Abstract
The modulation of the cytotoxic effects of an anthracyclin by CD40L was investigated in five non-Hodgkin’s lymphoma (NHL) cell lines (Daudi, Raji, BJAB, BL36, BL70). Incubation with doxorubicin (DOX) increased in a dose-dependent manner the percentage of apoptosis in NHL cells. Coculture with irradiated L cells expressing CD40L (CD40L L cells), but not CDw32 (CDw32 L cells), significantly reduced (33% to 89%) the percentage of apoptosis in all five cell lines treated with 0.1 to 0.5 μg/mL of DOX, but in only three cell lines at 1 μg/mL. Interleukin-10 (IL-10), IL-6, IL-2, or tumor necrosis factor (TNF) induced no additive protective effects with CD40L L cells. In all five cell lines, DOX induced a concentration-dependent increase of the activity of the cysteine-protease caspase 3. Coculture with CD40L L cells, but not with CDw32 L cells, inhibited (38% to 100%) the activation of caspase 3 induced by 0.1 to 0.5 μg/mL of DOX in all five NHL cell lines, but in only two cell lines at 1 μg/mL. Finally, the antiproliferative effect of 0.1 to 0.5 μg/mL concentrations of DOX was also partially abrogated on coculture with CD40L L cells in all five cell lines, but in only two cell lines at 1 μg/mL. Cytokines, either alone or in combination with CD40L L cells, did not affect DOX-induced inhibition of proliferation. These results indicate that CD40L inhibits the apoptosis and antiproliferative effect induced by DOX and interferes with caspase 3 activation in B NHL cell lines.
© 1998 by The American Society of Hematology.
ALTHOUGH NON-HODGKIN’S lymphoma (NHL) cells are sensitive to cytotoxic chemotherapy, primary or secondary chemoresistance frequently occurs and is the major cause of death of these patients.1,2 The biological mechanisms by which lymphoma cells acquire resistance to cytotoxic drugs are not fully understood.3 Although markers associated with the multi-drug resistance (MDR) phenotype, eg, P-glycoprotein (P-gp), are likely to play a role in this phenomenon, in vivo, chemoresistance of NHL is not consistently associated with P-gp expression indicating the existence of additional mechanisms.3
Apoptosis, or programmed cell death, is an active phenomenon dependent on RNA and protein synthesis, which plays an essential role in many normal processes, such as embryological development and immune cell selection.4-8 Apoptosis can be induced by ionizing radiation in cancer treatment, engagement of cell surface molecules such as Fas and tumor necrosis factor (TNF)-R, or deprivement in growth factors.7,9-12 Several cytotoxic agents act by inducing the apoptosis of tumoral cells.13-17 It has recently been shown that apoptosis caused by doxorubicin (DOX) in a human T-leukemia cell line is mediated via CD95L induction and subsequent activation of the CD95 pathway.17
CD40, a member of the TNF receptor superfamily, is expressed on B lymphocytes and interacts with a ligand (CD40L) expressed on activated T cells.18,19 CD40L exerts a complex modulation of B-cell apoptosis: it promotes the survival of germinal center B cell, but also induces Fas expression thereby rendering the cells sensitive to FasL or agonists.19-22 Several cytokines, in particular interleukin-10 (IL-10), IL-6, and IL-2, have also been reported to inhibit the apoptosis of normal or neoplastic B cells, in particular induced by cytotoxic agents, or FasL,23-27 a phenomenon associated with a modulation of the expression of bcl-2, bcl-xL, and/or BAG.28-30 CD40 is expressed at the surface of fresh B NHL cells and is able to trigger a proliferative signal in the presence of IL-6 and IL-10.19 31 The biological effect of a combination of cytokines and CD40L on the modulation of apoptosis of neoplastic B-cell lines induced by cytotoxic agents is not known.
In the present report, the capacity of CD40L to modulate the apoptosis of lymphoma cell lines induced by a cytotoxic agent was investigated. CD40L-expressing L cells were found capable of partially inhibiting the apoptosis and antiproliferative effect of DOX on lymphoma cells, as well as the activation of the cysteine protease caspase 3.32 33 These results uncover a new mechanism of resistance to cytotoxic agents conferred by adjacent nontumoral cells expressing CD40L.
MATERIALS AND METHODS
Cell lines and culture conditions.
Lymphoma cell lines (Daudi, Raji, BJAB, BL36, BL70) were grown at 106 cells/mL in RPMI 1640 (GIBCO-BRL, Gaithersburg, MD) containing 10% fetal calf serum (FCS), 100 U/mL penicillin, 100 mg/mL streptomycin (GIBCO) and 2 mmol/L L-glutamine. CDw32/FcγRII and CD40 ligand (CD40 L) transfected Ltk (-) cell line (CDw32 L cells and CD40L L cells) were kindly provided by Dr F. Rousset and Dr J. Banchereau (Schering-Plough, Dardilly, France). The CD40L L cells were previously published and are capable of inducing normal B-cell growth and Ig secretion.22 The expression of CD40L on the cell line used in the present report was evaluated before the initiation of these experiments using anti-CD40L labeling experiments. CD40L was detectable at the surface of 95% of CD40L L cells and 40% of phytohemagglutinin (PHA)-activated T-cell line Jurkatt used as control. CDw32 expressing L cells were also used in these experiments alone or in the presence of an anti-CD40 agonist antibody (monoclonal antibody [MoAb] 89) at 0.5 μg/mL to mimic the effect of CD40L. In some experiments, various concentrations of MoAb 89 were used, ranging from 0.05 μg/mL to 2 μg/mL.
DOX was added at the initiation of the culture during 24 hours (0.5 or 1 μg/mL) or 30 hours (0.1 μg/mL). A 24-hour exposure was chosen because of the long cellular half-life of this compound in vivo in B NHL cells; a prolonged exposure to DOX may be more relevant to the in vivo situation, as previously reported.17 34 After 24 hours, the medium was then removed and replaced with the same culture medium without DOX for 24 additional hours of culture. Irradiated (75 Gy) L-cell lines (ie, CD40L or CDw32 expressing L cells) were added at the initiation of DOX exposure at 105 cells/mL. Cytokines (IL-10, IL-6, IL-2, and TNF) were added at the initiation of the culture with or without DOX. Apoptosis and caspase 3 activation were tested 48 hours after the initiation of culture.
Drugs, cytokines, and antibodies.
DOX (Pharmacia, Paris, France) was dissolved in sterile distilled water before each experiment. Recombinant human (rh) IL-10 and MoAb 89 (anti-CD40 antibody) were provided by Dr F. Rousset and Dr J. Banchereau (Schering-Plough, Dardilly, France). The monoclonal anti-CD40L antibody was purchased from Immunotech (Marseilles, France). rhIL-10 was used at 100 ng/mL. rhIL-2 (Eurocetus, Amsterdam, the Netherlands) was used at 100 U/mL; rhIL-6 (Sandoz, Basel, Switzerland) was used at 40 ng/mL; rhTNFα (Eurocetus) was used at 10 ng/mL. The caspase 3 (Yama/CPP-32/apopain)-specific Asp-Glu-Val-Asp (DEVD) tetrapeptide labeled with a fluorogenic substrate (7 amino-4-trifluoro methyl coumarin, AFC), DEVD-AFC, and the inhibitor (DEVD-CHO) (Tebu, Le Perray-en-Yvelines, France) were used at 50 μmol/L.24 A cell permeable inhibitor of caspase 3 (Tebu, Le Perray-en-Yvelines, France) was used at 100 μmol/L and added at the initiation of cell culture.
Determination of apoptosis.
NHL cells were removed from 24-wells microtiter plates using gentle aspiration to avoid the removal of adherent CD40L L cells. The phenotype of the recovered cell population was consistently analyzed. The mean size of L cells was fourfold to fivefold higher than the mean size of the B NHL cell lines. In all experiments, the size and the level of CD20 expression was similar in noncocultured B NHL cell lines and in the recovered cell population, indicating that the contamination of recovered NHL cells by irradiated L cells is minimal, if it exists. The percentages of apoptotic NHL cell lines after removal from the CD40L or CD32 L cells layer ranged from 2% for the BL70 cell line to 23% for the Daudi cell line and were not found significantly different from that of cell lines cultured alone. For the quantification of apoptosis in the recovered cell population, DNA breaks characteristic of apoptotic cells were assessed by terminal deoxytransferase (TdT)-mediated deoxyuridine triphosphate (d-UTP) nick end labeling (TUNEL) assay (Boehringer Mannheim Corp, Indianapolis, IN). NHL cell lines were fixed with 1% paraformaldehyde, permeabilized with 0.1% Triton X100 in 0.1% sodium citrate and washed extensively. Incubation with TdT and fluorescein-labeled d-UTP provided visualization of DNA strand breaks by flow cytometry on a FACScan instrument (Becton Dickinson, Pont de Claix, France).
In each sample, 2,000 cells were analyzed for their content in fluorescein-labeled DNA strand breaks. The intensity of fluorescence was proportional to the number of fluorescein-labeled DNA strand breaks within the cell. The threshold level of fluorescence intensity beyond which cells were considered to be apoptotic was 101.
Proliferation assay.
NHL cell lines (3.5 × 104 cells in 200 μL) were cultured in 96-well flat-bottomed microtiter plates. DOX was added at the initiation of the culture during 24 hours (0.5 or 1 μg/mL) or 30 hours (0.1 μg/mL). After 24 hours, the medium was removed and replaced with the same culture medium without DOX for 24 additional hours of culture. Irradiated (75 Gy) L cell lines (ie, CD40L or CDw32 expressing L cells) were added at the initiation of DOX exposure at 2.5 × 104 cells/mL. After 72 hours (or more) of culture, cells were pulsed with 1 mCi/well of [3H]TdR (25 Ci/mmol, Amersham, Les Ulis, France) for 20 hours. [3H]TdR incorporation was measured by tritium detector using standard liquid scintillation counting techniques on a β counter (Packard, Rungis, France).
Assay for caspase 3 (Yama/CPP32/apopain) activity.
After 48 hours of culture, cells were harvested, washed in phosphate-buffered saline (PBS), and then resuspended in the lysis buffer (5× buffer Cold Spring Harbor [CSH], Triton 0.01%, orthovanadate 1×, protease inhibitor 1×) at 4°C for 30 minutes and finally centrifuged at 4°C for 15 minutes at 13,000g. For each cell lysate, three measures were performed.The negative control: cell lysates were preincubated at 30°C for 3 hours with DEVD-CHO in the reaction buffer (50 mmol/L HEPES, 10% sucrose, 0.1% CHAPS [pH, 7.5]) and 10 mmol/L dithiothreitol (dTT) before addition of the DEVD-AFC substrate. The blank group: DEVD-AFC substrate incubated in the reaction buffer only at 30°C for 1 hour. The assay group: cell lysates were directly incubated 1 hour at 30°C with DEVD-AFC substrate in the reaction buffer and dTT. Fluorescence measurements were performed after 1 (t0) and 2 hours (t60) of incubation. Caspase 3 activity was detected by AFC release monitored on a spectrofluorometer (Kontron Analytical SFM 25, Velizy, France), using an excitation wavelength of 400 nm and an emission wavelength of 505 nm. Caspase 3 activity was calculated with the following formula: 1 unit = (dFU/min) × (calibration curve slope)−1 × (1 U/1 × 10−6 mmol AFC/min), where dFU is the difference of fluorescence units: (FU of the assay group at t60 − FU of the blank group at t60) − (FU of the assay group at t0 − FU of the blank group at t0).
Statistics.
Statistical analyses were performed using paired Student’st-test.
RESULTS
Modulation of DOX-induced apoptosis by CD40L.
The capacity of CD40L expressed on L cells to modulate the apoptosis induced by DOX in B-lymphoma cell lines (BJAB, Daudi, BL70, BL36, Raji) was investigated. Incubation with DOX during 24 hours at therapeutic34 concentrations (0.1 to 1 μg/mL) induced a dose-dependent increase of apoptosis in the five cell lines tested (Fig 1). With a shorter duration (3 hours) of incubation, DOX at 1 to 2 μg/mL also induced the apoptosis of these cell lines, while lower concentrations (0.1 to 0.5 μg/mL) of DOX were not cytotoxic (not shown).
Dose-dependent induction of apoptosis by DOX in five lymphoma cell lines. Lymphoma cells were incubated with DOX for 24 hours (0.5 or 1 μg/mL) or 30 hours (0.1 μg/mL), then washed, and cultured without DOX, but otherwise in the same conditions during 24 additional hours, before quantification of the percentage of apoptotic cells. DOX concentrations: 0 μg/mL (1, ▪), 0.1 μg/mL (2, □), 0.5 μg/mL (3, ▧), 1 μg/mL (4, ). These results are the mean (and standard deviation [SD]) of five different experiments.
Dose-dependent induction of apoptosis by DOX in five lymphoma cell lines. Lymphoma cells were incubated with DOX for 24 hours (0.5 or 1 μg/mL) or 30 hours (0.1 μg/mL), then washed, and cultured without DOX, but otherwise in the same conditions during 24 additional hours, before quantification of the percentage of apoptotic cells. DOX concentrations: 0 μg/mL (1, ▪), 0.1 μg/mL (2, □), 0.5 μg/mL (3, ▧), 1 μg/mL (4, ). These results are the mean (and standard deviation [SD]) of five different experiments.
All five NHL cell lines tested express CD40 at their cell surface (>95% of positive cells, not shown). Coculture of lymphoma cell lines in the presence of irradiated (75 Gy) L cells expressing CD40L (CD40L L cells) resulted in a decrease of the percentage of lymphoma cell undergoing apoptosis (Fig 2A), as compared with lymphoma cells cultured with DOX alone or with L cells expressing CDw32, ie, the FcγRII receptor for IgG (Fig 2B). Coculture with CD40L L cells significantly (Student’s t-test, P< .05) reduced the intensity of TdT signal as well as the percentage (33% to 89%) of apoptotic cells after treatment with DOX as compared with no CD40L L cells or with CDw32 L cells (Fig 2B, Table 1), in all five cell lines at concentrations of 0.1 to 0.5 μg/mL. With 1 μg/mL of DOX, the inhibition of apoptosis was significant only for the Daudi, Raji, and BL36 cell lines (Fig 2C). Coculture with CDw32 L cells (without anti-CD40 Ab) did not significantly affect the apoptosis of the five NHL cell lines incubated with a 0.5-μg/mL concentration of DOX (Table1), or with 0.1 or 1 μg/mL (not shown). However, the addition of the agonist IgG anti-CD40 antibody (MoAb 89, 0.5 μg/mL) to the coculture of NHL cells with CDw32 expressing L cells, thus mimicking the effect of CD40L expressed at the cell surface, reduced the percentage of apoptotic NHL cells similarly to CD40L L cells (Table 1). Of note, a similar protective effect was observed with lower concentrations of anti-CD40 (0.05 μg/mL): for instance, in the BL70 cell line, the percentage of apoptotic cells was reduced from 57% ± 2% to 21% ± 1% with a 2-μg/mL concentration of MoAb 89 and to 19% ± 1% with a 0.05-μg/mL concentration of MoAb 89 in a representative experiment. The apoptosis induced by a shorter (3 hours) exposure to DOX (2 μg/mL) was inhibited similarly on coculture with CD40L L cells, with a reduction of the percentage of apoptotic cells ranging from 30% ± 2% for BL70 to 55% ± 2.5% for Daudi (not shown).
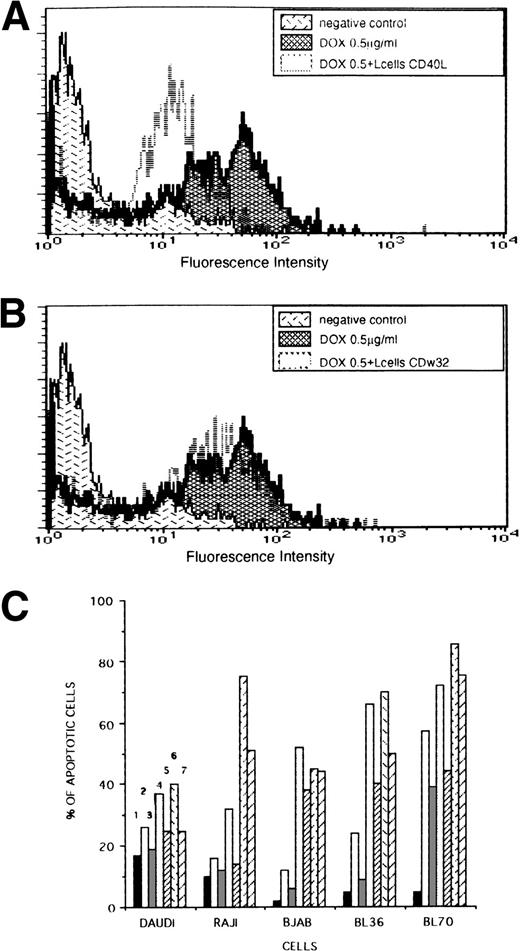
Inhibition of DOX-induced apoptosis by CD40L L cells in lymphoma cell lines. Lymphoma cells were incubated with DOX for 24 hours (0.5 or 1 μg/mL) or 30 hours (0.1 μg/mL) with or without irradiated (75 Gy) L cells expressing CD40L or CDw32, then washed, and cultured without DOX, but otherwise in the same conditions during 24 additional hours and tested for apoptosis using the TUNEL assay as indicated in Materials and Methods. (A and B) Inhibition of DOX-induced apoptosis of the BL36 cell line by CD40L. The intensity of fluorescence is proportional to the number of fluorescein-labeled DNA strand breaks within lymphoma cells. The threshold level of fluorescence intensity beyond which cells were considered to be in apoptosis was 101; 5% of lymphoma cells not treated with DOX were above this level. BL36 cells were cultured 48 hours without DOX (negative control) (5% ± 0.4% of apoptotic cells) or with 0.5 μg/mL of DOX during the first 24 hours (65% ± 1% of apoptotic cells) with irradiated CD40L L cells (40% ± 1% of apoptotic cells) (A) or CDw32 L cells (65% ± 1% of apoptotic cells) (B). (C) Inhibition of DOX-induced apoptosis by CD40L L cells in lymphoma cell lines. Cell lines were exposed to various concentrations of DOX during the first 24 hours: 0 μg/mL (1), 0.1 μg/mL (2) and (3), 0.5 μg/mL (4) and (5), 1 μg/mL (6) and (7) and cocultured in the presence of irradiated L cells expressing CD40L (3), (5), and (7). The SD of the percentages is under 2% in all conditions. This experiment is representative of seven different experiments.
Inhibition of DOX-induced apoptosis by CD40L L cells in lymphoma cell lines. Lymphoma cells were incubated with DOX for 24 hours (0.5 or 1 μg/mL) or 30 hours (0.1 μg/mL) with or without irradiated (75 Gy) L cells expressing CD40L or CDw32, then washed, and cultured without DOX, but otherwise in the same conditions during 24 additional hours and tested for apoptosis using the TUNEL assay as indicated in Materials and Methods. (A and B) Inhibition of DOX-induced apoptosis of the BL36 cell line by CD40L. The intensity of fluorescence is proportional to the number of fluorescein-labeled DNA strand breaks within lymphoma cells. The threshold level of fluorescence intensity beyond which cells were considered to be in apoptosis was 101; 5% of lymphoma cells not treated with DOX were above this level. BL36 cells were cultured 48 hours without DOX (negative control) (5% ± 0.4% of apoptotic cells) or with 0.5 μg/mL of DOX during the first 24 hours (65% ± 1% of apoptotic cells) with irradiated CD40L L cells (40% ± 1% of apoptotic cells) (A) or CDw32 L cells (65% ± 1% of apoptotic cells) (B). (C) Inhibition of DOX-induced apoptosis by CD40L L cells in lymphoma cell lines. Cell lines were exposed to various concentrations of DOX during the first 24 hours: 0 μg/mL (1), 0.1 μg/mL (2) and (3), 0.5 μg/mL (4) and (5), 1 μg/mL (6) and (7) and cocultured in the presence of irradiated L cells expressing CD40L (3), (5), and (7). The SD of the percentages is under 2% in all conditions. This experiment is representative of seven different experiments.
Inhibition of DOX-Induced Apoptosis by CD40L and IL-10
| Culture Conditions . | No. of Apoptotic Cells: Mean % of Control (SE) . | ||||||
|---|---|---|---|---|---|---|---|
| DOX (0.5 μg/mL) . | L Cells . | Cytokines . | Daudi . | Raji . | BJAB . | BL36 . | BL70 . |
| − | — | — | 24 (4) | 9 (11) | 0 (0) | 2 (0) | 3 (2) |
| + | — | — | 100-150 | 100-150 | 100-150 | 100-150 | 100-150 |
| + | CD40L | — | 36 (14)-151 | 57 (12)-151 | 54 (9)-151 | 52 (4)-151 | 65 (15)-151 |
| + | CDw32 | — | 90 (7) | 85 (10) | 95 (9) | 105 (10) | 101 (7) |
| + | Cdw32+Ab89 | 49 (11)-151 | 64 (9)-151 | 57 (8)-151 | NT | 77 (7)-151 | |
| + | — | IL-10 | 59 (18)-151 | 76 (14) | 53 (11)-151 | 85 (2)-151 | 76 (7)-151 |
| + | CD40L | IL-10 | 33 (10)-151 | 40 (7)-151 | 50 (3)-151 | 40 (5)-151 | 47 (11)-151 |
| Culture Conditions . | No. of Apoptotic Cells: Mean % of Control (SE) . | ||||||
|---|---|---|---|---|---|---|---|
| DOX (0.5 μg/mL) . | L Cells . | Cytokines . | Daudi . | Raji . | BJAB . | BL36 . | BL70 . |
| − | — | — | 24 (4) | 9 (11) | 0 (0) | 2 (0) | 3 (2) |
| + | — | — | 100-150 | 100-150 | 100-150 | 100-150 | 100-150 |
| + | CD40L | — | 36 (14)-151 | 57 (12)-151 | 54 (9)-151 | 52 (4)-151 | 65 (15)-151 |
| + | CDw32 | — | 90 (7) | 85 (10) | 95 (9) | 105 (10) | 101 (7) |
| + | Cdw32+Ab89 | 49 (11)-151 | 64 (9)-151 | 57 (8)-151 | NT | 77 (7)-151 | |
| + | — | IL-10 | 59 (18)-151 | 76 (14) | 53 (11)-151 | 85 (2)-151 | 76 (7)-151 |
| + | CD40L | IL-10 | 33 (10)-151 | 40 (7)-151 | 50 (3)-151 | 40 (5)-151 | 47 (11)-151 |
Abbreviation: NT, not tested.
Control.
P < .05 (Student’s paired t-test) as compared with DOX-treated cells in 7 experiments.
IL-10, IL-2, IL-6, and TNF were tested for their capacity to modulate CD40L-mediated inhibition of apoptosis. The percentage of apoptotic cells induced by DOX (0.5 μg/mL) was significantly reduced when NHL cells were incubated with IL-10 alone in four cell lines (Table 1). IL-2 and IL-6 also marginally reduced DOX-induced apoptosis in the BJAB cell line only, while TNF alone had no effect (not shown). No additive protective effect was observed between CD40L and IL-10 in any cell line tested (Table 1) or between CD40L and the other cytokines tested.
Activation of caspase 3 by doxorubicin: Modulation by CD40L.
Caspase 3 (Yama/CPP32/apopain) is a key enzyme involved in the cleavage of poly (ADP-ribose) polymerase (PARP) at the onset of apoptosis.32 33 DOX was found to induce a dose-dependent increase of caspase 3 activity in the five NHL cell lines (Table 2). This increase of caspase 3 activity was completely inhibited when cell lysates were preincubated with the caspase 3 inhibitor DEVD-CHO32 (Table 2). In all five cell lines, coculture with CD40L L cells, but not with CDw32 L cells, inhibited DOX-induced (0.1 or 0.5 μg/mL) caspase 3 activity by 38% to 100% (Table 2). With a 1-μg/mL concentration of DOX in contrast, coculture with CD40L L cells significantly reduced caspase 3 activity only in the BJAB and BL70 cell lines (Table 2).
Modulation of Caspase 3 Activity by DOX and CD40L
| Culture Conditions . | Caspase 3 Activity (U/2 × 105cells) . | |||||
|---|---|---|---|---|---|---|
| DOX (μg/mL) . | L Cells . | Daudi . | Raji . | BJAB . | BL36 . | BL70 . |
| — | — | 5.8 | 2.42 | 1.97 | 4.52 | 0.32 |
| 0.1 | — | 4.57 | 2.33 | 5.35* | 5.21* | 0.69* |
| 0.1 | CD40L | 4.49 | 1.85 | 2.62† | 1.77† | 0.43† |
| 0.5 | — | 7.22* | 5.33* | 12.07* | 8.6* | 2.71* |
| 0.5 | CD40L | 4.97† | 3.25† | 6.27† | 5.94† | 0.94† |
| 1 | — | 8.28* | 6.79* | 13.33* | 9.1* | 3.54* |
| 1 | CD40L | 7.69 | 5.53 | 5.92† | 8.68 | 1.45† |
| 0.5 | CDw32 | 7.26* | 8.56* | 14.12* | 8.71* | 4.34* |
| 0.5 | INH‡ | 0* | 0* | 0* | 0* | 0* |
| Culture Conditions . | Caspase 3 Activity (U/2 × 105cells) . | |||||
|---|---|---|---|---|---|---|
| DOX (μg/mL) . | L Cells . | Daudi . | Raji . | BJAB . | BL36 . | BL70 . |
| — | — | 5.8 | 2.42 | 1.97 | 4.52 | 0.32 |
| 0.1 | — | 4.57 | 2.33 | 5.35* | 5.21* | 0.69* |
| 0.1 | CD40L | 4.49 | 1.85 | 2.62† | 1.77† | 0.43† |
| 0.5 | — | 7.22* | 5.33* | 12.07* | 8.6* | 2.71* |
| 0.5 | CD40L | 4.97† | 3.25† | 6.27† | 5.94† | 0.94† |
| 1 | — | 8.28* | 6.79* | 13.33* | 9.1* | 3.54* |
| 1 | CD40L | 7.69 | 5.53 | 5.92† | 8.68 | 1.45† |
| 0.5 | CDw32 | 7.26* | 8.56* | 14.12* | 8.71* | 4.34* |
| 0.5 | INH‡ | 0* | 0* | 0* | 0* | 0* |
*P < .05 (Student’s paired t-test) as compared with culture without DOX in 7 experiments.
P < .05 (Student’s paired t-test) as compared with cells treated with DOX in 7 experiments.
Caspase 3 inhibitor (tetrapeptide DEVD-CHO, 50 μmol/L).
IL-10, IL-2, IL-6, and TNF alone also significantly inhibited DOX-induced caspase 3 activity in three cell lines, respectively (Table 3). IL-10 or IL-6 exerted a significant additive inhibitory effect on DOX-induced caspase 3 activity in the Daudi cell line only (Table 3).
Modulation of Caspase 3 Activity by Cytokines
| Culture Conditions . | Caspase 3 Activity (U/2 × 105cells) . | ||||||
|---|---|---|---|---|---|---|---|
| DOX (0.5 μg/mL) . | CD40L L Cells . | Cytokines . | Daudi . | Raji . | BJAB . | BL36 . | BL70 . |
| − | − | — | 1.38 | 1.48 | 1.3 | 5.82 | 2.25 |
| + | − | — | 5.2 | 14.8 | 9.3 | 8.6 | 6.4 |
| + | + | — | 3.2* | 7.9* | 4.3* | 5.9* | 0.9* |
| + | − | IL-10 | 3.3* | 8.9* | 7.5* | 6.9 | 9.2 |
| + | − | IL-2 | 3.9* | 6.4* | 6.9* | 7.9 | 5.3 |
| + | − | IL-6 | 3.2* | 10.0* | 7.0* | 6.2 | 5.2 |
| + | − | TNF | 3.2* | 10.9* | 4.7* | 6.8 | 3.6 |
| + | + | IL-10 | 1.1† | 6.5 | 5.5 | 6.7 | 0.8 |
| + | + | IL-2 | 2.8 | 6.9 | 6.1 | 6.7 | 0.6 |
| + | + | IL-6 | 2.3† | 6.8 | 5.5 | 5.3 | 0.6 |
| + | + | TNF | 2.7 | 9.0 | 6.0 | 6.5 | 2.5 |
| Culture Conditions . | Caspase 3 Activity (U/2 × 105cells) . | ||||||
|---|---|---|---|---|---|---|---|
| DOX (0.5 μg/mL) . | CD40L L Cells . | Cytokines . | Daudi . | Raji . | BJAB . | BL36 . | BL70 . |
| − | − | — | 1.38 | 1.48 | 1.3 | 5.82 | 2.25 |
| + | − | — | 5.2 | 14.8 | 9.3 | 8.6 | 6.4 |
| + | + | — | 3.2* | 7.9* | 4.3* | 5.9* | 0.9* |
| + | − | IL-10 | 3.3* | 8.9* | 7.5* | 6.9 | 9.2 |
| + | − | IL-2 | 3.9* | 6.4* | 6.9* | 7.9 | 5.3 |
| + | − | IL-6 | 3.2* | 10.0* | 7.0* | 6.2 | 5.2 |
| + | − | TNF | 3.2* | 10.9* | 4.7* | 6.8 | 3.6 |
| + | + | IL-10 | 1.1† | 6.5 | 5.5 | 6.7 | 0.8 |
| + | + | IL-2 | 2.8 | 6.9 | 6.1 | 6.7 | 0.6 |
| + | + | IL-6 | 2.3† | 6.8 | 5.5 | 5.3 | 0.6 |
| + | + | TNF | 2.7 | 9.0 | 6.0 | 6.5 | 2.5 |
*P < .05 (Student’s paired t-test) as compared with DOX-treated cells in 6 experiments.
P < .05 (Student’s paired t-test) as compared with DOX-treated cells cocultured with CD40L L cells.
CD40L increases the proliferation of doxorubicin-treated NHL cells.
DOX induced a dose-dependent inhibition of tritiated thymidine uptake by the five lymphoma cell lines at 72 hours of culture (Fig 3) and also after 96, 120, 144, and 240 hours of culture (not shown). When cocultured with CD40L L cells, but not with CDw32 L cells, the proliferation of the five cell lines treated with 0.1 to 0.5 μg/mL of DOX was partially restored at 72 hours (Fig 3) and also at 96, 120, 144, and 240 hours of culture (not shown). In contrast, CD40L L cells reversed the antiproliferative effect of a 1-μg/mL concentration of DOX in only two of the five cell lines (Daudi, BL70) (Fig 3). Thymidine incorporation by NHL cells cultured with DOX alone or with CDw32 L cells was similar (not shown). Cytokines (IL-10, IL-2, IL-6, or TNF) either alone or added to the coculture of lymphoma cells with CD40L L cells did not further increase the proliferative potential of these cell lines after exposure to DOX (not shown).
CD40L L cells stimulate the proliferation of DOX-treated lymphoma cells. Cells were incubated with DOX 0 μg/mL (1), 0.1 μg/mL (2) and (3), 0.5 μg/mL (4) and (5), 1 μg/mL (6) and (7) for 24 hours (0.5 or 1 μg/mL) or 30 hours (0.1 μg/mL) and with (3), (5), and (7) or without (1), (2), (4), and (6) irradiated CD40L L cells, then washed and cultured without DOX, but otherwise in the same conditions during 48 additional hours before measurement of [3H]TdR incorporation. [3H]TdR incorporation of all five lymphoma cell lines treated with DOX 0.1 μg/mL (2 v 3), 0.5 μg/mL (4 v 5) was significantly (Student’s t-test, P < .05) increased on coculture with irradiated CD40L L cells as compared with no L cells or L cells expressing CDw32 (not shown); with 1 μg/mL of DOX (6 v 7), the increase was significant only for the Daudi and BL70 cell lines. These results are the mean and SD of five different experiments.
CD40L L cells stimulate the proliferation of DOX-treated lymphoma cells. Cells were incubated with DOX 0 μg/mL (1), 0.1 μg/mL (2) and (3), 0.5 μg/mL (4) and (5), 1 μg/mL (6) and (7) for 24 hours (0.5 or 1 μg/mL) or 30 hours (0.1 μg/mL) and with (3), (5), and (7) or without (1), (2), (4), and (6) irradiated CD40L L cells, then washed and cultured without DOX, but otherwise in the same conditions during 48 additional hours before measurement of [3H]TdR incorporation. [3H]TdR incorporation of all five lymphoma cell lines treated with DOX 0.1 μg/mL (2 v 3), 0.5 μg/mL (4 v 5) was significantly (Student’s t-test, P < .05) increased on coculture with irradiated CD40L L cells as compared with no L cells or L cells expressing CDw32 (not shown); with 1 μg/mL of DOX (6 v 7), the increase was significant only for the Daudi and BL70 cell lines. These results are the mean and SD of five different experiments.
DISCUSSION
The results presented here show that drug resistance to a major cytotoxic agent can be induced by a ligand expressed on adjacent nontumoral cells. Coculture of lymphoma cells with CD40L-expressing L cells (or with L cells expressing CDw32, ie, FcγRII, in the presence of an IgG anti-CD40) was found capable (1) to partially inhibit the apoptosis of lymphoma cell lines induced by therapeutic concentrations of DOX, (2) to inhibit the activation of caspase 3 induced by DOX, and (3) to increase the proliferative potential of these cell lines after the end of exposure to DOX. An external signal provided by adjacent cells, L cells in the present case, is therefore capable of inducing the resistance of lymphoma cells to the proapoptotic and antiproliferative effects of cytotoxic drugs. Of note, the addition of the same concentrations of the anti-CD40 Ab alone without CDw32 L cells did not significantly protect these cell lines against DOX-induced apoptosis (not shown). CD40 ligand mRNA has been found detectable in lymphoma tumor samples by reverse transcriptase-polymerase chain reaction (RT-PCR).31 Recently, the presence of CD40L protein in NHL tumor samples has been reported in aggressive NHL and follicular NHL tumor samples.35 36 Conceivably, CD40L expressed on nontumoral infiltrating T cells may play a similar role in vivo, protecting tumor cells against the cytotoxic activity of anticancer drugs. However, the protective role of CD40L in vivo still remains to be demonstrated. It must indeed be noted that the level of CD40L expression at the surface of CD40L L cells is superior to that of T cells; for instance, CD40L was detectable at the surface of 95% of CD40L L cells as compared with 40% of PHA activated T-cell line Jurkatt. Therefore, we cannot exclude that the lower levels of CD40L expression in vivo may induce a less potent protective effect than CD40L L cells. However, it must be noted that the protective effect of CDw32 L cells with anti-CD40 Ab on DOX-induced apoptosis was similar when all five NHL cell lines were incubated with a 2-μg/mL of anti-CD40 Ab or 40-fold lower concentrations (0.05 μg/mL), suggesting that even low levels of expression of CD40L or CD40 agonist Ab are capable of protecting B NHL cells against drug-induced apoptosis.
Interestingly, at the highest (1 μg/mL) concentration of DOX tested, CD40L failed to protect some of the NHL cell lines against the apoptosis and antiproliferative effect of DOX, thereby providing a biological basis for the use of a high dose of cytotoxic agents to overcome this mechanism of drug resistance.2 Of note, the level of CD40 expression was high in all five cell lines and no correlation between the level of CD40 expression and the protective response to CD40L was observed (not shown), suggesting that the differential protective effect of CD40L in these cell lines may result from differences in the intracellular signaling pathways of CD40 in these cell lines.
The protective effect of CD40L L cells on DOX-induced apoptosis was not enhanced by the addition of IL-10, IL-2, IL-6, or TNF. Interestingly, however, IL-10 alone blocked the induction of apoptosis by 0.1 to 0.5 μg/mL concentrations of DOX in four lymphoma cell lines, whereas IL-6 and IL-2 exerted this effect in only one cell line. The magnitude of the protective effect of cytokines on DOX-induced apoptosis was less important than that of CD40L and the cytokines tested did not synergize with CD40L. Some of the NHL cell lines tested in this study produce IL-10 (BJAB) or IL-6 (BL70 and RAJI). However, the level of IL-10 and IL-6 are in the range of 100 to 300 pg/mL/106cells/24 hours (not shown), and therefore 100-fold to 200-fold lower than those used in the experiments. In addition, no correlation between cytokine production by NHL cell lines and the modulation of drug-induced apoptosis by the same cytokines was observed. These observations suggest that the endogenous production of IL-10 or IL-6 is unlikely to affect significantly the results presented in this study. Although IL-10 and IL-6 are frequently produced locally in NHL tumors in vivo,31 other biological phenomena may account for the negative prognostic value of the overproduction of these cytokines in vivo in lymphoma patients.37-39
Coculture with L cells expressing CD40L and/or in the presence of cytokines inhibited the activation of caspase 3 induced by DOX. The cytokines tested were found to inhibit caspase 3 activation alone and additively to CD40L for IL-6 and IL-10. The inhibition of caspase 3 activity has been reported to protect cells against the apoptosis induced by camptothecin and etoposide.32 40 The coexistence of the inhibitory effects of CD40L L cells on DOX-induced apoptosis and on caspase 3 activation suggests that caspase 3 inhibition is involved in the antiapoptotic effect of CD40L. However, the results shown here indicate that the cytotoxic activity of DOX is likely to involve other pathways because (1) the inhibition of caspase 3 activation was not consistently associated with a protective effect on apoptosis in these experiments, (2) a low (0.1 μg/mL) concentration of DOX induces the apoptosis in all five cell lines, but increases caspase 3 activity in only three of the five cell lines, (3) finally, the modulation of caspase 3 activity is not correlated with the protection from apoptosis by CD40L, in particular at a 1-μg/mL concentration of DOX.
The intracellular pathways involved in the protective effects of CD40L and cytokines on drug-induced apoptosis are currently under investigation. Several studies have shown that the apoptosis induced by TNF and cytotoxic agents is suppressed by NF-κB induction.41-43 CD40 induces the activation of NF-κB through a TNF associated factor-2 (TRAF2)-mediated mechanism suggesting a possible role of NF-κB in the antiapoptotic effect of CD40L.44 IL-10, IL-2, and IL-6 have also been reported to inhibit the apoptosis of B-lymphoid cells and other hematopoietic cell lines,23-27 by modulating the expression of genes of the bcl-2 family.28-30 Preliminary results indicate that bcl-2 expression is not affected by CD40L or cytokines in any of the five cell lines studied (Voorzanger et al, unpublished results). However, a recent report suggests that CD40L may upregulate bcl-xL expression in lymphoma cell lines suggesting a possible role of this protein in the above-described phenomenon.45
In conclusion, these results show a new mechanism of drug resistance of lymphoma cells triggered by CD40L expressed on adjacent nontumoral cells. The expression of CD40L by nontumoral cells in tumor microenvironment could play a role in drug resistance of lymphoma cells in vivo.
Supported by La Ligue contre le Cancer (Comités Départementaux du Rhône, de la Saône et Loire, de la Drôme, et de l’Ardèche), L’Association pour la Recherche sur le Cancer.
Address reprint requests to Jean-Yves Blay, MD, PhD, Unité Cytokine et Cancer, Unité INSERM U453, Centre Léon Bérard, 28, rue Laennec, 69008 Lyon, France; e-mail:blay@lyon.fnclcc.fr.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. Dose-dependent induction of apoptosis by DOX in five lymphoma cell lines. Lymphoma cells were incubated with DOX for 24 hours (0.5 or 1 μg/mL) or 30 hours (0.1 μg/mL), then washed, and cultured without DOX, but otherwise in the same conditions during 24 additional hours, before quantification of the percentage of apoptotic cells. DOX concentrations: 0 μg/mL (1, ▪), 0.1 μg/mL (2, □), 0.5 μg/mL (3, ▧), 1 μg/mL (4, ). These results are the mean (and standard deviation [SD]) of five different experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/9/10.1182_blood.v92.9.3381/5/m_blod42102001x.jpeg?Expires=1769224285&Signature=msBOuNIxcYG11ZMI2BXnnm4ypLYAYAXSZZtc2lxdUiiMdNXyXkEvbsKZVJi5JTbcNYzfvSuWzHG2f~Mkzv0awC4iPBzswNby5IB~CXByabap6VRHKyL0jm46GT03HXy82kzRYVt3FSUJ7jatH7ivjVutDGaboB3nMsIXepaB1LqXWqLTvzOG-xm9a4FLABHoTGilac9iu3rbOvEr7Tk5tVUvPlPNUbsYVyfETPcqZJqarNRaz1qepYNxXRERTLPrsgg3-Rr0h0xaY9ZvFoErdDH5ZPYlNg1fMYHqKfDTEaAhd9RF769gwrJAElA2uAFqfJ5jojXQtQ3VDEZQNuSeqw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. CD40L L cells stimulate the proliferation of DOX-treated lymphoma cells. Cells were incubated with DOX 0 μg/mL (1), 0.1 μg/mL (2) and (3), 0.5 μg/mL (4) and (5), 1 μg/mL (6) and (7) for 24 hours (0.5 or 1 μg/mL) or 30 hours (0.1 μg/mL) and with (3), (5), and (7) or without (1), (2), (4), and (6) irradiated CD40L L cells, then washed and cultured without DOX, but otherwise in the same conditions during 48 additional hours before measurement of [3H]TdR incorporation. [3H]TdR incorporation of all five lymphoma cell lines treated with DOX 0.1 μg/mL (2 v 3), 0.5 μg/mL (4 v 5) was significantly (Student’s t-test, P < .05) increased on coculture with irradiated CD40L L cells as compared with no L cells or L cells expressing CDw32 (not shown); with 1 μg/mL of DOX (6 v 7), the increase was significant only for the Daudi and BL70 cell lines. These results are the mean and SD of five different experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/9/10.1182_blood.v92.9.3381/5/m_blod42102003x.jpeg?Expires=1769224285&Signature=cBe4-t6hDcsv0u0xTNtZz3I6iaC-nJdxAUrhD20a0FSq0MrF3Rp7YrCo8LEhj6yPHB-~105uJbeZtZB3NFTv~zuK7D9xGNg~spaBYfS4G7oSeaDpWXpvfPU8KZbayNdbmXEmkxllo1aTtLhXrbgI956EF1hTOBrGUMhj2dY4-jpJWWNr7oh4tXTSGtjZioH5tHRURF7OPMzZovOE1Svc6-r8wmwynx59IH0nXH8jD89mwPFSPJEpLBaGk1zxPEmr1VsSYKAlOSUF5Lz9u25MG3YAxapFnTfHoi2fxwmmE6VdGnrzmNv9cJMuteFoKMz5UjRZYj8lpBx5CbVPhc~YPw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal