 MAPK was detected within 1 to 5 minutes after stimulation with each cytokine and was dependent on the concentrations of cytokines used. MEK inhibitor (PD98059) reduced tyrosine phosphorylation of ERK, but not p38 MAPK, induced by G-CSF, GM-CSF, or TNF. GM-CSF– or TNF-induced superoxide (O2−) release was inhibited by p38 MAPK inhibitor (SB203580) in a dose-dependent manner, suggesting the possible involvement of p38 MAPK in GM-CSF– or TNF-induced O2− release. The results indicate that G-CSF, GM-CSF, and TNF activate the overlapping but distinct MAPK subtype cascades in human neutrophils and suggest that the differential activation of ERK and p38 MAPK cascades may explain the differences of the effects of these cytokines on human neutrophil functions.
VARIOUS FUNCTIONS of mature human neutrophils are known to be activated or potentiated by hematopoietic growth factors or inflammatory cytokines, including granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), and tumor necrosis factor-α (TNF).1-3 Activation or potentiation of mature neutrophil functions by these cytokines may contribute not only to augmenting the host-defense against invading microorganisms, but also to increasing the tissue damage at the inflammatory sites. However, the mechanisms by which these cytokines activate or prime mature neutrophils are largely unknown.
Our previous studies show that there are some similarities in the characteristics of G-CSF–, GM-CSF–, and TNF-mediated activation or priming of human neutrophils1-3; ie, all these cytokines potentiate the responses triggered by receptor-mediated agonists such as N-formyl-methionyl-leucyl-phenylalanine (FMLP), but not by phorbol myristate acetate (PMA), a direct activator of protein kinase C, and do not by themselves stimulate any changes in cytoplasmic free Ca2+ and transmembrane potential. All of these cytokines induce intracellular alkalinization and tyrosine phosphorylation of a 42-kD protein.4 On the other hand, there are definite differences among the effects of these cytokines on human neutrophils. For example, superoxide (O2−) release in suspended human neutrophils is stimulated by GM-CSF or TNF, but not by G-CSF.1-3 NF-κB pathway in human neutrophils is activated by TNF, but not by G-CSF or GM-CSF.5 These previous studies suggest that these cytokines may not only share the common signaling pathways, but also use the cytokine-specific distinct signaling pathways in human neutrophils.
The mitogen-activated protein kinase (MAPK) cascade is a major signaling system that is shared by various types of cells.6 7 In mammalian cells, there are at least three MAPK subtypes; ie, extracellular signal-regulated kinase (ERK), p38 MAPK, and c-Jun amino-terminal kinase (JNK). The ERK cascade is activated in response to signals from receptor tyrosine kinases, hematopoietic growth factor receptors, or some heterotrimeric G-protein–coupled receptors and appears to mediate signals promoting cell proliferation or differentiation. The p38 MAPK and JNK cascades are activated in response to heat shock, hyperosmolarity, UV irradiation, protein synthesis inhibitors, or inflammatory cytokines and appear to be involved in the cell responses to stresses. Each MAPK subtype is activated by phosphorylation on threonine and tyrosine residues by an upstream dual-specificity kinase and phosphorylate substrates on serine or threonine adjacent to proline residues. Activation of the distinct MAPK subtype cascade is dependent on the types of cells and the stimuli used, and the functional role of each MAPK subtype may be different according to the types of cells.
Activation of the MAPK cascade is not restricted to immature cells, and this cascade is also activated in terminally differentiated cells such as neutrophils, suggesting that the MAPK cascade also plays an important role in some functions of terminally differentiated mature cells. We previously reported that tyrosine phosphorylation of a 42-kD protein, possibly a MAPK subtype, is closely associated with the priming effects of G-CSF, GM-CSF, and TNF on human neutrophils.4 By using the immunoprecipitation with specific antibodies against MAPK subtypes, we have recently demonstrated that the distinct MAPK subtype in human neutrophils is exclusively tyrosine-phosphorylated by GM-CSF and TNF.8 ERK was exclusively tyrosine-phosphorylated by GM-CSF, whereas p38 MAPK was exclusively tyrosine-phosphorylated by TNF.8 However, controversial results are reported about activation or tyrosine phosphorylation of MAPK subtypes in human neutrophils stimulated by cytokines. For example, it has been reported that TNF induces tyrosine phosphorylation of ERK in adherent, but not suspended, human neutrophils9; GM-CSF, but not TNF, activates ERK in human neutrophils10-12; and both TNF and GM-CSF induce tyrosine phosphorylation of p38 MAPK.13 In addition, it remains to be determined whether a tyrosine-phosphorylated 42-kD protein in human neutrophils stimulated by G-CSF also belongs to the MAPK family. In this report, we investigated phosphorylation of MAPK subtypes (ERK, p38 MAPK, and JNK) and upstream kinases of these MAPK subtypes (MAPK/ERK kinase [MEK] and MAPK kinase-3 or 6 [MKK3/MKK6]) in human neutrophils stimulated by G-CSF, GM-CSF, and TNF. The comparative studies show that G-CSF, GM-CSF, and TNF activate the overlapping but distinct MAPK subtype cascades in human neutrophils; ie, G-CSF exclusively tyrosine-phosphorylated ERK; GM-CSF tyrosine-phosphorylated ERK strongly and p38 MAPK weakly; TNF tyrosine-phosphorylated p38 MAPK strongly and ERK weakly; and JNK was not tyrosine-phosphorylated by any cytokine. The results suggest that the differential activation of the MAPK subtype cascades may explain the differences of the effects of these cytokines on human neutrophil functions and p38 MAPK may be involved in activation of O2− release.
MATERIALS AND METHODS
Reagents.
Highly purified recombinant human G-CSF, GM-CSF, and TNF produced byEscherichia coli were provided by Kirin Brewery Co Ltd (Tokyo, Japan), Schering-Plough Co Ltd (Osaka, Japan), and Dainippon Pharmaceutical Co Ltd (Osaka, Japan), respectively. The specific activity of TNF was 3 × 106 U/mg protein. Endotoxin contamination of each preparation was less than 100 pg/mg protein. Cytochrome c type III, superoxide dismutase, and all-trans retinoic acid (ATRA) were purchased from Sigma Chemical (St Louis, MO); Conray was purchased from Mallinckrodt (St Louis, MO); and Ficoll was purchased from Pharmacia Fine Chemicals (Piscataway, NJ). PD98059 (MEK inhibitor) and rabbit polyclonal antibodies against Ser217/221-phosphorylated MEK1/MEK2, Ser189/207-phosphorylated MKK3/MKK6, ERK1/ERK2, Tyr204-phosphorylated ERK1/ERK2, JNK/SAPK, Thr183/Tyr185-phosphorylated JNK/SAPK, p38 MAPK, and Thr180/Tyr182-phosphorylated p38 MAPK were purchased from New England Biolabs (Beverly, MA). SB203580 (p38 MAPK inhibitor) was provided by SmithKline Beecham Pharmaceuticals (King of Prussia, PA). The enhanced chemiluminescence (ECL) Western blotting system was purchased from Amersham (Arlington Heights, IL).
Cell culture.
Human myelogenous leukemia cell line HL-60 cells were grown in RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum, penicillin (100 U/mL), and streptomycin (100 μg/mL). For induction of differentiation of HL-60 to granulocytic cells, cells were seeded at 2 × 105 cells/mL and grown in the presence or absence of 1 μmol/L ATRA for 4 days.14
Preparation of cells.
Cultured HL-60 cells were harvested after 4 days of cultivation with or without ATRA, washed three times, and suspended in Hanks’ balanced salt solution (HBSS). Human neutrophils were prepared from healthy adult donors as described,1 using dextran sedimentation, centrifugation with Conray-Ficoll, and hypotonic lysis of contaminated erythrocytes. Neutrophil fractions were suspended in HBSS and contained more than 98% neutrophils.
Western blotting.
Human neutrophils (1 × 107/mL) suspended in HBSS were prewarmed for 10 minutes at 37°C and were then stimulated with cytokines for 1 to 40 minutes at 37°C. The reactions were terminated by rapid centrifugation, and the pellets were frozen in liquid nitrogen after aspiration of the supernatant. The cell-pellets were resuspended in ice-cold solution containing 50 mmol/L HEPES (pH 7.4), 1% Triton X-100, 2 mmol/L sodium orthovanadate, 100 mmol/L sodium fluoride, 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L phenylmethylsulfonyl fluoride, 100 μg/mL aprotinin, and 10 μg/mL leupeptin and were lysed for 60 minutes at 4°C. After rapid centrifugation, the supernatant was mixed 1:1 with 2× sample buffer (4% sodium dodecyl sulfate [SDS], 20% glycerol, 10% mercaptoethanol, and a trace amount of bromophenol blue dye in 125 mmol/L Tris-HCl, pH 6.8), heated at 100°C for 5 minutes, and then frozen at −80°C until use. Samples were subjected to 10% SDS gel electrophoresis. After electrophoresis, proteins were electrophoretically transferred from the gel onto a nitrocellulose membrane in a buffer containing 25 mmol/L Tris, 192 mmol/L glycine, and 20% methanol at 2 mA/cm2 for 4 hours at 25°C. Residual binding sites on the membrane were blocked by incubating the membrane in Tris-buffered saline (pH 7.6) containing 0.1% Tween 20 and 5% nonfat dry milk for 2 hours at 25°C. The blots were washed in Tris-buffered saline containing 0.1% Tween 20 (TBST) and then incubated with appropriate antibody overnight at 4°C. After washing three times with TBST, the membrane was incubated with anti-rabbit IgG antibody conjugated with horseradish peroxidase, and the antibody complexes were visualized by the ECL detection system as directed by the manufacturer.
Determination of O2− release.
O2− was assayed by superoxide-dismutase inhibitable reduction of ferricytochrome c, as described.15 The cell suspension in HBSS was added to a polypropylene tube (Falcon #2063; Falcon Labware, Becton Dickinson, Franklin Lakes, NJ) containing 100 μmol/L ferricytochromec with or without superoxide dismutase (200 U/mL) to obtain a final volume of 0.2 mL. The final cell concentration was 2 × 105 cells/0.2 mL. The reaction mixture was preincubated in the presence or absence of SB203580 (0.01 to 10 μmol/L) for 20 minutes at 37°C, and thereafter GM-CSF (5 ng/mL) or TNF (100 U/mL) was added. After incubation for 3 hours at 37°C, the reduction of ferricytochrome c was measured at 550 nm, with a reference wavelength at 540 nm.
Statistical analysis.
The Student’s t-test was used to determine statistical significance.
RESULTS
ERK1 and ERK2 were tyrosine-phosphorylated by G-CSF, GM-CSF, and TNF.
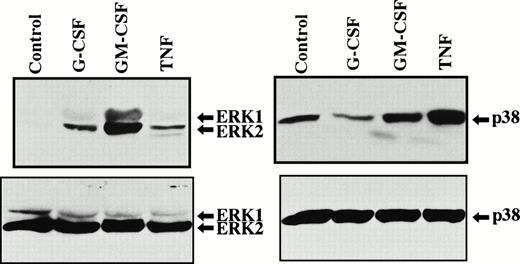
Human neutrophils in suspension were stimulated with G-CSF (50 ng/mL), GM-CSF (5 ng/mL), or TNF (100 U/mL) for 10 minutes at 37°C, and tyrosine phosphorylation of ERK1 and ERK2 was analyzed by immunoblotting using polyclonal antibody against tyrosine-phosphorylated ERK1 and ERK2. As shown in Fig 1, both ERK1 and ERK2 were strongly tyrosine-phosphorylated by GM-CSF with predominant phosphorylation of ERK2, in agreement with previous reports.10-12 Increased tyrosine phosphorylation of ERK1 and ERK2 was also detected in suspended human neutrophils stimulated by G-CSF and TNF, although it was much weaker than that induced by GM-CSF. ERK2 was predominantly tyrosine-phosphorylated by G-CSF and TNF. In TNF-stimulated neutrophils, an additional band was always detected just below the ERK2 band by this antibody, although it is unknown whether this band is related to ERK family or not (Fig 1; see also Figs 4 and 7).
Phosphorylation of ERK1, ERK2, and p38 MAPK in human neutrophils stimulated by G-CSF, GM-CSF, or TNF. Cells were stimulated with G-CSF (50 ng/mL), GM-CSF (5 ng/mL), or TNF (100 U/mL) for 10 minutes at 37°C. Phosphorylation of ERK1, ERK2, and p38 MAPK was analyzed by immunoblotting using antibody against phosphorylated form of each protein (upper panel). The equal loading of proteins onto each lane was confirmed by immunoblotting using antibody that recognizes both phosphorylated and unphosphorylated forms of ERK1/ERK2 or p38 MAPK (lower panel). The cell lysates equivalent to 3.8 × 106cells were loaded onto each lane. The results shown are representative of seven independent experiments. In this experiment, the exposure time was somewhat prolonged to determine whether G-CSF was able to phosphorylate p38 MAPK, which is responsible for higher baseline level of p38 MAPK phosphorylation as compared with that shown in Figs 3, 4, and 7.
Phosphorylation of ERK1, ERK2, and p38 MAPK in human neutrophils stimulated by G-CSF, GM-CSF, or TNF. Cells were stimulated with G-CSF (50 ng/mL), GM-CSF (5 ng/mL), or TNF (100 U/mL) for 10 minutes at 37°C. Phosphorylation of ERK1, ERK2, and p38 MAPK was analyzed by immunoblotting using antibody against phosphorylated form of each protein (upper panel). The equal loading of proteins onto each lane was confirmed by immunoblotting using antibody that recognizes both phosphorylated and unphosphorylated forms of ERK1/ERK2 or p38 MAPK (lower panel). The cell lysates equivalent to 3.8 × 106cells were loaded onto each lane. The results shown are representative of seven independent experiments. In this experiment, the exposure time was somewhat prolonged to determine whether G-CSF was able to phosphorylate p38 MAPK, which is responsible for higher baseline level of p38 MAPK phosphorylation as compared with that shown in Figs 3, 4, and 7.
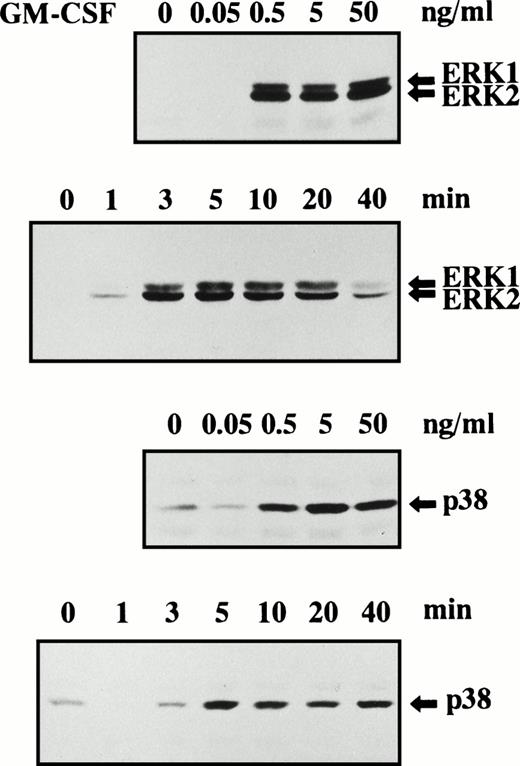
Tyrosine phosphorylation of ERK1 and ERK2 was dependent on the concentrations of G-CSF, GM-CSF, and TNF used as stimuli. When neutrophils were stimulated with G-CSF for 10 minutes, a significant increase of tyrosine phosphorylation of ERK1 and ERK2 was detected at 0.5 ng/mL G-CSF and maximal stimulation was obtained at 5 ng/mL G-CSF (Fig 2). G-CSF–induced tyrosine phosphorylation of ERK1 and ERK2 was rapid and already detected at 1 minute after stimulation with 50 ng/mL G-CSF, and the maximal level was observed at 3 minutes, followed by a gradual decrease of the level. The time-courses of tyrosine phosphorylation of ERK1 and ERK2 were similar to each other (Fig 2).
Phosphorylation of ERK1, ERK2, and p38 MAPK in human neutrophils stimulated by G-CSF. Cells were stimulated with indicated concentrations of G-CSF for 10 minutes at 37°C or stimulated with 50 ng/mL G-CSF for indicated periods at 37°C. Phosphorylation of ERK1, ERK2 (upper 2 panels), and p38 MAPK (lower panel) was analyzed by immunoblotting using antibody against phosphorylated form of each protein. The cell lysates equivalent to 2.7 × 106 cells were loaded onto each lane. The results shown are representative of five independent experiments. In this experiment, the exposure time was somewhat prolonged to determine whether G-CSF was able to phosphorylate p38 MAPK, which is responsible for higher baseline level of p38 MAPK phosphorylation as compared with that shown in Figs 3, 4, and 7.
Phosphorylation of ERK1, ERK2, and p38 MAPK in human neutrophils stimulated by G-CSF. Cells were stimulated with indicated concentrations of G-CSF for 10 minutes at 37°C or stimulated with 50 ng/mL G-CSF for indicated periods at 37°C. Phosphorylation of ERK1, ERK2 (upper 2 panels), and p38 MAPK (lower panel) was analyzed by immunoblotting using antibody against phosphorylated form of each protein. The cell lysates equivalent to 2.7 × 106 cells were loaded onto each lane. The results shown are representative of five independent experiments. In this experiment, the exposure time was somewhat prolonged to determine whether G-CSF was able to phosphorylate p38 MAPK, which is responsible for higher baseline level of p38 MAPK phosphorylation as compared with that shown in Figs 3, 4, and 7.
When neutrophils were stimulated with GM-CSF for 10 minutes, a significant increase of tyrosine phosphorylation of ERK1 and ERK2 was detected at 0.5 ng/mL GM-CSF. No further increase of tyrosine phosphorylation of ERK1 and ERK2 was observed when the concentrations of GM-CSF were increased up to 50 ng/mL (Fig 3). GM-CSF–induced tyrosine phosphorylation of ERK1 and ERK2 was rapid and already detected at 1 minute after stimulation with 5 ng/mL GM-CSF, and the maximal level was observed at 3 minutes, followed by gradual decrease of the level. The time-courses of tyrosine phosphorylation of ERK1 and ERK2 were similar to each other (Fig 3).
Phosphorylation of ERK1, ERK2, and p38 MAPK in human neutrophils stimulated by GM-CSF. Cells were stimulated with indicated concentrations of GM-CSF for 10 minutes at 37°C or stimulated with 5 ng/mL GM-CSF for indicated periods at 37°C. Phosphorylation of ERK1, ERK2, (upper 2 panels) and p38 MAPK (lower 2 panels) was analyzed by immunoblotting using antibody against phosphorylated form of each protein. The cell lysates equivalent to 1.9 × 106 cells were loaded onto each lane. The results shown are representative of three independent experiments.
Phosphorylation of ERK1, ERK2, and p38 MAPK in human neutrophils stimulated by GM-CSF. Cells were stimulated with indicated concentrations of GM-CSF for 10 minutes at 37°C or stimulated with 5 ng/mL GM-CSF for indicated periods at 37°C. Phosphorylation of ERK1, ERK2, (upper 2 panels) and p38 MAPK (lower 2 panels) was analyzed by immunoblotting using antibody against phosphorylated form of each protein. The cell lysates equivalent to 1.9 × 106 cells were loaded onto each lane. The results shown are representative of three independent experiments.
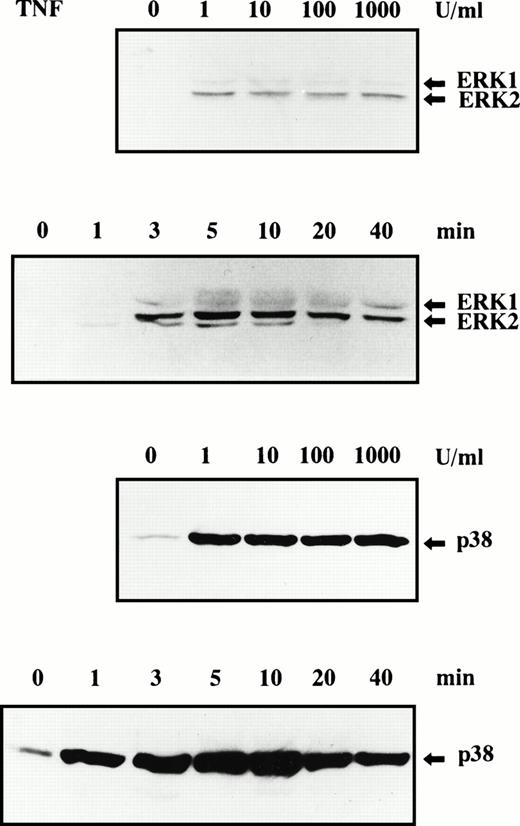
When neutrophils were stimulated with TNF for 10 minutes, a significant increase of tyrosine phosphorylation of ERK1 and ERK2 was detected at 1 U/mL TNF. No further increase of tyrosine phosphorylation of ERK1 and ERK2 was observed when the concentrations of TNF were increased up to 1,000 U/mL (Fig 4). TNF-induced tyrosine phosphorylation of ERK1 and ERK2 was clearly detected at 3 minutes after stimulation with 100 U/mL TNF, and the maximal level was observed at 5 minutes, followed by a gradual decrease of the level (Fig 4). The weak phosphorylation of ERK in TNF-stimulated neutrophils may be responsible for our previous failure to detect tyrosine phosphorylation of ERK in these cells by means of immunoprecipitation.8 The comparative studies showed that the potency of these cytokines to induce tyrosine phosphorylation of ERK1/ERK2 was GM-CSF > G-CSF > TNF (Fig 1). These findings are consistent with the previous report by Raines et al16 that the potency of GM-CSF to induce activation of MAPK is greater than that of G-CSF. The equal loading of proteins onto each lane in each experiment was confirmed by immunoblotting using antibody, which recognizes both phosphorylated and unphosphorylated forms of ERK1/ERK2 (Fig 1; data not shown for Figs 2,3, and 4). The retarded mobility of part of ERK2 observed in GM-CSF–stimulated cells (Fig 1, lower panel) may reflect strong tyrosine-phosphorylation of ERK2 by GM-CSF and is consistent with previous reports.11 12
Phosphorylation of ERK1, ERK2, and p38 MAPK in human neutrophils stimulated by TNF. Cells were stimulated with indicated concentrations of TNF for 10 minutes at 37°C or stimulated with 100 U/mL TNF for indicated periods at 37°C. Phosphorylation of ERK1, ERK2 (upper 2 panels), and p38 MAPK (lower 2 panels) was analyzed by immunoblotting using antibody against phosphorylated form of each protein. The cell lysates equivalent to 3 × 106 cells were loaded onto each lane. The results shown are representative of three independent experiments.
Phosphorylation of ERK1, ERK2, and p38 MAPK in human neutrophils stimulated by TNF. Cells were stimulated with indicated concentrations of TNF for 10 minutes at 37°C or stimulated with 100 U/mL TNF for indicated periods at 37°C. Phosphorylation of ERK1, ERK2 (upper 2 panels), and p38 MAPK (lower 2 panels) was analyzed by immunoblotting using antibody against phosphorylated form of each protein. The cell lysates equivalent to 3 × 106 cells were loaded onto each lane. The results shown are representative of three independent experiments.
A p38 MAPK was tyrosine-phosphorylated by GM-CSF and TNF, but not by G-CSF.
Human neutrophils were stimulated with G-CSF (50 ng/mL), GM-CSF (5 ng/mL), or TNF (100 U/mL) for 10 minutes at 37°C, and tyrosine phosphorylation of p38 MAPK was analyzed by immunoblotting using polyclonal antibody against tyrosine-phosphorylated p38 MAPK. As shown in Fig 1, p38 MAPK was significantly tyrosine-phosphorylated by GM-CSF and TNF, but not by G-CSF. G-CSF–induced increase in tyrosine phosphorylation of p38 MAPK was not detected even when the incubation time with G-CSF (50 ng/mL) was prolonged up to 40 minutes (Fig 2). The level of tyrosine phosphorylation of p38 MAPK was sometimes, but not always, reduced transiently in the early periods (within 10 minutes) after G-CSF stimulation (Fig 1).
When neutrophils were stimulated with GM-CSF for 10 minutes at 37°C, a significant increase of tyrosine phosphorylation of p38 MAPK was detected at 0.5 ng/mL GM-CSF and maximal stimulation was obtained at 5 ng/mL (Fig 3). As observed with G-CSF stimulation, the level of tyrosine phosphorylation of p38 MAPK was sometimes, but not always, reduced transiently in the early periods (within 3 minutes) after GM-CSF stimulation (Fig 3). A significant increase of tyrosine phosphorylation of p38 MAPK was detected at 5 minutes after GM-CSF stimulation, and the maximal level was observed at 5 to 10 minutes, followed by slight decrease of the level (Fig 3). The weak phosphorylation of p38 MAPK in GM-CSF–stimulated neutrophils may be responsible for our previous failure to detect tyrosine phosphorylation of p38 MAPK in these cells by means of immunoprecipitation.8
When neutrophils were stimulated with TNF for 10 minutes, a remarkable increase of tyrosine phosphorylation of p38 MAPK was already detected at 1 U/mL TNF. No further increase of tyrosine phosphorylation of p38 MAPK was observed when the concentrations of TNF were increased up to 1,000 U/mL (Fig 4). TNF-induced tyrosine phosphorylation of p38 MAPK was rapid and already detected at 1 minute after stimulation, and the maximal level was observed at 5 to 10 minutes, followed by a gradual decrease of the level (Fig 4). The comparative studies showed that the potency of these cytokines to induce tyrosine phosphorylation of p38 MAPK was TNF > GM-CSF (Fig 1). The equal loading of proteins onto each lane in each experiment was confirmed by immunoblotting using antibody, which recognizes both phosphorylated and unphosphorylated forms of p38 MAPK (Fig 1; data not shown for Figs 2, 3, and 4).
No tyrosine phosphorylation of JNK in human neutrophils stimulated by G-CSF, GM-CSF, and TNF.
Human neutrophils were stimulated with G-CSF (50 ng/mL), GM-CSF (5 ng/mL), or TNF (100 U/mL) for 10 minutes at 37°C, and tyrosine phosphorylation of JNK was analyzed by immunoblotting using polyclonal antibody against tyrosine-phosphorylated JNK. As shown in Fig 5, no significant increase of tyrosine phosphorylation of JNK was detected in neutrophils stimulated by G-CSF, GM-CSF, or TNF. G-CSF–, GM-CSF–, or TNF-induced increase in tyrosine phosphorylation of JNK was not detected even when the incubation time with each cytokine was prolonged up to 40 minutes (data not shown). The immunoblotting using antibody, which recognizes both phosphorylated and unphosphorylated forms of JNK, showed that two JNK isoforms (JNK1 and JNK2) were detected in human neutrophils (Fig 5). Thus, it appears that in human neutrophils JNK is not tyrosine-phosphorylated by any cytokine despite the existence of JNK proteins.
Phosphorylation of JNK in human neutrophils, undifferentiated HL-60 cells, and ATRA-differentiated HL-60 cells stimulated by G-CSF, GM-CSF, or TNF. Cells were stimulated with G-CSF (50 ng/mL), GM-CSF (5 ng/mL), or TNF (100 U/mL) for 10 minutes at 37°C. Phosphorylation of JNK was analyzed by immunoblotting using antibody against phosphorylated form of JNK (upper panel). The existence of JNK protein was analyzed by immunoblotting using antibody, which is produced by immunizing rabbits with a full-length JNK2 fusion protein and recognizes both phosphorylated and unphosphorylated forms of JNK (lower panel). Total extracts from human embryonic kidney 293 cells prepared with UV light treatment were used as phosphorylation-positive controls. The cell lysates equivalent to 4.7 × 106 neutrophils, 1.9 × 106undifferentiated HL-60 cells, or 1.9 × 106 differentiated HL-60 cells were loaded onto each lane. The results shown are representative of four independent experiments.
Phosphorylation of JNK in human neutrophils, undifferentiated HL-60 cells, and ATRA-differentiated HL-60 cells stimulated by G-CSF, GM-CSF, or TNF. Cells were stimulated with G-CSF (50 ng/mL), GM-CSF (5 ng/mL), or TNF (100 U/mL) for 10 minutes at 37°C. Phosphorylation of JNK was analyzed by immunoblotting using antibody against phosphorylated form of JNK (upper panel). The existence of JNK protein was analyzed by immunoblotting using antibody, which is produced by immunizing rabbits with a full-length JNK2 fusion protein and recognizes both phosphorylated and unphosphorylated forms of JNK (lower panel). Total extracts from human embryonic kidney 293 cells prepared with UV light treatment were used as phosphorylation-positive controls. The cell lysates equivalent to 4.7 × 106 neutrophils, 1.9 × 106undifferentiated HL-60 cells, or 1.9 × 106 differentiated HL-60 cells were loaded onto each lane. The results shown are representative of four independent experiments.
Tyrosine phosphorylation of JNK in undifferentiated and ATRA-differentiated HL-60 cells stimulated by TNF.
No tyrosine phosphorylation of JNK was also observed in human neutrophils stimulated by other agonists including FMLP (0.1 μmol/L), PMA (100 ng/mL), and ionomycin (1 μmol/L; data not shown). However, Rane et al17 have recently reported that FMLP induced tyrosine phosphorylation and activation of JNK in HL-60 cells differentiated by dimethyl sulfoxide (DMSO). This difference might be attributed to the difference of cells used. We then analyzed tyrosine phosphorylation of JNK by using undifferentiated and ATRA-differentiated HL-60 cells. As shown in Fig 5, significant tyrosine phosphorylation of JNK was detected in undifferentiated and differentiated HL-60 cells stimulated by TNF, but not by G-CSF or GM-CSF. Stimulation of KG-1 cells, another myelogenous leukemia cell line, with TNF also resulted in strong tyrosine phosphorylation of two JNK isoforms (data not shown). These findings indicate that, in contrast to normal human neutrophils, differentiated HL-60 cells retain the signaling pathway for tyrosine phosphorylation of JNK.
Effects of G-CSF, GM-CSF, and TNF on phosphorylation of MEK1/MEK2 and MKK3/MKK6.
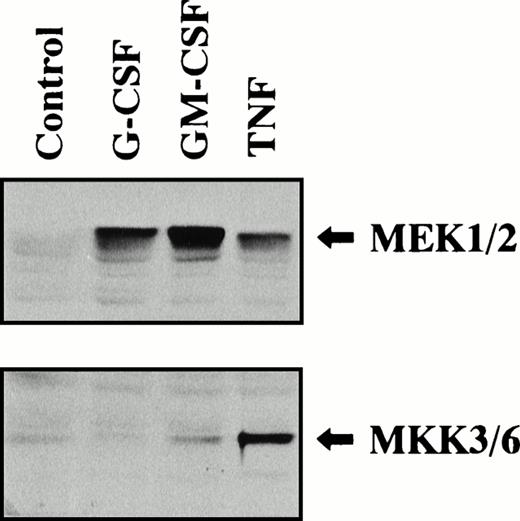
ERK and p38 MAPK are reported to be activated by the distinct signaling cascades.6,7 ERK1 and ERK2 are phosphorylated and activated by MEK1/MEK2, which is phosphorylated and activated by upstream serine/threonine kinases such as Raf and MEK kinase. On the other hand, p38 MAPK is phosphorylated and activated by MKK3/MKK6, which is also phosphorylated and activated by upstream serine/threonine kinases. However, it appears that these signaling cascades are not applicable to all types of cells. In fact, it has been reported that erythropoietin and interleukin-3 (IL-3) activate p38 MAPK in FDC-P2 cells, a murine hematopoietic progenitor cell line, without activating MKK3/MKK6.18 We then studied the effects of G-CSF, GM-CSF, and TNF on phosphorylation of MEK1/MEK2 and MKK3/MKK6 in human neutrophils by using antibodies against serine-phosphorylated MEK1/MEK2 and serine-phosphorylated MKK3/MKK6. As shown in Fig 6, stimulation of human neutrophils with G-CSF (50 ng/mL), GM-CSF (5 ng/mL), or TNF (100 U/mL) for 5 minutes at 37°C resulted in increased phosphorylation of MEK1/MEK2, and the potency of this effect was GM-CSF > G-CSF > TNF. The equal loading of proteins onto each lane was confirmed by immunoblotting using antibody, which recognizes both phosphorylated and unphosphorylated forms of ERK1/ERK2 (data not shown). This finding supports the idea that MEK1/MEK2 is an upstream kinase for ERK1 and ERK2. Similarly, significant increase of phosphorylation of MKK3/MKK6 was detected in neutrophils stimulated by GM-CSF or TNF, but not by G-CSF, and the potency of this effect was TNF > GM-CSF (Fig6). The equal loading of proteins onto each lane was confirmed by immunoblotting using antibody, which recognizes both phosphorylated and unphosphorylated forms of p38 MAPK (data not shown). Thus, this finding supports the idea that MKK3/MKK6 is an upstream kinase for p38 MAPK.
Phosphorylation of MEK1/MEK2 and MKK3/MKK6 in human neutrophils stimulated by G-CSF, GM-CSF, or TNF. Cells were stimulated with G-CSF (50 ng/mL), GM-CSF (5 ng/mL), or TNF (100 U/mL) for 5 minutes at 37°C. Phosphorylation of MEK1/MEK2 and MKK3/MKK6 was analyzed by immunoblotting using antibody against phosphorylated form of each protein. The cell lysates equivalent to 3 × 106cells were loaded onto each lane. The results shown are representative of three independent experiments.
Phosphorylation of MEK1/MEK2 and MKK3/MKK6 in human neutrophils stimulated by G-CSF, GM-CSF, or TNF. Cells were stimulated with G-CSF (50 ng/mL), GM-CSF (5 ng/mL), or TNF (100 U/mL) for 5 minutes at 37°C. Phosphorylation of MEK1/MEK2 and MKK3/MKK6 was analyzed by immunoblotting using antibody against phosphorylated form of each protein. The cell lysates equivalent to 3 × 106cells were loaded onto each lane. The results shown are representative of three independent experiments.
Effect of PD98059 on tyrosine phosphorylation of ERK1, ERK2, and p38 MAPK induced by G-CSF, GM-CSF, and TNF.
To clarify further the possible participation of MEK1/MEK2 in tyrosine phosphorylation of ERK1/ERK2, we studied the effect of PD98059, a potent inhibitor of MEK,19 on tyrosine phosphorylation of ERK1/ERK2 in human neutrophils stimulated by G-CSF, GM-CSF, and TNF. As shown in Fig 7, tyrosine phosphorylation of ERK1 and ERK2 induced by G-CSF (50 ng/mL), GM-CSF (5 ng/mL), or TNF (100 U/mL) was markedly reduced by the pretreatment of cells with PD98059 (100 μmol/L) for 30 minutes at 37°C. Under the same conditions, tyrosine phosphorylation of p38 MAPK induced by GM-CSF (5 ng/mL) or TNF (100 U/mL) was hardly reduced by PD98059. The equal loading of proteins onto each lane was confirmed by immunoblotting using antibody, which recognizes both phosphorylated and unphosphorylated forms of ERK1/ERK2 or p38 MAPK (data not shown).
Effect of PD98059 on tyrosine phosphorylation of ERK1, ERK2, and p38 MAPK in human neutrophils stimulated by G-CSF, GM-CSF, or TNF. Cells were pretreated with PD98059 (100 μmol/L) for 30 minutes at 37°C and thereafter stimulated with G-CSF (50 ng/mL), GM-CSF (5 ng/mL), or TNF (100 U/mL) for 10 minutes at 37°C. Phosphorylation of ERK1, ERK2, and p38 MAPK was analyzed by immunoblotting using antibody against phosphorylated form of each protein. The cell lysates equivalent to 2.8 × 106 cells were loaded onto each lane. The results shown are representative of three independent experiments.
Effect of PD98059 on tyrosine phosphorylation of ERK1, ERK2, and p38 MAPK in human neutrophils stimulated by G-CSF, GM-CSF, or TNF. Cells were pretreated with PD98059 (100 μmol/L) for 30 minutes at 37°C and thereafter stimulated with G-CSF (50 ng/mL), GM-CSF (5 ng/mL), or TNF (100 U/mL) for 10 minutes at 37°C. Phosphorylation of ERK1, ERK2, and p38 MAPK was analyzed by immunoblotting using antibody against phosphorylated form of each protein. The cell lysates equivalent to 2.8 × 106 cells were loaded onto each lane. The results shown are representative of three independent experiments.
Effect of SB203580 on O2− release stimulated by GM-CSF and TNF.
Tyrosine phosphorylation of p38 MAPK in human neutrophils was induced by GM-CSF and TNF, but not by G-CSF, and the potency of this effect was TNF > GM-CSF (Figs 1 through 4). These findings are consonant with our previous observations that O2−release in suspended human neutrophils is induced by GM-CSF and TNF, but not by G-CSF,1-3 and the potency of this effect is TNF > GM-CSF20 (Table 1). These findings raise the possibility that p38 MAPK may be involved in O2− release stimulated by GM-CSF and TNF. We then studied the effect of SB203580, a potent inhibitor of p38 MAPK,21 on O2− release in human neutrophils stimulated by GM-CSF or TNF. As shown in Table 1, when cells were pretreated with SB203580 (0.01 to 10 μmol/L) for 20 minutes at 37°C, GM-CSF– or TNF-induced O2− release was inhibited by SB203580 in a dose-dependent manner.
Effect of SB203580 on O2−Release in Human Neutrophils Stimulated by GM-CSF or TNF
| Pretreatment . | O2− Release (% control) . | |
|---|---|---|
| GM-CSF . | TNF . | |
| SB203580 | ||
| 0.01 μmol/L | 62.8 ± 7.3* | 66.2 ± 1.3* |
| 0.1 μmol/L | 35.2 ± 7.9* | 39.6 ± 3.4* |
| 1 μmol/L | 31.5 ± 9.5* | 32.2 ± 5.6* |
| 10 μmol/L | 32.6 ± 8.8* | 19.8 ± 3.8* |
| Pretreatment . | O2− Release (% control) . | |
|---|---|---|
| GM-CSF . | TNF . | |
| SB203580 | ||
| 0.01 μmol/L | 62.8 ± 7.3* | 66.2 ± 1.3* |
| 0.1 μmol/L | 35.2 ± 7.9* | 39.6 ± 3.4* |
| 1 μmol/L | 31.5 ± 9.5* | 32.2 ± 5.6* |
| 10 μmol/L | 32.6 ± 8.8* | 19.8 ± 3.8* |
Cells (2 × 105/0.2 mL) were preincubated with indicated concentrations of SB203580 for 20 minutes at 37°C and thereafter stimulated with GM-CSF (5 ng/mL) or TNF (100 units/mL) for 3 hours at 37°C. O2− release was determined as superoxide dismutase-inhibitable reduction of ferricytochromec during stimulation with GM-CSF or TNF. In this experiment, the amounts of O2− release in control cells stimulated by GM-CSF and TNF were 2.53 ± 0.16 and 4.92 ± 0.35 nmol/3 hours/ 2 × 105 cells, respectively. The data are expressed as the mean ± SD of three experiments.
Significantly inhibited by SB203580 (P < .001).
DISCUSSION
The present experiments show that stimulation of human neutrophils with G-CSF, GM-CSF, and TNF results in activation of the distinct MAPK subtype cascades in a cytokine-specific manner. G-CSF exclusively activates the ERK cascade; GM-CSF activates the ERK cascade strongly and the p38 MAPK cascade weakly; TNF activates the p38 MAPK cascade strongly and the ERK cascade weakly; and the JNK cascade is not activated by any cytokine. The differential activation of MAPK subtype cascades may explain the differences of the effects of these cytokines on human neutrophil functions.
G-CSF, GM-CSF, and TNF all induced tyrosine phosphorylation of ERK1 and ERK2 and phosphorylation of MEK1/MEK2, and the potency of these cytokines to induce phosphorylation of ERK1/ERK2 and MEK1/MEK2 was GM-CSF > G-CSF > TNF. In addition, tyrosine phosphorylation of ERK1 and ERK2 was markedly inhibited by PD98059, a potent inhibitor of MEK. These findings suggest that the signals provoked by the binding of G-CSF, GM-CSF, and TNF to their receptors converge to activation of MEK, which in turn phosphorylates and activates ERK1 and ERK2. Activation of this cascade appears to be independent of activation of the p38 MAPK cascade, because (1) G-CSF selectively induced tyrosine phosphorylation of ERK1 and ERK2 and did not induce tyrosine phosphorylation of p38 MAPK; (2) ERK1/ERK2 and p38 MAPK were differentially tyrosine-phosphorylated by GM-CSF and TNF (ie, the potency of tyrosine phosphorylation of ERK1/ERK2 was GM-CSF > TNF, whereas that of p38 MAPK was TNF > GM-CSF); (3) PD98059 markedly reduced tyrosine phosphorylation of ERK1 and ERK2 induced by GM-CSF or TNF, whereas it hardly reduced tyrosine phosphorylation of p38 MAPK induced by GM-CSF or TNF; and (4) tyrosine phosphorylation of p38 MAPK and phosphorylation of MKK3/MKK6 were induced by GM-CSF and TNF, but not by G-CSF, and the potency of these cytokines to induce phosphorylation of p38 MAPK and MKK3/MKK6 was TNF > GM-CSF. These findings also support the concept that GM-CSF and TNF, but not G-CSF, activate the MKK3/MKK6-p38 MAPK cascade in human neutrophils, as observed in other types of cells stimulated by appropriate agonists.6,7 However, these findings contrast with the observations obtained from FDC-P2 cells, a murine hematopoietic cell line, in which erythropoietin and IL-3 appear to activate p38 MAPK through a kinase other than MKK3/MKK6.18 The aberrant signaling pathways, which developed in FDC-P2 cells, might explain these differences.
In regard to tyrosine phosphorylation or activation of JNK in human neutrophils, controversial results are reported. It has been reported that FMLP does not activate JNK in normal human neutrophils,22 whereas a recent report shows that FMLP activates JNK in mature HL-60 cells differentiated by DMSO.17 In the present experiments, we could not detect tyrosine phosphorylation of JNK in normal human neutrophils stimulated by various agonists (G-CSF, GM-CSF, TNF, FMLP, PMA, and ionomycin), despite the existence of JNK proteins. On the other hand, JNK was tyrosine-phosphorylated in undifferentiated and ATRA-differentiated HL-60 cells stimulated by TNF, but not by G-CSF or GM-CSF. We also observed tyrosine phosphorylation of JNK in KG-1 cells, another myelogenous leukemia cell line, stimulated by TNF. Taken together, these findings suggest that the signaling pathway from TNF receptors to JNK exists in certain myeloid leukemia cell lines and still exists in granulocytic HL-60 cells differentiated by ATRA or DMSO, but this pathway does not work in normal mature human neutrophils. It is conceivable that certain signaling molecules mediating the signal transduction from TNF receptors to JNK may be defective in mature human neutrophils. Although it has been reported that JNK is activated in certain murine cell lines stimulated by G-CSF,23 we could not detect G-CSF–induced tyrosine phosphorylation of JNK in HL-60 cells or in leukemic cells obtained from some patients with acute myelogenous leukemia (data not shown).
Our previous study shows that stimulation of human neutrophils with G-CSF, GM-CSF, and TNF results in tyrosine phosphorylation of a 42-kD protein, which may be associated with the priming effect of these cytokines on O2− release.4The present experiments show that the tyrosine-phosphorylated 42-kD protein in human neutrophils stimulated by G-CSF is ERK1/ERK2, but not p38 MAPK and JNK, and ERK2 is predominantly tyrosine-phosphorylated by G-CSF. In considering that G-CSF never stimulates O2− release in human neutrophils,1,2 these findings indicate that ERK1 and ERK2 are unlikely to be involved in triggering of O2− release, but rather suggest that ERK1 and ERK2 may be involved in other functions, such as the priming effect or the inhibition of apoptosis.24 Consistent with this is the finding that PD98059, a MEK inhibitor, does not inhibit the release of O2− in human neutrophils stimulated by FMLP, which can activate the MEK-ERK cascade.25,26 It has been also demonstrated that cyclic AMP dissociates ERK activation from the oxidative burst in human neutrophils stimulated by FMLP or PMA.27 In myeloid cells, the activation of ERK cascade has been proposed to be associated with proliferative, but not nonproliferative, responses to G-CSF.28 However, the present study suggests that the ERK1/ERK2 cascade also plays a role in functional responses of mature human neutrophils to G-CSF. The present findings are consistent with the previous report by Colotta et al29 that stimulation of human neutrophils with G-CSF, GM-CSF, or TNF results in induction of c-fos mRNA expression, because ERK is known to be involved in induction of c-fos mRNA expression in various types of cells.30
One remarkable difference among the effects of G-CSF, GM-CSF, and TNF on human neutrophil functions is that both GM-CSF and TNF stimulate O2− release directly in suspended human neutrophils, whereas G-CSF does not.1-3 The potency for this effect is TNF > GM-CSF.20 These findings are consonant with the ability of these cytokines to stimulate tyrosine phosphorylation of p38 MAPK. In addition, increased tyrosine phosphorylation of p38 MAPK, which was detected within 1 to 5 minutes after stimulation with GM-CSF or TNF (Figs 3 and 4), preceded or occurred concomitantly with O2− release induced by each cytokine.2,3 These findings and the inhibition of GM-CSF– and TNF-induced O2− release by SB203580, a p38 MAPK inhibitor, when taken together, suggest that p38 MAPK plays a role in O2− release stimulated by GM-CSF and TNF. It has been also reported that p38 MAPK may be involved in O2− release in human neutrophils stimulated by FMLP, but not by platelet activating factor.22 The inability of G-CSF to stimulate O2− release in human neutrophils may be attributed to the inability of G-CSF to activate p38 MAPK.
The present experiments show that G-CSF, GM-CSF, and TNF activate the distinct MAPK subtypes (ERK1/ERK2 and p38 MAPK) in human neutrophils and suggest that the differential activation of ERK1/ERK2 and p38 MAPK may partly explain the differences of the effects of these cytokines on human neutrophil functions, as evidenced by the close relationship between activation of the p38 MAPK cascade and O2− release stimulated by G-CSF, GM-CSF, and TNF. The JNK cascade appears not to work in mature neutrophils. Further investigations are required to clarify the roles of each MAPK subtype cascade in cytokine-induced activation of human neutrophil functions as well as hematopoietic precursor cells.
ACKNOWLEDGMENT
The authors thank Dr A. Yuo (International Medical Center of Japan, Tokyo, Japan) for valuable discussions.
Supported by Grants-in-Aid from the Ministry of Education, Science and Culture, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Seiichi Kitagawa, MD, Department of Physiology, Osaka City University Medical School, Asahi-machi, Abeno-ku, Osaka 545-8585, Japan.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal