Abstract
Vascular endothelial-cadherin (VE-cadherin) is a calcium-dependent adhesive molecule, exclusively and constitutively expressed in endothelial cells. Analysis of the VE-cadherin promoter fused to a reporter gene in bovine aortic endothelial cells showed three major functional regions. The proximal region alone (−139, +24) promoted nonspecific transcription; the addition of the (−289, −140) and (−2226, −1190) domains abolished transcription in fibroblasts while expression in endothelial cells remained unchanged, suggesting that fragments (−2226, +24) and longer contain the full endogenous promoter activity. To study the transcriptional specificity of the promoter region in vivo, we generated transgenic mice carrying the chimeric construct containing the (−2486, +24) region. The promoter directed reporter expression in all examined organs of adult transgenic mice. During embryonic development, transgene expression was detected at the early steps of vasculogenesis. Later, the expression persisted during development of the vascular system and was restricted to the endothelial layer of the vessels. Together, these data provide evidence for specific regulatory regions within the VE-cadherinpromoter. Furthermore, the identification of DNA sequences restricting gene expression to the endothelium has many potential applications for the development of animal models of cardiovascular or angiogenic diseases or for the delivery of therapeutic molecules.
THE DEVELOPMENT of the vascular system is one of the earliest and most critical steps during vertebrate embryogenesis. In this process, morphogenic events, which lead to the formation of the mature vascular endothelium, are closely associated with successive differentiation stages. The acquisition of the endothelial phenotype by mesodermal precursors has been essentially documented by the expression of stable or transient markers.1 Currently, our understanding of the molecular cues that confer the endothelial phenotype is extremely limited. A useful starting point for gaining insights into the mechanisms that underlie endothelial differentiation is to characterize the regulatory regions of genes that are specifically expressed in this cell type.
An important objective of endothelial promoter study is to define molecular tools capable of directing endothelial-specific expression of proteins of interest. Such promoters can be useful in the development of transgenic animals in order to produce, for instance, animal models of human vascular diseases, endothelial-specific expression of dominant-negative mutants, or conditional gene targeting.
At present, the number of known endothelial specific (or at least restricted) proteins is small. These include, but are not limited to, von Willebrand factor,2 platelet/endothelial cell adhesion molecule-1 (CD31),3 preproendothelin-1,4tie-1,5 and P-selectin.6 However, these molecules are not exclusively expressed in the endothelium. Other markers such as E-selectin,7 vascular endothelial growth factor receptors 18,9 and 2,10 and a tyrosine-kinase receptor called tie-25 are specifically expressed in endothelial cells, but essentially after cell activation with inflammatory cytokines (for E-selectin) or during vascular proliferation (for the vascular endothelial growth factor receptors and tie-2).
In this report, we characterized the promoter activity of the VE-cadherin (CD144) gene. This molecule, which belongs to the cadherin family of adhesive receptors,11 is specifically localized at the interendothelial junctions, where it plays a crucial role in vascular assembly.12 This protein is exclusively and constitutively expressed by the endothelium of all types of vessels.13,14 Moreover, VE-cadherin is expressed by endothelial precursors as early as embryonic day 7.5 (E7.5) in the mouse embryo.14 The gene of this molecule thus represents a good candidate to study endothelial-specific transcriptional mechanisms. Therefore, we were prompted to investigate the role of the 5′-flanking region of mouse VE-cadherin gene in promoter activity. In previous work, we have identified and cloned the murine gene coding for VE-cadherin as well as 10 kb of upstream sequence.15 This gene contains 12 exons spanning more than 36 kb; the first exon is entirely untranslated and starts at one transcriptional site. In this report, we used a 5′-deletion strategy and transient transfection assay to delineate cis-acting DNA fragments in VE-cadherin promoter. Furthermore, we could define a promoter fragment sufficient to confer endothelial specific expression of a reporter gene in transgenic mice.
MATERIALS AND METHODS
Cloning procedures.
A 3.5-kb Sac I fragment isolated from clone λ1,15containing the promoter region, the first exon, and the beginning of the first intron of mouse VE-cadherin gene, was subcloned into pBluescript II (Stratagene, La Jolla, CA) and sequenced. (Sequence from position −2486 to +24 is available in the EMBL databank under accession no. Y10887). Constructs used for transient expression and transgenic animals were produced in a multistep process. As no unique restriction site was present within the first exon for subcloning into the chloramphenicol acetyl transferase (CAT) reporter plasmid, pBLCAT3,16 a polymerase chain reaction (PCR) strategy was developed to create an artificial Xho I site downstream of position +24. A PCR fragment was generated using clone λ1 as matrix and two primers, 5′-CCCGGAAAGATCTGCTCTCT-3′ and 5′-CTCCACTCGAGTCTGTCCAGGGCCGAGC-3′, containing the sequence centered around the BglII site at position −1190 and the sequence from position +6 to +24 of first exon followed by an Xho I site, respectively. This fragment was cut by BglII and Xho I and inserted into the corresponding sites of pBLCAT3. This construct, called −1190CAT, was verified by sequencing and constitutes the starting material for the other CAT constructs. −773CAT, −675CAT, −289CAT, and −139CAT were obtained by insertion of AccI/Xho I, BamHI/Xho I,HindIII/Xho I and Pst I/Xho I fragments, respectively, into their cognate sites in pBLCAT3. Constructs −515CAT and −187CAT were generated by ligation ofPvuII/Xho I and Apa I (blunted with T4 DNA polymerase)/Xho I fragments into Sal I site, filled-in with Klenow enzyme, and Xho I site of pBLCAT3. −900CAT was produced by inserting a filled-in Taq I fragment into the filled-inXho I site of pBLCAT3. For −5800CAT and −2226CAT, anEcoRI (filled-in)/BglII and an Nhe I (filled-in)/BglII fragments from clone λ1 were ligated to sites Sal I, filled-in, and BglII of −1190CAT. Finally, construct −2486CAT was obtained by insertion of SpeI/BglII fragment into Xba I and Bgl I sites of −1190CAT. The pBLCAT216 or pRSVCAT17 plasmids containing the CAT gene driven by the herpes simplex virus thymidine kinase (HSVTK) promoter or the long terminal repeats of the Rous sarcoma virus, respectively, were used as positive control.
Cell culture.
Bovine aortic endothelial cells (BAEC) were prepared as described previously18 and maintained in Dulbecco’s modified Eagle’s medium supplemented with 15% fetal calf serum (Seromed, Berlin, Germany), 100 U penicillin/mL, and 100 μg streptomycin/mL BAEC from passages 3 to 12 were used for transfection experiments. NIH-3T3, HeLa, HepG2, HEL, and Lin 175 cell lines were obtained from American Type Culture Collection (Rockville, MD) and cultured in conditions identical to those for BAEC except that we used 10% fetal calf serum.
Transfections, CAT assay, and luciferase assay.
BAEC, NIH-3T3, HeLa, and HepG2 cells were transfected by the calcium phosphate precipitation method as previously described.19HEL and Lin 175 cells were transfected by electroporation with a gene pulser (Bio-Rad, Hercules, CA) set at 400 V and 960 μF, in a total volume of 800 μL. All cell lines were transfected with 3.5 pmol of appropriate CAT construct, which is the equivalent of 10 μg of pBLCAT3, and 5 μg of luciferase reporter plasmid, pGL3-control (Promega, Madison, WI), to correct for variability in transfection efficiency. Cell extracts were prepared 48 hours later and luciferase activity was determined on an aliquot with a luciferase assay system (Promega, Madison, WI) and a luminometer (LKB, Bromma, Sweden). The CAT assays were performed as described20 with the equivalent of 1,200, 3,000, 600, 3,000, 150, and 200 arbitrary light units of extracts from BAEC, NIH-3T3, HEL, HepG2, HeLa, and Lin 175, respectively, to be in the linear range of the assay. Data were expressed as the percentage of acetylated chloramphenicol relative to total chloramphenicol.
Transgenic mice.
The DNA containing the promoter fragment of −2486CAT, the CATgene, and the small t intron and polyadenylation signal from SV40 was liberated from plasmidic sequences by digestion with Sal I andSac I. Insert isolated from agarose gels and further purified on Elutip-d columns (Schleicher & Schuell, Ecquevilly, France) was microinjected using established procedures21 into fertilized eggs resulting from mating between B6D2F1/JIco hybrids. The animals were screened by Southern blot with two different probes, one containing sequences of the CAT gene alone, the other containing sequences from the CAT gene and VE-cadherin promoter. Transgene copy number was estimated by comparative analysis with endogenous promoter on a phosphorimager system (Molecular Dynamics, Sunnyvale, CA). CAT assays of F1 animal tissues were performed with 2.5 μg of protein extract in 1 hour as previously described.20
RNA extraction and Northern blot analysis.
Total RNA were extracted from 2-month-old mouse tissues by the rapid total RNA isolation kit (5 Prime-3 Prime, Boulder, CO). Electrophoresis was performed by the formaldehyde method,22 using 20 μg of RNA. Northern blot was successively hybridized with probes for CAT (415 bp EcoRI-Sca I fragment),16VE-cadherin (1,740 bp EcoRI-Sph I fragment),14 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (full-length cDNA)23 transcripts.
In situ hybridization.
The techniques used were essentially as described.14 Frozen sections (10 μm) were hybridized to 35S-labeled RNA probes generated by in vitro transcription. Two probes were generated for hybridization with CAT transcripts either from a 256-bp XhoI-EcoRI fragment or from a 415-bp EcoRI-Sca I fragment.16 Nontransgenic tissues were used as negative control and did not show specific hybridization. ForVE-cadherin transcripts, a specific probe derived from the first 1,740 bp of mouse VE-cadherin cDNA was used as previously described.14
Immunohistological staining.
Embryos were fixed for 2 hours in 4% paraformaldehyde, incubated for 1 hour in 15% sucrose followed by 16 hours in 30% sucrose, and embedded in OCT (Miles Scientific, Elkart, IN) before freezing. Sections (10-μm thick) were postfixed for 30 minutes with 4% paraformaldehyde, rinsed for 5 minutes in phosphate-buffered saline (PBS), and incubated for 10 minutes with 1% bovine serum albumin, for 16 hours at 4°C with primary antibodies, and for 1 hour at 22°C with secondary antibodies, successively. Chicken antibody to CAT was purchased from Promega (Madison, WI) and used at 1:300 dilution. Rabbit anti-mouse VE-cadherin antiserum, raised against a specific region of VE-cadherin C-terminus,14 was diluted 1:300. As secondary antibodies, a fluorescein isothiocyanate rabbit anti-chicken IgY antibody (Promega), and a tetramethyl rhodamine isothiocyanate conjugated goat anti-rabbit IgG antibody (Jackson Laboratories, West Grove, PA) were used at 1:100 dilution.
RESULTS
In vitro expression of VE-cadherin promoter.
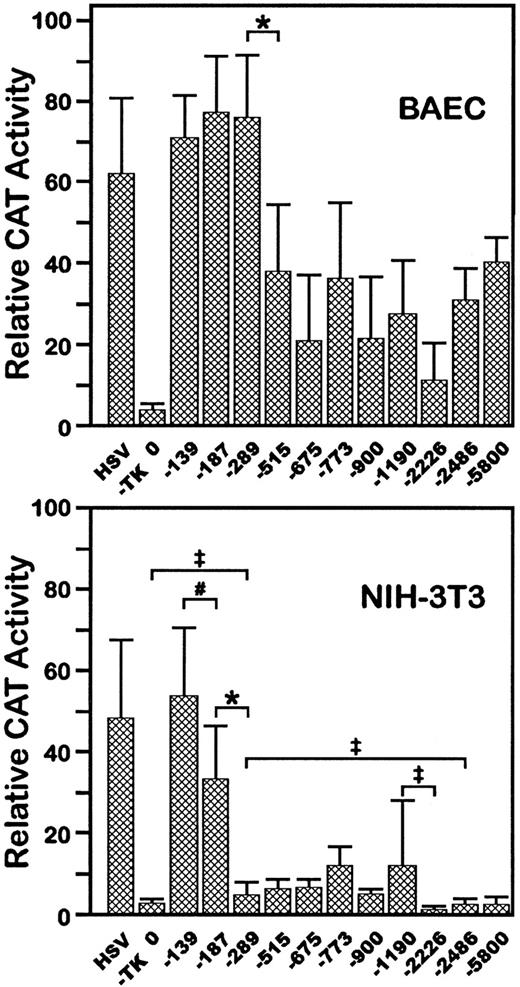
To test whether the 5′-flanking region of VE-cadherin gene had promoter activity, fragments of this region were inserted upstream from a promoterless CAT gene in the plasmid pBLCAT3. Eleven constructs of different sizes, from positions −5800 to −139 bp at the 5′-end to position +24 bp at the 3′-end, were analyzed by transient transfection assay, both in BAEC, which express VE-cadherin (data not shown), and in the fibroblastic cell line NIH-3T3, which does not.14 All of the constructs expressed the reporter gene in BAEC as indicated by CAT enzymatic assay of cellular extracts (Fig 1A). Constructs −139CAT, −187CAT, and −289CAT promoted similar high levels of CAT activity. A substantial decrease in CAT activity was observed following transfection with longer constructs from −515CAT to −5800CAT. These data suggest that at least one cis sequence responsible for promoter activity in BAEC is present between positions −139 and +24 and that a negative regulatory element is located between −515 and −290.
Functional analysis of VE-cadherin promoter by transient transfection. 5′-deleted fragments of the mouseVE-cadherin promoter were fused to the CAT gene as indicated in the text and transfected into BAEC (A) or NIH-3T3 cells (B). The HSVTK promoter was used as positive control. “0” states for the promoterless plasmid pBLCAT3. In each assay, the luciferase expression plasmid, pGL3, was cotransfected, and CAT assays were normalized according to the luciferase activity. The equivalent of 1,200 or 3,000 arbitrary light units of BAEC or NIH-3T3 cells extracts, respectively, was used to be in the linear range of the CAT assay. Each data is the average of 6 to 10 independent experiments. Standard deviations are indicated. *P < .01; #P < .02;‡P < .05.
Functional analysis of VE-cadherin promoter by transient transfection. 5′-deleted fragments of the mouseVE-cadherin promoter were fused to the CAT gene as indicated in the text and transfected into BAEC (A) or NIH-3T3 cells (B). The HSVTK promoter was used as positive control. “0” states for the promoterless plasmid pBLCAT3. In each assay, the luciferase expression plasmid, pGL3, was cotransfected, and CAT assays were normalized according to the luciferase activity. The equivalent of 1,200 or 3,000 arbitrary light units of BAEC or NIH-3T3 cells extracts, respectively, was used to be in the linear range of the CAT assay. Each data is the average of 6 to 10 independent experiments. Standard deviations are indicated. *P < .01; #P < .02;‡P < .05.
In NIH-3T3 cells, construct −139CAT exhibited strong promoter activity (Fig 1B). However, in contrast to BAEC, serial decreases in expression were observed following transfection with −187CAT and −289CAT. Constructs −289CAT to −1190CAT had minimal activity in these cells and constructs −2226CAT, −2486CAT, and −5800CAT had only background expression. Together, these data suggest that a basic and ubiquitous promoter is located between +24 and −139; in addition, in nonendothelial cells, at least two negative regulatory elements are present between −140 and −289 and between −1190 and −2226. Most importantly, these data also suggest that constructs −2226CAT and longer have endothelial-specific expression.
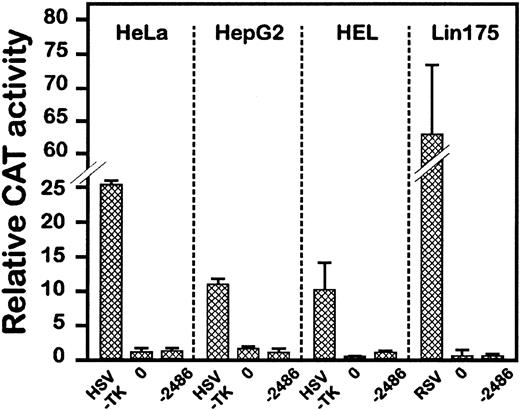
To substantiate the cell-specific expression capacity of these constructs, −2486CAT was transfected in other nonendothelial cell lines. As shown in Fig 2, −2486CAT, as opposed to viral promoters, had no promoter activity in cells of epithelial, hepatocytic, or erythro-megakaryocytic origin, which confirms the data obtained in NIH-3T3 cells. The fact that the promoter was silent in two hematopoietic cell lines is of particular interest, as endothelial and hematopoietic lineages have several common markers and a common origin, further suggesting the restricted expression potential of this promoter fragment.
Expression of the VE-cadherin −2486CAT fusion gene in various cell lines. Construct −2486CAT and pGL3 plasmid were cotransfected in cells of epithelial (HeLa), hepatocytic (HepG2), or erythro-megakaryocytic origin (HEL and Lin175). The HSVTK (see Fig 1) or the Rous sarcoma virus (RSV) promoters was used as positive control. CAT activities were determined with the equivalent of 150, 3,000, 600, or 200 light units of extracts from HeLa, HepG2, HEL, or Lin175, respectively. Data are the average of 3 independent experiments.
Expression of the VE-cadherin −2486CAT fusion gene in various cell lines. Construct −2486CAT and pGL3 plasmid were cotransfected in cells of epithelial (HeLa), hepatocytic (HepG2), or erythro-megakaryocytic origin (HEL and Lin175). The HSVTK (see Fig 1) or the Rous sarcoma virus (RSV) promoters was used as positive control. CAT activities were determined with the equivalent of 150, 3,000, 600, or 200 light units of extracts from HeLa, HepG2, HEL, or Lin175, respectively. Data are the average of 3 independent experiments.
Generation of VE-cadherin-CAT transgenic mice.
To definitely address the transcriptional specificity of theVE-cadherin gene 5′-flanking region, we developed a mouse transgenic model with −2486CAT. This construct, depleted of plasmidic sequences, was microinjected into fertilized eggs. Offspring were screened for integration of the transgene by Southern blot analysis with two probes hybridizing either with the CAT gene or the promoter region. Among 28 live-born offspring, two transgenic founders were obtained and were called mouse 23 and mouse 28. Copy number was estimated by comparing hybridization signal obtained for the transgene to that obtained for the endogenous promoter. Two transgenic lines were derived, line 23 and line 28, which contained 8 and 16 copies of the transgene, respectively. Analysis of the progeny of the founder animals showed that the transgene was transmitted at a frequency of 50%.
Transgene expression in adult tissue extracts.
To determine the expression of the transgene in adult mice, protein extracts from various tissues of offspring of transgenic founders were prepared and assayed for CAT enzymatic activity. For both lines, all solid tissues examined developed a CAT activity (Table 1), whereas no activity was detectable with tissues from normal mice despite long incubation times (data not shown). Data were higher for line 28 than for line 23, but in both cases, the activity profiles were similar, indicating the same expression pattern, which appeared to be positively correlated with the vascularization level of the different tissues. The activities were particularly high in lung and heart. Liver, brain, spleen, kidney, thymus, and skin expressed comparable and substantial amounts of CAT activity. As expected, blood cells did not express the enzyme but traces of activity could be detected in the plasma, suggesting either that some CAT molecules could enter the secretory pathway or more likely that this enzyme was liberated by lysed cells from endothelial cell turnover. In agreement with these data, 2% to 5% of CAT activity was detected in the supernatant of transfected BAEC.
Tissue CAT Activity in Two Transgenic Mice Derived From Independent Founders
| Tissue . | Mouse Line . | |
|---|---|---|
| 23* . | 28* . | |
| Heart | 85.3 (1) | 440 (1) |
| Lung | 275 (3.22) | 1298 (2.95) |
| Brain | 50.6 (0.59) | 252 (0.57) |
| Liver | 25.7 (0.30) | 162 (0.37) |
| Spleen | 21.2 (0.25) | 148 (0.34) |
| Kidney | 22.6 (0.26) | 168 (0.38) |
| Thymus | 12.9 (0.15) | 145 (0.33) |
| Skin | 19.7 (0.23) | 169 (0.38) |
| Blood cells | ND | ND |
| Plasma | 0.63 (0.007) | 0.50 (0.001) |
| Tissue . | Mouse Line . | |
|---|---|---|
| 23* . | 28* . | |
| Heart | 85.3 (1) | 440 (1) |
| Lung | 275 (3.22) | 1298 (2.95) |
| Brain | 50.6 (0.59) | 252 (0.57) |
| Liver | 25.7 (0.30) | 162 (0.37) |
| Spleen | 21.2 (0.25) | 148 (0.34) |
| Kidney | 22.6 (0.26) | 168 (0.38) |
| Thymus | 12.9 (0.15) | 145 (0.33) |
| Skin | 19.7 (0.23) | 169 (0.38) |
| Blood cells | ND | ND |
| Plasma | 0.63 (0.007) | 0.50 (0.001) |
Abbreviation: ND, not detectable.
Values are pmol product formed/μg of total protein extract in 1 hour (CAT activity relative to heart value).
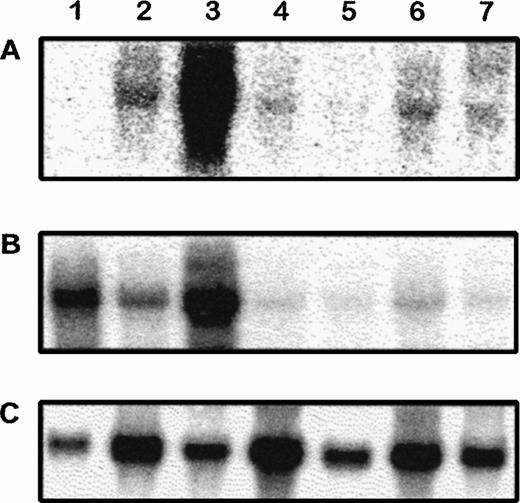
Northern blot analysis of transgenic tissue RNA further established transgene expression in adult organs and showed comparable RNA profile between CAT and VE-cadherin (Fig3).
Northern blot analysis of CAT, VE-cadherin, and GAPDH mRNA in adult transgenic tissues. RNA from wild-type lung (1) and transgenic heart (2), lung (3), brain (4), liver (5), kidney (6), and thymus (7) were hybridized to CAT (A), VE-cadherin (B), and GAPDH (C) probes. Similar expression profiles could be observed between CAT and VE-cadherin in transgenic tissues.
Northern blot analysis of CAT, VE-cadherin, and GAPDH mRNA in adult transgenic tissues. RNA from wild-type lung (1) and transgenic heart (2), lung (3), brain (4), liver (5), kidney (6), and thymus (7) were hybridized to CAT (A), VE-cadherin (B), and GAPDH (C) probes. Similar expression profiles could be observed between CAT and VE-cadherin in transgenic tissues.
In situ and immunohistologic detection of CAT gene expression in transgenic mice.
To analyze the expression of the reporter gene at the cellular level, in situ hybridization and immunohistologic stainings were performed on frozen tissue sections. Transgenic animals used for this study were obtained by mating transgenic males with wild-type females. The in situ experiments were conducted using two different probes derived from theCAT gene or a probe derived from mouse VE-cadherin cDNA (see Materials and Methods). Both CAT probes were found to be highly specific for CAT gene product and yielded identical results. No specific signal was observed when nontransgenic tissues or embryos were used (see Fig 6C). Both transgenic lines were investigated in this study and showed the same distribution pattern for CATexpression.
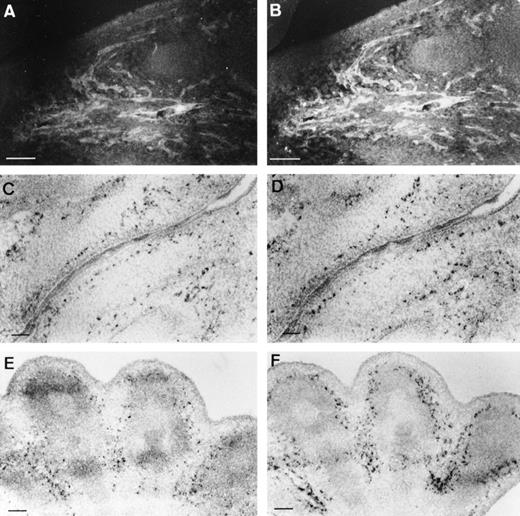
The endothelial-specific expression of CAT gene was first determined at the protein level by colabeling of tissue sections with CAT and VE-cadherin antibodies; a representative example shows identical staining pattern for both proteins (Fig 4A and B). Furthermore, in situ hybridizations performed on adjacent sections, using either theCAT or the VE-cadherin probes, indicated expression of both transcripts in the same vascular structures (Fig 4C through F) and confirmed the endothelial-specific expression of the transgene. Because of the high background level produced by the anti-CAT polyclonal antibody, subsequent analyses were performed by in situ hybridization.
Comparison of transgene and VE-cadherinexpression. Costaining of a E13.5 embryo section with anti-CAT (A) and anti–VE-cadherin (B) antibodies showed an identical expression pattern, as illustrated here for the oral area. In situ hybridizations, performed with either the CAT (C,E) or the VE-cadherin(D,F) probe on adjacent sections (10 μm), confirmed specific endothelial expression of CAT at the transcript level. (C,D) Oral area; (E,F) hindlimb; scale bars represent 100 μm.
Comparison of transgene and VE-cadherinexpression. Costaining of a E13.5 embryo section with anti-CAT (A) and anti–VE-cadherin (B) antibodies showed an identical expression pattern, as illustrated here for the oral area. In situ hybridizations, performed with either the CAT (C,E) or the VE-cadherin(D,F) probe on adjacent sections (10 μm), confirmed specific endothelial expression of CAT at the transcript level. (C,D) Oral area; (E,F) hindlimb; scale bars represent 100 μm.
Primary and progressive expression of CAT gene occurred in the vascular system of the developing embryo, perfectly matching the expression of the endogenous VE-cadherin gene.14 At E7.5,CAT expression was only observed in primitive blood islands consisting of hemangioblastic precursors and in allantois, at the sites of early vasculogenesis (Fig5)1 24; no signal could be detected in the embryo proper or in the wild-type maternal decidua. As the embryo developed further,CAT signal became more intense and colocalized with capillaries and larger vessels of embryonic and extraembryonic tissues.
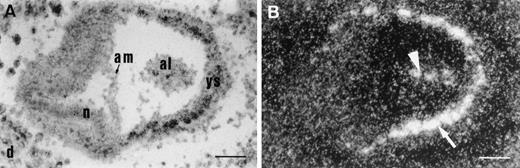
Transgene expression at E7.5. In situ hybridization of a transversal section with CAT probe, shown as bright-field (A) or dark-field image (B). The transgenic embryo is located in the wild-type maternal decidua. CAT transcripts were detected in the mesodermal aggregates of the yolk sac from which blood islands are derived (arrow), and in the inner part of allantois (arrowhead). Scale bar represents 100 μm; al, allantois; am, amnion; d, decidua; n, neuroectoderm; ys, yolk sac.
Transgene expression at E7.5. In situ hybridization of a transversal section with CAT probe, shown as bright-field (A) or dark-field image (B). The transgenic embryo is located in the wild-type maternal decidua. CAT transcripts were detected in the mesodermal aggregates of the yolk sac from which blood islands are derived (arrow), and in the inner part of allantois (arrowhead). Scale bar represents 100 μm; al, allantois; am, amnion; d, decidua; n, neuroectoderm; ys, yolk sac.
At E13.5, the transgene was strongly expressed in the vasculature of most organs, including lung, heart, gut, kidney, and limb buds (Fig 6). In comparison, expression in the liver was weaker, but still readily detectable. The head oral and neck areas also expressed high levels of CAT transcripts. The perineural vascular plexus and the choroid plexus were highly positive with CAT probes. Conversely, no signal could be detected within capillaries of the brain and spinal chord. This expression profile suggests a discrimination between the blood-brain barrier, which is already established at this stage, and the “permeable” endothelium of the brain.25 26 Higher magnification clearly shows silver grains over cells lining the luminal side of the aorta (Fig 7A) and the trabeculae of the endocardium (Fig 7B); capillaries within the myocardium also showed intense labeling. In the intestine, CAT transcripts were localized in the capillaries at the periphery of the epithelial barrier (Fig 7C). Lung (Fig 7D) contained a dense labeling in the microcirculation surrounding the bronchi. As shown Fig 7E, the transgene was expressed in the capillary network of the liver and the hepatic venous plexus. VE-cadherin RNA stainings, performed in parallel, showed expression pattern comparable to that of CAT RNA (Fig 7A′ through E′).
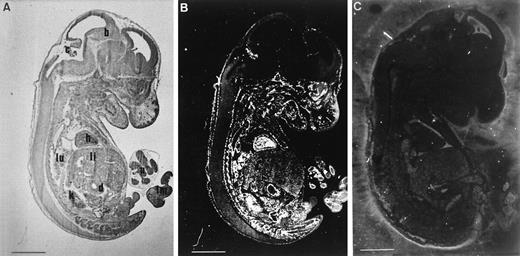
Transgene expression at E13.5. Parasagittal sections of E13.5 transgenic (A, bright field and B, dark field) or wild-type (C, dark field) animals, hybridized with CAT antisense probe. With the exception of the brain, specific signals were detected in the vasculature of all the embryo. Probe did not show any reactivity in nontransgenic mice. Scale bar represents 15 μm; b, brain; c, choroid plexus; d, duodenum; h, heart; k, kidney; li, liver; lm, limb; lu, lung; u, umbilic.
Transgene expression at E13.5. Parasagittal sections of E13.5 transgenic (A, bright field and B, dark field) or wild-type (C, dark field) animals, hybridized with CAT antisense probe. With the exception of the brain, specific signals were detected in the vasculature of all the embryo. Probe did not show any reactivity in nontransgenic mice. Scale bar represents 15 μm; b, brain; c, choroid plexus; d, duodenum; h, heart; k, kidney; li, liver; lm, limb; lu, lung; u, umbilic.
Endothelial expression of the CAT gene in various tissues of E13.5 embryos. Embryo sections were hybridized withCAT (A-E) or VE-cadherin (A′-E′) antisense transcripts. Comparison of gene expression in aorta (A-A′), heart (B-B′), intestine (C-C′), lung (D-D′), and liver (E-E′). Scale bars represent 50 μm (A-A′) or 100 μm (B-E, B′-E′); b, bronchi; c, capillary; e, endocardium; my, myocardium; ve, ventricle; vp, venous plexus.
Endothelial expression of the CAT gene in various tissues of E13.5 embryos. Embryo sections were hybridized withCAT (A-E) or VE-cadherin (A′-E′) antisense transcripts. Comparison of gene expression in aorta (A-A′), heart (B-B′), intestine (C-C′), lung (D-D′), and liver (E-E′). Scale bars represent 50 μm (A-A′) or 100 μm (B-E, B′-E′); b, bronchi; c, capillary; e, endocardium; my, myocardium; ve, ventricle; vp, venous plexus.
In 2-month-old mice, in situ analysis of sections from various tissues confirmed previous data obtained with E13.5 embryos (Fig 8 and not shown). In particular, functionality of organs like lung or liver, which really takes place after birth, did not affect CAT expression pattern (Fig 8A and B). As previously discussed for E13.5 embryos, expression was weaker in intrahepatic vessels in comparison to other visceral derivatives; however, this expression was similar among the different vessel types, namely, sinusoidal endothelium, centrilobular veins (Fig 8A), and portal vein (data not shown).
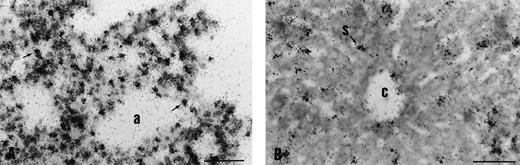
Endothelial expression of the transgene in adult organs. In the lung (A), silver grains are located over endothelial cells of capillaries (arrows) interspersed between pneumocytes. CATexpression in the sinusoids and centrilobular vein of the liver (B). Scale bar is 45 μm; a, alveolae; c, centrilobular vein; s, sinusoids.
Endothelial expression of the transgene in adult organs. In the lung (A), silver grains are located over endothelial cells of capillaries (arrows) interspersed between pneumocytes. CATexpression in the sinusoids and centrilobular vein of the liver (B). Scale bar is 45 μm; a, alveolae; c, centrilobular vein; s, sinusoids.
Taken together, these data show that the expression of the transgene is (1) endothelial-specific, (2) universal along the vascular tree, with the exception of the brain capillaries, and (3) constitutive during development from the early steps of vasculogenesis.
DISCUSSION
This report demonstrates that approximately 2,500 bp of the mouseVE-cadherin gene promoter region is sufficient to direct appropriate transcription in vivo in the endothelium of adult mice and during vascular development. Overall, the transgene expression pattern is similar to that of endogenous VE-cadherin.
In situ hybridization indicates that all types of vessels expressed the transgene, except brain capillaries, where highly specialized junctions are established at mid-gestation.25 EndogenousVE-cadherin expression was also shown to be downregulated in the blood-brain barrier, but to a lesser extent as transcripts were still detectable in brains of E17.5 embryos.14 This may represent a higher sensitivity of the promoter fragment to this organotypic regulation than the endogenous promoter. Interestingly, in vivo studies performed with the von Willebrand factor promoter identified a small DNA sequence (−487/+246) restricting reporter gene expression to endothelial cells of adult brain,27 whereas a larger fragment (containing 2,182 bp of 5′-flanking sequence, the first exon and the first intron) targeted expression within endothelial cells in the brain, heart, and skeletal muscle.28 These limited patterns of expression strongly suggest the existence of regional differences in endothelial regulatory factors, as well as subtype-specific cis-acting DNA domains. In this respect, the (−2486/+24) VE-cadherin and the (−487/+246) von Willebrand factor fragments are functionally complementary to target endothelial expression in adult mice. In the liver, differentiation of sinusoids from capillaries, which occurs between E17 and E19 in the mouse embryo,29 did not affect CATexpression. This result is in agreement with a previous study reported by Scoazec et al,30 who demonstrated that VE-cadherin is a homogenous marker of continuous and sinusoidal endothelia in the liver.
CAT transcripts were detected as early as E7.5 in blood islands and allantois of mice embryos, thus marking the early stages of vasculogenesis. Thereafter, the CAT gene was expressed in the vasculature of visceral, as well as parietal derivatives (ie, head, limbs, and kidney), which are vascularized by vasculogenic (the assembly of vessels from newly differentiated endothelial cells) and angiogenic (the sprouting of capillaries from preexisting vessels) mechanisms, respectively.1,31 Previous studies32 suggest the existence of different regulatory domains for directing expression during angiogenesis and vasculogenesis. If this hypothesis is correct, our data suggest thatVE-cadherin promoter contains both elements.
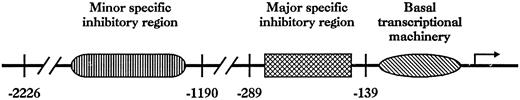
The 5′-deletion analysis, performed in BAEC, showed three important domains involved in the transcriptional regulation of theVE-cadherin gene: a proximal domain, located between positions −139 and +24, promoting transcription in a cell line-independent fashion and two negative domains between −289 and −140 bp and between −2226 and −1191 bp that abolish transcriptional rate in NIH-3T3 (Fig 9). Functional analysis of DNA sequences of the (−139/+24) region, as well as DNA-protein interaction studies, indicated several binding sites for transcription factors, such as Sp1, Sp3, and Ets factors.33 A fine functional dissection of the two other regions is underway to determine the transcription factors and the DNA elements that controlVE-cadherin expression and, more generally, to gain further insights in endothelial transcriptional mechanisms. Such a regulation with positively acting proximal domains and cell-type specific silencing domains is a unique feature among studied endothelial promoters, but is reminiscent of some promoters conferring tissue-specific expression in other cell-types, such as αIIbintegrin,34the immunoglobulin kappa gene,35 or the δ1-crystallin gene.36 However, it is conceivable that these silencing domains bind multiple positive and negative factors acting in a interdependent fashion.
Location of the major functional domains in the 5′-flanking region of the VE-cadherin gene.
Location of the major functional domains in the 5′-flanking region of the VE-cadherin gene.
A recent report indicates that a combination of the tie-2promoter with an intron fragment allows the expression of theLacZ reporter gene in all endothelial cells of transgenic mice, throughout embryogenesis and adulthood.37LacZstaining of adult vessels is surprising, as tie-2 expression is downregulated in quiescent endothelium.5 As evoked by the authors, this may be due to the loss of negative regulatory elements or it may represent β-galactosidase accumulation rather than active transcription. In this report, histologic investigations were preferentially performed by in situ hybridization to visualize transcripts rather than protein accumulation. Moreover, as CATmRNA is notoriously unstable in eukaryotic cells, the hybridization signal better reflects the transcriptional activity of the promoter.
VE-cadherin promoter may be widely used to target gene expression in the endothelium of transgenic mice in order to study various pathophysiologic conditions. Animal models where genes able to change the antithrombotic or hemostatic properties of endothelial cells, to decrease the neointima formation, or to limit angiogenic growth can be developed using the VE-cadherin promoter alone or in concert with cis-responsive elements that could modulate transcriptional activity.
Another potential application of the VE-cadherin promoter is gene therapy. An important problem with gene therapy is the specificity of gene transfer, and the possible deleterious side effects that might result from the expression of a therapeutic gene candidate in nonrelevant cells. The possibility of modulating endothelial cell responses by directing the expression of a desired gene within these cells via the VE-cadherin promoter is an attractive therapeutic goal.
ACKNOWLEDGMENT
We thank D. Vittet for helpful discussion. We are grateful to F. Aubouy for artwork, to T. Kortulewski and F. Breviario for excellent technical assistance, and to Y. Senis for careful reading of the manuscript.
Supported by the Commissariat à l’Energie Atomique, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to P. Huber, PhD, CEA-Grenoble, DBMS-TDC, 17, rue des Martyrs, 38054 Grenoble, France.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal