Abstract
To elucidate the mechanisms by which hematopoietic progenitor cells transmigrate via the bone marrow (BM) endothelial cells, we first established endothelial cell lines from BM and lung, and BM fibroblast cell lines; then we established an in vitro model of transendothelial migration of hematopoietic progenitor cells in the presence of chemoattractants secreted by BM fibroblast cells. The BM endothelial cells expressed vascular cell adhesion molecule-1 (VCAM-1), but the lung endothelial cells did not. The BM fibroblast cells secreted chemoattractants including stroma cell–derived factor (SDF)-1, which could attract hematopoietic progenitor cells to BM and activate the adhesion molecules expressed on hematopoietic progenitor cells after rolling along the endothelial cells. Anti–SDF-1 antibody inhibited the transendothelial migration of a hematopoietic progenitor cell line, FDCP-2. FDCP-2 that expressed very late activation antigen-4 (VLA-4) and normal progenitor cells transmigrated through BM endothelial cells but not lung endothelial cells, even if in the presence of chemoattractants produced by BM fibroblasts. Both anti–VLA-4 and anti–VCAM-1 antibodies inhibited the transendothelial migration of FDCP-2 cells and normal hematopoietic progenitor cells. These findings suggest that the transendothelial migration of hematopoietic progenitor cells is characteristic of BM endothelial cells, and that VLA-4/VCAM-1 and SDF-1 play important roles in the transendothelial migration and, consequently, homing of hematopoietic progenitor cells to BM.
MECHANISMS OF migration of leukocytes from peripheral blood (PB) to extra-vascular tissues have been extensively examined, and the transendothelial migration cascade system is now being proposed as a mechanism for leukocytes’ migration.1-7 This migration cascade system consists of rolling along the vessel wall, activation of adhesion molecules by cytokines and chemokines, firm adhesion to endothelial cells via the activation of adhesion molecules, and transendothelial migration. Transendothelial migration of hematopoietic progenitor cells occurs during mobilization of hematopoietic progenitor cells into PB, which is induced by cytokine-administration and/or chemotherapy, and it occurs when intravenously transplanted hematopoietic progenitor cells home to bone marrow (BM). It is believed that transendothelial migration of hematopoietic progenitor cells occurs through a similar cascade to that of leukocytes. It has recently been reported that the interaction of leukocyte function-associated antigen (LFA)-1 and intercellular adhesion molecule (ICAM)-1 played important roles in the transendothelial migration of CD34+ hematopoietic cells.8 In their study, the transendothelial migration was examined in the absence of any chemoattractant or chemokine in the lower chambers of transwell plates; in other words, the transendothelial migration of progenitor cells in their experimental model was not BM-oriented but rather random. Furthermore, it was not determined whether transendothelial migration of hematopoietic progenitor cells was characteristic of BM endothelial cells. Therefore, mechanisms of transendothelial migration of hematopoietic progenitor cells into BM are still poorly understood. In this study, we established endothelial cell lines from BM and lung, and BM fibroblast cell lines. Then, we established an in vitro transendothelial chemotactic migration model in the presence of chemoattractants produced by BM fibroblasts. Using this model, we examined whether hematopoietic progenitor cells selectively transmigrate through the BM endothelial cells compared with the lung endothelial cells, and then determined the roles of adhesion molecules and chemoattractants responsible for the transendothelial migration of hematopoietic progenitor cells.
MATERIALS AND METHODS
Reagents.
Anti-Thy 1.2, anti-mouse CD45R/B220 (Ly-5), anti-mouse granulocytes, and anti-mouse E-selectin antibodies were purchased from Pharmingen (San Diego, CA). Anti-mouse L-selectin antibody was purchased from Serotec Ltd (Oxford, UK). Anti-macrophage/CD11b antibody was purchased from Boehringer Mannheim Biochemica (Tokyo, Japan). Anti-mouse vascular cell adhesion molecule-1 (VCAM-1), anti-mouse very late activation antigen-4 (VLA-4) antibodies were purchased from Southern Biotechnology Associates, Inc (Birmingham, AL). Anti-mouse CD11a (LFA-1) and anti-mouse CD54 (ICAM-1) antibodies were purchased from Genzyme (Cambridge, MA). Anti–factor VIII antibody was purchased from Cosmo-Bio (Tokyo, Japan). Goat 1 F(ab′)2 anti-rat IgG-fluorescein was purchased from Leinco Technologies, Inc (Ballwin, MO). Anti–stroma cell–derived factor (SDF)-1 antibody was purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Dynabeads M-450 coated with sheep anti-rat IgG antibody was purchased from Dynal Inc (Great Neck, NY). Acetylated low-density lipoprotein labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyanine perchlorate (DiI-acetylated-LDL) was purchased from Paesel GmbH (Hanau, Germany).
Cell lines.
A murine interleukin-3 (IL-3)–dependent hematopoietic progenitor cell line, FDCP-2, was maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and 20% WEHI-3B (an IL-3–producing murine myelomonocytic cell line) cell-conditioned medium.
Establishment of BM endothelial cell lines, fibroblast cell lines, and a lung endothelial cell line.
Six- to 12-week-old female BDF1 mice were purchased from Japan Charles River (Kanagawa, Japan). Single-cell suspensions of the BM were obtained from femurs by flushing the marrow with ice-cold RPMI 1640 medium and 10% FCS using a 21-gauge needle. Primary stromal layers were established according to the method described previously.9 Briefly, the cells were plated at a density of 2 × 106 cells/mL in 25-cm2 flasks in RPMI 1640 medium supplemented with 10% FCS in a humidified incubator at 5% CO2 at 37°C. The cultures were fed every 3 to 4 days by changing half the medium. After 3 to 5 weeks of culture, confluent stromal layers were formed. The stromal cells were obtained by trypsinization, and 2 × 106 cells were seeded in 5 mL of RPMI 1640 medium supplemented with 10% FCS in 6-well culture plates. The cells were incubated at 37°C in a CO2 incubator for 2 days until the cells were 40% to 60% confluent. Transfections were performed using a LIPOFECTIN reagent (GIBCO-BRL Life Technologies, Tokyo, Japan) as previously described.10 SV40 vector (pMK16) constructed by Gluzman et al11 and pGEM-SRαNEO were mixed with the LIPOFECTIN reagent. The LIPOFECTIN reagent-DNA complexes were overlaid onto the sub-confluent stromal layers in serum-free RPMI-1640 medium. The cells were incubated for 24 hours at 37°C in a CO2 incubator and the medium was exchanged with fresh RPMI-1640 medium supplemented with 10% FCS and 800 μg/mL of G418. Four days after transfection, the cells were collected by EDTA/trypsin-treatment and then washed with RPMI-1640 medium supplemented with 10% FCS. Transformed BM adherent cells were maintained in RPMI-1640 medium with 10% FCS and G418. A single cell was suspended in 100 μL of fresh medium and cultured in 96-well plates. The medium was changed every 3 to 4 days. When the colonies developed in wells, they were removed by EDTA/trypsin-treatment and placed in 5 mL RPMI-1640 medium supplemented with 10% FCS in 25-cm2 plastic flasks. When the cells were confluent, aliquots of the cells were passed through three to four dilutions every week. The cells maintained for more than 20 passages were selected as immortalized cells and examined for expression of SV40 large T antigen. Established cell lines were designated as STR-1, STR-2, STR-3, and following ascending numbers up to STR-12. A lung endothelial cell line expressing SV40 large T antigen was also established from a lung endothelial cell line, LE1, by transfection with SV40 vector (pMK16) and designated as LE1SVO.12,13 LE1 cells have been reported to be factor VIII+ and able to uptake diI-acetylated LDL.12 LE1SVO cells, BM endothelial cell lines, and BM fibroblast cell lines were maintained in RPMI 1640 medium supplemented with 10% FCS.
Characteristics of BM endothelial and fibroblast cell lines.
BM endothelial and fibroblast cell lines were cytochemically analyzed for peroxidase, alkaline phosphatase, and a-naphthyl acetate esterase as described previously.14 Briefly, the established cell lines were cultured in chambered slides, washed with phosphate-buffered saline (PBS), and fixed with ethanol for 10 minutes and stained. Expression of SV40 large T antigen was examined with anti-T antigen antibody using a VECSTATIN ABC kit according to the manufacturer’s recommendation (Funakoshi, Tokyo, Japan). Expression of factor VIII was examined with anti–factor VIII antibody by the use of a confocal laser microscopy. Uptake of LDL was examined according to the previously described methods.15 Briefly, the cells were incubated with diI-acetylated LDL (10 mg/mL) at 37°C in the culture medium for 4 hours. The medium was removed, and after thorough washing the cells were fixed with 3% formaldehyde in PBS and evaluated for LDL uptake under a confocal laser microscopy. Expression of adhesion molecules and differentiation antigens was evaluated by fluorescence-activated cell sorter (FACS) analysis. Three (STR-4, STR-10, and STR-12) of the cell lines that were Mac-1−, factor VIII+, and able to uptake LDL were selected as BM endothelial cell lines, and another one (STR-2) that was Mac-1−, factor VIII−, and not able to uptake LDL was selected as a BM fibroblast cell line.
FACS analysis.
A total of 1 × 105 cells was incubated for 30 minutes on ice with first antibodies and then incubated with second antibodies for 45 minutes on ice. The cells were analyzed by the use of a FACScan (Becton Dickinson, San Jose, CA).
Preparation of conditioned medium.
STR-2 cells were grown to confluence in a tissue culture flask. After being washed twice with PBS, the cells were incubated for 48 hours in serum-free RPMI medium, and then conditioned medium was collected, filtrated through a 0.25-μm Milipore filter (Gelman Science, Ann Arbor, MI), stored at 4°C and used for the following experiments.
Semi-purification of normal progenitor cells.
Ten- to 15-week-old male BDF1 mice were obtained from Charles River Japan. Single-cell suspensions were prepared from pooled femurs of mice, which had been injected with 150 mg/kg of 5-fluorouracil (5-FU; Kyowa Hakko Kogyo Co, Tokyo, Japan) intravenously (IV) 2 days before examination (day 2 post-5-FU marrow cells) to enrich the noncycling hematopoietic primitive progenitor cells. Lineage-negative (Lin−) day 2 post 5-FU marrow cells were isolated as described previously.16 Briefly, mononuclear cells were separated by density centrifugation on Ficoll-Paque (specific gravity, 1.077) from the day 2 post 5-FU marrow cells. They were then incubated at 4°C for 45 minutes in a mixture of anti-Thy 1.2, anti-B220, anti–Gr-1, and anti–Mac-1/CD11b antibodies. After washing twice, the cells were incubated with sheep anti-rat IgG (Fc)-conjugated immunomagnetic beads at 4°C for 45 minutes. Lineage-specific antigen-positive cells were removed by a magnetic particle concentrator (Dynal), and Lin− cells were recovered.
Adhesion assay.
FDCP-2 cells were incubated with 5 μmol/L acetoxymethyl ester-2′,7′-bis-(2-carboxyethyl)-5-(and 6)-carboxyfluorescein (BCECF-AM) (Wako, Tokyo, Japan) for 1 hour. After washing three times with RPMI-1640 medium, BCECF-AM–labeled cells were placed on STR-4 cells and allowed to adhere for 2 hours. Nonadherent cells were removed by three washes with RPMI-1640 medium. After lysing with 1% Triton X-100 (Junsei Chemical Co, Ltd, Tokyo, Japan)–PBS, the plates were read on an ELISA reader (BioRad, Richmond, CA) at a wave length of 520 nm. Percentages of adherent cells were calculated by the following formula: % Adherent Cells = (Optical Density [OD] Values of Adherent Cells/OD Values of Total Cells) × 100.
Chemotactic migration assay.
To examine the chemotactic activities and random motility-stimulating activities in the conditioned medium of BM fibroblasts, the micropore filter assay was performed according to the previously described methods.17 In transwell plates, tissue-culture–treated micropore filters with 5-μm pores were placed to separate the upper and lower chambers. Six hundred microliters of STR-2 cell-conditioned medium was placed in the lower chambers (Transwell; Costar, Cambridge, MA). Suspensions (100 μL) of FDCP-2 cells at an appropriate concentration were placed in the upper chambers. After 4 hours of incubation, transmigrated cells into the lower chambers were counted under an inverted microscope at least in three areas. To evaluate the chemotactic activity for progenitor cells of SDF-1 in STR-2 cell-conditioned medium, anti–SDF-1 antibody was added in the upper chambers at up to 2 μg/mL.
Transendothelial migration assays.
For the transendothelial migration assay, 2 × 105 STR-4, STR-10, STR-12, and LE1SVO cells were seeded on micropore filters. After 4 days, the confluent monolayers were formed and were suitable for transendothelial migration assay. To assess the integrity of lung and BM endothelial cell monolayers grown on micropore filters, FDCP-2 cells (1 × 104) and semi-purified murine BM progenitor cells (5 × 104) were seeded in the upper chambers and incubated for 4 hours in the absence of STR-2 cell-conditioned medium in the lower chambers. Without any conditioned medium, the cells seeded on endothelial cell monolayers did not transmigrate into the lower chambers within 4 hours. We also examined the integrity of endothelial cell monolayers by diffusion assay using BCECF-AM. Diffusion of BCECF-AM through both STR-4 and LE1SVO cell monolayers was always less than 8% within 4 hours. FDCP-2 cells transmigrated into the lower chambers were counted under an inverted microscope at more than three areas. Normal BM progenitor cells transmigrated into the lower chambers were counted by colony assays using the total cells transmigrated into the lower chambers.
For blocking the migration, FDCP-2 cells or normal BM Lin− cells were simultaneously seeded on endothelial cell monolayers with various anti-adhesion molecule antibodies. To evaluate the role of SDF-1 in this transendothelial migration assays, anti–SDF-1 antibody was added in the upper chambers at up to 8 μg/mL.
Colony assay.
Colony assay was performed according to the previously described methods.18 Briefly, 1 × 104 cells or the whole transmigrated cells were plated in a mixture of Iscove’s modified Dulbecco’s medium (IMDM), 20% FCS, 0.3% agar, and colony-stimulating factors (CSFs) including 10 ng/mL of granulocyte-CSF (G-CSF), 10 ng/mL of IL-1 β, 10 ng/mL of IL-3, 10 ng/mL of IL-6, and 10 ng/mL of stem cell factor (SCF). Eleven to 14 days after incubation in a highly humidified CO2 incubator at 37°C, numbers of colonies (containing more than 40 cells) were counted.
Reverse transcriptase-polymerase chain reaction (RT-PCR).
Total RNA was extracted from STR-2, STR-4, STR-10, STR-12 cells, and LE1SVO cells using TRIzol (GIBCO-BRL, Gaithersburg, MD) and then treated with 0.1 U/mL of DNAse I Amp Grade (GIBCO-BRL) for 15 minutes at room temperature to exclude contaminated genomic DNA. Each RNA sample (5 μg) was subjected to cDNA synthesis in 50 μL of reaction mixture containing 75 mmol/L KCl, 50 mmol/L Tris-HCl (pH 8.3), 3 mmol/L MgCl2, 10 mmol/L dithiothreitol, 0.5 mmol/L each dNTP, 2 μmol/L random primer, and 1,000 U AMLV reverse transcriptase (GIBCO-BRL). PCR amplification of cDNA was performed in 50 μL of reaction mixture containing 50 mmol/L KCl, 10 mmol/L Tris-HCl (pH 9.0), 2.5 mmol/L MgCl2, 0.1% Triton X-100, 200 μmol/L each dNTP, 10 μmol/L each specific primers, and 1 U Taq polymerase (GIBCO-BRL). PCR was performed in a DNA thermal cycler (Barnstead/Thermolyne, Dubuque, IA) at 25, 30, and 35 cycles (94°C for 1 minute, 60°C for 1 minute, and 72°C for 2 minutes). The PCR product (9 μL) was subjected to electrophoresis on 1% agarose gels and stained with ethidium bromide. Primer sequences were as follows: SDF-1: forward primer: 5′-gctctgcatcagtgacggtaaac-3′, reverse primer: 5′-caatatcgtaccatatgctatggcc-3′; β-actin: forward primer: 5′-agggaaatcgtgcgtgacatcaaa-3′, reverse primer: 5′-actcatcgtactcctgcttgctga-3′.
RESULTS
Characteristics of established cell lines.
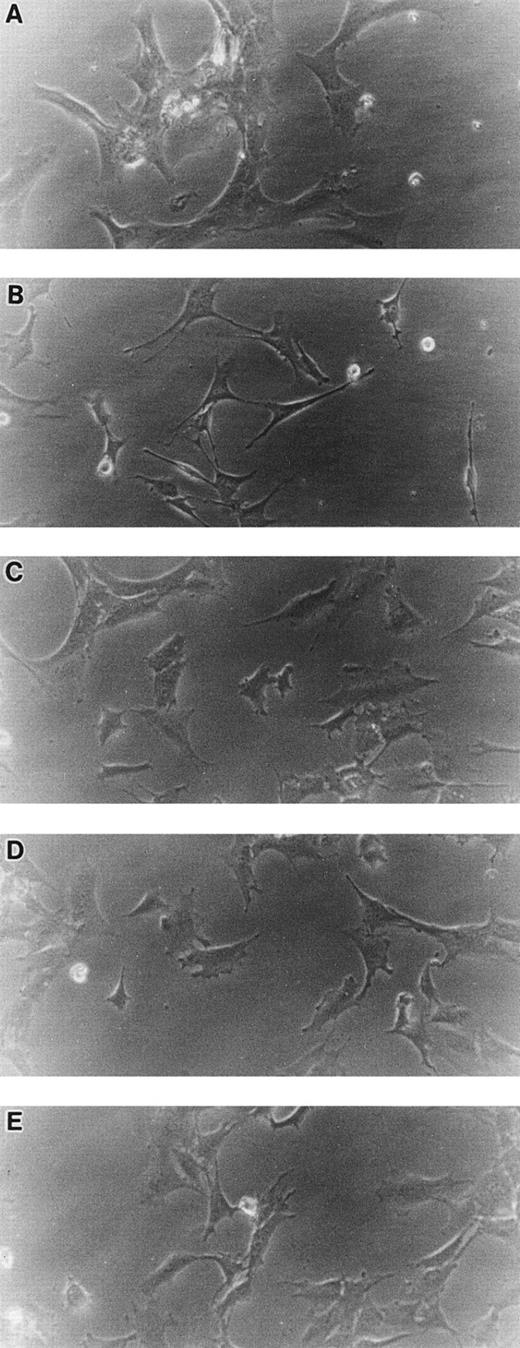
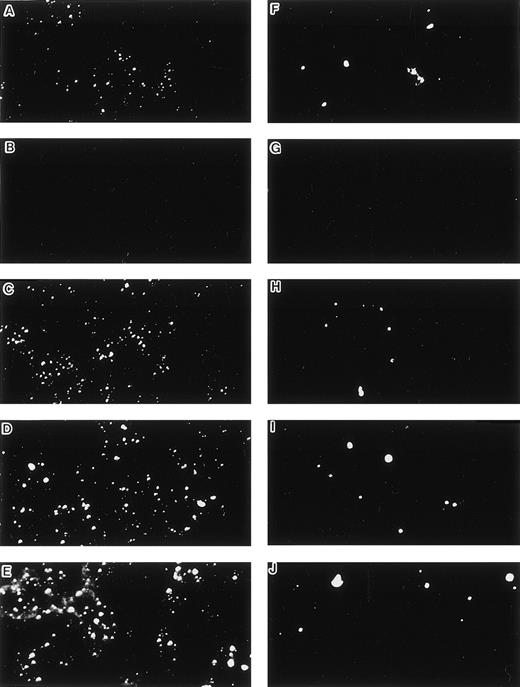
When cultured on tissue culture dishes in RPMI-1640 medium supplemented with 10% FCS, STR-4, STR-10, STR-12, and LE1SVO cells were initially spindle-shaped and then developed cobblestone monolayers (Fig1). Cytochemical staining showed that STR-2, STR-4, STR-10, and STR-12 cells were alkaline phosphatase+, α-naphtyl butyrate+, peroxidase−, and periodic acid-Schiff (PAS)− (Table 1). Staining with anti–factor VIII antibody showed that STR-4, STR-10, STR-12, and LE1SVO cells were mostly positive (Fig 2). Furthermore, STR-4, STR-10, STR-12, and LE1SVO cells demonstrated uptake of diI-acetylated-LDL after 4 hours of incubation (Fig 2). STR-2 cells were factor VIII− and did not uptake diI-1-acetylated-LDL (Fig 2).
Morphology of STR-2, STR-4, STR-10, STR-12, and LE1SVO cells. (A) LE1SVO; (B) STR-2; (C) STR-4; (D) STR-10; (E) STR-12 (original magnification × 400 under an inverted microscope).
Morphology of STR-2, STR-4, STR-10, STR-12, and LE1SVO cells. (A) LE1SVO; (B) STR-2; (C) STR-4; (D) STR-10; (E) STR-12 (original magnification × 400 under an inverted microscope).
Characteristics of STR Cell Lines
| . | STR-2 . | STR-4 . | STR-10 . | STR-12 . |
|---|---|---|---|---|
| Peroxidase | − | − | − | − |
| Alkaline phosphatase | + | + | + | + |
| PAS | − | − | − | − |
| α-Naphtyl butyrate | + | + | + | + |
| (NaF inhibition) | − | − | − | − |
| Factor VIII | − | + | + | + |
| Mac-1 | − | − | − | − |
| Dil-Ac-LDL uptake | − | + | + | + |
| . | STR-2 . | STR-4 . | STR-10 . | STR-12 . |
|---|---|---|---|---|
| Peroxidase | − | − | − | − |
| Alkaline phosphatase | + | + | + | + |
| PAS | − | − | − | − |
| α-Naphtyl butyrate | + | + | + | + |
| (NaF inhibition) | − | − | − | − |
| Factor VIII | − | + | + | + |
| Mac-1 | − | − | − | − |
| Dil-Ac-LDL uptake | − | + | + | + |
+, >30% of cells were positive.
Uptake of DiI-Ac-LDL and staining by anti–factor VIII antibody. (A and F) LE1SVO; (B and G) STR-2; (C and H) STR-4; (D and I) STR-10; (E and J) STR-12. (A through E) The cells were incubated with anti–factor VIII antibody and fluorescein-conjugated second antibody, and then examined under a confocal laser microscope (original magnification × 100). (F through J) The cells were incubated with DiI-acetylated LDL (10 mg/mL) at 37°C for 4 hours. After thorough washing, the cells were fixed and then evaluated for LDL uptake under a confocal laser microscope (original magnification × 100).
Uptake of DiI-Ac-LDL and staining by anti–factor VIII antibody. (A and F) LE1SVO; (B and G) STR-2; (C and H) STR-4; (D and I) STR-10; (E and J) STR-12. (A through E) The cells were incubated with anti–factor VIII antibody and fluorescein-conjugated second antibody, and then examined under a confocal laser microscope (original magnification × 100). (F through J) The cells were incubated with DiI-acetylated LDL (10 mg/mL) at 37°C for 4 hours. After thorough washing, the cells were fixed and then evaluated for LDL uptake under a confocal laser microscope (original magnification × 100).
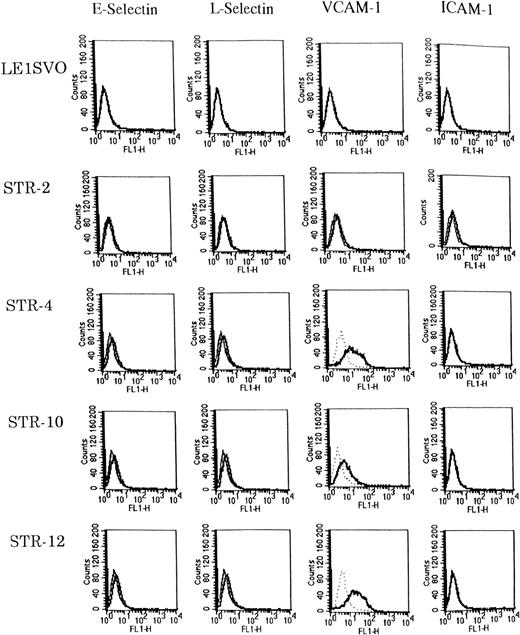
Expression of adhesion molecules was examined before and after stimulating with 100 ng/mL of IL-1β by FACS analysis (Fig3). Unstimulated STR-4, STR-10, and STR-12 cells expressed only VCAM-1 but not E-selectin, ICAM-1, or L-selectin. IL-1β induced the very weak expression of E-selectin and L-selectin on STR-4, STR-10, and STR-12 cells but no ICAM-1. Unstimulated LE1SVO cells expressed no VCAM-1, ICAM-1, L-selectin, or E-selectin.
Expression of adhesion molecules on LE1SVO and STR cell lines. (Dotted line), second antibody alone, (fine solid line), unstimulated, (solid line), stimulated with IL-1β.
Expression of adhesion molecules on LE1SVO and STR cell lines. (Dotted line), second antibody alone, (fine solid line), unstimulated, (solid line), stimulated with IL-1β.
Chemotactic activity in STR-2 cell-conditioned medium.
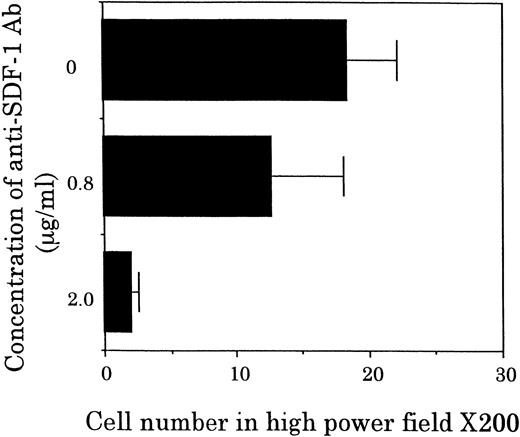
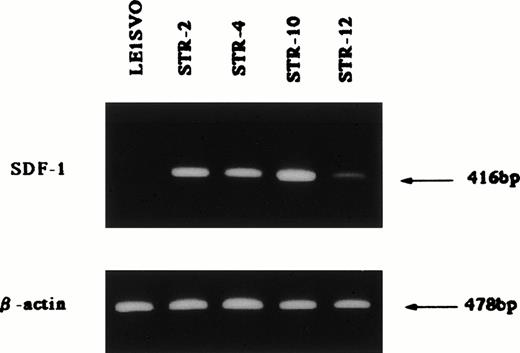
Chemotactic activity for FDCP-2 cells in STR-2 cell-conditioned medium was examined by checkerboard analysis. The results summarized in Table2 show that FDCP-2 cells migrated into the lower chamber when attracted by STR-2 cell-conditioned medium in a dose-dependent manner. Furthermore, even if concentrations of the conditioned medium in the upper chambers were intensified higher than those in the lower chambers, the numbers of migrated FDCP-2 cells increased. These results suggest that STR-2 cells secreted chemotactic factor(s) and also random motility-stimulating factor(s). Chemotactic activity of STR-2 cell-conditioned medium for FDCP-2 cells was abrogated by anti–SDF-1 antibody in a dose-dependent manner (Fig4). STR-2 cells as well as BM endothelial cells expressed SDF-1 mRNA (Fig 5). LE1SVO cells did not express SDF-1 mRNA. These findings suggest that chemotactic activity found in STR-2 cell-conditioned medium was mostly attributable to SDF-1.
Checkerboard Analysis
| Migration of FDCP-2 Cells Into Lower Chamber (no. of cells in high-power field × 200) . | |||
|---|---|---|---|
| STR-2 CM (%) in Lower Chamber . | STR-2 CM (%) in Upper Chamber . | ||
| 0 . | 50 . | 100 . | |
| 0 | 5.2 ± 3.2 | 8.7 ± 2.5 | 6.7 ± 2.8 |
| 50 | 38.2 ± 16.8 | 34.1 ± 4.9 | 42.4 ± 11.7 |
| 100 | 100.2 ± 7.9 | 43.2 ± 6.8 | 58.1 ± 9.8 |
| Migration of FDCP-2 Cells Into Lower Chamber (no. of cells in high-power field × 200) . | |||
|---|---|---|---|
| STR-2 CM (%) in Lower Chamber . | STR-2 CM (%) in Upper Chamber . | ||
| 0 . | 50 . | 100 . | |
| 0 | 5.2 ± 3.2 | 8.7 ± 2.5 | 6.7 ± 2.8 |
| 50 | 38.2 ± 16.8 | 34.1 ± 4.9 | 42.4 ± 11.7 |
| 100 | 100.2 ± 7.9 | 43.2 ± 6.8 | 58.1 ± 9.8 |
Results were shown as the mean ± SD of data obtained from three different experiments.
Effects of anti–SDF-1 antibody on chemotactic activity. FDCP-2 cells were placed in the upper chambers of Transwell plates with or without anti–SDF-1 antibody at the indicated concentrations. Six hundred microliters of STR-2 cell-conditioned medium was placed in the lower chambers. After 4 hours of incubation, transmigrated cells into the lower chambers were counted under an inverted microscope. Results were shown as the mean ± SD of data obtained from three different experiments.
Effects of anti–SDF-1 antibody on chemotactic activity. FDCP-2 cells were placed in the upper chambers of Transwell plates with or without anti–SDF-1 antibody at the indicated concentrations. Six hundred microliters of STR-2 cell-conditioned medium was placed in the lower chambers. After 4 hours of incubation, transmigrated cells into the lower chambers were counted under an inverted microscope. Results were shown as the mean ± SD of data obtained from three different experiments.
Expression of SDF-1 β-actin; 25 cycles amplified, SDF-1; 35 cycles amplified.
Transendothelial migration of FDCP-2.
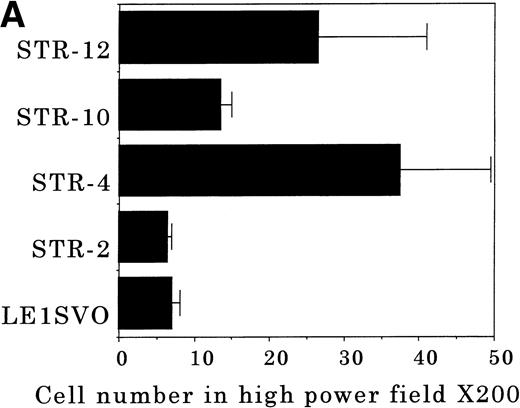
To elucidate the mechanisms by which hematopoietic progenitor cells in PB lodge in BM, we established an in vitro transendothelial migration model, where the conditioned medium of BM fibroblasts was placed in the lower chambers of transwell plates, and micropore filters coated with endothelial cell monolayers were placed in the interspace of the lower and upper chambers. The results are shown in Fig 6A through D. In this assay, FDCP-2 cells were attracted to the lower chambers by STR-2 cell-conditioned medium. We found that FDCP-2 cells transmigrated through BM endothelial cells (STR-4, STR-10, and STR-12 cells) but not STR-2 cells or LE1SVO cells (Fig 6A).
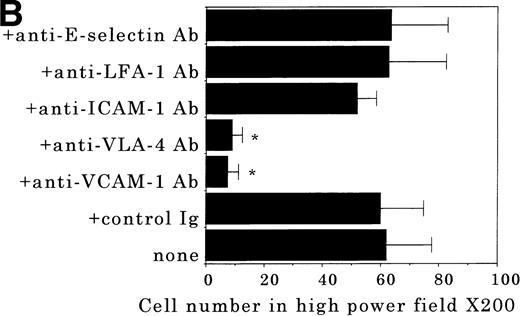
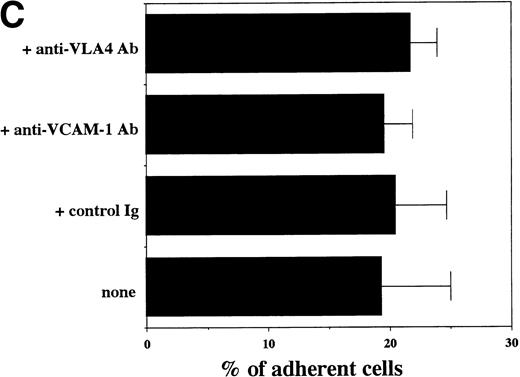
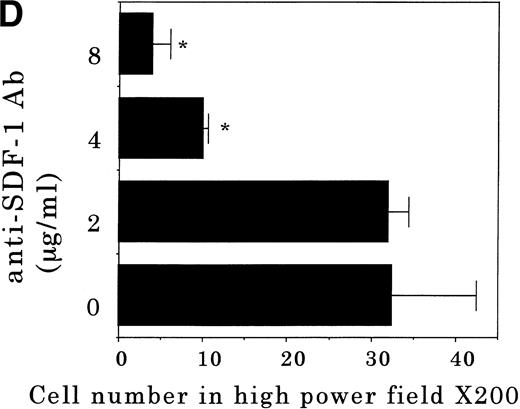
Transendothelial migration of FDCP-2 cells. (A) Transmigration of FDCP-2 cells through LE1SVO and STR cell lines; (B) effects of anti–VLA-4 and anti–VCAM-1 antibodies on the transmigration of FDCP-2 cells through STR-4 cells; (C) adhesion of FDCP-2 cells to STR-4 cells; (D) effects of anti–SDF-1 antibody on the transmigration of FDCP-2 cells through STR-4 cells. Results were shown as the mean ± SD of data obtained from three different experiments. *P < .01.
Transendothelial migration of FDCP-2 cells. (A) Transmigration of FDCP-2 cells through LE1SVO and STR cell lines; (B) effects of anti–VLA-4 and anti–VCAM-1 antibodies on the transmigration of FDCP-2 cells through STR-4 cells; (C) adhesion of FDCP-2 cells to STR-4 cells; (D) effects of anti–SDF-1 antibody on the transmigration of FDCP-2 cells through STR-4 cells. Results were shown as the mean ± SD of data obtained from three different experiments. *P < .01.
Anti–VCAM-1 antibody almost completely inhibited the transendothelial migration of FDCP-2 cells. Anti–VLA-4 antibody also inhibited the migration. Anti–E-selectin, anti–ICAM-1 antibodies, and control rat IgG showed no effect on the transendothelial migration of FDCP-2 cells through STR-4 cells (Fig 6B). Inhibition of transendothelial migration by anti–VLA-4 and anti–VCAM-1 antibodies was also observed in the experiments using STR-10 and STR-12 cells as monolayers. Because anti–VLA-4 and anti–VCAM-1 antibodies slightly but not significantly inhibited the adhesion of FDCP-2 cells to STR-4 cells (Fig 6C), inhibition of the transendothelial migration of FDCP-2 cells by these antibodies was not ascribed to the inhibition of adhesion. Anti–SDF-1 antibody inhibited the transendothelial migration of FDCP-2 cells through STR-4 cells in a dose-dependent manner (Fig 6D). Inhibition of transendothelial migration by anti–SDF-1 antibody was also observed in the experiments using STR-10 and STR-12 cells as monolayers (data not shown). These findings suggest that both SDF-1 and VCAM-1/VLA-4 are necessary for the transendothelial migration of hematopoietic progenitor cells.
Transendothelial migration of normal hematopoietic progenitor cells.
Table 3 shows the numbers of transmigrated Lin− cells and colony-forming unit granulocyte-macrophage (CFU-GM). Normal CFU-GM did not transmigrate through LE1SVO cell monolayers, whereas they transmigrated through STR-4, STR-10, and STR-12 cell monolayers in the presence of STR-2 cell-conditioned medium. Both anti–VLA-4 and anti–VCAM-1 antibodies significantly inhibited the transendothelial migration of normal CFU-GM (Table 3). Anti–ICAM-1 and anti–E-selectin antibodies as well as control rat IgG had no effect on the transendothelial migration (data not shown). Anti–SDF-1 antibody inhibited the tarnsendothelial migration of CFU-GM.
Transendothelial Migration of Normal Hematopoietic Progenitor Cells
| Cell Line . | Antibody . | No. of Transmigrated Cells (/5 × 104 semi-purified cells) . | No. of Transmigrated CFU-GM (/5 × 104 semi-purified cells) . |
|---|---|---|---|
| LE1SVO | 7.1 ± 1.0 | 0 | |
| STR-2 | 8.3 ± 3.2 | 0 | |
| STR-4 | +Control Ig | 82.6 ± 15.6 | 17.5 ± 13.0 |
| +Anti–VLA-4 antibody | 19.7 ± 0.8 | 0 | |
| +Anti–VCAM-1 antibody | 18.8 ± 7.3 | 0 | |
| STR-10 | +Control Ig | 36.6 ± 7.6 | 10.0 ± 5.3 |
| +Anti–VCAM-1 antibody | 4.6 ± 1.2 | 1.3 ± 2.1 | |
| STR-12 | +Control Ig | 54.3 ± 1.9 | 13.0 ± 2.6 |
| +Anti–VCAM-1 antibody | 5.0 ± 1.0 | 0 |
| Cell Line . | Antibody . | No. of Transmigrated Cells (/5 × 104 semi-purified cells) . | No. of Transmigrated CFU-GM (/5 × 104 semi-purified cells) . |
|---|---|---|---|
| LE1SVO | 7.1 ± 1.0 | 0 | |
| STR-2 | 8.3 ± 3.2 | 0 | |
| STR-4 | +Control Ig | 82.6 ± 15.6 | 17.5 ± 13.0 |
| +Anti–VLA-4 antibody | 19.7 ± 0.8 | 0 | |
| +Anti–VCAM-1 antibody | 18.8 ± 7.3 | 0 | |
| STR-10 | +Control Ig | 36.6 ± 7.6 | 10.0 ± 5.3 |
| +Anti–VCAM-1 antibody | 4.6 ± 1.2 | 1.3 ± 2.1 | |
| STR-12 | +Control Ig | 54.3 ± 1.9 | 13.0 ± 2.6 |
| +Anti–VCAM-1 antibody | 5.0 ± 1.0 | 0 |
DISCUSSION
Normal adult hematopoiesis is restricted within the BM extravascular spaces separated by BM endothelial cells and basement membrane from intravascular spaces.19 Hematopoietic progenitor cells in BM transmigrate through BM endothelial cells from extravascular spaces into intravascular spaces, and those transplanted into PB transmigrate through BM endothelial cells from intravascular spaces to extravascular spaces. Transendothelial migration of hematopoietic progenitor cells is thought to be through a similar transmigration cascade to the one proposed for leukocytes, such as rolling, secretion of cytokines and/or chemokines, activation of adhesion molecules, firm adhesion, and transendothelial migration.1,3 5
In this study, we showed that BM endothelial cells but not lung endothelial cells express VCAM-1 and that hematopoietic progenitor cells transmigrate through the BM endothelial cells, but not the lung endothelial cells, via the interaction between VLA-4 and VCAM-1. These results indicate that hematopoietic progenitor cells transmigrate into BM in an organ-specific manner, and that VLA-4/VCAM-1 play important roles in the homing of hematopoietic progenitor cells to BM. Both hematopoietic progenitor cells and endothelial cells express various adhesion molecules, including the integrin family and Ig super family.2,3,19,20 Previously it was reported that VLA-4, VLA-5, and LFA-1 expressed on hematopoietic progenitor cells were functionally important in hematopoiesis.21-24 Because VLA-5 is a classical fibronectin receptor and binds to the RGD site of fibronectin,25 it may play important roles in transmigration through basement membranes. VLA-4 binds to the CS-1 site of fibronectin as well as a member of the Ig superfamily, VCAM-1.26 Previous reports showed that the VLA4/VCAM-1 adhesion pathway played important roles in the homing of hematopoietic progenitor cells to BM.27-31 On the other hand, E-selectin, platelet endothelial cellular adhesion molecule (PECAM)-1, CD34, ELAM-1, ICAM-1, and VCAM-1 expressed on BM endothelial cells have been also reported to play important roles in hematopoiesis.32 The recent report by Schweitzer et al33 showed that E-selectin and VCAM-1 were expressed on endothelial cells of only hematopoietic tissues. Their findings are in accordance with ours that VCAM-1 was expressed only on the BM endothelial cells but not on the lung endothelial cells. Henninger et al34 reported in similar findings that there were significant differences in the expression of ICAM-1 and VCAM-1 among different tissues. In contrast to our findings, Mohle et al8 recently reported that LFA-1/ICAM-1 played important roles in transendothelial migration of hematopoietic progenitor cells. The discrepancy between their results and ours could be explained by the difference of adhesion molecules expressed on endothelial cells. The BM endothelial cells in our experiment expressed VCAM-1 constitutively but not ICAM-1, whereas their endothelial cells expressed ICAM-1 but not VCAM-1. Alternatively, all of our endothelial cell lines secreted chemoattractants including SDF-1 (Fig 3), which may have activated the adhesion molecules expressed on progenitors, especially VLA-4, and allowed the interaction between VLA-4 and VCAM-1 in our in vitro model. In fact, anti–SDF-1 antibody inhibited the transendothelial migration of FDCP-2 cells in a dose-dependent manner, suggesting that SDF-1 is necessary for transendothelial migration of hematopoietic progenitor cells. However, SDF-1 alone is not sufficient for the transendothelial migration of progenitor cells because FDCP-2 cells do not transmigrate through STR-2 cells that secrete SDF-1. Furthermore, chemoattractants secreted by BM endothelial cells may stimulate the expression of VCAM-1 on BM endothelial cells in an autocrine manner. This may explain why the BM endothelial cells but not the lung endothelial cells expressed VCAM-1 without any stimulation.
We examined the transendothelial migration in the presence of BM-fibroblast–conditioned medium that contained random motility-stimulating factor(s) and chemoattractant(s) including SDF-1 to make a similar situation in vitro to that of BM in vivo. Recently, Yong et al35 have reported that transmigration of CD34+ cells across endothelium was mediated by PECAM-1 (CD31). However, none of the chemoattractant was used in their transmigration experiments, which suggests that the transmigration of CD34+ cells in their experiment reflects the random migration rather than the chemotaxis. The difference in the assay system may account for the difference of adhesion molecules responsible for the transmigration of progenitor cells. STR-2 cells secreted SDF-1, which was reported to induce chemotaxis and transendothelial migration of progenitor cells.36-38 Aiuti et al37 have reported that SDF-1 induced the transendothelial migration of progenitor cells through mouse endothelial cells that did not express VCAM-1. Their experiments were performed by the use of human BM cells and a mouse endothelioma cell line. Therefore, the discrepancy between our results and their results may be explained by the differences of the endothelial cell lines used for the transendothelial migration assay. In addition, the motility-stimulating factor(s) other than SDF-1 contained in STR-2 cell-conditioned medium may influence the transendothelial migration. The nature of this random motility-stimulating activity is now under investigation. SDF-1 or SDF-1 plus random motility-stimulating activity secreted by BM fibroblasts may play important roles in attracting hematopoietic progenitor cells to BM.
ACKNOWLEDGMENT
We thank M. Yanome for assistance in preparing the manuscript.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Masanobu Kobayashi, MD, Laboratory of Pathology, Cancer Institute, Hokkaido University School of Medicine. Kita-15, Nishi-7, Kita-ku, Sapporo, 060 Japan; e-mail:mkobaya@med.hokudai.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal