Abstract
We investigated whether antithrombin (AT) can reduce ischemia/reperfusion (I/R)-induced injury of rat liver by promoting prostacyclin release from endothelial cells. Although intravenous administration of AT (250 U/kg) markedly reduced hepatic injury, neither dansyl-Glu-Gly-Arg-chloromethyl ketone-treated factor Xa (DEGR-Xa), a selective inhibitor of thrombin generation, nor Trp49-modified AT, which lacks affinity for heparin, had any effect. Hepatic levels of 6-keto-PGF1, a stable prostacyclin (PGI2) metabolite, were increased significantly after I/R of the rat liver. AT significantly increased the hepatic level of 6-keto-PGF1, whereas neither DEGR-Xa nor Trp49-modified AT increased it. Hepatic tissue blood flow was markedly reduced after I/R. Although AT significantly increased the hepatic tissue blood flow after I/R, neither DEGR-Xa nor Trp49-modified AT increased the blood flow. Hepatic levels of cytokine-induced neutrophil chemoattractant (CINC) and myeloperoxidase (MPO) were significantly increased after hepatic I/R. The levels of these two indicators were reduced by AT but were unaffected by either DEGR-Xa or Trp49-modified AT. Pretreatment of animals with indomethacin (IM) completely inhibited the protective effects of AT on the I/R-induced hepatic damage and the leukocyte activation as well as the AT-induced increase in hepatic 6-keto-PGF1 levels after I/R. Iloprost, a stable analog of PGI2, exhibited effects similar to those of AT and also significantly inhibited the exacerbation of liver injury, the decrease in hepatic tissue blood flow, and the increases in hepatic CINC and MPO levels seen in rats subjected to I/R but pretreated with IM. These findings suggest that AT may prevent I/R-induced hepatic injury by increasing the hepatic levels of PGI2 through the interaction of AT with cell-surface glycosaminoglycans, thus increasing hepatic tissue blood flow and inhibiting leukocyte activation in animals subjected to I/R.
ANTITHROMBIN (AT) inhibits the coagulation factors generated in the coagulation cascade. The inhibition of proteases by AT is markedly accelerated by its interaction with glycosaminoglycans on the endothelial cell surface.1
Prostacyclin (PGI2) is a well-known cytoprotective agent that is synthesized in endothelial cells.2 AT promotes the endothelial release of PGI2 in vitro and in vivo by interacting with cell surface glycosaminoglycans.3-5 One of the PGI2 activities is vasodilation.6PGI2 has also been shown to inhibit leukocyte activation by inhibiting tumor necrosis factor-α (TNF-α) production by monocytes,5 neutrophil activation,7 and neutrophil adhesion to endothelial cells.8 Because activated leukocytes release a variety of inflammatory mediators, including cytokines, neutrophil proteases, and reactive oxygen species, all of which can damage adjacent endothelial cells, they have been thought to play a role in tissue injury.9-13
The administration of AT has been found to reduce the mortality rate of animals challenged with lipopolysaccharide (LPS) or Escherichia coli.14 AT significantly increased the survival rate of rabbits exposed to LPS but did not improve coagulopathy.15Although heparin inhibited coagulopathy in baboons with endotoxin shock, it failed to reduce the mortality rate.16 These observations suggest that the beneficial effects of AT in sepsis may be due to both its anticoagulant activity and another unknown activity. Thus, we hypothesize that AT prevents tissue injury by inhibiting leukocyte activation through the promotion of endothelial release of PGI2. Consistent with this hypothesis, we have previously demonstrated that AT prevents endotoxin-induced pulmonary vascular injury by promoting PGI2 release from endothelial cells independent of its anticoagulant activity.17
Ischemia/reperfusion (I/R) is an important mechanism of tissue injury in which activated leukocytes are critically involved.18I/R-induced hepatic injury is an important pathologic process leading to hepatic damage after circulatory shock or major hepatic surgery.19-21 Because leukocytes are implicated in the pathology of I/R-induced hepatic injury,22-24 we hypothesize that AT prevents I/R-induced hepatic injury by promoting the endothelial release of PGI2.
In the present study, we examined whether AT reduces I/R-induced hepatic injury in rats by promoting the endothelial release of PGI2, thus maintaining the hepatic tissue blood flow and inhibiting leukocyte activation. To examine this hypothesis, we analyzed the effects of AT, an inactive derivative of factor Xa that inhibits thrombin generation selectively in vivo, and a chemically modified AT that lacks affinity for heparin on I/R-induced hepatic injury in rats.
MATERIALS AND METHODS
Reagents.
AT was kindly provided by Green Cross (Osaka, Japan). AT was purified from heat-treated, pooled human plasma by adsorption on fixed heparin according to a modified version of the technique of Miller-Anderson et al.25 The AT concentrate used in the experiments showed a single band in response to polyacrylamide gel electrophoresis with sodium dodecyl sulfate. Further characterization of the AT concentrate demonstrated that its heparin concentration was less than 0.01 U/mL and that it was free of pathogen. Iloprost was kindly provided by Eizai Pharmaceutical Co (Tokyo, Japan). Dimethyl(2-hydroxy-5-nitrobenzyl) sulfonium bromide (DHNBSB) and indomethacin (IM) were purchased from Sigma Chemical Co (St Louis, MO). All other reagents were of analytical grade.
Animal model of hepatic I/R.
Adult, pathogen-free, male Wistar rats (Nihon SLC, Hamamatsu, Japan), weighing 220 to 280 g, were used in each experiment. The care and handling of the animals were in accordance with the National Institute of Health guidelines. All experimental procedures described below were approved by the Kumamoto University Animal Care and Use Committee. All rats were deprived of food, but not water, for 24 hours before each experiment. The hepatic I/R protocol was performed as described previously.26,27 After induction of anesthesia in the animals with ketamine hydrochloride (100 mg/kg, intraperitoneally; Parke-Davis, Morris Plains, NJ), the liver of each was exposed through a midline laparotomy. Silk ligatures were placed around the right and left branches of the portal vein and the hepatic artery. Complete ischemia of the median and left hepatic lobes was produced by clamping the left branches of the portal vein and the hepatic artery for 60 minutes. The right hepatic lobe was perfused to prevent intestinal congestion. During the period of hepatic ischemia, the animal’s abdomen was covered with plastic wrap to prevent dehydration. After the period of ischemia, the ligatures around the left branches of the portal vein and hepatic artery were removed. To accurately evaluate blood flow of the median and left hepatic lobes after ischemia, the right branches of the portal vein and the hepatic artery were ligated to prevent shunting to the right lobe after reperfusion.26The wound was closed with 3-0 silk. This procedure directed all portal and hepatic blood flow, except for a small amount of flow to the caudal hepatic lobe, through the lobes of the liver previously made ischemic. Sham-operated animals were similarly prepared, except that no ligature was placed to obstruct the blood flow to the left and median hepatic lobes. Instead, the blood flow to the right lobe of the liver was occluded.
Measurement of serum liver enzymes.
Blood samples were taken 12 hours after reperfusion to measure the level of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST), as previously described.28 These blood samples were collected into test tubes from the anesthetized animals via withdrawal from the abdominal aorta with a 22-gauge needle. ALT and AST levels were measured by standard clinical automated analysis and the results are expressed in international units (IU) per liter.
Measurement of plasma fibrinogen concentration and serum fibrin and fibrinogen degradation product (E) [FDP (E)] levels.
Histological determination of fibrin deposits in the liver.
After 12 hours of reperfusion, liver specimens were fixed in 10% buffered formalin and then embedded in paraffin. Sections (4 μm) were prepared and stained with phosphotungstic acid-hematoxylin, as previously described.31
Measurement of hepatic tissue blood flow.
Hepatic tissue blood flow was measured by laser-Doppler flowmeter (ALF21N; Advance, Tokyo, Japan) for 3 hours after reperfusion, as described previously.32 After anesthesia with ketamine hydrochloride (100 mg/kg, intraperitoneally), the right jugular veins of these animals were cannulated with a PE-10 catheter for continuous infusion of normal saline or test drugs. The Doppler flowmeter probe was placed on the medial hepatic lobe. Hepatic tissue blood flow was measured from 30 minutes before ischemia until 3 hours after reperfusion. The results are expressed as the percentage of preischemia levels.
Determination of hepatic 6-keto-PGF1α levels.
Hepatic 6-keto-PGF1α levels were determined in animals subjected to I/R before and after 1, 2, 3, and 6 hours of reperfusion according to the methods described previously.33 In brief, the medial hepatic lobe in which the tissue blood flow had been measured was weighed and then immediately homogenized in 5 mL of 0.1 mol/L phosphate buffer (pH 7.4) containing 2 mmol/L IM at 5°C. The homogenate was first centrifuged at 2,000g for 10 minutes to remove minute amounts of solid tissue debris and the supernatant was then acidified with 1 mol/L HCl. 6-keto-PGF1α was extracted from the supernatant using columns packed with ethyl-bonded silica gel (ethyl C2; Amersham, Buckinghamshire, UK). The columns were prepared by washing them with 2 mL of methanol, followed by 2 mL of water. The acidified supernatant was applied to the column, and this was followed by sequential washes with 5 mL of water, 5 mL of 10% ethanol, and 5 mL of hexane. The elution of 6-keto-PGF1αwas performed with 5 mL of methyl formate, after which the solvent was evaporated under a stream of nitrogen gas. The concentration of 6-keto-PGF1α was assayed using a specific enzyme immunoassay kit (Amersham). This enzyme immunoassay was sensitive enough to detect 10 to 1,280 pg/mL of 6-keto-PGF1α. The results are expressed as ng of 6-keto-PGF1α per gram of tissue. The cross-reactivities of this assay with PGE2, PGF2α, thromboxane B2, and arachidonic acid were 2.8%, 1.4%, 0.03%, and 0.01%, respectively, according to the manufacturer’s data sheet. The maximum level of PGE2 in the liver of this animal model was about 4 ng per gram of tissue. The tissue level of PGE2 was assayed using a specific enzyme immunoassay kit (Amersham), as described previously.34
Determination of hepatic levels of cytokine-induced neutrophil chemoattractant (CINC).
Hepatic levels of CINC were determined by a modification of the method of Clark et al.35 In brief, the medial hepatic lobe in which the tissue blood flow had been measured was weighed and then homogenized in 5 mL of 0.1 mol/L phosphate buffer (pH 7.4) containing 0.05% (vol/wt) of sodium azide at 5°C. The homogenate was first centrifuged at 2,000g for 10 minutes to remove minute amounts of solid tissue debris. The supernatant was assayed using a Rat Interleukin-8 (CINC/gro) enzyme-linked immunosorbent assay (ELISA) system (Amersham). This ELISA was sensitive enough to detect 4.7 to 300 pg/mL of CINC/gro. The results are expressed as picograms of CINC per gram of tissue.
Determination of hepatic myeloperoxidase (MPO) activity.
After the indicated period of reperfusion, the livers were quickly removed, and accumulation of leukocytes was assessed by measuring MPO activity in the liver according to a previously described method.36 In brief, the livers were weighed and suspended in 6 mL of 50 mmol/L phosphate buffer (pH 6.0) containing 1% hexadecyltrimethylammonium bromide. The samples were homogenized and the homogenate was sonicated, freeze-thawed, and then centrifuged (4,500g for 15 minutes at 4°C). MPO activity in the supernatant (0.1 mL) was determined after the addition of 0.6 mL of phosphate buffer (pH 6.0) containing 0.167 mg/mL o-dianisidine dihydrochloride and 0.0005% hydrogen peroxide. The change in absorbance at 460 nm over 10 minutes was measured in a spectrophotometer (DU-54; Beckman, Irvine, CA). One unit of MPO activity was defined as the amount of enzyme able to reduce 1 μmol of peroxide per minute. Results are expressed as units of MPO activity per gram of tissue.
Five separate groups of animals (n = 40 per each group) were used to assess liver enzyme levels, tissue blood flow, hepatic 6-keto-PGF1α levels, hepatic CINC levels, and hepatic MPO activities. This procedure avoided problems associated with excessive blood loss due to blood sampling or associated with fluid loss due to blood flow measurement.
Preparation of Trp49-modified AT.
The Trp49 residue of AT was chemically modified by a version of the method of Karp et al.37 In brief, DHNBSB was mixed with a continuously stirred solution containing 40 μmol/L AT, 0.1 mol/L Tris-HCl (pH 8.0), and 0.15 mol/L NaCl. The final concentration of DHNBSB was calculated as 8 mmol/L. After stirring for 15 minutes at 22°C, the insoluble hydroxynitrobenzyl alcohol, which formed as a hydrolysis product, was removed by centrifugation. The resulting solution was next subjected to chromatography on a column (2.6 × 60 cm) of Sephacryl S-200HR that had been equilibrated with 0.1 mol/L Tris-HCl (pH 8.0) and 0.15 mol/L NaCl and then subjected to chromatography on a column (3 × 6 cm) of heparin-Sepharose CL6B, as previously described.38 The extent of derivatization of AT was determined spectrophotometrically in 2 mol/L NaOH at a wave length of 410 nm (molar extinction coefficient, 1.85 × 104 mol/L−1).39 The progressive antithrombin activity of the Trp49-modified AT in the absence of heparin was virtually identical to that of intact AT (data not shown). The study of thrombin inhibition by Trp49-modified AT was performed as previously described.39
Preparation of active site-blocked factor Xa.
Factor X was purified from human plasma40 and then activated with Russell’s viper venom, as described previously.41 The activated factor Xa was then inactivated by incubating it with a 20-fold molar excess of dancyl glutamylglycylarginyl chloromethyl ketone (DEGR) for 30 minutes at 25°C, after which the mixture was subjected to extensive dialysis in a solution containing 20 mmol/L TrisHCl (pH 7.4) and 100 mmol/L NaCl. Such DEGR-treated factor Xa (DEGR-Xa) selectively inhibits thrombin generation by competing with intact factor Xa in the formation of the prothrombinase complex.42 The remaining factor Xa activity, assayed by using the chromogenic substrate S-2222,43 was less than 0.01% of the untreated factor Xa. The DEGR-Xa prepared in this manner prolonged the activated partial thromboplastin time (APTT) in a concentration-dependent manner (0 to 300 μg/mL; data not shown). APTT was measured using actin FS reagent (Baxter, Deerfield, IL) according to the manufacturer’s instructions.
Administration of AT, Trp49-modified AT, and DEGR-Xa.
We have previously reported that the plasma concentration of 6-keto-PGF1α, a stable metabolite of PGI2, begins to increase 30 minutes after the intravenous (IV) administration of AT (250 U/kg) in intact rats.44 Thus, AT was administered 30 minutes before reperfusion in this study. Trp49-modified AT (250 U/kg) and DEGR-Xa (3 mg/kg) were also injected IV 30 minutes before reperfusion. At the dosage used in the present study, Trp49-modified AT and DEGR-Xa have antithrombin activities comparable to that of 250 U/kg of native AT.17
IM and iloprost administration.
IM (20 mg/kg) was suspended in bicarbonate-buffered saline and administered subcutaneously for 30 minutes before ischemia. Control animals received the same volume of bicarbonate-buffered saline instead of IM.
Iloprost was dissolved in normal saline and continuously infused (100 ng/kg/min) into the animals via the right jugular vein. For those groups of animals in which the tissue blood flow was measured, this infusion began before the onset of hepatic ischemia and continued until the rats were killed 3 hours after onset of reperfusion. For those groups of animals in which the liver enzyme levels were measured, iloprost was infused continuously for 6 hours after the onset of reperfusion to prevent any respiratory problems associated with prolonged anesthesia. Control animals received continuous infusion of the same volume of normal saline instead of iloprost.
Statistical analysis.
Data are expressed as the mean ± SD. The results were compared using either an analysis of variance followed by Scheffé’s post hoc test or an unpaired t-test. A level of P < .05 was considered statistically significant.
RESULTS
Effects of AT, DEGR-Xa, and Trp49-modified AT on I/R-induced hepatic injury.
Serum levels of ALT and AST were significantly increased after 1 hour of reperfusion compared with levels in sham-operated animals, peaking at 12 hours after reperfusion.28 Plasma levels of fibrinogen and serum levels of FDP (E) were not significantly increased 1, 3, 6, and 12 hours after reperfusion compared with those of sham-operated animals (data not shown). Fibrin deposition was not observed histologically in the livers of rats subjected to hepatic I/R 12 hours after reperfusion (data not shown).
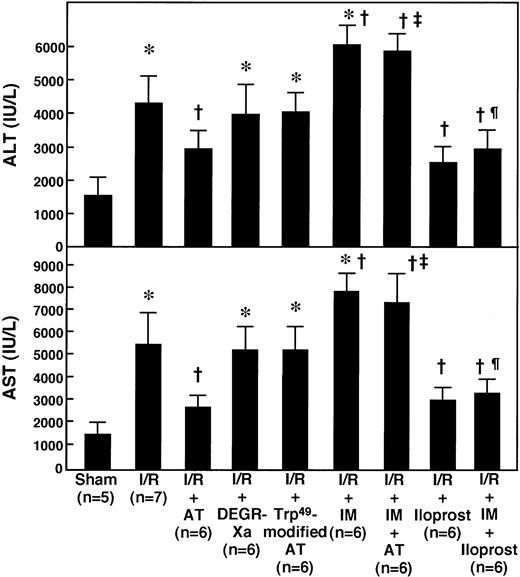
Although the IV administration of 250 U/kg of AT significantly inhibited the increase in serum aminotransferase levels 12 hours after reperfusion (Fig 1), the lower doses of AT (50 and 100 U/kg) did not (data not shown). Neither DEGR-Xa (3 mg/kg, IV), a selective inhibitor of thrombin generation, nor Trp49-modified AT (250 U/kg, IV), which lacks affinity for heparin, inhibited the increase in serum aminotransferase levels observed at 12 hours after reperfusion (Fig 1).
Effects of AT and various agents on serum levels of aminotransferases in rats after hepatic I/R. Animals were subjected to 60 minutes of hepatic ischemia followed by reperfusion as described in Materials and Methods. Animals were injected IV with AT (250 U/kg) 30 minutes before reperfusion or subcutaneously with IM (20 mg/kg) 30 minutes before ischemia. DEGR-Xa (3 mg/kg) or Trp49-modified AT (250 U/kg) was injected intravenously 30 minutes before reperfusion. Iloprost was infused continuously starting just before the onset of ischemia at a rate of 100 ng/kg/min with or without subcutaneous injection of IM (20 mg/kg). Serum levels of ALT and AST were determined after 12 hours of reperfusion. Each bar represents the mean ± SD. *P < .01 versus sham. †P < .01 versus I/R. ‡P < .01 versus AT-treated I/R. ¶P < .01 versus IM-treated I/R.
Effects of AT and various agents on serum levels of aminotransferases in rats after hepatic I/R. Animals were subjected to 60 minutes of hepatic ischemia followed by reperfusion as described in Materials and Methods. Animals were injected IV with AT (250 U/kg) 30 minutes before reperfusion or subcutaneously with IM (20 mg/kg) 30 minutes before ischemia. DEGR-Xa (3 mg/kg) or Trp49-modified AT (250 U/kg) was injected intravenously 30 minutes before reperfusion. Iloprost was infused continuously starting just before the onset of ischemia at a rate of 100 ng/kg/min with or without subcutaneous injection of IM (20 mg/kg). Serum levels of ALT and AST were determined after 12 hours of reperfusion. Each bar represents the mean ± SD. *P < .01 versus sham. †P < .01 versus I/R. ‡P < .01 versus AT-treated I/R. ¶P < .01 versus IM-treated I/R.
Effects of AT, DEGR-Xa, and Trp49-modified AT on hepatic levels of 6-keto-PGF1α.
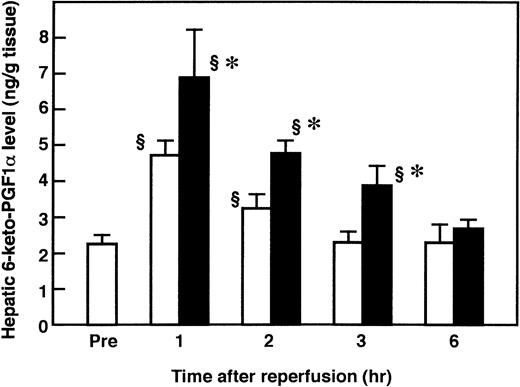
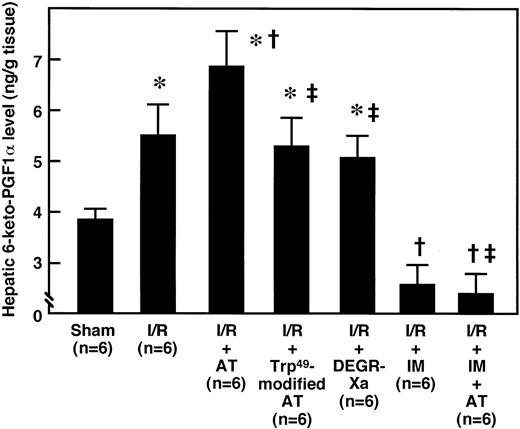
To determine whether AT promoted the endothelial release of PGI2 in the liver after I/R by interacting with glycosaminoglycans on the endothelial cell surface, we examined the effects of AT and Trp49-modified AT on hepatic levels of 6-keto-PGF1α after I/R. The hepatic levels of 6-keto-PGF1α were significantly increased after reperfusion, compared with those of the sham-operated animals, and peaked 1 hour after reperfusion (Fig 2). AT (250 U/kg) significantly enhanced the I/R-induced increase in hepatic 6-keto-PGF1α levels 1 hour after reperfusion (Fig 3), whereas neither Trp49-modified AT (250 U/kg) nor DEGR-Xa (3 mg/kg) had an effect (Fig 3).
Changes in hepatic 6-keto-PGF1 levels in rats subjected to hepatic I/R. Animals were subjected to 60 minutes of hepatic ischemia followed by reperfusion as described in Materials and Methods. Sham-operated animals were prepared in a similar manner, except that blood flow to the left and median hepatic lobes was not obstructed. Mean values of hepatic 6-keto-PGF1 levels after various periods of hepatic reperfusion are shown. Data represent the mean ± SD derived from six animal experiments. (□) Sham-operated animals; (▪) I/R animals. §P < .01 versus preischemia. *P < .01 versus sham.
Changes in hepatic 6-keto-PGF1 levels in rats subjected to hepatic I/R. Animals were subjected to 60 minutes of hepatic ischemia followed by reperfusion as described in Materials and Methods. Sham-operated animals were prepared in a similar manner, except that blood flow to the left and median hepatic lobes was not obstructed. Mean values of hepatic 6-keto-PGF1 levels after various periods of hepatic reperfusion are shown. Data represent the mean ± SD derived from six animal experiments. (□) Sham-operated animals; (▪) I/R animals. §P < .01 versus preischemia. *P < .01 versus sham.
Effects of AT, Trp49-modified AT, DEGR-Xa, and AT plus IM pretreatment on changes in hepatic 6-keto-PGF1 levels in rats subjected to hepatic I/R. Animals were subjected to 60 minutes of hepatic ischemia followed by reperfusion as described in Materials and Methods. Animals were either injected IV with AT (250 U/kg), Trp49-modified AT (250 U/kg), or DEGR-Xa (3 mg/kg) 30 minutes before reperfusion or injected subcutaneously with IM (20 mg/kg) 30 minutes before ischemia. After 1 hour of reperfusion, hepatic 6-keto-PGF1 levels were measured by enzyme immunoassay (EIA). Each bar represents the mean ± SD. *P < .01 versus sham. †P < .01 versus I/R. ‡P < .01 versus AT-treated I/R.
Effects of AT, Trp49-modified AT, DEGR-Xa, and AT plus IM pretreatment on changes in hepatic 6-keto-PGF1 levels in rats subjected to hepatic I/R. Animals were subjected to 60 minutes of hepatic ischemia followed by reperfusion as described in Materials and Methods. Animals were either injected IV with AT (250 U/kg), Trp49-modified AT (250 U/kg), or DEGR-Xa (3 mg/kg) 30 minutes before reperfusion or injected subcutaneously with IM (20 mg/kg) 30 minutes before ischemia. After 1 hour of reperfusion, hepatic 6-keto-PGF1 levels were measured by enzyme immunoassay (EIA). Each bar represents the mean ± SD. *P < .01 versus sham. †P < .01 versus I/R. ‡P < .01 versus AT-treated I/R.
Effects of AT, DEGR-Xa, and Trp49-modified AT on the changes in hepatic tissue blood flow in rats subjected to hepatic I/R.
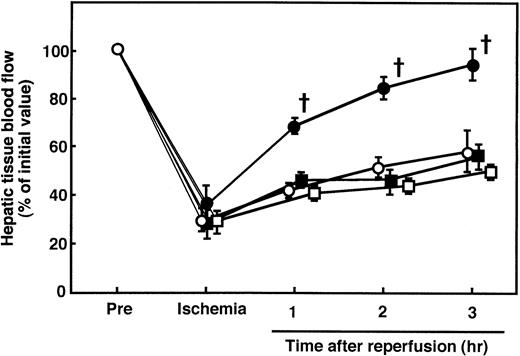
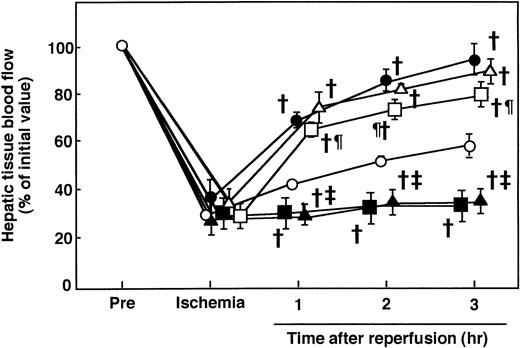
During hepatic ischemia, the hepatic tissue blood flow decreased to approximately 30% of the preischemia level and then increased to 50% of the preischemia level 3 hours after reperfusion (Fig 4). Although AT (250 U/kg) significantly increased the hepatic tissue blood flow after 1 to 3 hours of reperfusion, neither DEGR-Xa nor the Trp49-modified AT increased the blood flow (Fig 4).
Effects of AT, DEGR-Xa, and Trp49-modified AT on the changes in hepatic tissue blood flow in rats subjected to hepatic I/R. Animals were subjected to 60 minutes of hepatic ischemia followed by reperfusion as described in Materials and Methods. Animals were injected IV with AT (250 U/kg), DEGR-Xa (3 mg/kg), or Trp49-modified AT (250 U/g) at 30 minutes before reperfusion. Hepatic tissue blood flow was continuously measured by a laser Doppler flowmeter starting 30 minutes before ischemia until 3 hours after reperfusion. Each value represents the mean ± SD derived from 5 animals. (○) I/R animals; (•) AT-treated I/R animals; (▪) DEGR-Xa-treated I/R animals; (□) Trp49-modified AT-treated animals. †P < .01 versus I/R. ‡P< .01 versus AT-treated I/R.
Effects of AT, DEGR-Xa, and Trp49-modified AT on the changes in hepatic tissue blood flow in rats subjected to hepatic I/R. Animals were subjected to 60 minutes of hepatic ischemia followed by reperfusion as described in Materials and Methods. Animals were injected IV with AT (250 U/kg), DEGR-Xa (3 mg/kg), or Trp49-modified AT (250 U/g) at 30 minutes before reperfusion. Hepatic tissue blood flow was continuously measured by a laser Doppler flowmeter starting 30 minutes before ischemia until 3 hours after reperfusion. Each value represents the mean ± SD derived from 5 animals. (○) I/R animals; (•) AT-treated I/R animals; (▪) DEGR-Xa-treated I/R animals; (□) Trp49-modified AT-treated animals. †P < .01 versus I/R. ‡P< .01 versus AT-treated I/R.
Effects of AT, DEGR-Xa, and Trp49-modified AT on I/R-induced changes in hepatic levels of CINC and MPO in rats.
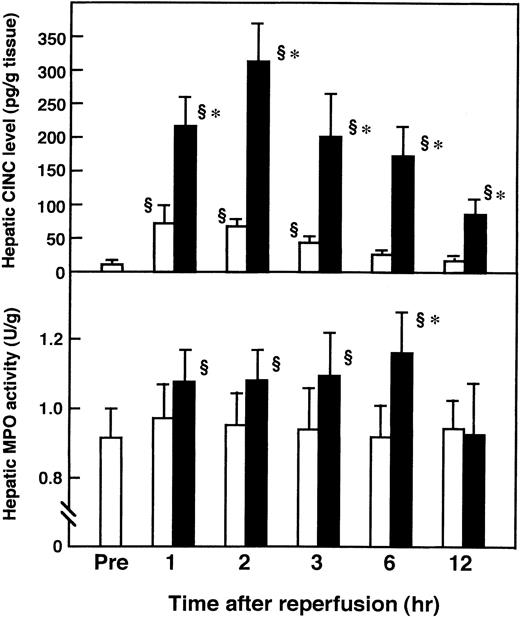
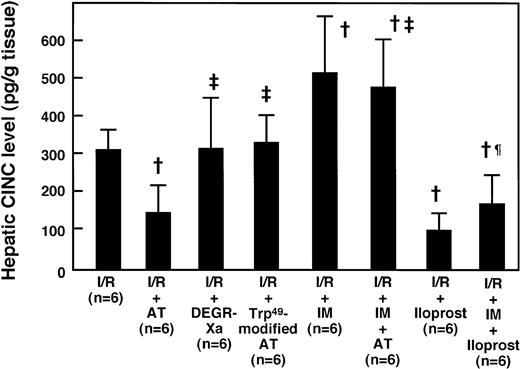
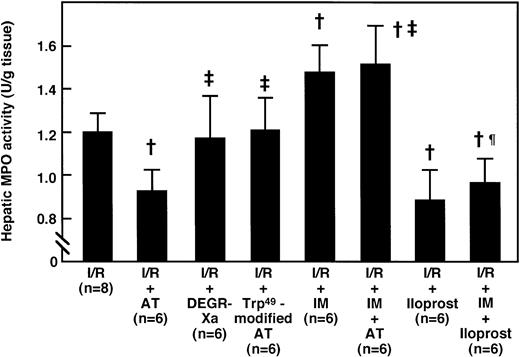
Hepatic levels of CINC, a member of the interleukin-8 (IL-8) family, were significantly increased after reperfusion, compared with those of the sham-operated animals, and peaked 2 hours after reperfusion (Fig 5A). Administration of AT (250 U/kg) significantly inhibited this increase 2 hours after reperfusion (Fig 6), whereas the administration of Trp49-modified AT (250 U/kg) and DEGR-Xa (3 mg/kg) had no effect. Hepatic MPO activity was increased significantly after reperfusion, compared with that of the sham-operated animals, and peaked 6 hours after reperfusion (Fig 5B). Administration of AT (250 U/kg) significantly inhibited this increase 6 hours after reperfusion, whereas the administration of Trp49-modified AT (250 U/kg) and DEGR-Xa (3 mg/kg) had no effect (Fig7).
Changes in hepatic CINC levels (A) and MPO activity (B) in rats subjected to hepatic I/R. Animals were subjected to 60 minutes of hepatic ischemia followed by reperfusion as described in Materials and Methods. Sham-operated animals were prepared in a similar manner, except that blood flow to the left and median hepatic lobes was not obstructed. Mean values of the hepatic CINC level and MPO activity after various periods of hepatic reperfusion are shown. Data represent the mean ± SD derived from six animal experiments. (□) Sham-operated animals; (▪) I/R animals. §P < .01 versus preischemia. *P < .01 versus sham.
Changes in hepatic CINC levels (A) and MPO activity (B) in rats subjected to hepatic I/R. Animals were subjected to 60 minutes of hepatic ischemia followed by reperfusion as described in Materials and Methods. Sham-operated animals were prepared in a similar manner, except that blood flow to the left and median hepatic lobes was not obstructed. Mean values of the hepatic CINC level and MPO activity after various periods of hepatic reperfusion are shown. Data represent the mean ± SD derived from six animal experiments. (□) Sham-operated animals; (▪) I/R animals. §P < .01 versus preischemia. *P < .01 versus sham.
Effects of AT and various agents on the I/R-induced increase in hepatic CINC levels 2 hours after reperfusion. Animals were subjected to 60 minutes of hepatic ischemia followed by reperfusion as described in Materials and Methods. Mean values of the hepatic CINC levels after 2 hours of hepatic reperfusion are shown. Data represent the mean ± SD. †P < .01 versus I/R. ‡P < .01 versus AT-treated I/R. ¶P < .01 versus IM-treated I/R.
Effects of AT and various agents on the I/R-induced increase in hepatic CINC levels 2 hours after reperfusion. Animals were subjected to 60 minutes of hepatic ischemia followed by reperfusion as described in Materials and Methods. Mean values of the hepatic CINC levels after 2 hours of hepatic reperfusion are shown. Data represent the mean ± SD. †P < .01 versus I/R. ‡P < .01 versus AT-treated I/R. ¶P < .01 versus IM-treated I/R.
Effects of AT and various agents on the I/R-induced increase in hepatic MPO activity 6 hours after reperfusion. Animals were subjected to 60 minutes of hepatic ischemia followed by reperfusion as described in Materials and Methods. Mean values of hepatic MPO activity after 6 hours of hepatic reperfusion are shown. Data represent the mean ± SD. †P < .01 versus I/R. ‡P < .01 versus AT-treated I/R. ¶P < .01 versus IM-treated I/R.
Effects of AT and various agents on the I/R-induced increase in hepatic MPO activity 6 hours after reperfusion. Animals were subjected to 60 minutes of hepatic ischemia followed by reperfusion as described in Materials and Methods. Mean values of hepatic MPO activity after 6 hours of hepatic reperfusion are shown. Data represent the mean ± SD. †P < .01 versus I/R. ‡P < .01 versus AT-treated I/R. ¶P < .01 versus IM-treated I/R.
Effect of IM pretreatment on the AT-induced effects in I/R of the rat liver.
Pretreatment of animals with IM (20 mg/kg, subcutaneously) significantly reduced hepatic 6-keto-PGF1α levels in rats subjected to I/R and it completely inhibited the increase in hepatic 6-keto-PGF1α levels seen in response to AT administration (Fig 3). AT did not inhibit the increase in serum aminotransferase levels seen 12 hours after reperfusion in animals pretreated with IM (Fig 1). IM itself significantly enhanced the increase in serum aminotransferase levels 12 hours after reperfusion (Fig 1). AT did not increase the hepatic tissue blood flow in animals pretreated with IM (Fig 8). IM itself significantly reduced hepatic tissue blood flow (Fig 8). AT did not prevent the I/R-induced increases in hepatic levels of CINC and MPO in animals pretreated with IM (Figs 6 and 7). IM itself significantly enhanced the I/R-induced increases in these indicators for leukocyte activation (Figs 6 and 7).
Effects of IM pretreatment and continuously infused iloprost on I/R-induced changes in hepatic tissue blood flow in rats receiving AT. Animals were subjected to 60 minutes of hepatic ischemia followed by reperfusion as described in Materials and Methods. Animals were injected IV with AT (250 U/kg) 30 minutes before reperfusion or subcutaneously with IM (20 mg/kg) 30 minutes before ischemia. Iloprost was infused continuously starting before the onset of ischemia at a rate of 100 ng/kg/min with or without subcutaneous injection of IM (20 mg/kg) 30 minutes before ischemia. Hepatic tissue blood flow was continuously measured by laser Doppler flowmeter starting 30 minutes before ischemia until 3 hours after reperfusion. Each value represents the mean ± SD derived from 5 animals. (○) I/R animals; (▪) IM-treated I/R animals; (•) AT-treated I/R animals; (▴) AT-treated I/R animals pretreated with IM; (▵) iloprost-treated I/R animals; (□) iloprost-treated I/R animals pretreated with IM. †P< .01 versus I/R. ‡P < .01 versus AT-treated I/R. ¶P < .01 versus IM-treated I/R.
Effects of IM pretreatment and continuously infused iloprost on I/R-induced changes in hepatic tissue blood flow in rats receiving AT. Animals were subjected to 60 minutes of hepatic ischemia followed by reperfusion as described in Materials and Methods. Animals were injected IV with AT (250 U/kg) 30 minutes before reperfusion or subcutaneously with IM (20 mg/kg) 30 minutes before ischemia. Iloprost was infused continuously starting before the onset of ischemia at a rate of 100 ng/kg/min with or without subcutaneous injection of IM (20 mg/kg) 30 minutes before ischemia. Hepatic tissue blood flow was continuously measured by laser Doppler flowmeter starting 30 minutes before ischemia until 3 hours after reperfusion. Each value represents the mean ± SD derived from 5 animals. (○) I/R animals; (▪) IM-treated I/R animals; (•) AT-treated I/R animals; (▴) AT-treated I/R animals pretreated with IM; (▵) iloprost-treated I/R animals; (□) iloprost-treated I/R animals pretreated with IM. †P< .01 versus I/R. ‡P < .01 versus AT-treated I/R. ¶P < .01 versus IM-treated I/R.
Effect of iloprost, a stable analog of PGI2, on hepatic injury and the other events induced by I/R of the rat liver.
The continuous IV infusion of iloprost (100 ng/kg/min) significantly inhibited the increase in serum aminotransferase levels observed 12 hours after reperfusion (Fig 1) and increased the hepatic tissue blood flow after hepatic I/R (Fig 8). The continuous IV infusion of iloprost significantly inhibited the I/R-induced increase in hepatic levels of CINC (Fig 6) and MPO activity (Fig 7) 2 and 6 hours after reperfusion, respectively. The increases in serum aminotrasferase levels (Fig 1), hepatic CINC levels (Fig 6), and MPO activity (Fig 7) and the reduction of hepatic tissue blood flow (Fig 8) in rats subjected to hepatic I/R, but pretreated with IM, were significantly inhibited by the continuous IV infusion of iloprost.
DISCUSSION
In the present study, AT prevented hepatic injury induced by I/R, whereas Trp49-modified AT did not. Because Trp49 plays a critical role in AT’s interaction with heparin,39 Trp49-modified AT retains its progressive antithrombin activity, but lacks affinity for heparin.17 Because the inhibition of thrombin by AT is markedly accelerated by its interaction with glycosaminoglycans on the endothelial cell surface,1 the lack of efficacy of Trp49-modified AT seen in this model may be due to a decrease in its inhibitory activity against thrombin. However, this possibility seems unlikely, because DEGR-Xa, a selective inhibitor of thrombin generation, did not prevent I/R-induced liver injury in the present study. DEGR-Xa (3 mg/kg) inhibited endotoxin-induced coagulation abnormalities to the same extent as AT (250 U/kg).17 These results parallel earlier studies that were performed on the primate (baboon) in which DEGR-Xa also inhibited coagulation abnormalities after infusion of LD100E coli, without protecting against its lethal effects.45In addition, we have previously demonstrated that Trp49-modified AT (250 U/kg) alleviates endotoxin-induced coagulation abnormalities to the same extent as native AT (250 U/kg),17 suggesting that the antithrombin activity of Trp49-modified AT may be similar to that of AT in respect to the alleviation of coagulation abnormalities induced by endotoxin. This observation also suggests that the failure of Trp49-modified AT to prevent the I/R-induced liver injury was not due to its decreased anticoagulant activity, but probably due to a lack of affinity for heparin. These observations suggest that the preventive effect of AT on I/R-induced hepatic injury may not be a result of its anticoagulant activity, but may be dependent on its interaction with glycosaminoglycans on the endothelial cell surface.
AT promotes the release of PGI2 from endothelial cells by interacting with glycosaminoglycans on the endothelial cell surface.3-5 In the present study, AT significantly increased the hepatic level of 6-keto-PGF1α 1 hour after hepatic I/R. Trp49-modified AT does not promote the release of PGI2 from endothelial cells.44 Neither Trp49-modified AT nor DEGR-Xa increased the hepatic tissue level of 6-keto-PGF1α in animals subjected to I/R, suggesting that AT could increase hepatic production of PGI2 not through its antithrombin activity, but by interaction with glycosaminoglycans on the endothelial cell surface. Because neither Trp49-modified AT nor DEGR-Xa did not prevent the I/R-induced hepatic injury, we postulate that AT may prevent I/R-induced hepatic injury by promoting the endothelial release of PGI2.
In the present study, the critical role of hepatic PGI2 in the prevention of I/R-induced hepatic injury was illustrated by the following observations. (1) Iloprost, a stable analog of PGI2, prevented the hepatic injury induced by hepatic I/R. (2) Pretreatment of animals with IM, a potent inhibitor of PGI2 synthesis, significantly exacerbated the I/R-induced hepatic injury. (3) Iloprost significantly inhibited the IM-induced exacerbation of hepatic injury. Also, the preventive effects of AT were not observed in the animals pretreated with IM.
Taken together, these observations further support the hypothesis that the protective effect of AT against I/R-induced hepatic injury could be mediated primarily by the actions of PGI2.
Because PGI2 exhibits vasodilator activity, the hepatic production of PGI2 induced by AT after reperfusion may increase the hepatic tissue blood flow. Consistent with this hypothesis is the observation made in the present study that the I/R-induced decrease in hepatic tissue blood flow was markedly inhibited by AT administration. This inhibitory effect was not observed in animals pretreated with IM. Iloprost inhibited the I/R-induced decrease in hepatic tissue blood flow and also prevented the IM-induced decrease in hepatic tissue blood flow induced by hepatic I/R. These observations suggest that AT inhibits the I/R-induced decrease in hepatic tissue blood flow by increasing the hepatic production of PGI2. These effects contribute to the attenuation of hepatic injury after I/R.
Activated leukocytes are considered to play a pivotal role in I/R-induced hepatic injury by releasing various inflammatory mediators that are capable of damaging endothelial cells.46,47 We have previously demonstrated the involvement of neutrophil elastase in an animal model of hepatic I/R.28 Consistent with these observations are the findings in the present study that hepatic levels of CINC, a member of the IL-8 family that promotes the accumulation of neutrophils,46 as well as hepatic MPO activity were increased after I/R. Because PGI2 inhibits the production of TNF-α, which enhances the production of IL-8, a potent activator of neutrophils,48,49 it is possible that AT may also prevent I/R injury in rats by inhibiting leukocyte activation through the promotion of PGI2 production. This hypothesis is supported by the observation in the present study that AT inhibited the I/R-induced increase in hepatic levels of CINC and MPO. These effects of AT were not observed in animals pretreated with IM. Iloprost inhibited the I/R-induced increase in hepatic levels of CINC and MPO. It also inhibited the IM-induced enhancement of CINC and MPO levels in animals subjected to hepatic I/R. These observations suggest that PGI2 plays an important role in preventing hepatic I/R-induced activation of leukocytes. Thus, it is likely that AT may also prevent I/R-induced hepatic injury by inhibiting the hepatic accumulation of neutrophils through an increase in the hepatic PGI2 levels. This hypothesis is consistent with our previous report demonstrating that AT prevents in vivo endotoxin-induced endothelial cell injury by inhibiting the activation of leukocytes by promoting the endothelial PGI2release.17 Taken together, these observations strongly suggest that AT prevents I/R-induced hepatic injury by maintaining tissue blood flow and by inhibiting leukocyte activation, both of which can be mediated by the actions of PGI2.
AT appears to prevent I/R-induced hepatic injury independent of its anticoagulant effect in the present study. Although neither an increase in the serum levels of FDP nor hepatic fibrin formation was observed in rats subjected to I/R, these observations cannot completely rule out thrombin generation in the hepatic microcirculation in this model. In hepatic injury induced by I/R of whole liver in rats, AT has been shown to prevent hepatic injury by inhibiting coagulation abnormalities.50 Thus, it is possible that AT can prevent I/R-induced hepatic injury by inhibiting thrombin activity as well as by inhibiting leukocyte activation through the induction of PGI2 release.
Emerson et al14 has reported that AT prevents hepatic damage induced by endotoxin in rats without inhibiting endotoxin-induced coagulation abnormalities. Jochum51 has demonstrated that an infusion of AT concentrate significantly inhibits hepatic dysfunction in patients with multiple trauma by inhibiting leukocyte activation.
We conclude that the beneficial effects of AT on hepatic injury can be explained partly by its promotion of the release of PGI2 by endothelial cells independent of its antithrombin activity.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Kenji Okajima, MD, Department of Laboratory Medicine, Kumamoto University School of Medicine, Honjo 1-1-1 Kumamoto 860, Japan; e-mail: whynot@kaiju.medic.kumamoto-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal