Abstract
Arsenic trioxide (As2O3) induces clinical remission in acute promyelocytic leukemia (APL) with minimal toxicity and apoptosis in APL-derived NB4 cells at low (1 to 2 μmol/L) concentration. We examined the basis for NB4 cell sensitivity to As2O3 to identify experimental conditions that would render other malignant cells responsive to low concentrations of As2O3. The intracellular glutathione (GSH) content had a decisive effect on As2O3-induced apoptosis. Highly sensitive NB4 cells had the lowest GSH and the sensitivity of other cell lines was inversely proportional to their GSH content. The t(14;18) B-cell lymphoma cell line had low GSH levels and sensitivity to As2O3 at levels slightly higher than in APL cells. Experimental upmodulation of GSH content decreased the sensitivity to As2O3. Ascorbic acid and buthionine sulfoxide (BSO) decreased GSH to a greater extent, and rendered malignant cells more sensitive to As2O3. As2O3-induced apoptosis was not enhanced by ascorbic acid in normal cells, suggesting that the combination of ascorbic acid and As2O3may be selectively toxic to some malignant cells. Ascorbic acid enhanced the antilymphoma effect of As2O3 in vivo without additional toxicity. Thus, As2O3alone or administered with ascorbic acid may provide a novel therapy for lymphoma.
ARSENIC TRIOXIDE (AS2O3), in a protocol originally developed in Harbin, China, has recently been confirmed to be an effective treatment for acute promyelocytic leukemia (APL) in patients who relapsed after chemotherapy and all-trans retinoic acid (tRA) treatment.1-4 The peak As2O3 plasma concentration in patients was 5 to 7 μmol/L and rapidly diminished to a more sustained level of 1 to 2 μmol/L, which is thought to be the therapeutic range in treating APL.3As2O3 (1 to 2 μmol/L) induces apoptosis in the t(15;17) APL cell line NB4 and APL cells in vitro and, to some extent, in vivo in patients without significant myelosuppression.5,6 As2O3-induced apoptosis in NB4 cells was associated with rapid degradation of PML/RAR-α protein,6 the oncogenic protein that is the product of the t(15;17) translocation characteristic of APL.7,8 The fact that, among a series of 37 patients, the two APL patients who failed to respond to As2O3lacked measurable PML/RAR-α message by polymerase chain reaction (PCR) suggests an important role for PML/RAR-α protein in As2O3-induced apoptosis. Additional clinical experience obtained in Harbin, China by Ting Dong Zhang (personal communication, 1996) showed As2O3 to be effective in patients with conventional chemotherapy-resistant lymphoproliferative and myeloproliferative disorders. Because As2O3 is reported to be clinically effective in patients previously treated with alkylating agents and anthracyclines,3 4 there should be lack of cross resistance between As2O3 and these chemotherapeutic agents.
Organic arsenic compounds are significantly more toxic than inorganic arsenic because of high binding affinity to vicinal SH group-containing proteins.9 Organic arsenicals such as melasporal, although more potent than As2O3 in inducing apoptosis in NB4 cells, are clinically too toxic to be used in the treatment of APL.10 Most previous studies that investigated the cellular and biochemical effects of arsenic were performed using concentrations greater than 5 μmol/L, often 50 μmol/L, and the relevance to therapeutic levels (1 to 2 μmol/L) remains to be determined. These high concentrations may initiate gene transcription by altering the phosphorylation state of signal transduction proteins such as tyrosine kinases.11 Arsenic effects phosphorylation by activating specific kinases, by inhibiting thiol-dependent phosphatases, or by interfering with phosphotransferase reactions.12 The protective effects of thiols, such as glutathione, cysteine,13 and dithiols, such as dithiotreitol, against the toxic effects of arsenic suggests that arsenic toxicity results from forming reversible bonds with the thiol groups of regulatory proteins.
The glutathione (GSH) redox system is known to modulate the growth-inhibitory effect of arsenicals.14-18 Until recently, the effect of this intracellular defense system has not been studied in arsenic-induced apoptosis. As5+, cadmium, thalium, selenium, zinc, or mercury did not induce apoptosis at 1 μmol/L in NB4 cells, suggesting a unique effect of As3+(as in As2O3) in this process.19The findings that arsenites (40 μmol/L) can induce apoptosis in hamster CHO cells,20 that hydrogen peroxide-resistant CHO cells are less responsive,21 and that catalase-deficient CHO cells are hypersensitive suggests an important role for hydrogen peroxide as a mediator of arsenic-induced apoptosis.21
There is a need to understand better the cellular response to As2O3 at 1 to 2 μmol/L concentrations because it appears to be cancer-selective and therapeutically achievable. Accordingly, we investigated whether the GSH content and its modulation can be correlated with the sensitivity of As2O3in NB4 and other malignant cells. We found that the sensitivity to As2O3-induced apoptosis was inversely related to the intracellular GSH content and that pharmacologic modulation of intracellular GSH content modulates sensitivity to As2O3.
MATERIALS AND METHODS
Reagents.
A 0.1% As2O3 solution was kindly supplied by Dr Ting Dong Zhang (Harbin, China). Buthionine sulfoxide (BSO), N-acetylcysteine (NAC), ascorbic acid, catalase, and lipoic acid were purchased from Sigma Chemical (St Louis, MO). All of the agents were dissolved in phosphate-buffered saline (PBS), except lipoic acid, which was dissolved in 100% ethanol at a stock solution of 0.1 mol/L. The final ethanol concentration in the medium was not greater than 0.1%.
Cell lines.
NB4 t(15;17) (obtained from Dr M. Lanotte),22 HL-60 cells (American Type Culture Collection [ATCC], Rockville, MD), and t(14;18) B-cell lymphoma su-DHL-4 cell lines, which overexpress Bcl-2 (obtained from Dr M. Cleary),23were cultured in RPMI-1640 medium, supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, 1 mmol/L L-glutamine, and 10% fetal bovine serum. Human breast cancer cell lines T47D and MDA-MB-468 were obtained from ATCC and cultured as previously reported.24 RM5.21 human fibroblasts from normal breast tissue were isolated from reduction mammaplasty specimen and human embryo fibroblasts (HEF) were cultured as described.24 Cell viability was determined by trypan-blue exclusion. The cells were in logarithmic growth when seeded at 1 × 105 cells/mL for studies performed in duplicate and repeated at least three times.
Quantitation of apoptotic cells.
Apoptotic cells stained with acridine orange (AO) and ethidium bromide (EB) were assessed by fluorescence microscopy. Briefly, 1 μL of stock solution containing 100 μg/mL AO and 100 μg/mL EB was added to 25 μL of cell suspension. Total cells, as well as apoptotic cells that showed nuclear shrinkage, blebing, and apoptotic bodies, were counted. DNA fragmentation analysis was performed as described previously.25
Measurement of intracellular GSH.
Intracellular GSH contents were measured using Glutathione Assay Kit (Calbiochem, San Diego, CA). In brief, 5 × 106 cells were homogenized in 5% metaphosphoric acid using a Teflon pestle (Racine, WI). Particulate matter was separated by centrifugation at 4,000g. Supernatant was used for GSH measurement according to the manufacturer’s instruction, while the pellet was dissolved in 1 mol/L NaOH and analyzed for protein by Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA). The GSH content was expressed as nanomoles per milligram protein.
Western blot analysis.
Protein extracts (50 μg) prepared with RIPA lysis buffer (50 mmol/L Tris-HCl, 150 mmol/L NaCl, 0.1% sodium dodecyl sulfate [SDS], 1% NP-40, 0.5% sodium deoxycholate, 1 mmol/L phenylmethylsulfonyl fluoride [PMSF], 100 μmol/L leupeptin, and 2 μg/mL aprotinin, pH 8.0) were separated through an 8% or 12% SDS-polyacrylamide gel and transferred to nitrocellulose membranes. The membranes were stained with 0.2% Ponceau red to assure equal protein loading and transfer. After blocking with 10% nonfat milk, the membrane was incubated with polyclonal antibody to PARP (Boehringer Mannheim, Indianapolis, IN), and monoclonal antibodies to Cpp32 and Bcl-2 (Oncogene Research Products, Cambridge, MA). The immunocomplex was visualized by chemoluminescence (ECL kit; Amersham, Buckinghamshire, UK).
Colony-forming assays of human bone marrow and peripheral blood cells.
Mononuclear cells (MNC) from blood and/or bone marrow from normal adults collected into syringes containing 50 U heparin were prepared following dilution 1:1 with α medium, layered onto an equal volume of Ficoll-Paque, and centrifuged at 450g for 30 minutes at 18°C. For methylcellulose cultures, MNC were cultured according to methods previously described.26 Each milliliter of culture contained 300,000 MNC, 0.8% methylcellulose in alpha medium, 30% fetal bovine serum, 1% bovine serum albumin (BSA; COHN fraction IV), 0.1 mmol/L 2-mercaptoethanol, 0.1 U penicillin, and 0.1 μg/mL streptomycin. Erythroid cultures contained erythropoietin (2 U/mL). Myeloid cultures contained granulocyte-macrophage colony-stimulating factor (GM-CSF; 2 ng/mL) and interleukin-3 (IL-3; 50 U/mL). Cultures were performed in triplicate in 0.3-mL vol in Nunc four-well dishes (Intermed, Roskilde, Denmark) and incubated at 37°C in 4% CO2 with or without As2O3, ascorbic acid, or the combination. Colonies derived from colony-forming units—erythrocyte (CFU-E) were counted on day 7 and those from burst-forming units—erythrocyte (BFU-E) and CFU-GM on day 13 using a Stereozoom dissecting microscope (Bausch & Lomb, Rochester, NY).
3H-thymidine incorporation in phytohemagglutinin-activated lymphocytes.
Lymphocytes were isolated from normal adult blood and3H-thymidine incorporation was measured in phytohemagglutinin (PHA)-activated lymphocytes according to the reported method.27
In vivo experiments.
BDF1 female mice were obtained from Charles River Laboratories (Wilmington, MA). All procedures confirmed to the National Institutes of Health (NIH) guidelines for the care and use of laboratory animals. Transplantable P388D1 cells were obtained from ATCC and carried intraperitoneally in BDF1 mice. For experiments, 0.1 mL containing 2 × 106 cells obtained from ascites after 7 days of transplantation were inoculated intraperitoneally. Mice were randomly divided into four groups each with five mice. After 24 hours, each group was given saline, As2O3 (5 mg/kg), and ascorbic acid (500 mg/kg) alone or in combination intraperitoneally every other day for seven times. The percentage increase in lifespan over control (ILS) was calculated as follows: ILS% = T/C% minus 100, where T is the test mean survival time, and C is the control mean survival time. Pairedt-test was used to determine significance.
RESULTS
The growth inhibitory and apoptotic effect of As2O3 inversely correlates with GSH content of NB4, su-DHL-4, and HL-60 cells.
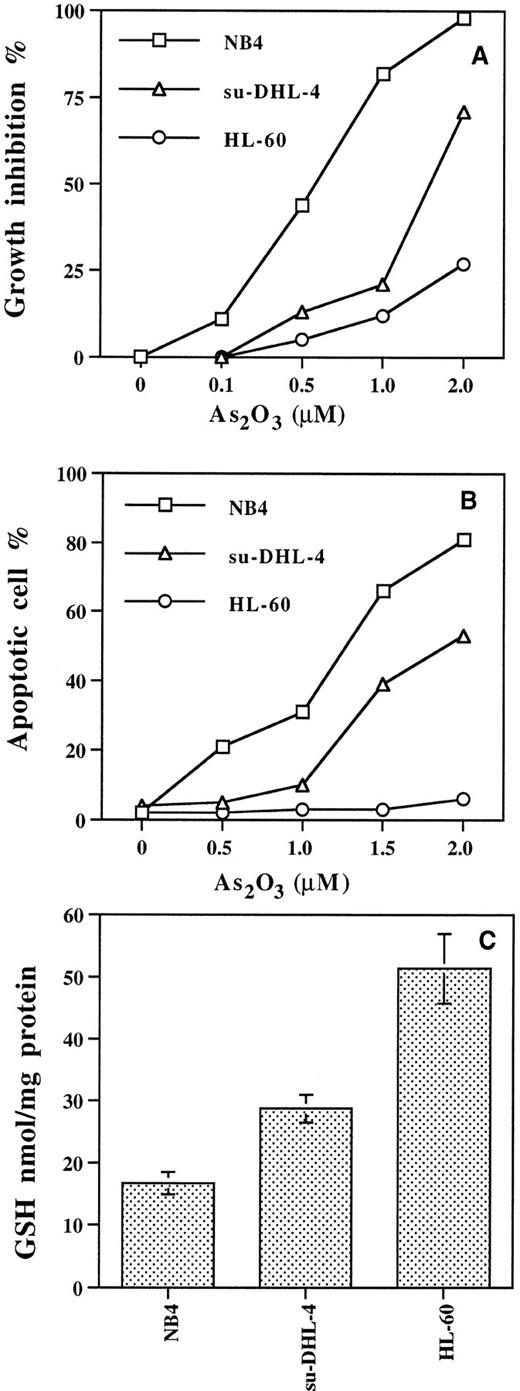
The 50% inhibitory concentration (IC50) after 3 days of As2O3 treatment for NB4 cells was 0.5 μmol/L, for su-DHL-4 cells 1.5 μmol/L, and for HL-60 cells greater than 3 μmol/L (Fig 1A). As2O3 1.2 μmol/L induced apoptosis at day 3 in 50% of the NB4 cells, whereas 1.8 μmol/L was required in su-DHL-4 cells (Fig 1B), while even 5 μmol/L As2O3 was insufficient to induce 50% apoptosis in HL-60 cells. The GSH content was three times greater in HL-60 cells than in NB4 cells, whereas in su-DHL-4 cells GSH content fell in between these two values (Fig 1C).
Growth inhibition (A) and apoptosis (B) induced by As2O3 in NB4, su-DHL-4, and HL-60 cells, and basal GSH levels (C). Cells were treated with different concentrations of As2O3 for 3 days. Cell growth and apoptotic cell number were determined with fluorescence staining as described in Materials and Methods. Values are the mean of three independent experiments.
Growth inhibition (A) and apoptosis (B) induced by As2O3 in NB4, su-DHL-4, and HL-60 cells, and basal GSH levels (C). Cells were treated with different concentrations of As2O3 for 3 days. Cell growth and apoptotic cell number were determined with fluorescence staining as described in Materials and Methods. Values are the mean of three independent experiments.
Cell growth inhibition and apoptotic effect of As2O3 is modulated by experimentally changing the GSH content.
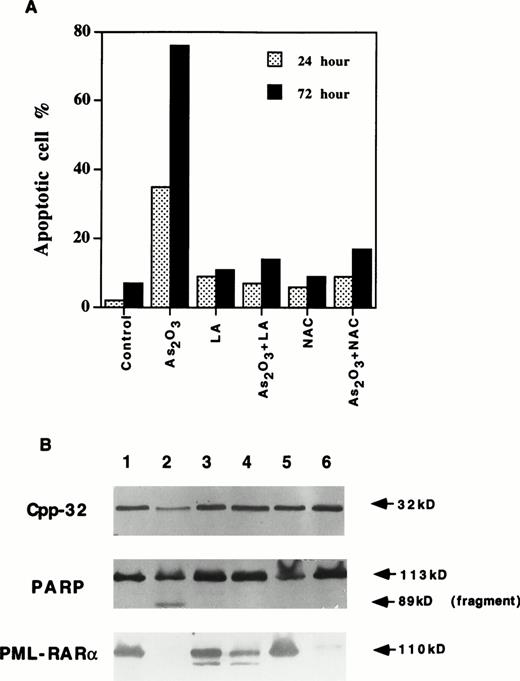
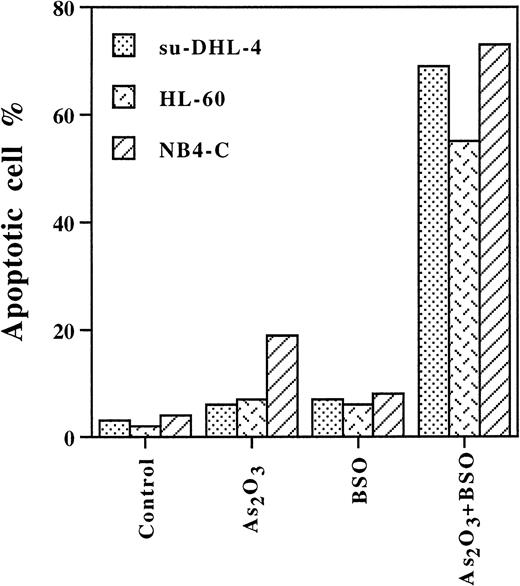
Increasing the GSH content by NAC treatment28 in NB4 cells (Table 1) prevented 2 μmol/L As2O3 induction of apoptosis (Fig2A). Similarly, lipoic acid completely blocked As2O3-induced apoptosis, perhaps by binding As2O3 to its vicinal thiol groups and increasing GSH content.29 Both NAC and lipoic acid treatment inhibited As2O3-induced activation of CPP32 and apoptosis. However, NAC did not and lipoic acid only partially inhibited the degradation of PML/RAR-α protein (Fig 2B, lanes 4 and 6). Thus, the degradation of PML/RAR-α alone was not sufficient for the As2O3 induction of apoptosis (Fig 2B). In contrast, BSO is known to decrease intracellular GSH levels by inhibition of γ-glutamylcysteine synthase activity.30 During a 24-hour treatment, BSO alone reduced the GSH level to approximately 10 nmol/mg protein in all three cell lines (Table 1), while it did not affect cell growth or apoptosis (Fig3). Treatment with BSO for 4 hours followed by 1 μmol/L As2O3 for 12 hours sensitized the three cell lines to As2O3 since 75% of the NB4 cells, 70% of the su-DHL-4 cells, and 55% of the HL-60 cells underwent apoptosis (Fig 3). Thus, leukemic cell lines devoid of PML/RAR-α can be made as sensitive to As2O3 as NB4 cells.
GSH Levels in NB4, su-DHL-4, and HL-60 Cells Treated With Different Agents for 24 Hours
| Cell . | Treatment . | GSH (nmol/mg protein) (mean ± SE) . |
|---|---|---|
| HL-60 | Basal | 51.4 ± 5.6 |
| BSO (100 μmol/L) | 13.7 ± 2.3 | |
| As2O3 (1 μmol/L) | 52.0 ± 3.1 | |
| AA (62.5 μmol/L) | 36.6 ± 4.0 | |
| As2O3 (1 μmol/L) + AA (62.5 μmol/L) | 34.6 ± 1.8 | |
| NB4 | Basal | 16.7 ± 1.8 |
| BSO (100 μmol/L) | 10.1 ± 1.4 | |
| AA (62.5 μmol/L) | 17.2 ± 2.7 | |
| As2O3 (1 μmol/L) | 16.5 ± 2.9 | |
| As2O3 (1 μmol/L) + AA (62.5 μmol/L) | 15.6 ± 1.0 | |
| NAC (10 mmol/L) | 27.2 ± 3.7 | |
| su-DHL-4 | Basal | 28.7 ± 2.2 |
| BSO (100 μmol/L) | 7.9 ± 1.3 | |
| AA (62.5 μmol/L) | 6.9 ± 2.6 | |
| As2O3 (1 μmol/L) | 24.1 ± 3.1 | |
| AA (62.5 μmol/L) + As2O3 (1 μmol/L) | 6.1 ± 1.8 |
| Cell . | Treatment . | GSH (nmol/mg protein) (mean ± SE) . |
|---|---|---|
| HL-60 | Basal | 51.4 ± 5.6 |
| BSO (100 μmol/L) | 13.7 ± 2.3 | |
| As2O3 (1 μmol/L) | 52.0 ± 3.1 | |
| AA (62.5 μmol/L) | 36.6 ± 4.0 | |
| As2O3 (1 μmol/L) + AA (62.5 μmol/L) | 34.6 ± 1.8 | |
| NB4 | Basal | 16.7 ± 1.8 |
| BSO (100 μmol/L) | 10.1 ± 1.4 | |
| AA (62.5 μmol/L) | 17.2 ± 2.7 | |
| As2O3 (1 μmol/L) | 16.5 ± 2.9 | |
| As2O3 (1 μmol/L) + AA (62.5 μmol/L) | 15.6 ± 1.0 | |
| NAC (10 mmol/L) | 27.2 ± 3.7 | |
| su-DHL-4 | Basal | 28.7 ± 2.2 |
| BSO (100 μmol/L) | 7.9 ± 1.3 | |
| AA (62.5 μmol/L) | 6.9 ± 2.6 | |
| As2O3 (1 μmol/L) | 24.1 ± 3.1 | |
| AA (62.5 μmol/L) + As2O3 (1 μmol/L) | 6.1 ± 1.8 |
Values are the mean of 3 independent experiments.
Abbreviations: BSO, buthionine sulfoxide; AA, ascorbic acid; NAC,N-acetylcysteine.
(A) NAC and lipoic acid (LA) blocked As2O3-induced apoptosis but not PML/RAR- degradation in NB4 cells. NB4 cells were treated with 2 μmol/L As2O3 alone or together with 10 mmol/L NAC or 100 μmol/L LA for 24 and 72 hours. Apoptotic cells were determined by fluorescence staining as described in Materials and Methods. Values are the mean of three independent experiments. (B) NAC and LA blocked As2O3-induced activation of Cpp32 and cleavage of PARP, but not PML/RAR- degradation in NB4 cells. Lane 1, control; lane 2, As2O3; lane 3, LA; lane 4, As2O3 + LA; lane 5, NAC; lane 6, As2O3 + NAC. Cells were treated with concentrations described above for 24 hours.
(A) NAC and lipoic acid (LA) blocked As2O3-induced apoptosis but not PML/RAR- degradation in NB4 cells. NB4 cells were treated with 2 μmol/L As2O3 alone or together with 10 mmol/L NAC or 100 μmol/L LA for 24 and 72 hours. Apoptotic cells were determined by fluorescence staining as described in Materials and Methods. Values are the mean of three independent experiments. (B) NAC and LA blocked As2O3-induced activation of Cpp32 and cleavage of PARP, but not PML/RAR- degradation in NB4 cells. Lane 1, control; lane 2, As2O3; lane 3, LA; lane 4, As2O3 + LA; lane 5, NAC; lane 6, As2O3 + NAC. Cells were treated with concentrations described above for 24 hours.
BSO enhanced As2O3-induced apoptosis in NB4, su-DHL-4, and HL-60 cells. Cells were pretreated with BSO 100 μmol/L for 4 hours, then with or without As2O3 1 μmol/L for 12 hours. Values are the mean of three independent experiments.
BSO enhanced As2O3-induced apoptosis in NB4, su-DHL-4, and HL-60 cells. Cells were pretreated with BSO 100 μmol/L for 4 hours, then with or without As2O3 1 μmol/L for 12 hours. Values are the mean of three independent experiments.
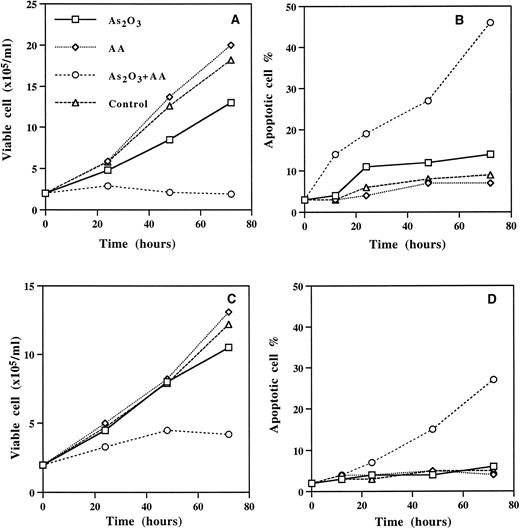
In HL-60 and su-DHL-4 cells, ascorbic acid acts as an oxidizing agent decreasing the GSH content of the cells and synergizing with the growth-inhibitory and apoptotic effect of As2O3(Fig 4 and Table 1). The apoptotic morphology and DNA ladder, as well as Cpp32 activation, in su-DHL-4 cells treated with 1 μmol/L As2O3 and 62.5 μmol/L ascorbic acid are shown in Fig 5. In contrast to ascorbic acid, dehydroascorbic acid did not potentiate the effect of As2O3 (Table2). It is plausible that the autooxidation of ascorbic acid that results in the formation of H2O231 is responsible for the synergistic effect obtained by ascorbic acid. This suggestion is substantiated by our findings that (1) H2O2synergized the effect of As2O3 (Table 2), and (2) catalase abolished the synergistic effects obtained by ascorbic acid.
Ascorbic acid (AA) enhanced As2O3-induced apoptosis in su-DHL-4 (A,B) and HL-60 cells (C,D). Cells were treated with 1 μmol/L As2O3, 62.5 μmol/L AA alone or together. Cell viability and apoptotis were determined as described in Materials and Methods. Values are the mean of three independent experiments.
Ascorbic acid (AA) enhanced As2O3-induced apoptosis in su-DHL-4 (A,B) and HL-60 cells (C,D). Cells were treated with 1 μmol/L As2O3, 62.5 μmol/L AA alone or together. Cell viability and apoptotis were determined as described in Materials and Methods. Values are the mean of three independent experiments.
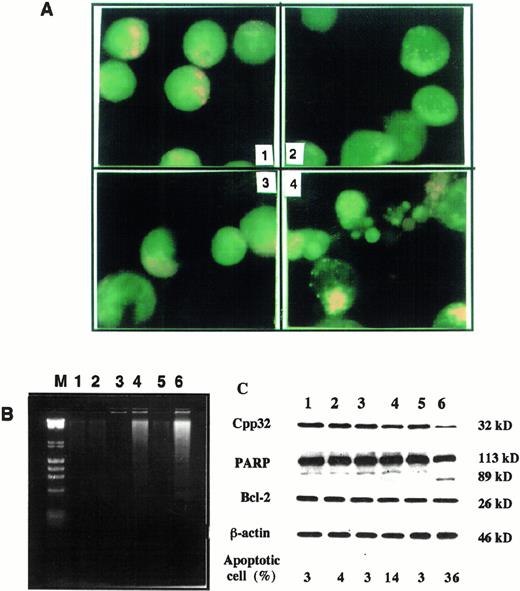
As2O3 combined with AA induces apoptosis in su-DHL-4 cells. (A) Morphologic features of apoptosis. Cells were treated for 24 hours. 1, Without treatment; 2, As2O3 (1 μmol/L); 3, AA (62.5 μmol/L); 4, As2O3 (1 μmol/L) with AA (62.5 μmol/L). Apoptotic cells were determined with fluorescence staining as described in Materials and Methods. (B) DNA fragmentation. Cells were treated for 24 hours and total DNA was isolated and separated on agarose gel. DNA ladder was visualized with EB as described in Materials and Methods. Lane M, size markers, λ DNA HindIII digest and φx174 RF DNAHaeII digest; lane 1, control; lane 2, As2O3 (1 μmol/L); lane 3, AA (62.5 μmol/L); lane 4, As2O3 (1 μmol/L) + AA (62.5 μmol/L); lane 5, AA (125 μmol/L); lane 6, As2O3 (1 μmol/L) + AA (125 μmol/L). (C) Activation of CPP32 and effect on Bcl-2 by treatments as in B.
As2O3 combined with AA induces apoptosis in su-DHL-4 cells. (A) Morphologic features of apoptosis. Cells were treated for 24 hours. 1, Without treatment; 2, As2O3 (1 μmol/L); 3, AA (62.5 μmol/L); 4, As2O3 (1 μmol/L) with AA (62.5 μmol/L). Apoptotic cells were determined with fluorescence staining as described in Materials and Methods. (B) DNA fragmentation. Cells were treated for 24 hours and total DNA was isolated and separated on agarose gel. DNA ladder was visualized with EB as described in Materials and Methods. Lane M, size markers, λ DNA HindIII digest and φx174 RF DNAHaeII digest; lane 1, control; lane 2, As2O3 (1 μmol/L); lane 3, AA (62.5 μmol/L); lane 4, As2O3 (1 μmol/L) + AA (62.5 μmol/L); lane 5, AA (125 μmol/L); lane 6, As2O3 (1 μmol/L) + AA (125 μmol/L). (C) Activation of CPP32 and effect on Bcl-2 by treatments as in B.
Catalase and H2O2 on the Synergistic Effects of Ascorbic Acid With As2O3 in HL-60 Cells
| . | 1 Day . | 3 Days . | ||
|---|---|---|---|---|
| Growth Inhibition (%) . | Apoptosis (%) . | Growth Inhibition (%) . | Apoptosis (%) . | |
| Control | 0 | 2.3 ± 0.4 | 0 | 4.0 ± 1.3 |
| As2O3 | 3.0 ± 0.7 | 3.0 ± 1.7 | 15.2 ± 2.1 | 9.0 ± 1.7 |
| AA | 0 | 2.7 ± 1.3 | 0 | 5.0 ± 1.3 |
| AA + As2O3 | 27.0 ± 3.3 | 10.3 ± 3.0 | 69.7 ± 10.3 | 29.5 ± 1.7 |
| Dehydroascorbic acid + As2O3 | 8.3 ± 3.9 | 3.7 ± 2.1 | 11.6 ± 4.6 | 8.3 ± 0.9 |
| Catalase | 1.7 ± 1.0 | 3.0 ± 1.7 | 2.7 ± 1.3 | 4.3 ± 0.7 |
| Catalase + AA + As2O3 | 5.7 ± 1.3 | 3.3 ± 1.3 | 11.3 ± 3.0 | 10.0 ± 2.3 |
| H2O2 | 1.7 ± 0.3 | 3.3 ± 1.0 | 9.4 ± 3.3 | 11.0 ± 1.7 |
| H2O2 + As2O3 | 39.0 ± 3.3 | 16.3 ± 1.7 | 79.3 ± 5.1 | 44.6 ± 6.0 |
| . | 1 Day . | 3 Days . | ||
|---|---|---|---|---|
| Growth Inhibition (%) . | Apoptosis (%) . | Growth Inhibition (%) . | Apoptosis (%) . | |
| Control | 0 | 2.3 ± 0.4 | 0 | 4.0 ± 1.3 |
| As2O3 | 3.0 ± 0.7 | 3.0 ± 1.7 | 15.2 ± 2.1 | 9.0 ± 1.7 |
| AA | 0 | 2.7 ± 1.3 | 0 | 5.0 ± 1.3 |
| AA + As2O3 | 27.0 ± 3.3 | 10.3 ± 3.0 | 69.7 ± 10.3 | 29.5 ± 1.7 |
| Dehydroascorbic acid + As2O3 | 8.3 ± 3.9 | 3.7 ± 2.1 | 11.6 ± 4.6 | 8.3 ± 0.9 |
| Catalase | 1.7 ± 1.0 | 3.0 ± 1.7 | 2.7 ± 1.3 | 4.3 ± 0.7 |
| Catalase + AA + As2O3 | 5.7 ± 1.3 | 3.3 ± 1.3 | 11.3 ± 3.0 | 10.0 ± 2.3 |
| H2O2 | 1.7 ± 0.3 | 3.3 ± 1.0 | 9.4 ± 3.3 | 11.0 ± 1.7 |
| H2O2 + As2O3 | 39.0 ± 3.3 | 16.3 ± 1.7 | 79.3 ± 5.1 | 44.6 ± 6.0 |
As2O3 (1 μmol/L), ascorbic acid (62.5 μmol/L), catalase (500 U/mL), and H2O2 (4 μmol/L) were used for the experiments.
The synergistic growth-inhibitory effect of As2O3 and ascorbic acid was observed not only in cell lines, but also in primary cultures of chronic lymphocytic leukemia (CLL) cells (Table 3). The data in Table 3 show that a 5-day exposure of the cells to 1 μmol/L As2O3 or 125 μmol/L ascorbic acid alone had minimal effect on cell growth, but the combination of the two agents reduced the viable cell number by approximately 60% (Table3).
Effect of Combined Treatment of As2O3 and Ascorbic Acid of Primary Cultures of Chronic Lymphocytic Leukemia Cells
| Patient No. . | Viability (%) . | ||
|---|---|---|---|
| 1 Day . | 3 Days . | 5 Days . | |
| 1 | |||
| Control | 93 | 93 | 78 |
| As2O3 (1 μmol/L) | 92 | 86 | 68 |
| AA (125 μmol/L) | 85 | 78 | 74 |
| As2O3 (1 μmol/L) + AA (125 μmol/L) | 74 | 53 | 32 |
| 2 | |||
| Control | 88 | 86 | 80 |
| As2O3 (1 μmol/L) | 85 | 84 | 76 |
| AA (125 μmol/L) | 87 | 73 | 72 |
| As2O3 (1 μmol/L) + AA (125 μmol/L) | 62 | 41 | 36 |
| 3 | |||
| Control | 91 | 89 | 81 |
| As2O3 (1 μmol/L) | 93 | 81 | 69 |
| AA (125 μmol/L) | 71 | 70 | 70 |
| As2O3 (1 μmol/L) + AA (125 μmol/L) | 63 | 46 | 41 |
| Patient No. . | Viability (%) . | ||
|---|---|---|---|
| 1 Day . | 3 Days . | 5 Days . | |
| 1 | |||
| Control | 93 | 93 | 78 |
| As2O3 (1 μmol/L) | 92 | 86 | 68 |
| AA (125 μmol/L) | 85 | 78 | 74 |
| As2O3 (1 μmol/L) + AA (125 μmol/L) | 74 | 53 | 32 |
| 2 | |||
| Control | 88 | 86 | 80 |
| As2O3 (1 μmol/L) | 85 | 84 | 76 |
| AA (125 μmol/L) | 87 | 73 | 72 |
| As2O3 (1 μmol/L) + AA (125 μmol/L) | 62 | 41 | 36 |
| 3 | |||
| Control | 91 | 89 | 81 |
| As2O3 (1 μmol/L) | 93 | 81 | 69 |
| AA (125 μmol/L) | 71 | 70 | 70 |
| As2O3 (1 μmol/L) + AA (125 μmol/L) | 63 | 46 | 41 |
Lymphocytes (>95 of the cells) were isolated as described in the Methods from peripheral blood obtained from patients with CLL at various stages of disease. Values are the mean of 3 independent experiments.
As2O3 (1 μmol/L) inhibited cell viability in two human breast cancer cells (Table 4). MDA-MB-468, a breast cancer cell line with low glutathione-S-transferase (GST) activity, was remarkably sensitive to 1 μmol/L As2O3 (97% inhibition of growth), while T47D with higher GST activity was less sensitive to As2O3 (Table 4). The sensitivity to As2O3 was inversely proportional to GSH levels in both breast carcinoma cell lines. Ascorbic acid increased T47D sensitivity to As2O3. In contrast, normal human breast fibroblasts or HEF were more resistant to As2O3 alone or in combination with ascorbic acid.
Growth Inhibition of Human Breast Cancer Cell Lines and Fibroblasts by As2O3 and Ascorbic Acid
| Cell Line . | GST . | GSH . | Viable Cell (% of control) . | ||
|---|---|---|---|---|---|
| As2O3 . | AA . | As2O3 + AA . | |||
| T47D | 4.9 | 95.1 | 62.2 | 87.7 | 26.1 |
| MDA-MB-468 | 2.5 | 59.2 | 1.8 | 95.5 | 2.1 |
| RM5.21 | — | — | 103.0 | 89.2 | 100.9 |
| HEF | — | — | 101.0 | 93.2 | 105.0 |
| Cell Line . | GST . | GSH . | Viable Cell (% of control) . | ||
|---|---|---|---|---|---|
| As2O3 . | AA . | As2O3 + AA . | |||
| T47D | 4.9 | 95.1 | 62.2 | 87.7 | 26.1 |
| MDA-MB-468 | 2.5 | 59.2 | 1.8 | 95.5 | 2.1 |
| RM5.21 | — | — | 103.0 | 89.2 | 100.9 |
| HEF | — | — | 101.0 | 93.2 | 105.0 |
Cells were treated for 6 days with 1 μmol/L As2O3 or 62.5 μmol/L AA alone or combination. Viable cells number was determined by the MTT method.51 GST (nmol/mg protein/min) was determined according to reported method.14 GSH (nmol/mg protein) was determined as described in the Methods. Values are the mean of 3 independent experiments.
Abbreviations: RM5.21, human breast fibroblast; HEF, human embryo fibroblast.
Effect of As2O3 and ascorbic acid on normal hematopoietic cells.
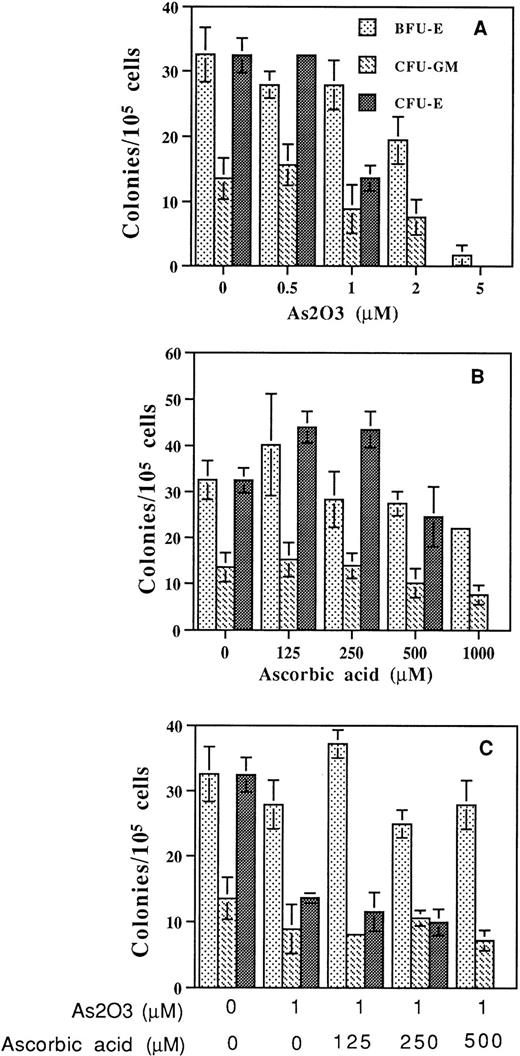
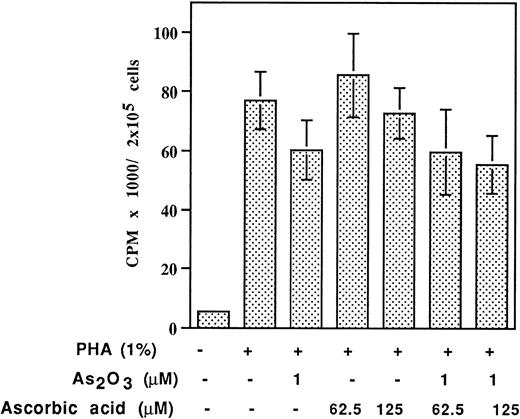
Human bone marrow or peripheral blood MNC grown in methylcellulose were treated with As2O3 and ascorbic acid alone or in combination for up to 2 weeks. A 1 μmol/L quantity of As2O3 inhibited CFU-E cells by approximately 60%, but had minimal effect on CFU-GM or BFU-E colony formation. As2O3 significantly inhibited colony-forming ability at concentrations greater than 2 μmol/L (Fig6A). Treatment with ascorbic acid did not inhibit colony formation at concentrations less than 500 μmol/L (Fig6B). Ascorbic acid did not enhance As2O3inhibition of CFU-GM or BFU-E, but at high concentration (500 μmol/L) enhanced As2O3 inhibition of CFU-E (Fig 6C). Similarly, the mitogenic response to PHA by normal lymphocytes as measured by 3H-thymidine incorporation was not significantly affected by As2O3 and the effect was not augmented by cotreatment with up to 125 μmol/L ascorbic acid (Fig 7). Thus, it seems that malignant cells are more sensitive to the combined treatment of ascorbic acid and As2O3 than normal cells.
Effect of As2O3 and AA on colony-forming ability of human bone marrow or peripheral blood progenitor cells. (A) As2O3, (B) AA alone, or (C) together at the indicated concentrations. Colony-forming ability was determined as described in Materials and Methods. Results are representative of three independent experiments each performed in triplicate.
Effect of As2O3 and AA on colony-forming ability of human bone marrow or peripheral blood progenitor cells. (A) As2O3, (B) AA alone, or (C) together at the indicated concentrations. Colony-forming ability was determined as described in Materials and Methods. Results are representative of three independent experiments each performed in triplicate.
Effect of As2O3 and AA on3H-thymidine incorporation of PHA activated lymphocytes. Lymphocytes in primary culture were treated with indicated drugs for 3 days.
Effect of As2O3 and AA on3H-thymidine incorporation of PHA activated lymphocytes. Lymphocytes in primary culture were treated with indicated drugs for 3 days.
Ascorbic acid enhances As2O3 antilymphoma effect in vivo.
In vivo studies to evaluate the effect of ascorbic acid and As2O3 in the treatment of lymphoma were initiated. Mouse lymphoma P388D1 cells, which demonstrate in vitro ascorbic acid enhancement of As2O3growth inhibition, were implanted (2 × 106 cells) into the peritoneum of BDF1 mice. On the second day As2O3 (5 mg/kg) and ascorbic acid (500 mg/kg) alone or in combination was given every other day for seven times. The combination treatment improved survival time, with an ILS of 40%, whereas single-agent treatment at these nontoxic concentrations did not influence survival (Table 5). The significantly prolonged survival was without additive toxicity as compared with As2O3 or ascorbic acid treatment alone.
Antitumor Effects of As2O3 and Ascorbic Acid Alone or in Combination in Mice Bearing P388D1 Lymphoma
| Treatment . | Weight Change (g) . | Survival Time (d) . | ILS (%) . |
|---|---|---|---|
| Control | 1.7 | 23.7 ± 1.3 | 0 |
| As2O3 | 4.9 | 22.8 ± 2.0 | −3.8 |
| AA | 2.5 | 25.0 ± 4.3 | 5.4 |
| As2O3 + AA | 1.6 | 33.2 ± 8.25-150 | 40.1 |
| Treatment . | Weight Change (g) . | Survival Time (d) . | ILS (%) . |
|---|---|---|---|
| Control | 1.7 | 23.7 ± 1.3 | 0 |
| As2O3 | 4.9 | 22.8 ± 2.0 | −3.8 |
| AA | 2.5 | 25.0 ± 4.3 | 5.4 |
| As2O3 + AA | 1.6 | 33.2 ± 8.25-150 | 40.1 |
As2O3 5 mg/kg or AA 500 mg/kg was given intraperitoneally every other day for 7 times. Weight was measured before any treatment and after the last treatment.
Abbreviation: ILS, increase in lifespan over control.
P < .05.
DISCUSSION
The therapeutic efficacy of As2O3 in APL2-4 prompted our investigations to elucidate the mechanism of action of As2O3 in APL derived NB4 cells. Our data indicate that the modulation of the redox system, and particularly the GSH content, determines the sensitivity of the cells toward As2O3. We found that 1 to 2 μmol/L As2O3, the therapeutically effective concentrations of As2O3 which induce remission in APL with minimal toxicity,3 induces apoptosis within 3 days in NB4 cells while other leukemic cells are less sensitive to As2O3 (Fig 1). The effect of As2O3 is associated with the morphologic changes characteristic of apoptosis, with activation of CPP32, cleavage of PARP, and fragmentation of DNA (Figs 2 and 5). However, degradation of Bcl-2 previously reported during As2O3-induced apoptosis of NB4 cells5 was not observed in su-DHL-4 cells even in the presence of ascorbic acid (Fig 5C) and may be cell-type specific.
We investigated whether PML/RAR-α, the oncogenic protein of APL, is responsible for the exquisite sensitivity of NB4 toward As2O3. We have confirmed6 that As2O3 at concentrations less than 0.5 μmol/L for 3 days of treatment induces degradation of PML/RAR-α in NB4 cells without inducing apoptosis. In the presence of NAC or lipoic acid, As2O3, even at high concentration, does not induce apoptosis, but it is still effective in inducing degradation of the PML/RAR-α protein (Fig 2). That lipoic acid, a vicinal SH group-containing compound that may directly bind arsenic,9was more effective (Fig 2, lane 4) than NAC (Fig 2, lane 6) in preventing PML/RAR-α degradation suggests that lipoic acid competes with PML/RAR-α in binding to As2O3 and may prevent As2O3-induced structural changes in PML/RAR-α. The high sensitivity to As2O3degradation of the nucleoplasmic fraction of PML and PML/RAR-α, but not other proteins in the nuclear dense body,32 may be due to their high content of vicinal cysteines in ring fingers and B-box motifs7 to which As2O3 may bind with high affinity.9
GSH is the major autooxidant of the cells and functions to scavenge free radicals and to detoxify toxins and chemotherapeutic agents.33,34 GSH can bind arsenic from attacking its target by formation of a transient As(GS)3 complex.18Many enzymes and factors modulate the level of GSH. It is known that the glutathione transferase GST-π, an enzyme involved in metabolic detoxification of a variety of xenobiotics, is increased in an arsenic-resistant CHO cell line.14,35 We also found that the MDA-MB-468, a breast cancer line with low GST activity, is sensitive to very low concentrations of As2O3(Table 4).36 Glutathione peroxidase is lower in another CHO subclone than in the parent cells and is less resistant to arsenite-induced genotoxicity.37 Drugs that inhibit the activity of GSH peroxidase increase the efficacy of the apoptotic activity of As2O3 in malignant lymphocytic cell lines (manuscript in preparation). Multidrug-resistant protein (MRP) is important in determining the sensitivity of many natural toxins, including sodium arsenite, and cotransports GSH and xenobiotics from the cell.38,39 The relative importance of adenosine triphosphate (ATP)-dependent membrane export by MRP and the GSH detoxification pathway in determining sensitivity to As2O3 remains to be determined. Meanwhile, using subtractive hybridization, it was found that metallothionine and thioredoxin reductase, two other detoxification system-related factors,40 41 are upregulated in arsenic treated NB4 cells (M. Mao, personal communication, 1997).
We have shown that compounds that lower the GSH content potentiate the apoptotic effect of As2O3, whereas protecting or increasing the GSH level protects the cells from the apoptotic effect of As2O3 (Figs 2 and 3). NB4 cells are protected from the apoptotic effect of As2O3 if treated with the antioxidant NAC, which preserves the GSH content (Fig2A). On the other hand, BSO, which inhibits the synthesis of glutamyl-cysteine, lowers the GSH content of NB4 cells and potentiates the apoptotic effect of As2O3. In BSO-treated cells, 1 μmol/L As2O3 induces apoptosis in 70% of the cells in only 12 hours of treatment, and less sensitive cell lines like su-DHL-4 and HL-60 become as sensitive as NB4 (Fig 3).
Ascorbic acid, which can act as a major antioxidant can protect cells from oxygen radicals formed by 4-HPR,42curcumin,43 and other agents44,45 that induce apoptosis. In contrast, ascorbic acid can induce apoptosis alone46 or in combination with agents in other cell systems.47 In HL-60 cells, dehydroascorbic acid but not ascorbic acid is transported via glucose transporters and is coupled to its intracellular reduction to ascorbic acid.48 In our studies, ascorbic acid in combination with As2O3 has a potentiating effect due to its capacity to undergo autooxidation resulting in the formation of H2O2 which potentiates the effect of As2O3 (Table 1). This assumption is fortified by our findings that in the presence of catalase, ascorbic acid does not enhance As2O3 and the combined treatment of As2O3 and H2O2 is synergistic for the induction of apoptosis (Table 2). Thus, the balance between GSH and H2O2 may determine the apoptotic effect of As2O3 in leukemic cells. However, ascorbic acid did not potentiate the effect of As2O3 on the colony formation capacity of normal hematopoietic cells (Figs 6 and 7). The synergistic effect of As2O3 with ascorbic acid manifested itself also in one epithelial mammary carcinoma line T47D, whereas normal breast or embryo fibroblasts were insensitive to As2O3and not sensitized by the addition of ascorbic acid (Table 4). This selective action of ascorbic acid may be partially due to the low concentration of catalase in some malignant cells.49 50Thus, it should be possible to use low concentration of As2O3 with ascorbic acid to selectively induce apoptosis in some malignant cells without causing severe side effect in normal tissues. We tested this possibility in an in vivo mouse model. We found that the combined effect of As2O3 and ascorbic acid increased the survival time of mice injected with P388D-lymphoma cells, whereas single-agent treatment did not influence survival (Table 5).
In summary the redox system of the cell and the capacity to eliminate reactive oxygen species determine the efficacy of As2O3. It is noteworthy that normal cells in general are more efficient in eliminating reactive oxygen species than malignant cells. Thus, it seems that malignant cells with low GSH levels or GST activity should be sensitive to As2O3 or the combined treatment of As2O3 and ascorbic acid.
ACKNOWLEDGMENT
We appreciate the technical assistance of Yelena Galperin for bone marrow colony assays and the guidance of Dr George Acs throughout these studies.
Supported by National Institutes of Health Grant No. 5RO1CA59936-03-05, the Gloria and Sidney Danziger Foundation, and the Samuel Waxman Cancer Research Foundation.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Samuel Waxman, MD, Division of Neoplastic Diseases, Department of Medicine, Mount Sinai Medical Center, Box 1178, One Gustave L. Levy Place, New York, NY 10029-6547.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal