Abstract
Factor VIII and von Willebrand factor (vWF) circulate in the plasma as a noncovalent protein complex. Circulating levels of factor VIII are coordinately regulated with circulating levels of vWF in which the ratio is maintained at 1 molecule of factor VIII for 50 to 100 vWF subunits. Infusion of vWF into vWF-deficient animal models and human patients yields a secondary increase in circulating levels of factor VIII. We have studied the mechanism of the secondary rise in factor VIII in a porcine model of vWF deficiency. On infusion of vWF into a vWF-deficient pig there was an approximately fivefold increase in circulating factor VIII activity. Liver biopsies were taken pre- and post-vWF infusion for isolation of total messenger RNA (mRNA). Factor VIII–specific mRNA was measured by an RNAse protection assay. The results showed no difference in the liver-specific factor VIII mRNA on vWF infusion. These results indicate that the secondary rise in factor VIII levels in response to exogenous vWF infusion is not dependent on increased steady-state levels of factor VIII mRNA in the liver.
FACTOR VIII IS THE X chromosome gene product that is deficient or defective in the bleeding disorder hemophilia A. Factor VIII functions in the intrinsic pathway of blood coagulation as a cofactor to accelerate the activation of factor X by factor IXa that occurs on a phospholipid surface in the presence of calcium ions. The factor VIII amino acid sequence deduced from the cloned complementary DNA (cDNA) identified that the molecule is synthesized as a single-chain polypeptide having the domain structure A1-A2-B-A3-C1-C21 2 and on secretion from the cell is processed to a heterodimer consisting of a carboxy-terminal–derived light chain of 80 kD in a metal ion–dependent association with a 200 kD amino-terminal–derived heavy-chain fragment.
In plasma, factor VIII is bound and stabilized by noncovalent interactions with von Willebrand factor (vWF).3-8 In addition, in vitro studies showed that vWF regulates factor VIII activity through additional mechanisms: vWF prevents activation of factor VIII by factor Xa9; vWF prevents inactivation of factor VIII by activated protein C10-12; and vWF prevents binding of factor VIII to phospholipids13,14 and to thrombin-activated platelets.15 In addition, vWF can also stimulate the stable accumulation of factor VIII on secretion from transfected mammalian cells in culture.16,17 A primary factor VIII binding site was identified within the amino-terminus of mature vWF (residues 1 to 27218-20), and recent studies suggest that both the amino-terminus and the carboxy-terminus of the factor VIII light chain mediate interaction with vWF.21-25
Although each subunit of a vWF multimer contains one factor VIII binding site, in vitro binding studies yielded conflicting data for factor VIII:vWF subunit ratios of 1:1,26,271:4,28,29 and 1:10,15 to as low as 1:70 high-affinity binding sites.30 The source for the difference remains unknown because different reagents, protein concentrations, and assays were used for these studies. At least one factor that influences this ratio is the conformation of vWF in the assay.31 However, the ratio of circulating factor VIII to vWF observed in vivo is tightly maintained at 1:50.7 Any change in plasma vWF level is coupled with a concordant change in the factor VIII level. The infusion of vWF into vWF-deficient animal models and human patients immediately elevates factor VIII levels and the factor VIII levels exhibit a sustained rise, even while vWF levels decline as a result of clearance.3,4,7,8,32-34 In addition, 1-desamino-8-D-arginine vasopressin is used to treat bleeding episodes in mild von Willebrand’s disease (vWd) and mild hemophilia A and is proposed to stimulate the endogenous release of vWF, thereby increasing plasma levels of factor VIII.35 The mechanism by which vWF stimulates factor VIII levels in plasma is not known. The presence of vWF increases the plasma half-life of factor VIII from 2 to 3 hours to 12 to 14 hours.5 6 However, it is not known if vWF also stimulates factor VIII synthesis as well. We have studied whether plasma vWF levels influence factor VIII messenger RNA (mRNA) expression.
A porcine model for severe homozygous vWF deficiency in humans exists. In this porcine model of homozygous vWF deficiency the vWF antigen level is 0.25% and the factor VIII activity is 15% to 30% of normal.36 The genetic lesion responsible for the porcine defect is thought to be a mutation outside of the coding region influencing vWF mRNA stability.37 Infusion of vWF into the circulation8 or perfusion of the porcine liver with vWF38 elicits an increase in circulating factor VIII activity. Thus, this model mimics the severe human vWF deficiency. In this study, we have measured the effect of vWF infusion on the level of factor VIII mRNA in the liver. The results show that infusion of vWF elicits a rise in circulating factor VIII at a post-transcriptional level.
MATERIALS AND METHODS
Infusion of vWF into vWF-deficient pig and liver biopsy.
Surgery was performed on a 35 kg female vWd pig 3 months and 3 weeks of age. The animal was anesthetized intramuscularly with ketamine-xylazine-torbugesic (17.5 mg/kg, 2.5 mg/kg, and .025 mg/kg, respectively) in divided doses. To provide vascular access, a Hickman catheter was placed in the left carotid artery with the port tunneled under the skin to the dorsal neck. The animal received 1 gm Ancef and 80 mg gentocin during catheter placement. A 5 cm incision below the diaphragm to the right of the midline exposed the liver. A segment of the margin of the liver was isolated using two Cooley aortic clamps (Miltex Surgical Instruments, Lake Success, NY) applied from opposite directions. This tissue was removed and placed immediately into liquid nitrogen. An infusion of porcine vWF concentrate was initiated to assist in hemostasis and to stimulate the appearance of factor VIII in the circulation. While still secured with the aorta clamps, the cut edge of the organ was sutured with No. 0 silk using a purse-string stitch. As the suture was drawn tight the clamps were removed. Gelfoam was applied until bleeding stopped, the liver was replaced, and the incision closed. Porcine vWF concentrate was infused every 8 hours with a target plasma concentration of 50%. The animal was placed under constant human observation for the following 99 hours (72 hours past the second procedure). After 27 hours, a second similar procedure was performed on the same animal after it had received four infusions of porcine vWF totaling 3,000 U (ristocetin vWF activity). After the second biopsy, the animal was provided additional infusions of porcine vWF three times a day to prevent hemorrhage during recovery. At the time of the second biopsy the factor VIII over-response was at twice the level of the ristocetin vWF activity and five times the level of the vWF antigen measurements.
vWF preparation and vWF and factor VIII assay.
vWF concentrate was prepared by precipitation of porcine plasma with 1 mol/L potassium phosphate, pH 6.5, as described.39 The precipitated proteins were suspended in approximately .08 plasma volumes and dialyzed against 0.02 mol/L Na Citrate, 0.14 mol/L NaCl, pH 7.4. This procedure yields a product that contains on average 10 to 15 U/mL of ristocetin-vWF factor activity and 6 to 10 U/mL of vWF antigen. Material prepared in this manner for infusion was frozen at −20°C for up to 5 months before its administration.
vWF-dependent, ristocetin-induced platelet agglutination was measured using gel-filtered porcine platelets in the assay previously described.40 vWF antigen was determined by the method of Laurell,41 using a rabbit antiporcine vWF antibody produced in our laboratory.
Factor VIII activity was measured using a clotting assay with human factor VIII-deficient plasma as substrate. In this modified activated partial thromboplastin time assay normal pooled porcine plasma was used as the standard. Units are therefore expressed as porcine units, with 100% (1 U/mL) being the activity found in the frozen (−70°C) porcine plasma pool.
Preparation of RNAse protection probe.
A HindIII-SmaI 441 base-pair fragment from the porcine factor VIII B domain42 was subcloned into theHindIII and SmaI sites of pGEM4. Plasmid DNA was prepared and linearized by digestion with EcoRI restriction endonuclease and 400 ng was used for in vitro transcription with bacteriophage T7 RNA polymerase (Promega Biotech, Madison WI) in 50 mmol/L Tris-HCl pH 8.3, 75 mmol/L KCl, 3 mmol/L MgCl2, 10 mmol/L dithiothreitol, RNAsin (Promega Biotech, Madison WI), 100 μmol/L guanosine triphosphate (GTP), and 2 mmol/L of each nucleotide adenosine triphosphate (ATP), cytidine triphosphate (CTP), and uridine triphosphate (UTP), in the presence of 100 μmol/L Ci [32P]-αGTP (New England Biolabs, Beverly, MA). After incubation at 40°C for 1 hour, RQ1 DNase (Promega Biotech) was added and incubated another 15 minutes at 37°C. The mixture was extracted with phenol:chloroform (1:1) and 5 μg of yeast transfer RNA (tRNA) was added for ethanol precipitation. After centrifugation, the RNA pellet was dried and resuspended in diethylpyrocarbonate-treated H2O.
Total RNA isolation and RNAse protection.
Total RNA was extracted from 0.2 gm of frozen porcine liver biopsy samples. The frozen tissue was pulverized using a mortar and pestle, placed in guanidine thiocyanate, and homogenized in a dounce homogenizer.43 The RNA was then purified over a cesium chloride gradient.44 For control, RNA was isolated from Chinese hamster ovary (CHO) cells as previously described.45 Aliquots of RNA (10 μg and 15 μg) were mixed with 1 × 106 cpm of 32P-labeled T7 transcript and dried down under vacuum. Samples were resuspended in 80% formamide, 40 mmol/L PIPES pH 6.4, 400 mmol/L NaCl, 1 mmol/L EDTA, and heated to 85°C for 15 minutes. Samples were incubated at 45°C overnight. After incubation, samples were treated with pancreatic ribonuclease (0.5 ug/mL) and RNAse T1 (50 ng/mL) in the presence of 30 mmol/L Tris-HCl pH 8.0, 200 mmol/L NaCl, 100 mmol/L LiCl, and 1 mmol/L EDTA for 30 minutes at 37°C. Reactions were stopped by addition of sodium dodecylsulfate (SDS) to 0.5% and proteinase K (130 μg/mL) and incubation 15 minutes at 37°C. Samples were extracted with phenol:chloroform and ethanol precipitated after addition of 5 μg yeast tRNA. Precipitated samples were resuspended in formamide buffer for electrophoresis on a polyacrylamide gel. Radioactive gels were exposed for autoradiography. Band intensities were quantitated by NIH Image software (public domain).
Measure of poly(A)+ RNA.
A poly(T) probe was synthesized as first-strand cDNA by reverse transcription of HeLa cell poly(A)+ RNA. A 100 μL reaction contained 500 ng/μL poly(A)+ RNA, 10 mmol/L dithiothreitol, 50 mmol/L Tris-HCl pH 8.3, 75 mmol/L KCl, 3 mmol/L MgCl2, 0.5 U/μL RNAguard (Pharmacia, Piscataway, NJ), 1 mmol/L thymidine triphosphate (TTP), 10 μg/mL oligo (dT)12-18, 100 μCi α-32P dTTP (3000 Ci/mmol, Amersham, Arlington Heights, IL/US Biochemicals, Cleveland, OH), and 5 U/μL Moloney murine leukemia virus reverse transcriptase (GIBCO-BRL, Gaithersburg, MD). The reaction was incubated at 42°C for 90 minutes and then terminated with the addition of 100 mmol/L NaOH and 10 mmol/L EDTA. The reaction was incubated at 65°C for 30 minutes to hydrolyze the poly(A)+ template. The alkaline reaction was neutralized with 100 mmol/L Tris-HCL, pH 7.5 for 15 minutes at room temperature. The probe was separated from unincorporated radionucleotides by ethanol precipitation after addition of ammonium acetate to 0.3 mol/L. The average length of the probe was 200 to 300 bases.
RNA samples were denatured with 500 μL of ice-cold 10 mmol/L NaOH and 1 mmol/L EDTA and were immediately applied to a nitrocellulose membrane using a dot-blot apparatus. Wells were rinsed with 500 μL cold 10 mmol/L NaOH and 1 mmol/L EDTA and dried under vacuum. Filters were rinsed in 2× SSC (150 mmol/L NaCl, 15 mmol/L sodium citrate) with 0.1% SDS and cross-linked using a GSG gene linker (Bio-Rad Laboratories, Richmond, VA). Filters were pretreated and hybridized with the poly(T) probe in 5× SSCPE (0.9 mol/L NaCl, 50 mmol/L Na2HPO4, 50 mmol/L EDTA) with 5× Denhardt’s solution,46 0.1% SDS, 100 μg/mL denatured salmon sperm DNA, and 50% formamide. Band intensities were quantitated by NIH Image software.
RESULTS
To measure the effect of vWF infusion on circulating levels of factor VIII in the plasma and factor VIII mRNA levels in the liver, plasma samples were taken from a homozygous vWF-deficient pig to obtain baseline levels of factor VIII activity, vWF antigen, and vWF activity levels. At 1 day and 4 days (not shown) before vWF infusion the levels of vWF were measured for ristocetin cofactor activity and were not detectable. The level of vWF antigen measured by quantitative immunoelectrophoresis was also not detectable, although factor VIII activity was detected at approximately 25% (Fig 1). Before infusion of vWF, a liver biopsy was performed for extraction of RNA. Purified porcine vWF (approximately 700 U) was infused into the homozygous vWF-deficient pigs and subsequently every 8 hours. After infusion of porcine vWF, plasma samples were obtained and monitored for factor VIII activity, vWF antigen, and ristocetin cofactor activity (Fig 1). vWF levels, measured by ristocetin cofactor activity, increased to approximately 30% immediately after infusion and with additional infusions, subsequently increased over the next 24 hours up to approximately 60% and vWF antigen up to 20%. These levels roughly correlated with the ristocetin cofactor and vWF antigen determinations previously reported for this form of porcine concentrate.47 Factor VIII activity levels increased steadily over the 24-hour period coincident when the vWF infusions were initiated and after 24 hours the plasma factor VIII activity was 131%. At 27 hours postinfusion, another liver biopsy was performed. After the second biopsy, continued infusion of porcine vWF was maintained (2,100 ristocetin cofactor Units per 24 hours) to provide effective hemostasis. The elevation in factor VIII activity in the plasma was sustained throughout the 72-hour period after the second biopsy, a time during which the animal was under constant human observation.
Factor VIII and vWF levels pre- and post-vWF infusion. Plasma samples were isolated at the indicated periods of time from a vWF-deficient pig. The times that vWF infusions were performed are indicated by the “I” arrows. vWF ristocetin cofactor activity and antigen and factor VIII activity were measured as described in Materials and Methods and presented as a percent of normal porcine values. Liver biopsies were performed at the times indicated by the “B” arrows. Factor VIII, □; Ristocetin cofactor, •; vWF antigen, ▴.
Factor VIII and vWF levels pre- and post-vWF infusion. Plasma samples were isolated at the indicated periods of time from a vWF-deficient pig. The times that vWF infusions were performed are indicated by the “I” arrows. vWF ristocetin cofactor activity and antigen and factor VIII activity were measured as described in Materials and Methods and presented as a percent of normal porcine values. Liver biopsies were performed at the times indicated by the “B” arrows. Factor VIII, □; Ristocetin cofactor, •; vWF antigen, ▴.
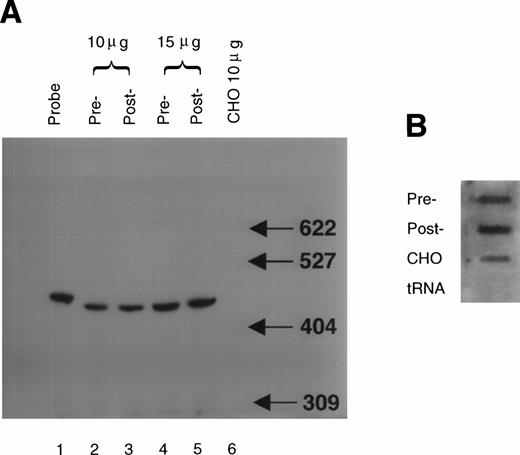
Factor VIII mRNA in liver biopsy samples was quantified by an RNAse protection assay. The probe was an antisense RNA transcript containing 441 nucleotides of the porcine factor VIII B domain. The total length of the purified probe was 471 nucleotides due to the presence of 30 nucleotides derived from the pGEM4 polylinker site. The vector-derived sequences allowed the clear separation of undigested probe from the RNAse protected fragment. Analysis by polyacrylamide gel electrophoresis showed a protected fragment migrating at approximately 440 nucleotides in both RNA samples from liver biopsies isolated pre- and post-vWF infusion (Fig 2A). Two concentrations of RNA were used to confirm that the probe was in excess. The protected fragment was not detected in RNA isolated from CHO cells, showing specificity for the protection. This analysis did not detect a significant difference in the amount of protected fragment observed in RNA isolated before vWF infusion compared with RNA isolated 27 hours after vWF infusion. RNA dot blot analysis of the same samples using a poly(T) probe (as a measure of poly(A)+ mRNA) indicated that the inability to detect a difference in factor VIII mRNA was not due to differences in the amount of poly(A)+containing RNA (Fig 2B). Hybridization to poly(T) was not detected with yeast tRNA, showing specificity of the hybridization. At the time point of RNA analysis, serum levels of factor VIII were significantly elevated. This result indicates that factor VIII mRNA levels were not elevated at this time point in the liver.
Factor VIII mRNA levels in the liver pre- and post-vWF infusion. Total RNA was isolated from samples of porcine liver biopsied pre- and post-vWF infusion. The total RNA samples (10 and 15 mg) were assayed by RNAse protection as described in Materials and Methods (Panel A). Analysis of a 1/50 aliquot of the probe (lane 1) showed that under these assay conditions the radiolabeled probe was in vast excess. Densitometry of the autoradiogram indicated that levels of factor VIII mRNA did not significantly differ between pre-vWF infusion (relative band intensities were: 1.0 U/10 mg RNA and 1.8 U/15 mg RNA) and post-vWF infusion (1.1 U/10 mg RNA and 2.0 U/15 mg RNA). The amount of poly(A)+ RNA was determined by dot-blot hybridization to a poly(T) probe (panel B). For controls total CHO cell RNA and yeast tRNA were analyzed as indicated. Densitometry of the dot-blot showed that pre- (area = 4.5) and post-vWF (area = 4.8) infusion samples did not significantly differ in their hybridization to the poly(T) probe.
Factor VIII mRNA levels in the liver pre- and post-vWF infusion. Total RNA was isolated from samples of porcine liver biopsied pre- and post-vWF infusion. The total RNA samples (10 and 15 mg) were assayed by RNAse protection as described in Materials and Methods (Panel A). Analysis of a 1/50 aliquot of the probe (lane 1) showed that under these assay conditions the radiolabeled probe was in vast excess. Densitometry of the autoradiogram indicated that levels of factor VIII mRNA did not significantly differ between pre-vWF infusion (relative band intensities were: 1.0 U/10 mg RNA and 1.8 U/15 mg RNA) and post-vWF infusion (1.1 U/10 mg RNA and 2.0 U/15 mg RNA). The amount of poly(A)+ RNA was determined by dot-blot hybridization to a poly(T) probe (panel B). For controls total CHO cell RNA and yeast tRNA were analyzed as indicated. Densitometry of the dot-blot showed that pre- (area = 4.5) and post-vWF (area = 4.8) infusion samples did not significantly differ in their hybridization to the poly(T) probe.
DISCUSSION
The observation that hemophilia A offers protection from ischemic heart disease48 and evidence that elevated factor VIII is associated with thrombotic disease49 provides an incentive to understand the mechanism by which factor VIII levels are regulated in plasma. It has long been known that plasma levels of vWF can regulate factor VIII levels. At least one mechanism involves stabilization of factor VIII in the blood.5,6 However, on infusion of vWF into vWF-deficient patients, factor VIII levels exhibit a sustained increase, even in the presence of decreasing levels of vWF due to its clearance.33 34 These observations prompted us to evaluate whether factor VIII mRNA expression is also regulated by circulating levels of vWF. Our studies did not detect any increase in factor VIII-specific mRNA in the liver, although circulating levels of factor VIII in the plasma were greatly elevated. Therefore, if vWF elicits an increase in factor VIII synthesis, it does not exert this effect through a change in the steady-state level of factor VIII mRNA in the liver.
To date, most evidence supports that factor VIII is synthesized in the liver. Factor VIII mRNA and protein is detected in many tissues including liver, spleen, lymph node, kidney, and placental extracts.50-53 Transplantation of liver as well as reticuloendothelial tissue have successfully sustained physiologically functional levels of factor VIII in the plasma.54-59Therefore, we cannot rule out that factor VIII mRNA levels do rise in tissues other than the liver in response to vWF infusion.
Our findings support that vWF regulates factor VIII levels by protecting factor VIII from clearance and also possibly by stabilizing factor VIII on secretion from the site of synthesis. The results of these studies suggest that the regulation of factor VIII transcription in the liver is not a contributing factor in the regulation of plasma factor VIII levels by vWF. Therefore, strategies for liver-directed gene therapy that use promoter elements other than factor VIII, would be justified if the expression vector provides sufficient level of factor VIII in the plasma. Because vWF appears to be a significant factor in the regulation of factor VIII plasma levels, it is possible that an increase in the synthesis of factor VIII above normal may not result in excess factor VIII in the plasma because vWF binding would be limiting. Further studies are required to directly test this hypothesis. If this turns out to be true, then a thrombotic concern for the overexpression of factor VIII mRNA in gene therapy may be minimized.
ACKNOWLEDGMENT
We thank Mariot Varban, Micheline Moussalli, Joseph Nowak, and Luigina Tagliavacca for technical assistance and Steven Pipe for critical comments on the manuscript.
Supported by National Institutes of Health grant HL52173 (R.J.K.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal