Abstract
Heparin/heparan sulfate proteoglycans (HSPGs) have the potential to bind and directly regulate the bioactivity of hematopoietic growth factors including interleukin-7 (IL-7), a cytokine critical for murine B-cell development. We examined the consequence of manipulating soluble heparin and cell-surface heparan sulfate to IL-7–dependent responses of B-cell precursors. Soluble heparin was found to inhibit production of lymphoid, but not myeloid, cells in long-term bone marrow cultures. Analysis of pro-B cells lacking plasma membrane HS suggests that this glycosaminoglycan is required for efficient binding and responsiveness to IL-7. By contrast, responses of hematopoietic cells to other cytokines were not influenced by heparin addition or HS removal. Therefore, HSPGs on B-lineage precursors may function as IL-7 receptor components similar to HSPGs known to be important for the bFGF receptor. Other experiments suggest that HSPGs on the surface of stromal cells provide a weakly associating docking site for IL-7, possibly controlling availability of this cytokine to B-cell precursors. Together these data demonstrate a direct role for heparinlike molecules in regulating the IL-7–dependent stages of murine B lymphopoiesis.
GLYCOSAMINOGLYCANS (GAGs) present in the bone marrow (BM) are well situated to participate in the regulation of lympho-hematopoiesis. Proteoglycans tethered to the plasma membrane of lymphocytes and stromal cells, as well as GAGs synthesized as part of the extracellular matrix, may influence hematopoietic processes. Recent attention has been focused on heparin/heparan sulfate proteoglycans (HSPGs) as potential regulators of hematopoietic cell behavior. By mediating adhesive interactions and modulating cytokine bioactivity these glycosaminoglycans may contribute to the biological activity of specific BM microenvironments.
Heparin/HSPG-dependent interactions between hematopoietic precursors and the microenvironment may be important for cell anchorage as well as maturation processes. For example, heparan sulfate (HS) has been shown to be involved in adhesion and long-term maintenance of hematopoietic cells.1,2 Cell-surface molecules such as CD45, Mac-1, PECAM-1, and Thy-1, which are known to be heparin-binding proteins, may contribute to this interaction.3-5 Similarly, heparin/HSPGs may influence proliferation and differentiation of various hematopoietic lineages. Association with BM-derived HS induced morphological changes characteristic of differentiation in myeloid leukemic cells.6 Furthermore, interaction of pre-B cells with the STIM-Ig fusion protein, a stromal-derived molecule shown to augment interleukin-7 (IL-7)–driven proliferation, was blocked by heparin.7
Heparinlike GAGs are recognized to bind and potentially modulate the bioactivity of several hematopoietic regulatory factors including IL-7, granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-1, IL-3, and transforming growth factor-β (TGF-β).8-13 Interaction with heparin/HSPGs may serve to compartmentalize individual growth factors to specific niches of the microenvironment, regulate degradation of bioactive factors during transport, and provide an immobilized matrix upon which such factors can be presented to target cells.11,12,14 Heparin/HSPGs may also serve as coreceptors that facilitate interaction of growth factors with their receptors. For example, basic fibroblast growth factor (bFGF) does not bind to its high-affinity receptor in HS-deficient mutants but binding can be restored by addition of exogenous heparin.15 The syndecan family of cell-surface HSPGs, which has been shown to promote functional FGF:FGF-receptor complexing and signaling,16,17 may serve as such coreceptors. Interestingly, not all growth factors are positively influenced by interaction with GAGs. Binding of interferon-γ (IFN-γ) to heparin competitively inhibits IFN-γ:IFN-γ–receptor complex formation,18 highlighting the potential for selective regulation of cytokine bioactivity.
Murine B-lymphocyte development is critically dependent on IL-7 availability. Early B-cell precursors that do not receive this growth signal rapidly undergo apoptosis.19 Low concentrations of IL-7 may then favor the maturation of more differentiated cells.20 Despite observations that heparinlike molecules can bind to IL-7,8 9 the role of these GAGs in regulating IL-7 bioavailability/bioactivity at this point in development is unknown. Therefore, we examined the contribution of soluble heparin and cell surface HS to the IL-7–dependent stages of B lymphopoiesis.
EXPERIMENTAL PROCEDURES
Animals.
Male BALB/c mice 6 to 8 weeks old were obtained from the Oklahoma Medical Research Foundation Laboratory Animal Resources Center. Animals were housed in a room apart from other colonies and were maintained on a 12:12 light:dark cycle with food and water available ad libitum.
Media and reagents.
RPMI-1640, Fischer’s medium, minimal essential media (MEM)-α (α-MEM), Dulbecco’s phosphate-buffered saline (PBS; free of Ca2+ and Mg2+), and horse serum were purchased from GIBCO-BRL (Grand Island, NY). Fetal bovine serum (FBS) tested to be permissive for B-lineage lymphocyte growth was from Intergen Co (Purchase, NY). Heparin from two different sources (Sigma, St Louis, MO, and ICN Pharmaceuticals Inc, Costa Mesa, CA) was found to have similar activity and data using the Sigma preparation are presented. Chondroitin sulfate A was from Sigma. Recombinant murine GM-CSF and IL-7 were from R&D Systems (Minneapolis, MN).
Cell lines.
The cell lines DW34,21 2E8,22 F10 (T. Shimozato and P.W. Kincade, manuscript in preparation), BC7.12 (derived in our laboratory from BALB/c BM, unpublished), DA/GM and NFS-60 (responsive to GM-CSF and G-CSF, respectively; kindly provided by Dr Donna Rennick, DNAX Research Institute, Palo Alto, CA), FDC-P1,23 and CTLL-2 (clone TIB 214; American Type Culture Collection [ATCC], Rockville, MD) were maintained in RPMI-1640 complete medium (5% FBS, 2 mmol/L L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 5 × 10−5 mol/L β-mercaptoethanol). The rat lymphoma Nb2 (a gift of Dr Li-Yuan Yu-Lee, Baylor College of Medicine, Houston, TX) was maintained in Fischer’s medium containing 10% horse serum, 10−4mol/L β-mercaptoethanol, 0.5% gentamicin, 2 mmol/L L-glutamine. Factor-dependent cell lines were maintained in the excess of purified recombinant (r) GM-CSF, conditioned medium (CM) from IL-7–transfected NIH-3T3 cells, CM from IL-3–transfected Chinese hamster ovary (CHO) cells, or lung CM as a source of G-CSF24 as indicated (see Fig 2).
Long-term BM cultures (LTBMCs).
Whole BM (WBM) cells were cultured under lymphoid-permissive25 or myeloid-permissive26conditions. In lymphoid-supportive cultures, WBM cells resuspended in RPMI-1640 complete medium were aliquotted at 8.0 × 106 cells/6 mL/flask into 25-cm2 tissue culture flasks (Costar Corp, Cambridge, MA) and cultured at 37°C in 7% CO2. For establishment of myeloid-supportive cultures, WBM cells resuspended in α-MEM containing 20% horse serum, 10−6 mol/L hydrocortisone sodium succinate, 100 U/mL penicillin, and 100 μg/mL streptomycin were aliquotted at 12.0 × 106 cells/8 mL/flask and maintained at 33°C in 5% CO2. For establishment of switch cultures,27 flasks maintained under myeloid-supportive conditions for 5 weeks were gently rinsed and the medium replaced with lymphoid-supportive medium. Cultures were subsequently maintained under lymphoid-permissive conditions. In each LTBMC system, half of the medium was replaced with fresh medium weekly. Heparin or chondroitin sulfate A was added at the weekly feedings as described in the figure legends. Lineage identity of nonadherent cells was confirmed using fluorescently labeled antibodies specific to Gr-1 for myeloid-permissive cultures and CD19 or CD45 for lymphoid-permissive cultures (data not shown). Viability in all cultures was typically greater than 98%.
Removal of cell-surface HS.
Digestion with heparitinase (Seikagaku, Ijamsville, MD) was performed as previously described28 except that the reaction was performed in PBS (pH 7.0) containing 0.1% bovine serum albumin (BSA). The efficacy of heparitinase treatment was confirmed by flow cytometric analysis using the monoclonal antibodies (MoAbs) 10E4 and 3G10 (both from Seikagaku), which react with native HS and heparitinase-digested HS, respectively.29 SB/14 (rat IgG2a), a newly characterized antibody to the IL-7 receptor α chain (Y. Yamashita and P.W. Kincade, manuscript in preparation), was similarly used to analyze treated cells.
Immunofluorescence analysis of IL-7 binding.
Staining of cells with biotinylated IL-7 (kit no. NF700; R&D Systems) was performed according to the manufacturer’s instructions. In brief, washed cells were incubated with biotinylated IL-7 or the negative control reagent (biotinylated soybean trypsin inhibitor) for 45 minutes followed by incubation with avidin-fluorescein for another 30 minutes on ice. The specificity of biotinylated IL-7 was confirmed using the supplied neutralizing antibody. We also determined that biotinylated IL-7 retained biological activity as measured by the capacity to stimulate proliferation of F10 lymphocytes (data not shown). Identical procedures were followed for staining with biotinylated IL-2 (kit no. NF200; R&D Systems).
Proliferation assays.
WBM cells resuspended in RPMI-1640 complete medium or factor-dependent cells resuspended in the complete medium used to maintain each line were aliquotted at 1.0 to 2.0 × 104 cells/well to 96-well plates. Nonsaturating amounts of growth factor (as determined in titration experiments for each cell population) were pre-incubated with heparin for 30 minutes at room temperature after which the mixture was added to wells, in triplicate. The final concentration of heparin was 100 μg/mL in a total volume of 200 μL/well.
For proliferation experiments involving heparitinase, mock- or enzyme-digested cells were resuspended in RPMI-1640 complete medium and aliquotted at 1.0 to 2.0 × 104 cells/well. Fresh heparitinase (0.01 U/mL) or vehicle was added in the presence or absence of growth factor to a final volume of 100 μL. Flow cytometric analysis of a separate aliquot of cells confirmed the continued efficacy of heparitinase for at least 50 hours (data not shown).
In all assays, plates were incubated at 37°C in 7% CO2for 3 days. [3H]thymidine (65 Ci/mmol; ICN Pharmaceuticals Inc) was added at 1.0 μCi/well during the last 6 hours of incubation. Incorporated radioactivity was detected using a Beckman LS 6000SE liquid scintillation counter.
Determination of soluble sulfated glycosaminoglycan.
Confluent monolayers of BMS2,22 BMS2.4,30 or OP4231 stromal cells were cultured for 5 to 7 days in RPMI-1640 complete medium. Supernatant was collected, passed through a 0.2-μm filter to remove debris, and concentrated 10× using a spin freezer concentrator. Samples were assayed for sulfated glycosaminoglycan content using the Blyscan kit (Accurate Chemical & Scientific Corp, Westbury, NY) as described by the manufacturer except that the assay was scaled to 300 μL final volume. Aliquots of concentrated culture supernatant or concentrated RPMI-1640 complete medium were added to 300 μL of the Blyscan dye reagent and vortexed for 30 minutes. In some experiments, before addition of the Blyscan dye, concentrated supernatants were treated with 0.1 U/mL heparitinase for 30 minutes at 37°C after which another 0.1 U/mL heparitinase was added and the incubation repeated. Mock-treated controls received diluent during the incubation period. Samples were centrifuged at 8,000g for 10 minutes and the pellet solubilized in 300 μL of the dissociation reagent. After 30 minutes of vortexing optical density was read at 656 nm. The glycosaminoglycan concentration of samples was determined using a standard curve established with chondroitin-4-sulfate.
Bioassay of stromal cell-bound IL-7.
Stromal cells derived from IL-7 knockout mice (kindly provided by Dr Pamela Witte, Loyola University Medical Center, Maywood, IL) were resuspended in RPMI-1640 complete medium and seeded at ≈5.0 × 104/0.4 mL/well in 24-well tissue-culture plates. Cultures were incubated at 37°C in 7% CO2 for 1 day before use. Adherent stromal cells treated with or without heparitinase as described above were then incubated with purified murine rIL-7 (5 ng/well) for 30 minutes at 37°C followed by gentle washing to remove unbound cytokine. BC7.12 pro-B cells were added at 2 × 104 cells/well and cultures were incubated at 37°C in 7% CO2 for 3 days after which viable lymphocytes were enumerated by trypan blue exclusion.
RESULTS
Heparin abrogates lymphopoiesis but not myelopoiesis in LTBMC.
The capacity of heparin/HS to interact with essential growth factors such as IL-7 and the CSFs points to a specific mechanism by which these glycans can participate in hematopoiesis. Therefore, we investigated the ability of heparin to impact hematopoietic development using LTBMCs under conditions described for lymphopoiesis (Whitlock-Witte type)25 or myelopoiesis (Dexter type).26 LTBMCs established from BALB/c BM were treated with heparin (50 μg/mL) beginning at culture initiation and thereafter weekly. While control (vehicle alone) Whitlock-Witte cultures commenced lymphocyte production by 3 weeks, heparin-treated flasks did not produce detectable numbers of lymphocytes by 5 weeks (Fig 1A, left panel, solid lines). Lymphocyte development was not perturbed in LTBMCs treated with an equivalent amount of chondroitin sulfate A, a GAG shown not to block IL-7 bioactivity.8 9 In contrast to the inhibitory effect on lymphocyte outgrowth, heparin did not impair establishment of myeloid-permissive cultures (Fig 1A, left panel, dashed lines).
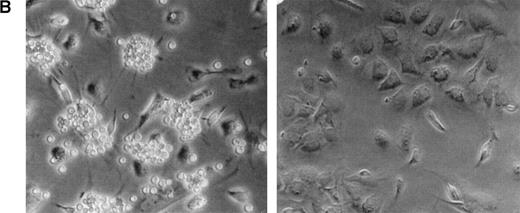
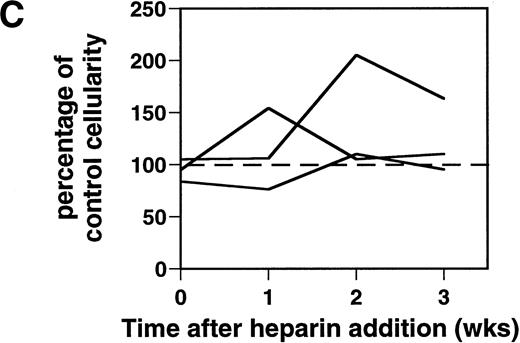
Effect of heparin on lymphopoiesis and myelopoiesis in LTBMCs. (A, left) WBM cells were seeded using lymphoid-supportive (solid line) or myeloid-supportive (dashed line) conditions as described in Experimental Procedures. Heparin (•; 50 μg/mL), chondroitin sulfate (▵; 50 μg/mL), or vehicle (○) was added weekly beginning at culture initiation. (Right) LTBMCs cultured under myeloid-permissive conditions for 5 weeks were switched (arrow) to lymphoid-permissive conditions. Heparin (50 μg/mL) or vehicle was added weekly throughout the entire assay. (B) LTBMCs maintained under lymphoid-supportive conditions were cultured in the absence (left) or presence (right) of 25 μg/mL heparin for 6 weeks. Original magnification × 100. (C) Lymphoid-permissive LTBMCs were established for 4 to 6 weeks in the absence of treatment and then treated weekly with 50 μg/mL heparin or vehicle. The data are presented relative to the number of lymphocytes recovered from flasks receiving vehicle alone and results from three independent experiments are shown.
Effect of heparin on lymphopoiesis and myelopoiesis in LTBMCs. (A, left) WBM cells were seeded using lymphoid-supportive (solid line) or myeloid-supportive (dashed line) conditions as described in Experimental Procedures. Heparin (•; 50 μg/mL), chondroitin sulfate (▵; 50 μg/mL), or vehicle (○) was added weekly beginning at culture initiation. (Right) LTBMCs cultured under myeloid-permissive conditions for 5 weeks were switched (arrow) to lymphoid-permissive conditions. Heparin (50 μg/mL) or vehicle was added weekly throughout the entire assay. (B) LTBMCs maintained under lymphoid-supportive conditions were cultured in the absence (left) or presence (right) of 25 μg/mL heparin for 6 weeks. Original magnification × 100. (C) Lymphoid-permissive LTBMCs were established for 4 to 6 weeks in the absence of treatment and then treated weekly with 50 μg/mL heparin or vehicle. The data are presented relative to the number of lymphocytes recovered from flasks receiving vehicle alone and results from three independent experiments are shown.
As shown in Fig 1B, Whitlock-Witte LTBMCs receiving heparin were completely devoid of lympho-hematopoietic foci. Despite the paucity of B-cell precursors, microscopic examination of heparin-treated cultures showed a normal adherent cell monolayer. This adherent layer was found to be competent to support propagation of foster lymphocytes, albeit at a reduced level (data not shown).
The differential effects of heparin on lymphopoiesis versus myelopoiesis were even more striking in a switch culture. In this culture system, BM cells propagated under myeloid-permissive conditions for 5 weeks were subsequently switched to lymphoid-supportive conditions. Heparin (50 μg/mL) or vehicle was added weekly throughout the entire experiment. Although heparin treatment had no discernible effect on myelopoiesis, lymphopoiesis in those same cultures was completely inhibited (Fig 1A, right panel). These data show that soluble heparin inhibits B lymphopoiesis in LTBMCs.
Temporal effects of heparin on lymphopoiesis.
Although heparin inhibited lymphopoiesis in flasks treated with this GAG beginning at the first week of culture (Fig 1A and B), heparin did not block LTBMCs that were well established before treatment. LTBMCs cultured for 4 to 6 weeks before commencement of weekly heparin treatment had normal-to-elevated levels of lymphopoiesis (Fig 1C). Heparin likely affects several qualitative aspects of the cell layer and the consequence to lymphopoiesis may vary, for example, according to whether the marrow is well established or remodeling (eg, during fetal development or regenerating after wounding).
Differential effects of heparin on factor-dependent proliferation.
In addition to lymphocytes and myeloid cells, hematopoietic tissue supports the outgrowth of several other cytokine-dependent lineages. We explored the effects of heparin using a panel of factor-responsive cells of hematopoietic origin (Fig 2). WBM cells or the indicated cell lines were stimulated with limiting amounts of growth factor in the presence of heparin or vehicle. While heparin blocked the growth of each IL-7–dependent population tested, this GAG did not noticeably affect the proliferation of cells dependent on several other growth factors including IL-3, G-CSF, GM-CSF, and lactogen. These data point to a regulatory effect of heparinlike GAGs that may be specific to IL-7.
Comparison of heparin effects on growth factor–dependent cells. WBM (105 cells/well) or cell lines (104cells/well) were stimulated with the indicated growth factors in the presence or absence of 100 μg/mL heparin and proliferation was assessed by [3H]thymidine incorporation. Growth factors were added in an amount that produced half-maximal stimulation for each population. The data are presented as mean ± SD of triplicate wells.
Comparison of heparin effects on growth factor–dependent cells. WBM (105 cells/well) or cell lines (104cells/well) were stimulated with the indicated growth factors in the presence or absence of 100 μg/mL heparin and proliferation was assessed by [3H]thymidine incorporation. Growth factors were added in an amount that produced half-maximal stimulation for each population. The data are presented as mean ± SD of triplicate wells.
IL-7 binds to native cell surface HS on B-cell precursors.
A prominent reservoir of heparinlike GAGs exists tethered to the plasma membrane of BM cells in the form of HS.32 33 Cell-surface HS present on lymphocytes and stromal cells could potentially participate in regulation of IL-7 bioavailability/bioactivity. To directly examine whether this cytokine interacts with GAGs on B-lymphocyte precursors, plasma membrane HS was destroyed using heparitinase. The efficacy of enzyme treatment was assessed using the MoAbs 10E4 and 3G10 which recognize native HS and a neo-epitope revealed by heparitinase treatment, respectively. Before enzymatic digestion, 60% of F10 lymphocytes were stained by 10E4 while less than 1% displayed the 3G10 epitope. After digestion, 99% of this population was stained by 3G10 indicating essentially complete removal of HS (Fig 3A and B).
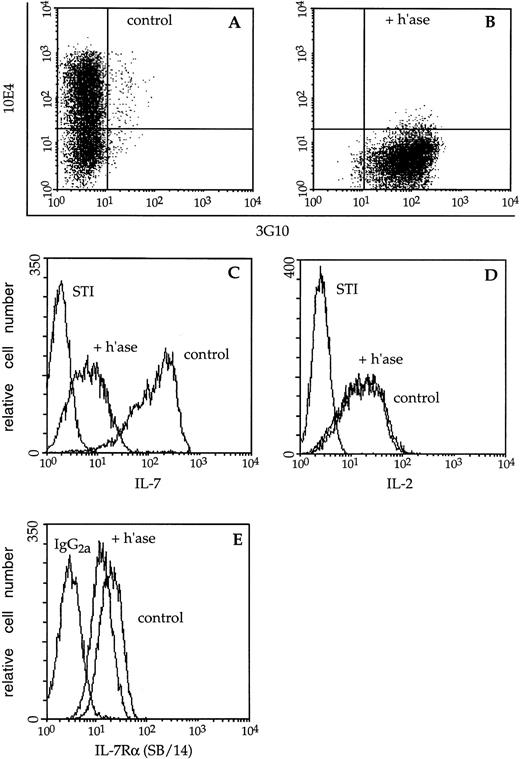
Immunofluorescence analysis of cytokine binding to lymphocyte cell-surface HS. (A) Mock- (control) or (B) heparitinase-digested (+ h’ase) F10 cells were stained and analyzed by two-color flow cytometry. Biotinylated 10E4 (followed by streptavidin-red 613) recognizes native cell-surface HS while 3G10 (FITC) detects heparitinase-cleaved HS. (C) The capacity of these B-cell precursors to bind biotinylated IL-7 or the negative control reagent, biotinylated soybean trypsin inhibitor (STI), followed by avidin-fluorescein, was subsequently assessed. (D) Binding of biotinylated IL-2 to control or heparitinase-treated CTLL-2 cells; the binding profiles directly overlap. (E) Control or heparitinase-digested F10 lymphocytes were stained with an antibody to the IL-7 receptor chain (SB/14) or rat IgG2a followed by goat anti-rat Ig-FITC.
Immunofluorescence analysis of cytokine binding to lymphocyte cell-surface HS. (A) Mock- (control) or (B) heparitinase-digested (+ h’ase) F10 cells were stained and analyzed by two-color flow cytometry. Biotinylated 10E4 (followed by streptavidin-red 613) recognizes native cell-surface HS while 3G10 (FITC) detects heparitinase-cleaved HS. (C) The capacity of these B-cell precursors to bind biotinylated IL-7 or the negative control reagent, biotinylated soybean trypsin inhibitor (STI), followed by avidin-fluorescein, was subsequently assessed. (D) Binding of biotinylated IL-2 to control or heparitinase-treated CTLL-2 cells; the binding profiles directly overlap. (E) Control or heparitinase-digested F10 lymphocytes were stained with an antibody to the IL-7 receptor chain (SB/14) or rat IgG2a followed by goat anti-rat Ig-FITC.
The bright staining of control F10 pro-B cells by biotinylated IL-7 was significantly diminished after digestion with heparitinase (Fig 3C). Heparitinase treatment similarly reduced IL-7 binding to BC7.12 (pro-B) and DW34 (pre-B) cells, as well as primary B-lymphocyte precursors derived from Whitlock-Witte LTBMCs (Table1). Despite the dramatic decrease in IL-7 binding, expression of the IL-7 receptor α chain on F10 cells was only slightly diminished after digestion with heparitinase (Fig 3E). Heparitinase treatment did not dramatically affect expression of the CD19, CD45, CD43, BP-1, or M1/69 antigens, confirming the specificity of this enzyme for HS (data not shown). In contrast to effects on IL-7 binding, the capacity of biotinylated IL-2 to interact with CTLL-2 cells was not affected by heparitinase (Fig 3D). These data suggest that membrane-associated HS may play an essential role in IL-7–dependent interactions.
IL-7 Binding to Cells Lacking Cell-Surface HS
| . | Mean Fluorescence Intensity of IL-7 Binding* . | |
|---|---|---|
| Control . | Heparitinase-Treated (% of control) . | |
| Hematopoietic cells | ||
| LTBMC lymphocytes | 159.70 | 31.24 (19.56) |
| F10 | 238.65 | 24.26 (10.16) |
| BC7.12 | 71.36 | 15.43 (21.62) |
| DW34 | 103.41 | 15.21 (14.71) |
| FDC-P1 | 10.15 | ND |
| Stromal cells | ||
| OP42 | 45.78 | 10.72 (23.42) |
| BMS2 | 54.06 | 13.16 (24.34) |
| BMS2.4 | 34.83 | ND |
| TEC | 36.02 | ND |
| NIH-3T3 | 66.26 | ND |
| . | Mean Fluorescence Intensity of IL-7 Binding* . | |
|---|---|---|
| Control . | Heparitinase-Treated (% of control) . | |
| Hematopoietic cells | ||
| LTBMC lymphocytes | 159.70 | 31.24 (19.56) |
| F10 | 238.65 | 24.26 (10.16) |
| BC7.12 | 71.36 | 15.43 (21.62) |
| DW34 | 103.41 | 15.21 (14.71) |
| FDC-P1 | 10.15 | ND |
| Stromal cells | ||
| OP42 | 45.78 | 10.72 (23.42) |
| BMS2 | 54.06 | 13.16 (24.34) |
| BMS2.4 | 34.83 | ND |
| TEC | 36.02 | ND |
| NIH-3T3 | 66.26 | ND |
Abbreviation: ND, not determined.
Undigested or heparitinase-digested cells were stained with biotinylated IL-7 as described in Experimental Procedures. Data are representative of 2 to 10 independent experiments, and the mean fluorescence intensity of the negative control reagent was <10 U for each cell type.
Loss of cell-surface HS diminishes responsiveness to IL-7.
A reduction in the capacity of lymphocytes to bind to IL-7 may have direct biological implications. To investigate if the interaction of IL-7 with cell-surface HS is necessary for efficient cytokine stimulation, we examined the capacity of lymphocytes which lack plasma membrane HS to respond to limiting amounts of IL-7. F10 cells treated in the presence or absence of heparitinase were cultured for 3 days with IL-7 and proliferation was assessed (Fig 4, solid bars). Digestion with heparitinase reduced IL-7–driven lymphocyte proliferation 50% to 75% compared with controls, a magnitude of inhibition similar to the effects of heparin. By contrast, heparitinase treatment had no discernible effect on the IL-3–dependent responses of FDC-P1 cells (Fig 4, hatched bars). These data directly demonstrate the importance of cell-surface HS to IL-7–dependent stages of B lymphopoiesis.
Cytokine responsiveness of heparitinase-treated hematopoietic cell lines. Mock- or heparitinase-digested F10 or FDC-P1 cells were cultured with the indicated growth factor in the presence or absence of heparin (100 μg/mL) and proliferation was assessed after 3 days by [3H]thymidine incorporation. The data are reported as the average ± SD of triplicate samples and are representative of five (F10) or two (FDC-P1) independent experiments.
Cytokine responsiveness of heparitinase-treated hematopoietic cell lines. Mock- or heparitinase-digested F10 or FDC-P1 cells were cultured with the indicated growth factor in the presence or absence of heparin (100 μg/mL) and proliferation was assessed after 3 days by [3H]thymidine incorporation. The data are reported as the average ± SD of triplicate samples and are representative of five (F10) or two (FDC-P1) independent experiments.
IL-7 binds to native cell-surface HS on stromal cells.
Stromal cells may support B lymphopoiesis in part by regulating local growth factor availability, for example, through selective compartmentalization.11 12 GAGs tethered to the plasma membrane as well as those released by stromal cells have the potential to regulate bioavailability of IL-7. Sulfated GAGs were detected in the CM of several different stromal cell lines (μg/mL sulfated GAG: cell-free medium, 0.29 ± 0.24; OP42 CM, 3.52 ± 0.06; BMS2.4 CM, 4.79 ± 0.14; BMS2 CM, 4.23 ± 0.03). However, only 20% to 30% was sensitive to heparitinase (heparitinase-digested BMS2 CM, 3.02 ± 0.18). Therefore, we focused on the potential of IL-7 to interact with membrane-associated HS.
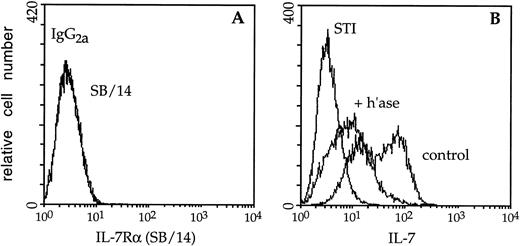
Fluorescence analysis showed that biotinylated IL-7 readily bound to BM-derived lymphocyte-supportive stromal cells (ie, BMS2 and OP42) (Table 1). By contrast, the myeloid cell line FDC-P1 was not detectably stained by IL-7. These results are particularly striking in that neither BMS2 nor OP42 expresses the IL-7 receptor α chain (Fig 5A, and data not shown), and point to a unique regulatory role for GAGs on the surface of these stromal cells. However, the binding of IL-7 to stroma appears to be of low avidity as much of the biotin-labeled cytokine could be removed by extensive washing (data not shown).
To directly confirm that cell-surface HS enabled IL-7 binding to stroma, BMS2 cells were treated with heparitinase. Before enzymatic treatment, BMS2 cells displayed a bimodal binding profile when stained with biotinylated IL-7 while subsequent to digestion, only a single dim fluorescent peak was observed (Fig 5B). A similar reduction in the capacity to bind IL-7 was observed upon heparitinase digestion of the OP42 stromal cell line (Table 1). The specificity of the heparitinase was confirmed in that no significant changes in CD44 or CD9 expression were detected upon treatment with this enzyme (data not shown).
Immunofluorescence analysis of IL-7 binding to stromal cell plasma membrane HS. (A) BMS2 stromal cells were stained with an antibody (SB/14) to the IL-7 receptor chain or rat IgG2a followed by goat anti-rat Ig-FITC; the binding profiles directly overlap. (B) Mock- (control) or heparitinase-digested (+ h’ase) BMS2 cells were stained with biotinylated IL-7 or the negative control reagent (STI) as described in the legend to Fig 3. Control BMS2 cells displayed a bimodal profile (rightmost peaks). Binding of biotinylated IL-7 was readily blocked using a neutralizing antibody to IL-7 and efficacy of the digestion procedure was confirmed using the 10E4 and 3G10 MoAbs (data not shown).
Immunofluorescence analysis of IL-7 binding to stromal cell plasma membrane HS. (A) BMS2 stromal cells were stained with an antibody (SB/14) to the IL-7 receptor chain or rat IgG2a followed by goat anti-rat Ig-FITC; the binding profiles directly overlap. (B) Mock- (control) or heparitinase-digested (+ h’ase) BMS2 cells were stained with biotinylated IL-7 or the negative control reagent (STI) as described in the legend to Fig 3. Control BMS2 cells displayed a bimodal profile (rightmost peaks). Binding of biotinylated IL-7 was readily blocked using a neutralizing antibody to IL-7 and efficacy of the digestion procedure was confirmed using the 10E4 and 3G10 MoAbs (data not shown).
Stromal cell-bound IL-7 stimulates B-lymphocyte precursors.
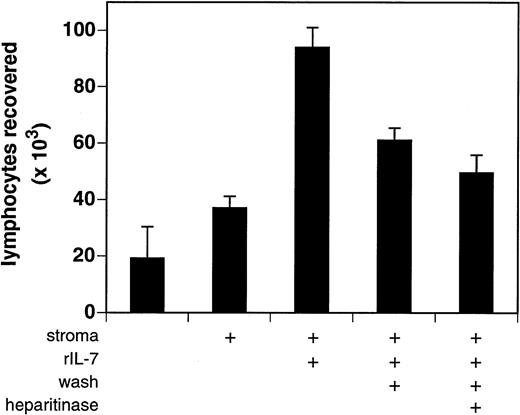
The bioactivity of stromal cell–bound IL-7 was assessed using the IL-7–dependent indicator cell line BC7.12. Stromal cells were incubated with 5 ng of purified rIL-7, gently washed to remove unbound cytokine, and cocultured with BC7.12 pro-B lymphocytes. Stromal cells that had been washed of excess IL-7 retained low but detectable amounts of bioactive cytokine (Fig 6). After 3 days of culture, 61,000 ± 4,500 BC7.12 lymphocytes were recovered in wells containing mock-digested IL-7–laden stroma versus only 37,000 ± 4,100 in wells containing mock-digested vehicle-treated stroma (P = .006; mean ± SD of four independent experiments). This capacity of stromal cells to retain bioactive IL-7 was significantly reduced by treatment with heparitinase (49,500 ± 6,400 BC7.12 lymphocytes; P = .0347 v mock-digested controls). Maximal proliferation was obtained in cultures of BC7.12 lymphocytes incubated with stroma and IL-7 in the absence of washing, indicating that only a fraction of the available soluble cytokine is bound by the stromal cell plasma membrane. These data indicate that the low avidity interaction of IL-7 with stromal cells is partially dependent on cell-surface HS.
Bioactivity of stromal cell–bound IL-7. Stromal cells (≈5 × 104/well) derived from IL-7 knockout mice were treated in the presence or absence of heparitinase and susbequently incubated with rIL-7 (5 ng/well) for 30 minutes at 37°C. Cells were washed to remove unbound IL-7 and then 2 × 104 BC7.12 lymphocytes were added to each well. Cultures were incubated for 3 days after which viable lymphocytes were enumerated. To assess maximal IL-7 bioactivity, the washing step was omitted in one treatment group (central bar). The results are presented as the average ± SD of four independent experiments.
Bioactivity of stromal cell–bound IL-7. Stromal cells (≈5 × 104/well) derived from IL-7 knockout mice were treated in the presence or absence of heparitinase and susbequently incubated with rIL-7 (5 ng/well) for 30 minutes at 37°C. Cells were washed to remove unbound IL-7 and then 2 × 104 BC7.12 lymphocytes were added to each well. Cultures were incubated for 3 days after which viable lymphocytes were enumerated. To assess maximal IL-7 bioactivity, the washing step was omitted in one treatment group (central bar). The results are presented as the average ± SD of four independent experiments.
DISCUSSION
Local availability of IL-7 reflects not only synthesis and stability of this growth factor but also compartmentalization to specialized sites within the BM. Such niches are shaped by the molecules displayed on stroma and stroma-derived matrix, as well as those present on hematopoietic cells. We examined the ability of heparinlike molecules to regulate the bioavailability and bioactivity of IL-7. We demonstrated lineage-specific effects of heparin manipulation in long-term culture of normal lympho-hematopoietic cells. In addition, we specifically focused on the role of native HSPGs on the plasma membrane of BM cells. Not only did HSPGs present on both B-lymphocyte progenitors and lymphocyte-supportive stroma direct binding of IL-7 to the cell surface, but this association was critical for efficient proliferative stimulation of B-cell precursors. This study establishes a direct role for HS in regulating the IL-7–dependent stages of B lymphopoiesis.
Heparinlike GAGs have been shown to serve as coreceptors for targeting growth factors such as FGF to plasma membrane receptors.15,34 In this case, interaction with GAGs is essential for binding of FGF to its high-affinity receptor. That HSPGs may similarly participate in directing IL-7 to the plasma membrane of target cells is suggested by two lines of evidence: the competitive affinity of IL-7 for heparin and heparan sulfate (kd of 25 nmol/L and 70 to 80 nmol/L,9 respectively, v kd of 6 to 11 nmol/L35 for binding of IL-7 to the IL-7 receptor), and the ability of each GAG to regulate IL-7 bioactivity. Our observations directly demonstrate that cell-surface HS is required for efficient labeling of B-cell precursors by IL-7. Removal of plasma membrane HS was associated with a dramatic inhibition of IL-7 binding and a consequent reduction in proliferative stimulation. There is some size variation in the IL-7 receptor that might be glycosylation-dependent,35 but HS has not been reported on the α or γ chains of the IL-7 receptor. During development in the BM, B-lineage precursors express different proteoglycans at different stages of development. For example, the HSPG syndecan-1 is expressed on B-cell precursors but is lost before extravasation to the peripheral compartment.36 Therefore, we consider it possible that HSPGs on B-lymphocyte precursors and/or stromal cells participate in IL-7–dependent events.
Positioning or “compartmentalization” of growth factors in the BM microenvironment is recognized to directly influence hematopoiesis. For example, localization of GM-CSF to GAGs on marrow-derived extracellular matrix drives granulocyte-macrophage colony formation,12and adsorption of GM-CSF and IL-3 on matrix HS serves to present these factors in biologically active form to multipotent precursors.11 Studies with T-lymphocyte lineage cells indicate that matrix immobilized IL-7 can deliver proadhesive signals,37,38 as can hepatocyte growth factor and MIP-1β.39 40 Our finding that heparinlike molecules enable binding of IL-7 not only to B-cell precursors, but also to BM-derived stromal cells implies an additional mechanism by which specific microenvironments may be generated at the plasma membrane.
HSPG-dependent targeting of cytokines to the surface of lymphocyte-supportive stromal cells may enable immobilization and presentation of specific growth factors to dependent hematopoietic cells. We found that IL-7 could bind to marrow-derived stromal cells (OP42, BMS2) as well as adherent cells derived from other tissues (TEC, NIH-3T3). These data correlate with our previous observations that several types of adherent cells could support the short-term survival of B-lymphocyte precursors,41 and suggest one mechanism by which these positive effects might occur. Interestingly, BMS2.4, a cell line shown to suppress the outgrowth of B-cell precursors,30 41 similarly binds IL-7. It will be interesting to determine if these stromal cells have an equivalent capacity to present IL-7 to dependent B-cell precursors or if, in some instances, the stroma-bound IL-7 is retained in a form unavailable to lymphocytes.
That the interaction of IL-7 with heparinlike molecules is unique is suggested by our observations that (1) heparin abrogates lymphopoiesis but not myelopoiesis in LTBMCs; (2) heparin inhibits IL-7–dependent proliferation but not that mediated by other hematopoietic cytokines including IL-3, G-CSF, and GM-CSF; (3) removal of HS with heparitinase blocks binding of IL-7 but not, for example, IL-2 to the plasma membrane; and (4) cells lacking HS have significantly diminished proliferative responses to IL-7 whereas responses to IL-3 are unaffected. It is important to note that while our studies used soluble heparin as a surrogate GAG, the specificity of the interaction between IL-7 and heparinlike molecules may be further refined in the marrow by local expression of different species of proteoglycans6,33,42,43 as well as by the fine structure of HS on each proteoglycan core. The MS-5 murine stromal cell, for example, synthesizes at least seven different HSPGs including syndecans.33 The proteoglycan core of syndecans can be uniquely sulfated by individual cells in a manner that alters the functional capacity of the molecule.44 These differences may determine whether the interaction of IL-7 with a particular cell-surface GAG is inhibitory, enhancing, or neutral to cytokine bioavailability/bioactivity.
In this study we defined the contribution of heparinlike molecules to the IL-7–dependent stages of B-cell development and described one way in which specific microenvironments may be created within the BM. Clearly, the potential exists not only for HSPGs to regulate additional hematopoietic processes within the marrow, but also for such mechanisms to operate in other tissue compartments.
ACKNOWLEDGMENT
We thank Dr Ralph Sanderson for helpful advice on removing cell-surface heparan sulfate; Viji Dandapandi and John Rummage for excellent technical work; and Shelli Wasson for secretarial assistance.
Supported by Grants No. AI20069 and AI33085 from the National Institutes of Health awarded to P.W.K. L.A.B. was supported by Training Grant No. HL07207-21.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Paul W. Kincade, PhD, Immunobiology and Cancer Program, Oklahoma Medical Research Foundation, 825 NE 13th St, Oklahoma City, OK 73104; e-mail: paul-kincade@omrf.ouhsc.edu.


![Fig. 2. Comparison of heparin effects on growth factor–dependent cells. WBM (105 cells/well) or cell lines (104cells/well) were stimulated with the indicated growth factors in the presence or absence of 100 μg/mL heparin and proliferation was assessed by [3H]thymidine incorporation. Growth factors were added in an amount that produced half-maximal stimulation for each population. The data are presented as mean ± SD of triplicate wells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/1/10.1182_blood.v93.1.140/5/m_blod40140002x.jpeg?Expires=1767827260&Signature=YERbP-XrEhy8gEIODdib9Z67o8j0n38AUpr7lakEv5QshHRSU4-w5ilzCuDMhuq9md8XX6GmeCAFYkKQzabcdU5hpUmwGVlOzfOfzVdbb06s9iAGlLxy1e~JDSqSa6nRT~xKOZ0ILje7OphaHO3GCbJokAAAQpvOh9uOa-DVNnqTWf3aLcMPz-IeXXCSn67zVfMsSjnx-S7eK~oHkIwe~xMugNXEqisNykWrLacDS9QCxsldvPZU-QKAR3DGzeQLiNRPT0RF8j7udMCQrsuS8brQFeorJyouku1oGhViL0~nxklr2k0nQgmmXJGQLSZaobU1RlyzbGW6LNeQZh6Rzg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Cytokine responsiveness of heparitinase-treated hematopoietic cell lines. Mock- or heparitinase-digested F10 or FDC-P1 cells were cultured with the indicated growth factor in the presence or absence of heparin (100 μg/mL) and proliferation was assessed after 3 days by [3H]thymidine incorporation. The data are reported as the average ± SD of triplicate samples and are representative of five (F10) or two (FDC-P1) independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/1/10.1182_blood.v93.1.140/5/m_blod40140004x.jpeg?Expires=1767827260&Signature=KyJapf94olmr6E8DmJUVJxFbti~dClBVrEDpdZFbYWP442vjNQYOdTAnTGR1P6uaNbkY4wr~fK1ThAPsUL3cKxKeNoAAq8hgWBKAoOmvVkfzkNL~WF~hqZw~FgsdjRZi6UzX~FdxcG~f6p917hD5FSxxmspQyi~MMGKIEdey0h6uKJhqXo-IQhnbLyy4dT~Fy3VJtAZRMxEmNWb3~tRLj-ZvrFNKkxoaDuCexuPAGcZKqB1YVZL4J55ibAWubSKvxrDQLflgTyTt7taX~Xq2kTFDg4dyOduPCKwoqFpxmxnkbPKwYOpvHlmI8gRVeyC~q2JpXFf~xhQialpyXA1s4A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal