Abstract
The adenosine deaminase (ADA) inhibitor 2′-deoxycoformycin (dCF) significantly inhibits the proliferation of leukemia and lymphoma cell lines. When cells were incubated in the presence of both dCF and 2′-deoxyadenosine (dAd), the concentration of dCF required to induce apoptosis of monocytoid leukemia cells was much lower than that required for myeloid, erythroid, or lymphoma cell lines. Among the cell lines tested, U937 cells were the most sensitive to this treatment. The concentration of dCF that effectively inhibited the proliferation of U937 cells was 1/1,000 of that required for lymphoma cell lines, on a molar basis. However, the uptake of dCF or dAd in U937 cells was comparable with that in other leukemia and lymphoma cell lines. The intracellular accumulation of dATP in U937 cells was only slightly higher than that in other leukemia cells in dCF-treated culture. Treatment with dCF plus dAd induced apoptosis in U937 cells at low concentrations, and this apoptosis was reduced by treatment with caspase inhibitors. Induction of caspase-3 (CPP32) activity accompanied the apoptosis induced by dCF plus dAd. No activation of CPP32 was observed in cytosol prepared from exponentially growing leukemia and lymphoma cells. However, dATP effectively induced CPP32 activation in cytosol from monocytoid cells, but not in that from nonmonocytoid cells, suggesting that dATP-dependent CPP32 activation is at least partly involved in the preferential induction of apoptosis in monocytoid leukemia cells. The combination of dCF and dAd may be useful for the clinical treatment of acute monocytic leukemia.
© 1998 by The American Society of Hematology.
WITH PROGRESS in chemotherapy, the frequency of complete remission of acute myelogenous leukemia (AML) has increased. However, acute monocytic leukemia is more resistant to intensive chemotherapy than other types of AML, and its prognosis is poor. Even when remission is achieved by treatment with conventional cytotoxic antileukemic drugs, the median duration of remission is only about 6 months.1 These results in acute monocytic leukemia clearly call for improved therapies.
2′-Deoxycoformycin (dCF), a nucleoside analog produced byAspergillus nidulans Y176-2,2 is a specific and potent inhibitor of adenosine deaminase (ADA). dCF has been effectively used to treat hairy cell leukemia,3 adult T-cell leukemia/lymphoma,4 cutaneous T-cell lymphoma,5acute lymphoblastic leukemia,6 chronic lymphocytic leukemia,7 and other lymphoid malignancies. The mechanism of action of dCF, either as an antitumor agent in vivo or as an antiproliferative agent when combined with the ADA substrate 2′-deoxyadenosine (dAd) in vitro, is not completely understood. However, it is generally believed to be mediated through the accumulation of dAd after ADA inhibition. These changes have been reported to result in elevated levels of dATP,8 inhibition of S-adenosyl homocysteine (SAH) hydrolase,9 and single-strand breaks in DNA.10
Recently, we showed that dCF induced functional and morphological differentiation of myeloid leukemia HL-60 and NB4 cells in combination with dAd, but not alone.11 Differentiation of these cells was effectively induced by clinically applicable concentrations of dCF in the presence of dAd. Although dCF has been reported to be less effective in clinical trials against myeloid malignancies than against lymphoid ones, dCF might be useful for treating some myeloid malignancies. Preliminary results showed that human monocytic leukemia U937 cells were much more sensitive to dCF than human lymphoma cell lines with regard to the inhibition of cell proliferation. Therefore, in the present study, we examined the growth-inhibitory effect of dCF on several human myeloid, monocytoid, and lymphoid cell lines and the possible mechanism(s) of selective apoptosis of monocytoid leukemia cells.
MATERIALS AND METHODS
Materials.
dCF was obtained from The Chemo-Sero-Therapeutic Research Institute (Kumamoto, Japan). dAd, thymidine (dT), deoxyguanosine (dG), deoxycytidine (dC), 5′-amino-5′-dAd, 1-[β-D-arabinofuranosyl] cytosine (Ara C) and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium (MTT) were purchased from Sigma Chemical Co (St Louis, MO). Polyethyleneimine (PEI)-cellulose thin-layer plastic sheets were purchased from Merck (Darmstadt, Germany). [G-3H]dCF (179 GBq/mmol) and [8-14C]dAd (2072 MBq/mmol) were obtained from Moravek Biochemicals, Inc (Brea, CA). Acetyl-Tyr-Val-Ala-Asp-aldehyde (YVAD), acetyl-Asp-Glu-Val-Asp-aldehyde (DEVD), and benzyloxycarbonyl-Asp-CH2OC(O)-2,6,-dichlorobenzene (Z-Asp-CH2-DCB) were purchased from Peptide Laboratories (Osaka, Japan). Monoclonal antibodies against human CPP32 (caspase-3) and cytochrome c were purchased from Transduction Laboratories (Lexington, KY) and PharMingen (San Diego, CA), respectively.
Cell lines and cell culture.
Human myeloid (HL-60 and NB4), monocytoid (U937, THP-1, and HEL/S), erythroid (K562, KU812, and HEL) leukemia, and B-cell lymphoma (BALM3, SKW-4, and U-698-M) cell lines were cultured in suspension in RPMI 1640 medium supplemented with 10% fetal bovine serum at 37°C in a humidified atmosphere of 5% CO2 in air.12 The B-cell lymphoma cell lines were donated by Dr Yoshinobu Matsuo (Hayashibara Biochemical Laboratories, Okayama, Japan).
Assay of cell growth and apoptosis.
Cells (105/mL) were suspended in 2 mL of culture medium and cultured with or without test compounds in multidishes (Costar, Cambridge, MA). Cell numbers were counted with a Model ZM Coulter Counter (Coulter Electronics, Luton, UK) after culture for the indicated times. Cell viability in the above experiments was examined by MTT assay, as described previously.13 Morphological changes were examined in cell smears stained with May-Grünwald-Giemsa stain. The cellular DNA content was analyzed using propidium iodide–stained nuclei.14
Measurement of ADA activity.
Cells were washed three times with phosphate-buffered saline (PBS) and cell suspension (107/0.1 mL) was lysed by sonication for 30 seconds. After centrifugation at 2,000g for 10 minutes, the supernatant fluid was used as a crude cell extract for assay of ADA activity. Activity was measured in terms of the conversion of dAd to deoxyinosine in the presence of cell extract.14
Uptake of dAd and dCF.
Cells were incubated with [14C]dAd or [3H]dCF at final external concentrations of 10 to 50 μmol/L or 0.1 to 10 μmol/L for various periods of time ranging from 0.25 to 24 hours, respectively. At each time point, an aliquot of cells was obtained by centrifugation and washed twice with PBS. The radioactivity of the cell pellets was counted in a liquid scintillation counter.
Measurement of dATP accumulation.
Cells (3 × 106 cells/5 mL) were preincubated with or without 0.1 μmol/L dCF at 37°C for 2 hours. Triplicate cultures were incubated with 50 μmol/L [14C]dAd for 1 hour. The incubation was stopped by adding 5 vol of cold PBS and washed three times with cold PBS. Nucleosides and nucleotides were then extracted with tetrahydrofuran at 4°C for 2 hours.15 Ascending chromatography on a PEI-cellulose plate was used to separate dAd and its metabolites. Authentic compounds were added to the supernatant and an aliquot was spotted on a chromatography sheet, which was developed in a solvent system of 0.1 mol/L LiCl. The zones corresponding to authentic compounds were evaluated by autoradiography with a Fuji Bio-Image Analyzer BSA2000 (Fuji Film Co, Ltd, Tokyo, Japan).
Assay for ICE and CPP32 activity.
ICE (caspase-1) and CPP32 (caspase-3) activities were assayed with the fluorogenic substrates acetyl-Tyr-Val-Ala-Asp-7-amino-4-methylcoumarin (YVAD-MCA) and acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin (DEVD-MCA) (Peptide Institute, Inc, Osaka, Japan).16Briefly, 107 cells were extracted with 1 mL of 10 mmol/L Tris-HCl (pH 8.1) containing 9 mmol/L 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate, 5 mmol/L dithiothreitol, 1 mmol/L phenylmethanesulfonyl fluoride, 100 μmol/L leupeptin, 1.5 μmol/L pepstatin, 18.4 μmol/mL phosphoramidon, and 5 mmol/L EDTA at 0°C for 30 minutes, and then centrifuged at 12,000 rpm for 2 minutes. Extracts were stored at −80°C until use. For assay, the extracts were mixed with the substrate (final, 100 μmol/L) and Tris-HCl (final, 10 mmol/L; pH 7.4), and incubated at 37°C for 90 minutes. An equal volume of 1 mol/L acetic acid was added and the supernatants were analyzed by a fluorescence spectrophotometer (excitation at 370 nm and emission at 460 nm). Enzyme activity was expressed as pmol aminomethylcoumarin/min/mg protein.17
Preparation of cytosol and immunoblot analysis.
Exponentially growing cells were obtained and washed three times with cold PBS. The cells were suspended in 5 vol of cold extraction buffer (20 mmol/L HEPES-KOH [pH 7.5], 10 mmol/L KCl, 1.5 mmol/L MgCl2, 1 mmol/L sodium EDTA, 1 mmol/L sodium EGTA, 1 mmol/L dithiothreitol, and 1 mmol/L phenylmethylsulphonyl fluoride). After sitting on ice for 15 minutes, the cells were broken by passing 10 times through a G28 needle. After centrifugation in a microcentrifuge for 15 minutes at 4°C, the supernatants were further centrifuged at 105g for 60 minutes in a table-top ultracentrifuge (Beckman, Palo Alto, CA). The resulting supernatants were used to assay activation of CPP32. An aliquot (10 μL) of cytosol (50 μg protein) was incubated in the presence of dATP at 37°C for 1 hour in a final volume of 20 μL of extraction buffer. Samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to Immobilon polyvinylidene difluoride membrane (Millipore, Bedford, MA). Western blot analysis was performed as described previously.18 19
RESULTS
Effects of dCF and dAd on the growth of several human leukemia and lymphoma cell lines.
We examined the growth-inhibitory effects of dCF and dAd on several leukemia and lymphoma cell lines (Fig 1). dCF alone did not inhibit the growth of any of the cell lines tested, even at a high concentration (data not shown), and dAd had only a modest growth-inhibitory effect. When the cells were treated with 10 μmol/mL of dAd for 4 days, growth was inhibited by 0% to 5%. Cell growth was dose-dependently inhibited by dCF in the presence of 10 μg/mL dAd (Fig 1). Monocytic leukemia U937, THP-1, and HEL/S cells were much more sensitive to the growth-inhibitory activity of dCF and dAd than other cells: 9.2 to 9.7 nmol/L dCF in the presence of 10 μmol/mL dAd inhibited growth by 50% (IC50). The cells were cultured with various concentrations of dCF in the presence of 10, 30, and 50 μmol/mL of dAd for 4 days, and IC50concentrations of dCF were calculated. The IC50s of monocytoid leukemia cells were three orders of magnitude lower than those of the other leukemia and lymphoma cells. The synergistic effect of dCF and dAd was also clearly observed in the treatment of U937 cells for 2 days (data not shown).
Combined effects of dCF and dAd on the growth of several human leukemia and lymphoma cells. Cells were cultured with various concentrations of dCF in the presence of 10 μmol/L dAd for 4 days. Monocytic: U937 (•), THP-1 (○), HEL/S (◐); myeloid: HL-60 (▪), NB4 (□); erythroid: K562 (▴), KU812 (▵), HEL (); B-lymphoma: BALM3 (▾), SKW-4 (▿), U-698-M ().
Combined effects of dCF and dAd on the growth of several human leukemia and lymphoma cells. Cells were cultured with various concentrations of dCF in the presence of 10 μmol/L dAd for 4 days. Monocytic: U937 (•), THP-1 (○), HEL/S (◐); myeloid: HL-60 (▪), NB4 (□); erythroid: K562 (▴), KU812 (▵), HEL (); B-lymphoma: BALM3 (▾), SKW-4 (▿), U-698-M ().
Reduction of the growth-inhibitory effect of dCF plus dAd on U937 cells by other nucleosides.
dCF effectively inhibits ADA, which catalyzes the conversion of adenosine and dAd into inosine and deoxyinosine, respectively, by deamination. If dCF is administered, the dAd level in the blood increases as a result of the inhibition of ADA activity, and dAd is incorporated into cells where it undergoes triphosphorylation and is converted into dATP. This strongly inhibits ribonucleotide reductase (RNR) activity, leading to the inhibition of DNA synthesis.20 In addition, dAd can inhibit the methylation of DNA and RNA by inhibiting SAH hydrolase, an enzyme that is essential for methylation.21,22 When U937 cells were treated with 10 μmol/L each of dC, dT, and dG in the presence of dCF, no significant inhibition of growth was observed. Adenosine was also ineffective in inhibiting cell growth in combination with dCF, suggesting that the combination of dAd and dCF is specific for the growth-inhibitory effect. The growth-inhibitory effect of dAd plus dCF was decreased by the addition of dC, dT, and dG (Fig 2). The addition of 10 μmol/L dC alone did not reduce the growth-inhibitory effect of dCF plus dAd (data not shown). When neplanocin A, a potent inhibitor of SAH hydrolase inhibitor,22 was applied along with various concentrations of dAd and/or dCF, dAd and/or dCF did not significantly enhance the growth inhibition induced by neplanocin A, suggesting that the growth-inhibitory effect of dCF plus dAd on U937 cells is not primarily mediated by the inhibition of SAH hydrolase activity.
Effects of other nucleosides on the growth of monocytoid (U937, THP-1), K562 erythroid, or HL-60 myeloid cell lines treated with dCF and dAd. Cells were cultured with various concentrations of dCF in the presence of 0 (▪), 10 μmol/L dAd (•), 10 μmol/L each of dC + dG + dT (▴), or 10 μmol/L each of dAd + dC + dG + dT (⧫) for 4 days.
Effects of other nucleosides on the growth of monocytoid (U937, THP-1), K562 erythroid, or HL-60 myeloid cell lines treated with dCF and dAd. Cells were cultured with various concentrations of dCF in the presence of 0 (▪), 10 μmol/L dAd (•), 10 μmol/L each of dC + dG + dT (▴), or 10 μmol/L each of dAd + dC + dG + dT (⧫) for 4 days.
Effects of dAd analogs on the growth of several human leukemia cells.
Treatment with 5′-amino-5′-dAd, an inhibitor of adenosine kinase,14 dose-dependently reduced the growth inhibition induced by the combination of dCF and dAd in monocytoid U937 and THP-1 cells (Fig 3). A low concentration of this drug (1 μmol/L) reversed the growth inhibition in the monocytoid cells treated with 1 μmol/L dCF and 50 μmol/L dAd, suggesting that adenosine phosphorylating activity may be involved in the growth-inhibitory effect of dCF plus dAd.
Reduction of the growth-inhibitory effect of dCF plus dAd by 5′-amino-5′-dAd. Cells were cultured with 50 μmol/L dAd and various concentrations of dCF in the presence of 0 (▪), 1 (•), or 10 (▴) μmol/L 5′-amino-5′-dAd for 4 days.
Reduction of the growth-inhibitory effect of dCF plus dAd by 5′-amino-5′-dAd. Cells were cultured with 50 μmol/L dAd and various concentrations of dCF in the presence of 0 (▪), 1 (•), or 10 (▴) μmol/L 5′-amino-5′-dAd for 4 days.
Ara C is phosphorylated by deoxycytidine kinase, and it similarly suppressed the growth of U937, THP-1, HL-60, K562, and BALM3 cells (data not shown). Fludarabine and cladribine are ADA-resistant analogs of dAd and need intracellular phosphorylation by deoxycytidine kinase to form intracellular active metabolites.23 24 These drugs similarly suppressed the growth of monocytoid and nonmonocytoid cells. Hydroxyurea, a potent inhibitor of RNR activity, also similarly inhibited the growth of these cells (data not shown), suggesting that RNR in U937 and THP-1 cells is as sensitive to hydroxyurea as that in other leukemia cells.
Cellular uptake of dCF, ADA activity, and the accumulation of dATP in cells treated with dCF.
Conversion of dAd to deoxyinosine is prevented when ADA is substantially inhibited by dCF. dAd is converted via phosphorylation catalyzed by adenosine kinase into dATP, which effectively inhibits RNR activity. Thus, this inhibition reduces the production of dGTP, dCTP, and dTTP and causes derangement of DNA synthesis. dCF has been considered to suppress cell growth through these processes. We measured the dATP level in several leukemia cells and the effect of dCF on dATP synthesis in the cells. U937 and K562 cells exhibited similar kinetics in their uptake of [14C]dAd with respect to time (data not shown). dCF was taken up much more slowly than natural nucleosides. With [3H]dCF at an extracellular concentration of 10 nmol/L, a steady intracellular level was reached after 3 hours; ie, 4.98 and 2.47 pmol/107 cells for U937 and K562 cells, respectively. However, in the presence of 50 μmol/L dAd this level was 7.32 and 2.71 pmol/107 cells, respectively (Fig 4). The intracellular uptake of dCF by other monocytic leukemia cells was also slightly higher than that by other types of leukemia cells (Fig 4).
Uptake of dCF into monocytoid and nonmonocytoid cells. Cells were incubated with 10 nmol/L [3H]dCF in the presence or absence of 50 μmol/L dAd. U937 cells with (•) or without (▪) dAd, THP-1 cells with (○) or without (□) dAd, HL-60 cells with (▵) or without (◊) dAd, and K562 cells with (▴) or without (⧫) dAd. Values are the mean for three separate experiments.
Uptake of dCF into monocytoid and nonmonocytoid cells. Cells were incubated with 10 nmol/L [3H]dCF in the presence or absence of 50 μmol/L dAd. U937 cells with (•) or without (▪) dAd, THP-1 cells with (○) or without (□) dAd, HL-60 cells with (▵) or without (◊) dAd, and K562 cells with (▴) or without (⧫) dAd. Values are the mean for three separate experiments.
ADA activity of untreated U937 cells was similar to that of K562 cells, and there was no significant difference in the sensitivity of ADA activity in the cell lysates to dCF between U937 and K562 cells, suggesting that ADA of U937 cells was quantitatively and qualitatively similar to that of K562 cells (data not shown). Treatment with dCF for 24 hours concentration-dependently inhibited ADA activity of leukemia cells, and ADA activity in dCF-treated U937 and THP-1 cells was slightly lower than that in nonmonocytoid cells (Fig 5).
ADA activity of dCF-treated cells. U937 (▪), THP-1 (□), HL-60 (○), NB4 (▴), and K562 (•) cells were treated with various concentrations of dCF for 24 hours. The cell extracts were incubated with [14C]dAd at 37°C for 60 minutes. Values are the mean for four separate experiments.
ADA activity of dCF-treated cells. U937 (▪), THP-1 (□), HL-60 (○), NB4 (▴), and K562 (•) cells were treated with various concentrations of dCF for 24 hours. The cell extracts were incubated with [14C]dAd at 37°C for 60 minutes. Values are the mean for four separate experiments.
The amount of dATP in several leukemia cells treated with dCF was investigated. The formation of dATP in untreated U937 and K562 cells was 22.3 and 26.2 pmol/106 cells/h, respectively, when incubated with 50 μmol/L [14C]dAd. Treatment with dCF resulted in the concentration-dependent accumulation of large amounts of dATP in these cells. After incubation with 0.1 μmol/L dCF for 1 hour, the amount of dATP was 78.6 and 54.5 pmol/106 cells in U937 and K562 cells, respectively. Lower levels of dATP accumulated in BALM3, NB4, HL-60, and HEL cells (53.4, 49.8, 53.1, and 61.2 pmol/106 cells), and a higher accumulation of dATP was observed in THP-1 and HEL/S cells (71.5 and 79.2 pmol/106cells), although these differences were modest. Similar results were obtained when intracellular dATP levels were measured by the DNA polymerase assay method (data not shown).
Induction of apoptosis of U937 cells treated with dCF and dAd.
When exposed to dCF in the presence of10 μmol/L dAd for 2 days, the number of viable U937 cells decreased in a dose-dependent manner, whereas the viability of K562 cells was not affected. dCF effectively inhibited the viability of U937 cells at a concentration of less than 5 nmol/L. After exposure to dCF for 2 days, a morphological analysis showed shriveled cells, chromatin condensation, nuclear fragmentation and cytoplasmic blebbing (data not shown). Induction of apoptosis in treated U937 cells was confirmed by an analysis of DNA histograms (Fig 6). When U937 cells were cultured with various concentrations of dCF in combination with 10 μmol/L dAd for 2 days, cells in sub G1 phase (apoptotic cells) appeared dose dependently. A significant increase in apoptotic cells was observed in U937 cells treated with 1 nmol/L dCF in the presence of 10 μmol/L dAd (Fig 6). Similar findings were observed in other monocytic leukemia cells at this concentration, but not in K562 or HL-60 cells (Fig 6).
DNA histogram of U937, HL-60, and K562 cells after treatment with dCF and dAd. Cells were cultured with 1 (2) and 10 (3) nmol/L dCF in the presence of 10 μmol/L dAd for 2 days. 1, Untreated control cells. Cells were fixed and stained with propidium iodide, and the DNA content was analyzed by flow cytometry. The apoptotic cell population is shown by the first peak (Apo).
DNA histogram of U937, HL-60, and K562 cells after treatment with dCF and dAd. Cells were cultured with 1 (2) and 10 (3) nmol/L dCF in the presence of 10 μmol/L dAd for 2 days. 1, Untreated control cells. Cells were fixed and stained with propidium iodide, and the DNA content was analyzed by flow cytometry. The apoptotic cell population is shown by the first peak (Apo).
Untreated U937 cells expressed Fas-antigen (22.5% positive cells), and this expression increased to 60.3% after treatment with the combination of 10 nmol/L dCF and 10 μmol/L dAd (data not shown). However, THP-1 cells did not express the antigen, and expression was not affected by the combined treatment.
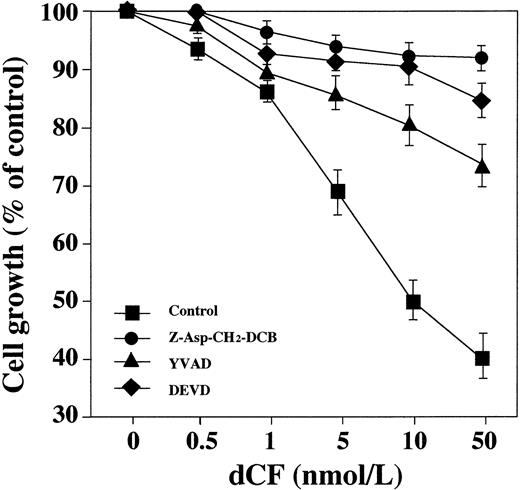
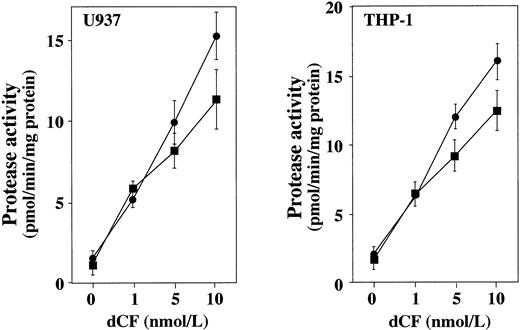
An apoptotic signal requires activation of ICE and CPP32, members of a family of cysteine proteases that are evolutionarily conserved determinants of cell death.25 26 Using tetrapeptide inhibitors of ICE and CPP32, we examined the extent to which these proteases participate in the apoptosis of U937 cells caused by dCF plus dAd. U937 cells were treated with YVAD-CHO, DEVD-CHO, or Z-Asp-CH2-DCB for 2 hours, and then with dCF+dAd. Cell viability was measured by the MTT assay after 48 hours. Figure 7 shows that these peptides suppressed apoptosis caused by dCF plus dAd, suggesting that ICE and CPP32 are involved in the apoptosis induced by dAd plus dCF. YVAD had the weakest suppressive effect. Next, we examined the activities of ICE and CPP32 using YVAD-MCA and DEVD-MCA as substrates in the extract of U937 or THP-1 cells treated with dAd plus dCF. Treatment with dCF in the presence of 10 μmol/L dAd dose-dependently induced both proteolytic activities, and the activity of DEVD-MCA cleavage was slightly greater (Fig 8). Assessment of the expression of apoptosis-associated mRNAs showed that bcl-2,bcl-XL, and c-myc mRNA expression began to decrease 24 hours after treatment with 10 nmol/L dCF plus 10 μmol/L dAd, but bax mRNA expression did not significantly change (data not shown).
Effect of caspase inhibitors on dCF-induced cell death. U937 cells were incubated with culture medium alone (▪), 20 μmol/L YVAD (▴), 20 μmol/L DEVD (⧫), or 100 μg/mL Z-Asp-CH2-DCB (•) for 2 hours and then further incubated with various concentrations of dCF in the presence of 10 μmol/L dAd for 2 days. Values are the mean SD for three separate experiments.
Effect of caspase inhibitors on dCF-induced cell death. U937 cells were incubated with culture medium alone (▪), 20 μmol/L YVAD (▴), 20 μmol/L DEVD (⧫), or 100 μg/mL Z-Asp-CH2-DCB (•) for 2 hours and then further incubated with various concentrations of dCF in the presence of 10 μmol/L dAd for 2 days. Values are the mean SD for three separate experiments.
Induction of caspase activity by dCF and dAd. Cell lysates from U937 and THP-1 cells treated with various concentrations of dCF and 10 μmol/L dAd for 2 days were assayed for protease activity toward Ac-YVAD-MCA (▪) or Ac-DEVD-MCA (•). Values are the mean SD for three separate experiments.
Induction of caspase activity by dCF and dAd. Cell lysates from U937 and THP-1 cells treated with various concentrations of dCF and 10 μmol/L dAd for 2 days were assayed for protease activity toward Ac-YVAD-MCA (▪) or Ac-DEVD-MCA (•). Values are the mean SD for three separate experiments.
dATP-dependent activation of CPP32 in cytosol of leukemia cells.
CPP32 in cytosol can be activated by dATP, but not by other nucleotides.18 We prepared 100,000g cytosolic supernatants from exponentially growing leukemia and lymphoma cells. Because the activation of CPP32 is the result of cleavage of its 32-kD precursor into a 20-kD NH2-terminal fragment and an 11-kD COOH-terminal fragment,27 the activation of CPP32 in the cytosol was monitored by Western blot analysis using a monoclonal antibody against the 20-kD fragment of CPP32. Figure 9 shows that dATP markedly accelerates the activation of CPP32 in monocytic leukemia cell cytosol, whereas it does not effectively activate CPP32 in nonmonocytic leukemia cell cytosol.
dATP-dependent activation of CPP32 in vitro. An aliquot (10 μL) of cytosol was incubated with 0, 0.1, or 1 mmol/L dATP at 37°C for 60 minutes. HL-60 cells (lanes 1 through 3), K562 cells (lanes 4 through 6), BALM3 cells (lanes 7 through 9), U937 cells (lanes 10 through 12), THP-1 cells (lanes 13 through 15), HEL/S cells (lanes 16 through 18), ML-1 cells (lanes 19 through 21), and NB4 cells (lanes 22 through 24). P32; CPP32, and P20; 20-kD fragment of CPP32 (active form).
dATP-dependent activation of CPP32 in vitro. An aliquot (10 μL) of cytosol was incubated with 0, 0.1, or 1 mmol/L dATP at 37°C for 60 minutes. HL-60 cells (lanes 1 through 3), K562 cells (lanes 4 through 6), BALM3 cells (lanes 7 through 9), U937 cells (lanes 10 through 12), THP-1 cells (lanes 13 through 15), HEL/S cells (lanes 16 through 18), ML-1 cells (lanes 19 through 21), and NB4 cells (lanes 22 through 24). P32; CPP32, and P20; 20-kD fragment of CPP32 (active form).
Recent reports link the release of mitochondrial cytochrome c to the induction of apoptosis.19 Cytochrome c was detected in the cytosol of monocytic leukemia cells, and the amounts were similar to those in nonmonocytoid cells (data not shown). We also examined the total amount of cytochrome c per cell in U937 and K562 cells. The results indicated that these cells contain comparable amounts of cytochrome c. Cytochrome c is a protein located in the intermembrane space of mitochondria.28 Therefore, the presence of cytochrome c in the cytosolic fraction may be the result of a ruptured outer mitochondrial membrane caused by hypotonic shock during preparation. To test this hypothesis, we also prepared cytosol from U937 and K562 cells in the presence of 0.25 mol/L sucrose to preserve mitochondrial integrity. The cells were gently broken by douncing in a teflon homogenizer. Cytosol prepared this way contained little cytochrome c compared with that used in the previous experiments, and there was no significant difference between U937 and K562 cells (data not shown). These results suggest that the release of cytochrome c does not play a dominant role in the selective activation of CPP32 in the cytosol of monocytoid cells, although cytochrome c is required for activation of CPP32.
DISCUSSION
dCF, a heterocyclic purine analog of antimicrobial origin, is an irreversible inhibitor of ADA with an inhibition constant (Ki) value of 0.1 nmol/L. As a result of the high ratio of the phosphorylating enzyme (deoxy)adenosine kinase to the dephosphorylating enzyme 5-nucleotidase in lymphocytes, adenosine and dAd are converted to triphosphate metabolites. The accumulation of dATP inhibits RNR, which in turn inhibits DNA synthesis. This is believed to be the mechanism of action of dCF in dividing cells. However, treatment with dCF plus dAd is toxic to both resting lymphocytes and phytohemagglutinin (PHA)-stimulated lymphocytes, which do not synthesize DNA when their ADA is inhibited by dCF.20Sylwestrowicz et al29 suggested that there may be two mechanisms for the toxicity of dCF toward blastic cells. First, the accumulation of dATP may inhibit RNR by reducing the supply of the three other deoxynucleoside triphosphates needed for DNA synthesis. Second, the accumulation of SAH may inhibit S-adenosylmethionine-mediated reactions. The present study showed that the growth-inhibitory effect by dCF plus dAd is decreased in the presence of dC, dG, and dT, suggesting that the inhibition of RNR may be involved in this growth-inhibitory effect. However, there was no significant difference in the growth-inhibitory effects of hydroxyurea on monocytoid and nonmonocytoid leukemia cells, suggesting that the inhibition of RNR is not involved in the preferential effect of dCF plus dAd on monocytoid cells. Neplanocin A (an SAH hydrolase inhibitor) did not affect the growth inhibition produced by dCF plus dAd, and the growth suppression produced by neplanocin A alone was similar among the various cell lines, suggesting that the inhibition of SAH hydrolase does not play a role in the preferential suppression of cell growth. Therefore, the antiproliferative effect of dCF plus dAd on monocytoid cells may be based on some mechanism(s) other than the suppression of RNR or SAH hydrolase.
ADA, a key enzyme in the purine salvage pathway, regulates intracellular adenosine and dAd levels through the irreversible deamination of adenosine and dAd. ADA is widely distributed in mammalian tissues, with the highest activity found in lymphoid tissues, including circulating lymphocytes, spleen, and thymus, although significant activity is also found in the intestine and pancreas.30 ADA levels are higher in normal circulating T cells than in B cells, and are much higher in the blast cells of patients with thymic phenotype of acute lymphocytic leukemia than in those of common non–B-cell and non–T-cell acute lymphocytic leukemia.31 ADA activity is low in normal bone marrow cells, whereas myeloid leukemic blast cells express high levels.32 T lymphoblasts show high ADA activity and contain a high concentration of dATP, and are thus very sensitive to the cytotoxic effects of dAd.33,34 According to Camici et al,14 the sensitivity of colon carcinoma cells increases with adenosine kinase activity. In the present study, we investigated the mechanism by which dCF + dAd produces marked suppression of the growth of monocytic leukemia cell lines. First, we measured ADA activity in the cells. The ADA activity of U937 cells was not significantly different from that of nonmonocytoid K562 cells, nor was there any remarkable difference in the suppression of ADA activities after treatment with dCF + dAd. We also measured intracellular levels of dAd and dATP in the cells. The uptake of dAd and accumulation of dATP in dCF-treated K562 were comparable to those in U937 cells. dAd kinase has been considered to have no role in the suppression of cell growth by fludarabine or cladribine.23 24 These drugs are also adenosine analogs, but are metabolized by deoxycytidine kinase to triphosphates. Their cytotoxicity has been considered to be based on the inhibition of RNR by these triphosphates. Fludarabine and cladribine exert a cytotoxic effect of comparable strength against a variety of cells. These results are consistent with the hypothesis that the inhibition of RNR is not involved in the preferential suppression of cell growth in monocytoid cells, although dATP formation is required for the action of dAd and dCF on monocytoid cells.
Monocytic leukemia U937 cells have been reported to undergo apoptotic cell death when treated with various agents, including anticancer drugs. Although these agents act on different cellular targets, they induce a similar pattern of apoptosis, suggesting a common signaling pathway for apoptosis.35-37 CPP32 normally exists as an inactive precursor that becomes activated proteolytically in cells undergoing apoptosis.38,39 More direct evidence to support the concept that the activity of CPP32 is required for apoptosis came from an experiment in which a tetrapeptide aldehyde inhibitor that specifically inhibits CPP32 activity was shown to block apoptosis.35 40 The present experiments showed that YVAD-CHO, DEVD-CHO, and Z-Asp-CH2-DCB effectively suppressed the apoptosis caused by dCF plus dAd, and the activities of both ICE and CPP32 were increased in U937 cells 48 hours after treatment with dCF plus dAd. These results suggest that caspase activation is at least partly involved in the apoptosis of monocytoid cells induced by dCF plus dAd.
Binding of Fas ligand to Fas/APO-1 receptor transduces an apoptotic signal that requires activation of ICE and CPP32.25 U937 cells significantly expressed Fas-antigen, but the other leukemia cells examined did not. Therefore, Fas-mediated signal transduction may be not associated with the apoptosis induced by dCF plus dAd.
Recent reports indicate that caspase-9 is the most upstream member of the apoptotic protease cascade that is triggered by dATP and cytochrome c.18,41 In etoposide-induced apoptosis, the spectrum and subcellular distribution of active caspase species differ between K562 and HL60 leukemia cells.42 In the present study, a caspase-1 inhibitor blocked the dCF + dAd–induced apoptosis of U937 cells. Therefore, selective activation of several caspases, including caspase-3 (CPP32), may be involved in the preferential induction of apoptosis in monocytoid cells. The bcl-2 family of proteins consists of members with both survival-enhancing and proapoptotic activities.43 44 Expression of members of the bcl-2 family was also affected by treatment with dCF plus dAd. The differential expression of bcl-2 family genes might contribute to the difference in the sensitivity to dCF + dAd between monocytoid and nonmonocytoid cells.
Acute monocytic leukemia is more refractory to conventional chemotherapy than other types of acute myeloid leukemia.1When dCF is administered along with dAd, the concentration required to induce apoptosis of human monocytic leukemia cell lines is much lower than that required to induce apoptosis of human lymphoma or other leukemia cell lines. We are beginning to examine the apoptosis-inducing effect of dAd and dCF in monocytoid and nonmonocytoid leukemia cells in primary culture (4 cases of AML-M4 and M5, 6 cases of other AML, and 5 cases of lymphoid leukemia and lymphoma). In the presence of 10 μmol/L dAd, the IC50s of dCF were 0.03, 4,600, and 92 μmol/L, respectively. These results may provide useful information regarding the value of this combination for the clinical treatment of acute monocytic leukemia.
Supported in part by Grants for Cancer Research from the Ministry of Education, Science, Sports and Culture and the Ministry of Health and Welfare, Japan.
Address reprint requests to Yoshio Honma, PhD, Department of Chemotherapy, Saitama Cancer Center Research Institute, Ina, Kita-adachi, Saitama 362, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.




![Fig. 4. Uptake of dCF into monocytoid and nonmonocytoid cells. Cells were incubated with 10 nmol/L [3H]dCF in the presence or absence of 50 μmol/L dAd. U937 cells with (•) or without (▪) dAd, THP-1 cells with (○) or without (□) dAd, HL-60 cells with (▵) or without (◊) dAd, and K562 cells with (▴) or without (⧫) dAd. Values are the mean for three separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/9/10.1182_blood.v92.9.3368/5/m_blod42127004x.jpeg?Expires=1763464254&Signature=lTo4NCoVrz1Ul2cI2OOZnbvCWpt02~P35X0gckOrlilKxwtDJ~gHcuZ-59Dp5gbb34cP3RwDWzBD-Px7AvMO32AWoS8O~GHY7l-4zOQbqyaZFK8-o7gQs48ll-piP4uU68mX9XjYc9hrM4BzKZcJ8EqWx38ivrK~LiDoXGNz1apL5mtHa4rgK1ZjEMVe-hB-L4Nn45u1a0dQlALk6uNFoyTNEHl7G1gG2GMlyJoSprVfHrl61w3EtzI3GT1369LbUZdYrhEch3FzIeol6V0CFAwtL4ZWmJxqpFzMmD1FW~vtVuAXHTob~RND99XCkrV7Mdam7BGnB~oYNkQh4j08jg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. ADA activity of dCF-treated cells. U937 (▪), THP-1 (□), HL-60 (○), NB4 (▴), and K562 (•) cells were treated with various concentrations of dCF for 24 hours. The cell extracts were incubated with [14C]dAd at 37°C for 60 minutes. Values are the mean for four separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/9/10.1182_blood.v92.9.3368/5/m_blod42127005x.jpeg?Expires=1763464254&Signature=Vbuw79qVgnjhoUMjk1ywekAdqQ~dnNciWWmAitZqL6JyiAf1KVAgwkHvj5bBqBgjtqk4YHEKPhzDXh0scQA~4G8HEsnxKkzgb-cpeWDHICOP2Nj7BHy4WBPx3ym7snM7CkwJxLNpFjrAxCA4ooFF-eOED7W-1~xoUeI7WOPHyQrQt7BHzslxGGUdWecwKbk3rVLmhTwyZ84dkVQLpZ-7SqdNG3Ylio1xUHr1mTv~VsB51U2lw1~noyO9bGUMGYgU2sldZ7bnr9NAPHxp6fn6WC8Y2TZMoojvAhN8EsTj54jg-57Byk5FzFvUXkkUo1wEw-CP1XfI8z6My9X2VF1ceg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal