Abstract
We have developed culture conditions for the efficient expansion of cytotoxic effector cells from peripheral blood mononuclear cells (PBMNCs) by the timed addition of interferon-γ (IFN-γ), interleukin-2 (IL-2), and the monoclonal antibody (MoAb) OKT3. These cells, termed cytokine-induced killer (CIK) cells, are composed primarily of T cells, and the population of cells with the greatest cytotoxic activity is an otherwise rare population of CD3+CD56+ cells that expand dramatically under these culture conditions. CIK cells were expanded from PBMNCs from 13 patients with chronic myeloid leukemia (CML). These cultures contained a variable number of T cells at the start of the culture (median 44%, range 1% to 64%), yet after 21 to 28 days of culture, virtually all of the cells were CD3+ T cells (median 97%, range 90% to 99%). The CD3+CD56+subset of cells expanded significantly (median 25-fold, range 2.2- to 525-fold). CIK cells from all patients showed cytotoxicity against the tumor cell lines OCI-LY8 and K562. In four patients the expanded CIK cells suppressed colony growth of autologous CML blast cells and myeloid progenitor cells. Allogeneic CIK cells from normal donors also suppressed CML colony growth but did not inhibit growth of normal hematopoietic colonies. Twelve of the 13 cultures were exclusively composed of Philadelphia (Ph)-negative cells and one culture had 1 out of 20 Ph-positive metaphases after 4 weeks in culture. Intracellular cytokine production was assayed by fluorescence-activated cell sorter (FACS), and the expanded T-cell cultures produced IL-2, IFN-γ, and tumor necrosis factor- (TNF-), but not IL-4. Both the CD4+ and CD8+ subsets secreted this cytokine profile. To test the in vivo activity of the expanded CIK cells, CML was engrafted into severe combined immunodeficiency disease (SCID) mice using matrigel. After 4 weeks, 4 × 107autologous CIK cells were injected intravenously by tail vein injection into groups of mice, and the animals were sacrificed after a total of 18 weeks. Bcr-abl was detected in the bone marrow or spleen of 5 out of 6 control mice and only 2 out of 13 mice who received the autologous CIK cells (P = .02). In an additional series of animals, the mice did not engraft with CML but instead developed large human Epstein-Barr virus–associated lymphomas by 12 weeks. The mice who received autologous CIK cells at 4 weeks had either no tumor (5) or small tumors (5), whereas all 10 mice that received CIK cells at week 8 developed lymphomas; however, these were not as large as in the 10 control mice who did not receive CIK cells (P = .03). This study shows that CIK cells, which are Ph chromosome–negative, can be expanded from patients with CML and have potent in vitro and in vivo efficacy against autologous tumor cells.
© 1998 by The American Society of Hematology.
CHRONIC MYELOID leukemia (CML) is a fatal myeloproliferative disorder that is characterized by the presence of the Philadelphia (Ph) chromosome leading to the transcription of an abnormal fusion gene termed bcr-abl. At present CML is only curable by allogeneic bone marrow transplantation (BMT),1,2 although autologous BMT (ABMT)3,4 and treatment with interferon- (IFN-) may prolong survival.5 Allogeneic BMT is a highly effective treatment for CML due to the high dose chemoradiation conditioning regimen and immune effects known as graft-versus-leukemia (GVL). The importance of immune effects in CML is shown by the high rate of relapse of CML following T-cell–depleted BMT6 and identical twin transplants.7 A GVL reaction has been directly shown by the success of donor buffy coat infusions to treat patients who have relapsed following allogeneic BMT.8 9Despite the use of unrelated donors, the majority of patients with CML will not have an allogeneic bone marrow donor or will be too old to pursue aggressive therapy. Alternative strategies are, therefore, needed.
In recent years, a number of centers have reported studies of autologous BMT for chronic-phase CML, using a variety of conditioning regimens and purged and unpurged stem cells.3,4,10-12 Many patients became Ph negative shortly after ABMT, and a few have remained Ph negative for long periods of time. In some studies, survival was prolonged compared with historical conventionally treated patients. Survival and possibly cure may be further improved if immunotherapy could be developed that is effective in the autologous setting. Toward this end, HLA-restricted T-cell clones have been generated that recognize bcr-abl–specific peptides and autologous leukemic cells but not normal cells.13,14 The generation of T-cell clones from individual patients is difficult, time consuming, and probably not a viable option for routine therapeutic use. Natural killer (NK) cells and lymphokine-activated killer (LAK) cells have been shown to have in vitro cytotoxicity against autologous and allogeneic CML cells,15,16 which has been corelated with an improved clinical outcome.17 However, NK cells do not expand well in vitro and need exogenous interleukin-2 (IL-2) for maximum activity in vivo.
We have defined culture conditions, using IFN-γ, IL-2, and a monoclonal antibody directed against CD3 (OKT3), where a subset of T cells with the phenotypes of CD3+CD16−CD56+ and CD3+CD16−CD56− can be expanded ex vivo. These expanded cells, which have been termed cytokine-induced killer (CIK) cells, have non–major histocompatibility complex (MHC)—restricted cytotoxicity against a variety of tumor cell targets.18,19 The cells with the greatest cytotoxic activity in these cultures are the otherwise rare population of CD3+CD56+ cells, which expand up to 1,000-fold after 3 to 4 weeks in culture. This population of CD3+CD56+ cells was initially described in 1986 by Ritz20 and Lanier21 and further characterized by Ortaldo,22 who showed that CD3+CD56+ cells are capable of lysing a broad array of tumor cell targets in a non-MHC–restricted manner. In CIK cell cultures, the expanded CD3+CD56+ cells are derived from CD3+CD56− T cells and not CD3−CD56+ NK cells.19 CIK cells have been shown to protect animals from a lethal challenge of a non-Hodgkin’s lymphoma cell line in vivo using mice with severe combined immunodeficiency (SCID). Approximately 30% to 40% of animals treated with CIK cells survived long term as compared with none of the control animals injected with the human non-Hodgkin’s lymphoma cell line SU-DHL-4.19,23 In addition, the expanded CIK cells have been shown to be superior to IL-2–activated NK cells in this SCID mouse model and do not require exogenous administration of IL-2 for in vivo activity.19 In vitro, the cytotoxicity of CD3+CD56+ cells is inhibited by antibodies to ICAM-1, LFA-1, and CD18 but not by antibodies to TCR, CD3, CD4, CD8, CD56, MHC class I, or MHC class II.18
In this study we show that CIK cells can be generated from CML patients. The expanded CIK cells are Ph chromosome negative and have both in vitro and in vivo cytotoxicity against tumor cell lines and autologous leukemic cells. CIK cells may be a useful therapeutic option, for example, following an autologous transplant procedure.
MATERIALS AND METHODS
Patients.
Peripheral blood progenitor cells (PBPCs) or bone marrow (BM) cells were obtained after informed consent from 11 chronic-phase CML patients and 2 blast crisis patients before allogeneic or autologous BMT. For autografting, PBPCs were mobilized with idarubicin, cytarabine, and etoposide chemotherapy.11 Details on individual patients from whom CIK cells were generated are presented in Table 1. For the in vivo CIK experiments in mice, fresh BM was obtained from patient K.D. at the time of a back-up BM harvest; patient M.R. underwent leukopheresis; and blast cells from patient R.G. were collected after 6 cGy of radiation when the white blood cell count rose suddenly to 165 × 109/L. All cells injected into the animals were 100% Ph+, as assessed by cytogenetics.
Clinical Details of the Patients Included in This Study
| Patient . | Time from Diagnosis (mo) . | Disease Status . | WBC × 106/mL* . | Previous Treatment . | Current Treatment . |
|---|---|---|---|---|---|
| G.H. | 50 | CML-CP | 6.5 | IFN-α, ABMT | IFN-α |
| K.D. | 14 | CML-CP | 4.1 | HU, IFN-α | HU |
| M.R. | 12 | CML-CP | 20 | HU, IFN-α | None |
| M.B.U. | 3 | CML-CP | 4.9 | HU | HU |
| M.B.A. | 10 | CML-CP | 3.4 | HU | HU |
| S.T. | 2 | CML-CP | 40 | HU, IFN-α | IFN-α |
| L.C. | 1 | CML-CP | 90 | None | None |
| A.Z. | 18 | CML-BC | 40 | HU, IFN-α | HU, IFN-α |
| P.S. | 9 | CML-CP | 14 | HU, IFN-α | HU |
| R.D. | 36 | CML-CP | 21 | HU, IFN-α, ICE | None |
| S.S. | 3 | CML-CP | 12.5 | HU | HU |
| R.G. | 18 | CML-BC | 160 | IFN-α, HU | HU |
| G.T. | 39 | CML-CP | 51 | IFN-α, HU | HU |
| Patient . | Time from Diagnosis (mo) . | Disease Status . | WBC × 106/mL* . | Previous Treatment . | Current Treatment . |
|---|---|---|---|---|---|
| G.H. | 50 | CML-CP | 6.5 | IFN-α, ABMT | IFN-α |
| K.D. | 14 | CML-CP | 4.1 | HU, IFN-α | HU |
| M.R. | 12 | CML-CP | 20 | HU, IFN-α | None |
| M.B.U. | 3 | CML-CP | 4.9 | HU | HU |
| M.B.A. | 10 | CML-CP | 3.4 | HU | HU |
| S.T. | 2 | CML-CP | 40 | HU, IFN-α | IFN-α |
| L.C. | 1 | CML-CP | 90 | None | None |
| A.Z. | 18 | CML-BC | 40 | HU, IFN-α | HU, IFN-α |
| P.S. | 9 | CML-CP | 14 | HU, IFN-α | HU |
| R.D. | 36 | CML-CP | 21 | HU, IFN-α, ICE | None |
| S.S. | 3 | CML-CP | 12.5 | HU | HU |
| R.G. | 18 | CML-BC | 160 | IFN-α, HU | HU |
| G.T. | 39 | CML-CP | 51 | IFN-α, HU | HU |
*WBC day CIK cultured started.
Abbreviations: CML-CP, chronic-phase CML; ABMT, autologous bone marrow transplant; CML-BC, blast crisis CML; HU, hydroxyurea; ICE, idarubicin, cytosine arabinoside, etoposide; WBC, white blood cell.
Generation of CIK cells.
BM or peripheral blood mononuclear cells (PBMNCs) were isolated by ficoll density gradient centrifugation, washed, and resuspended at 2 to 5 × 106 cells/mL in complete RPMI (cRPMI) containing 10% fetal calf serum (FCS; Hyclone labs, Logan, UT), penicillin 100 U/mL, streptomycin 100 mg/mL, 2 mmol/L l-glutamine, and 50 μmol/L 2-mercaptoethanol. On day 1, IFN-γ (Genentech, South San Francisco, CA) was added to a final concentration of 1,000 U/mL. On day 2, IL-2 (Chiron, Inc, Emeryville, CA) at a final concentration of 300 U/mL and a monoclonal antibody to CD3 (OKT3; Ortho Biotech, Raritan, NJ) at 25 ng/mL were added. Cultures were fed every 5 to 7 days with cRPMI and IL-2. Expansion was assessed between days 21 and 30.
Cytogenetic studies.
An aliquot of cells in cRPMI and IL-2 was removed from the cultures at various time points from 7 to 30 days, and colchicine 50 ng/mL was added for 1 hour (termed lymphoid cytogenetics). Cells were stained with Giemsa, G banded, and 20 cells on average were analyzed for the presence of the Ph chromosome. In some patients an aliquot of cells was removed, washed 3 times to remove any IL-2, and resuspended in Myelocult (Terry Fox Labs, Vancouver, Canada). Cytogenetics was performed after 2 to 4 days in culture (termed myeloid cytogenetics).
Hematopoietic colony assays of CIK cells.
A total of 2 × 105 cells was washed 3 times in phosphate-buffered saline (PBS) to remove any IL-2 and plated in triplicate in 1mL of methocult (Terry Fox Labs). Colonies were counted after 14 days of culture in 5% CO2. Clusters of more than 50 cells were scored as colonies.
Fluorescence-activated cell sorter (FACS) analysis.
A total of 1 × 106 ficolled MNC or CIK cells was washed once in PBS containing 1% bovine serum albumin (BSA) and resuspended in 100 μL PBS/BSA. The cells were incubated with various conjugated monoclonal antibodies for 60 minutes at 4°C, washed twice in PBS, and resuspended in 200 μL of PBS. Ten milliliters of propridium iodide (PI) was added to each tube shortly before analysis. Flow cytometric analysis was performed on the FACScanner (Becton Dickinson, San Jose, CA), and data on 10,000 cells were acquired. Dead cells were excluded by PI after acquisition, and results reported are for live cells only. The monoclonal antibodies (Becton Dickinson) used were CD3, CD4, CD16 (FITC conjugated); CD8, CD34, CD56 (phycoerythrin [PE] conjugated); and CD4, CD8 (PerCP conjugated).
Intracellular cytokine production.
Day-21 to -28 CIK cells known to be greater than 98% CD3+were analyzed for intracellular expression of IL-2, TNF, IFN-γ, and IL-4. Ten micrograms brefeldin A was added to two tubes containing 5 × 106 CIK. The cells in one tube were stimulated for 4 hours at 37°C with 25 ng Phorbol 12-Myristate 13-Acetate (PMA) and 1 μg ionomycin (activated), and then both tubes were stained with CD8 PCP and CD56 FITC (as above) and washed once in PBS. A total of 1 × 106 cells was permeabilized in separate tubes using 500 μL of 1× FACS permeabilizing solution (Becton Dickinson) for 10 minutes at room temperature and washed once in PBS/1% BSA. Cells were then incubated with 20 μL of PE-conjugated antibodies to IL-2, TNF, IFN-γ, or IL-4 for 30 minutes at room temperature. Cells were then washed and fixed in 1% paraformaldehyde. Data were acquired on 50,000 cells using a FACS vantage analyzer and analyzed using LYSYS II software (Becton Dickinson).
Cr51 cytotoxicity assay.
Two tumor cell lines, OCI-Ly8, a human lymphoma cell line, and K562, a Ph+ erythroleukemia cell line, were selected as targets. A total of 1 × 106 target cells was labeled with 100 μCi of Cr51 (Dupont-NEN, Boston, MA) for 1 to 2 hours at 37°C. The labeled cells were washed three times in PBS and resuspended in 10 mL of cRPMI. A total of 1 × 104targets in 100 μL cRPMI was plated in triplicate into U-bottomed 96-well plates and incubated for 4 hours with 100 μL of effector cells at various effector-to-target ratios. Cells were then collected by centrifugation and an aliquot of supernatant counted in a gamma counter. The percentage of specific lysis was calculated according to the equation:
Spontaneous release was obtained by incubating cells in medium alone and maximal release after treatment with 2% Nonidet P-40 (Sigma, St Louis, MO).
Preparation of mice.
CB17 SCID mice 8 to 12 weeks old were obtained from the colony at Stanford University. From birth, mice were housed in a clean isolated environment with autoclaved food and water containing trimethoprim sulfamethoxazole (Biocraft, Elmwood Park, NJ). To eradicate NK cells,24 the mice received 20 μL of antiasialo GM1 rabbit antiserum (Wako Chemicals, Dallas, TX) by intraperitoneal injection (IP) on the day of injection of the CML cells. Antiasialo GM1 antiserum was not given at the time of injection of the CIK cells.
Preparation and injection of CP-CML cells.
Fresh BM cells from CML patients were layered over percoll (Pharmacia, Uppsala, Sweden) of density of 1.068 g/L to remove red cells and mature myeloid cells. Cells were washed twice in PBS and resuspended in 100 to 200 μL of cRPMI. The cells (in 200 μL) were then added to 1 mL of cold matrigel (Becton Dickinson) on ice, vortexed, and aspirated into a 1-mL syringe using a 21-gauge needle for injection into the mice. For each patient sample, colony-forming unit–granulocyte-macrophage (CFU-GM) and burst-forming unit–erythroid (BFU-E) assays were performed and the CD34 content assayed by FACS using the PE-conjugated antibody HPCA (Becton Dickinson).
In vivo assessment of cytotoxicity of CIK cells.
To test the in vivo activity of expanded CIK cells, 20 mice were injected with 9 × 106 low-density BM cells, containing 1.5 × 106 CD34+ cells from patient K.D., in matrigel subcutaneously. The human cytokines stem cell factor (SCF) 100 ng/mL (Amgen, Thousand Oaks, CA), IL-3 100 ng/mL (Sandoz, E Hanover, NJ), and granulocyte-macrophage colony-stimulating factor (GM-CSF) 50 ng/mL (Immunex, Seattle, WA) were added to the matrigel, and the mice did not receive any additional cytokines. After 4 weeks, seven mice received 4 × 107autologous CIK cells grown from the PB, and seven mice received 4 × 107 CIK cells grown from the BM, by tail vein injection. Exogenous IL-2 was not given.
An additional 30 mice were injected with 1 mL of matrigel containing 1 × 107 MNC cells (2 × 106CD34+ cells) from patient M.R. and 1 × 106 irradiated M210B4 cells that were genetically engineered to secrete human IL-3 (gift from Dr D. Hogge, Vancouver, Canada). Cytokines were added to the matrigel as described previously. After 4 and 8 weeks, 10 mice in each group were injected with 2 × 107 autologous CIK cells derived from the PB by tail vein injection.
A total of 2 × 106 blast cells from patient R.G. was injected subcutaneously (SC) in matrigel into 30 mice. After 4 weeks, 12 mice were injected intravenously (IV) with 2 × 107fresh PB from the allogeneic donor (K.S.) and 7 mice with 2 × 107 CIK cells grown from the allogeneic donor.
Detection of CML in SCID mice.
To detect engraftment of primitive hematopoietic stem cells rather than a committed progenitor, individual mice were sacrificed after 12 weeks unless they became ill. The spleen (SP) was removed, half of which was made into a single-cell suspension. BM was extracted from the femurs. Excess red blood cells (RBCs) were lysed with NH4Cl, and the cells were washed twice in PBS. A total of 2 × 105 BM or SP cells was plated separately in 1 mL of methyl cellulose containing SCF (100 ng/mL), IL-3 (100 ng/mL), GM-CSF (50 ng/mL), and erythropoietin (3 U/mL) and incubated at 37°C in 5% CO2 for 14 to 21 days. An aliquot of cells from the BM and SP was used for reverse transcription polymerase chain reaction (RT-PCR), and cytospins were prepared.
RT-PCR.
RNA was extracted using either the RNeasy kit (Qiagen Inc, Santa Clarita, CA), Trizol (GIBCO, Grand Island, NY), or RNAzol B (TM Cinna Scientific Inc, Friendswood, TX). The extracted RNA was dissolved in 30 μL DEPC-treated water. Amplification of the mouse housekeeping gene hypoxanthine phosphoribosyltransferase (HPRT) was used for ensuring the integrity of the RNA.25 To perform RT-PCR, the RNA was incubated with 1 μL of 5′RNAse inhibitor (5 Prime → 3 Prime Inc, Boulder, CO) and 3 μL of random hexamers 200 ng/mL (Pharmacia, Piscataway, NJ) for 5 minutes at 70°C and then rapidly chilled. Sixteen microliters of the reaction mixture containing 5 U of MLV reverse transcriptase (GIBCO) was added followed by an incubation at 37°C for 2 hours and 90°C for 5 minutes. One fifth (10 μL) of the reaction product was added to 20 μL of PCR reaction mixture containing 1U of TAQ polymerase (GIBCO) and appropriate primers (BCR-1A, ABL-2C, or HPRT-S, HPRT-AS). After overlayering with oil, 33 cycles of PCR was performed using a Perkin Elmer thermocycler (Chiron, Emeryville, CA). To increase the sensitivity of detection of bcr-abl, a second round of PCR was performed using the nested primers BCR-1B and ABL-2D.26 The sequence of the primers used is as follows. For amplification of bcr-abl, ABL2C 5′ TTATCTCCACTGGCCACAAA 3′, BCR1A 5′ AGTTACACGTTCCTGATCTC 3′, ABL2D 5′ AGTGCAACGAAAAGGTTGGG 3′, BCR1B 5′ TCTGACTATGTGCGTGCAGA 3′. The expected product size is either 363 bp (b3a2 transcript) or 288 bp (b2a2 transcript). For detection of the mouse HPRT gene the primers HPRT-S 5′ GTAATGATCAGTCAACGGGGGAC 3′ and HPRT-AS 5′ CCAGCAAGCTTGCAACCTTAACCA 3′ were used.25 The expected product size is 214 bp. For detection of normal human cells, β-actin was amplified using the primers ACTIN-F 5′ CCGCAAAGACCTGTACGCCA 3′ and ACTIN-I 5′TGGACTTGGGAGAGGACTGG 3′.27
To confirm that appropriate sequences had been amplified, some gels were blotted with Zetaprobe membranes and hybridized overnight with appropriate P32-labeled internal oligonucleotides. The sequences of the internal oligonucleotides used are as follows: for detection of bcr-abl 5′AGCCTCAGGGTCTGAGTGAAGCCGCTCGTT 3′, for HPRT-5′GCTTTCCCTGGTTAAGGACAGTACAGCCCC 3′,25and for β-actin 5′GCCATCCTAAAAGCCACCCCACTTCTCTCTA 3′. The hybridization blot was washed in 2× SSC and subjected to autoradiography.
VDJ analysis of IgH gene by PCR.
DNA was extracted from 5 μ thick paraffin-embedded tissue sections using proteinase K as described previously28 and 35 cycles of PCR performed using the primers VH-FR3: 5′GACACGGCCGTGTATTACTG and JH:5′-ACCTGAGGAGACGGTGACCAGGGT. Three control samples were included in each experiment: (1) B-lineage non-Hodgkin’s lymphoma, (2) tonsil showing reactive hyperplasia, and (3) “no DNA” control sample. Epstein-Barr virus (EBV)–negative samples were also amplified for the mouse TCR gene under similar conditions to show integrity of the DNA preparations. The primers for the mouse TCR introns between JB2.6 and CB2, termed J2CII (5′ TCCTAGCTTGCGAGAGAGCGA) and J2CIV (5′AACAGTACTTCGGTCCCGGCA) were kindly provided by Dr S. Strober (Stanford University, Stanford, CA).
Detection of EBV in mouse tissues.
DNA was extracted from mouse tumor, BM, or spleen using the Instangene matrix (Biorad, Hercules, CA) or precipitated from the Trizol/RNAsol using ethanol. EBV latent membrane protein (LMP) was amplified using the primers LMP 9 5′AGCGACTCTGCTGGAAATGAT and LMP 11 5′TGATTAGCTAAGGCATTCCCA.29 PCR products were run on a 1.5% agarose gel, blotted, and subjected to autoradiography using the probe LMP 10 5′CATGTCATAGGCTTGCTGAC-3′.
Histological examination.
The spleen, BM, and remnants of the matrigel and surrounding tissues were examined histologically along with any organs thought to be infiltrated with leukemic cells. Immunohistochemistry was performed on both fresh cytospins and paraffin sections. For each cytospin a tris-buffered saline (TBS) control was performed in parallel. Slides were fixed in acetone, then blocked with human AB serum, washed in TBS and CD15 (Becton Dickinson), CD43 (Pharmingen, San Diego, CA), or CD45 (Dako Chemicals, Carpinteria, CA) applied for 45 minutes at room temperature. Positive cells were detected using biotinylated goat anti-mouse (Caltag, San Francisco, CA) followed by streptavidin-conjugated to alkaline phosphatase or peroxidase (GIBCO).
Statistical analysis.
The Fisher’s exact test was used to compare the incidence of bcr/abl positivity in treated versus untreated groups of mice. The statistical analysis of the colony killing data was performed on the logarithm of the number of colonies, comparing the geometric means between groups using the unpaired t-test.
RESULTS
Expansion and phenotype of CIK cells from CML patients.
The starting population of PBMNCs contained a variable number of CD3+ T cells with a median of 44% (range 1% to 64%) and a median of 1% CD3+CD56+ cells (range 0% to 4%). After 21 to 28 days in culture, the median content of CD3+ cells was 97% (range 90% to 99%) and for CD3+CD56+ was 12% (range 3% to 45%). This represents an expansion of CD3+ cells of 20.2-fold (range 2.5- to 74-fold) and of CD3+CD56+ 26.5-fold (range 2- to 525-fold). Individual patient expansions are presented in Table 2. The two cultures grown from frozen cells of patients M.R. and R.D. showed much poorer expansion.
Expansion of T Cells and Cytogenetics of the CIK Cell Cultures
| Patient . | CD3+ Day 1 (%) . | CD3+CD56+ Day 1 (%) . | CD3+ Day 21 (%) . | CD3+CD56+ Day 21 (%) . | Exp CD3+ . | Exp CD3+CD56+ . | Cyto Ph+/ total . |
|---|---|---|---|---|---|---|---|
| G.H. | 44 | 1 | 97 | 41 | 28 | 525 | ND |
| K.D.* | 12 | 1 | 97 | 24 | 74 | 363 | 0/20 |
| M.R.† | 52 | 4 | 99 | 3 | 6 | 2.5 | 0/20 |
| M.B.U. | 63 | 2 | 99 | 11 | 26 | 109 | 0/20 |
| M.B.A. | 64 | 3 | 92 | 13 | 8.3 | 26.5 | 0/50 |
| S.T. | 4 | 0 | 93 | 12 | 32 | 85 | 1/25 |
| L.C. | 1 | 0 | 94 | 16 | 20.2 | 19.5 | 0/20 |
| A.Z. | 5 | 0 | 90 | 6 | 20 | 19.5 | 0/20 |
| S.S. | 45 | 2 | 96 | 21 | 17.8 | 111 | 0/20 |
| P.S. | 51 | 1 | 97 | 8 | 5.4 | 24.2 | 0/20 |
| R.D.† | 40 | 4 | 99 | 4 | 2.5 | 2 | 0/20 |
| R.G. | 2 | 1 | 99 | 45 | 67 | 117 | 0/20 |
| G.T. | 34 | 4 | 99 | 3 | 11 | 7 | 0/20 |
| Median | 44 | 1 | 97 | 12 | 20.2 | 26.5 | |
| Range | 1-64 | 0-4 | 90-99 | 3-45 | 2.5-74 | 2-525 |
| Patient . | CD3+ Day 1 (%) . | CD3+CD56+ Day 1 (%) . | CD3+ Day 21 (%) . | CD3+CD56+ Day 21 (%) . | Exp CD3+ . | Exp CD3+CD56+ . | Cyto Ph+/ total . |
|---|---|---|---|---|---|---|---|
| G.H. | 44 | 1 | 97 | 41 | 28 | 525 | ND |
| K.D.* | 12 | 1 | 97 | 24 | 74 | 363 | 0/20 |
| M.R.† | 52 | 4 | 99 | 3 | 6 | 2.5 | 0/20 |
| M.B.U. | 63 | 2 | 99 | 11 | 26 | 109 | 0/20 |
| M.B.A. | 64 | 3 | 92 | 13 | 8.3 | 26.5 | 0/50 |
| S.T. | 4 | 0 | 93 | 12 | 32 | 85 | 1/25 |
| L.C. | 1 | 0 | 94 | 16 | 20.2 | 19.5 | 0/20 |
| A.Z. | 5 | 0 | 90 | 6 | 20 | 19.5 | 0/20 |
| S.S. | 45 | 2 | 96 | 21 | 17.8 | 111 | 0/20 |
| P.S. | 51 | 1 | 97 | 8 | 5.4 | 24.2 | 0/20 |
| R.D.† | 40 | 4 | 99 | 4 | 2.5 | 2 | 0/20 |
| R.G. | 2 | 1 | 99 | 45 | 67 | 117 | 0/20 |
| G.T. | 34 | 4 | 99 | 3 | 11 | 7 | 0/20 |
| Median | 44 | 1 | 97 | 12 | 20.2 | 26.5 | |
| Range | 1-64 | 0-4 | 90-99 | 3-45 | 2.5-74 | 2-525 |
*Cultures initiated with bone marrow cells.
Cultures initiated with cryopreserved peripheral blood cells.
The expanded cultures contained both CD4+ and CD8+ T cells. The median percentage of CD4+cells was 51% (range 17% to 88%) and of CD8+ cells was 59% (range 12% to 82%). In some cultures there were large numbers of CD4+CD8+ T cells, median 6% (range 1% to 41%). In the majority of the cultures CD16+CD56+ NK cells were not present except for one patient (A.Z.), who had CML with a myeloid blast crisis and had significant numbers of NK cells (7%).
Ph chromosome status of CIK cells.
All of the CIK cultures were evaluated by cytogenetic analysis for the presence of the Ph chromosome at different time points in culture and after 21 to 28 days. At least 20 metaphases were analyzed for each patient. Cells from all patients were Ph chromosome–positive at the beginning of the culture period. After 21 to 28 days of culture, cells from 12 of the 13 patients were Ph-negative (Table 2). One patient (S.T.) had 1 of 25 metaphases that were Ph positive. At day 7, 4 out of 6 cultures tested were Ph negative, and at day 14, 6 out of 8 cultures were Ph negative. To test whether dormant myeloid cells may still be present in these expanded cell cultures, aliquots of cells were evaluated from two CP-CML patients who were Ph negative on day 7, by culturing the cells in myelocult (Stem Cell Technology, Vancouver, Canada), which favors the growth of myeloid cells. Indeed, myeloid cells that were Ph positive could be grown early on in these cultures. Similar aliquots of cells taken from the culture of patient S.T. on days 14, 21, and 35 and recultured with myelocult showed 20 out of 20 Ph-positive metaphases on day 14, revealed no analyzable metaphases on day 21 and 0 out of 5 Ph-positive cells on day 35 of culture. In 14- to 21-day CIK cultures derived from an additional five patients (two myeloid blast crisis and three chronic-phase patients), no metaphases were observed after switching to conditions that favored the growth of myeloid cells. At day 28 all CIK cultures remained bcr-abl positive by RT-PCR using nested primers.
Hematopoietic colony growth.
Two patients had CFU-GM assays performed on day 1 of culture, which showed greater than 800 and greater than 1,000 colonies per 1 × 105 cells. In 9 out of the 13 patients, no myeloid colonies could be grown from the CIK cultures at days 21 to 28. In four patients a small number of colonies (2 to 6 per 105 cells) were detected. The colonies from three of these patients were small, with less than 100 cells per colony. The 4th patient (L.C., with untreated CP-CML with a white blood cell count of 90 × 109/L) grew large multilineage colonies that were Ph negative by cytogenetics.
Cytotoxicity assays.
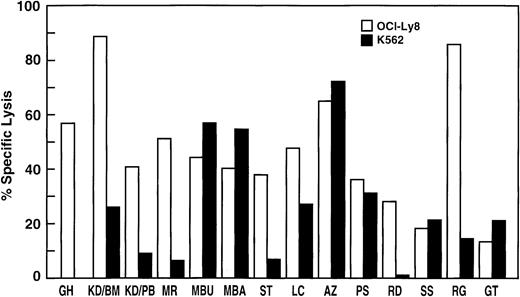
Cytotoxicity of the expanded CIK cells was evaluated in all patients by Cr51-release assays against the tumor cell lines OCI-Ly8 and K562. Specific lysis of tumor cell lines could be shown in all patients. In general the killing of OCI-Ly8 by CIK cells was superior to that of K562 (Fig 1). Although there was no direct correlation between the percentage of CD3+CD56+ cells in the cultures from different patients and the degree of specific lysis, repeated analysis of individual patients showed superior killing with an increasing percentage of CD3+CD56+ cells in that culture (data not shown). The percentage of CD4+ or CD8+ cells did not correlate with the degree of specific lysis, although there was a trend toward better killing with a predominance of CD4+ cells or the presence of CD4+CD8+ double-positive cells in the culture. The patient A.Z. with 7% NK cells showed superior killing of K562, an NK-sensitive target.
Cytotoxicity of expanded CIK cells from CML patients. The tumor cell lines OCI-Ly8 and K562 were labeled with Cr51and incubated for 4 hours with CIK cells at an effector-to-target ratio of 40:1. The percentage specific lysis for each patient’s CIK cells at day 21 to 28 is shown.
Cytotoxicity of expanded CIK cells from CML patients. The tumor cell lines OCI-Ly8 and K562 were labeled with Cr51and incubated for 4 hours with CIK cells at an effector-to-target ratio of 40:1. The percentage specific lysis for each patient’s CIK cells at day 21 to 28 is shown.
In a standard 4-hour Cr51 release assay, no consistent lysis of patient CML cells could be shown using both allogeneic and autologous CIK cells. However, these target cell populations were a heterogenous mixture of different populations of cells and were not preselected for progenitor cells. Therefore, the effect of CIK cells on the growth of hematopoietic colonies was evaluated.
Colony inhibition assays.
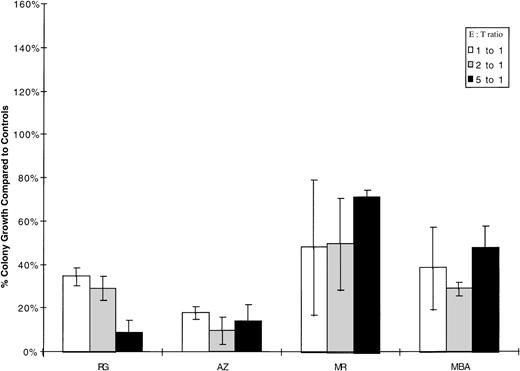
To further evaluate the in vitro cytotoxicity of autologous CIK cells, colony assays were performed. A total of 1 × 105cryopreserved blast cells from patients R.G. and A.Z. was incubated for 4 hours at 37°C in 5% CO2 with autologous CIK cells at effector:target ratios ranging from 1:1 to 5:1. The cells were then washed once and plated in 1mL of methocult in triplicate. Similarly, low-density cells from two chronic-phase patients (M.R. and M.B.A.) were incubated with autologous CIK cells at effector:target ratios ranging from 1:1 to 10:1. In patient M.R., the experiment was complicated by the fact that the CIK cells alone grew approximately 17 colonies /105 cells. There was reduction in the number of colonies in all patients, which was statistically significant (P < .01) in patients A.Z., R.G., and M.R. Colony growth, after incubation with autologous CIK cells at various effector:target ratios, is shown compared to targets alone in Fig 2. Colonies grown from patient M.R. after incubation with autologous CIK cells at a ratio of 5:1 were picked and RT-PCR performed, all colonies were bcr-abl positive.
Inhibition of autologous hematopoietic colony growth by CML CIK cells. 1 × 105 myeloid progenitor cells were incubated for 4 hours with autologous CIK cells at ratios of 1:1, 2:1, and 5:1; washed once; and then replated in 1mL of methyl cellulose. Each incubation was performed in triplicate, and colonies were scored after 14 days’ incubation in 5% CO2. Results are expressed as the percentage growth compared with control target cells incubated in medium alone. The mean number of colonies for 1 × 105 targets alone were MR-325, MBA-21, RG-35, and AZ-73. Patient M.R. grew 17 colonies/105 CIK cells, but this was not adjusted for in calculating the percentage growth compared to targets alone.
Inhibition of autologous hematopoietic colony growth by CML CIK cells. 1 × 105 myeloid progenitor cells were incubated for 4 hours with autologous CIK cells at ratios of 1:1, 2:1, and 5:1; washed once; and then replated in 1mL of methyl cellulose. Each incubation was performed in triplicate, and colonies were scored after 14 days’ incubation in 5% CO2. Results are expressed as the percentage growth compared with control target cells incubated in medium alone. The mean number of colonies for 1 × 105 targets alone were MR-325, MBA-21, RG-35, and AZ-73. Patient M.R. grew 17 colonies/105 CIK cells, but this was not adjusted for in calculating the percentage growth compared to targets alone.
To evaluate the effect of CIK cells on normal hematopoietic colony growth as compared to CML cells, CIK cells were generated from buffy coats obtained from normal donors and incubated with 1 × 105 targets at an effector target ratio of 5:1 for 4 hours then replated in methyl cellulose. Targets used were PBSCs obtained from a normal donor (M.B.), lymphoma (P.S.), and CML (M.R.). Colonies were counted 14 days later. All experiments were performed in triplicate and controls included both CIK cells and targets incubated in medium alone. Results are expressed as the percentage growth relative to that of targets alone (Table3). As can be seen the allogeneic CIK cells from normal donors did not effect overall colony growth of hematopoietic stem cells from these individuals. These same cultures of CIK cells derived from normal donors also showed significant inhibition of CFU-GM colonies from CML patient M.R. (33% to 50% inhibition, P < .05). This level of inhibition of colony growth was comparable to that observed with CIK cells against autologous hematopoietic targets.
Growth of Normal and Leukemic CFU-GM Colonies in the Presence and Absence of Allogeneic CIK Cells
| Cells . | Percent CD3+CD56+ . | M.B. (normal) . | P.S. (NHL) . | M.R. (CML) . |
|---|---|---|---|---|
| Targets alone | — | 100 | 100 | 100 |
| CIK-1 | 32 | 93.7 | 218.2 | 50.8* |
| CIK-2 | 40 | 92.6 | 213.6 | 62.7† |
| CIK-3 | 27 | 86.3 | 218.2 | 55.6* |
| Cells . | Percent CD3+CD56+ . | M.B. (normal) . | P.S. (NHL) . | M.R. (CML) . |
|---|---|---|---|---|
| Targets alone | — | 100 | 100 | 100 |
| CIK-1 | 32 | 93.7 | 218.2 | 50.8* |
| CIK-2 | 40 | 92.6 | 213.6 | 62.7† |
| CIK-3 | 27 | 86.3 | 218.2 | 55.6* |
Results are representative of three experiments and are expressed as the percentage of CFU-GM growth to that of targets alone. The number of CFU-GM colonies for MB (normal donor) was 95 ± 12.7, for P.S. (non-Hodgkin’s lymphoma) 22 ± 4.6 colonies, and for M.R. (CML patient) 333 ± 71.9 colonies. All experiments were performed in triplicate. CIK cells were prepared from buffy coats of three normal donors.
P < .05.
P = .07.
Cytokine production by expanded cells in CIK cultures.
CIK cells generated from six patients and the allogeneic donor (K.S.) for patient R.G. were assessed by intracellular staining and FACS analysis for production of the cytokines IFN-γ, TNF-α, IL-2, and IL-4. Cytokine expression was examined in CD3+56+ cells and CD3+56− cells. None of the CIK cells produced IL-4, whereas IL-2, IFN-γ, and TNF-α was detected in a variable percentage of both CD3+56+ and CD3+56− cells (Fig 3). Two cultures (one from a CML patient and from the normal donor K.S.) on repeated testing at different time points showed a consistent cytokine profile.
Intracellular cytokine production of expanded CIK cells from CML patients. CIK cells from different patients on various days of culture were stimulated with PMA and ionomycin for 4 hours then stained with surface antibodies against CD3 (PCP) and CD56 (PE), followed by permeabilization and intracellular staining with FITC-labeled antibodies against TNF-, IFN-γ, IL-2, and IL-4. The percentage of CD3+56+ cells (light shaded bars) and CD3+56− cells (dark bars) expressing TNF-, IFN-γ, IL-2, and IL-4 is shown.
Intracellular cytokine production of expanded CIK cells from CML patients. CIK cells from different patients on various days of culture were stimulated with PMA and ionomycin for 4 hours then stained with surface antibodies against CD3 (PCP) and CD56 (PE), followed by permeabilization and intracellular staining with FITC-labeled antibodies against TNF-, IFN-γ, IL-2, and IL-4. The percentage of CD3+56+ cells (light shaded bars) and CD3+56− cells (dark bars) expressing TNF-, IFN-γ, IL-2, and IL-4 is shown.
In vivo activity of autologous CIK cells in SCID mice engrafted with CML.
To test the in vivo efficacy of the expanded CIK cells from patients with CML, we established an animal model of this disease in SCID mice.30 To do this chronic-phase CML cells were obtained from the PB and enriched for immature progenitor cells by percoll gradient centrifugation. These CD34+-enriched cells were admixed with matrigel. Matrigel is a soluble extract of basement membranes material containing laminin, collagen type IV, proteoglycans, and a variety of growth factors that is liquid below 22°C and gels rapidly at 37°C. In these experiments, SCID mice were first injected with CML cells in matrigel and CIK cells were expanded in vitro from the same patient material. After 4 weeks the autologous CIK cells were injected IV into groups of mice. No exogenous IL-2 was administered to any of the animals because in our prior experiments expanded CIK cells did not require the administration of IL-2 to the animals for in vivo activity.19
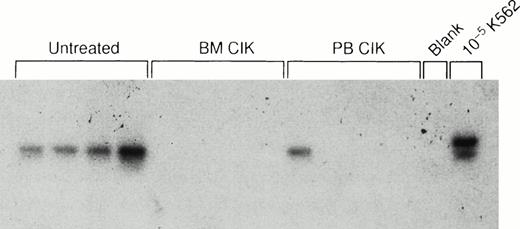
In the first experiment cells from patient K.D. were used and injected into 20 animals to establish disease. Autologous CIK cells were expanded from either the BM or PBMNCs and 4 × 107 CIK cells were injected IV into groups of mice 4 weeks later. At week 7, one control mouse became ill (cause unknown) and was killed; in this animal bcr-abl was detected in the BM by RT-PCR, but this mouse was not included in the overall analysis. A second mouse (given BM CIK cells) died unexpectedly at week 11, and no RNA could be recovered. The remaining mice were sacrificed after 18 to 20 weeks. Bcr-abl was detected in the BM or spleen of 4 out of 5 of the remaining control mice. In marked contrast, only 1 out of 6 mice who received CIK cells derived from the PB and 1 out of 7 mice who received CIK cells expanded from the BM were PCR positive for bcr-abl (Table 4; P = .02). Detection of bcr-abl in the spleens of a representative 12 mice is shown in Fig 4.
Detection of bcr/abl in SCID Mice at 18 Weeks After Injection
| Control | 4/5 (80%) |
| CIK cells derived from bone marrow | 1/7 (14.3%)3-150 |
| CIK cells derived from peripheral blood | 1/6 (16.7%)3-150 |
| Control | 4/5 (80%) |
| CIK cells derived from bone marrow | 1/7 (14.3%)3-150 |
| CIK cells derived from peripheral blood | 1/6 (16.7%)3-150 |
SCID mice were first injected subcutaneously with low-density CML cells containing 1.5 × 106 CD34+ cells per animal admixed with matrigel. Four weeks later some animals received 4 × 107 autologous CIK cells either derived from bone marrow or peripheral blood. Animals were killed 14 weeks later and analyzed for the presence of bcr/abl transcripts by RT-PCR from RNA extracted from PB, bone marrow, and spleen.
Indicates that the CIK-treated animals were less likely to have positive RT-PCR results (P = .02).
In vivo activity of autologous CIK cells. Southern hybridization of bcr-abl RT-PCR products from the spleens of 12 mice injected with CML cells in matrigel followed by autologous CIK cells. Lanes 1 through 4, control mice not receiving CIK cells; lanes 5 through 8, mice treated with autologous BM-derived CIK cells; lanes 9 through 12, mice treated with autologous CIK cells derived from the peripheral blood; lane 13, buffer control; lane 14, 1 in 105 dilution of chronic-phase CML cells in murine cells.
In vivo activity of autologous CIK cells. Southern hybridization of bcr-abl RT-PCR products from the spleens of 12 mice injected with CML cells in matrigel followed by autologous CIK cells. Lanes 1 through 4, control mice not receiving CIK cells; lanes 5 through 8, mice treated with autologous BM-derived CIK cells; lanes 9 through 12, mice treated with autologous CIK cells derived from the peripheral blood; lane 13, buffer control; lane 14, 1 in 105 dilution of chronic-phase CML cells in murine cells.
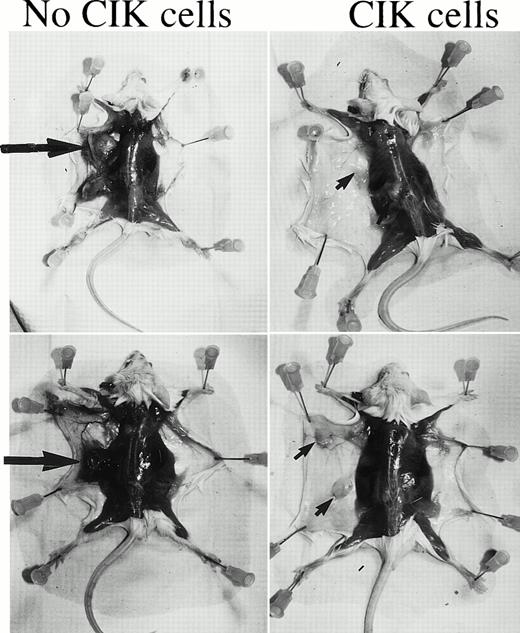
In a second in vivo experiment 30 mice were injected with CML cells admixed with matrigel from patient M.R. After 4 and 8 weeks, 10 mice per group were injected with 2 × 107 autologous CIK cells expanded from PB. In one mouse the matrigel ulcerated the skin at day 5, and this mouse was killed and excluded from further analysis. By 10 weeks all of the control mice had developed subcutaneous tumors at the site of injection of the matrigel. In seven animals these tumors were large (>1.5 cm diameter). At week 11 one mouse died, and the remainder of the mice were sacrificed at week 12 to 13. Histological examination showed that the tumors were an intermediate grade CD20+ lymphoma. The lymphoma was confirmed to be of human origin by cytogenetics and PCR of human immunoglobulin JHregion and to be EBV derived by PCR detection of the latent membrane protein of EBV. The tumors were Ph negative, but the tumor from one mouse had a t(4:10) chromosomal translocation. PCR of the human immunoglobulin JH region showed that the lymphomas were both clonal and oligoclonal as is typical of EBV-transformed lymphomas in SCID mice.31 Figure 5 shows the macroscopic appearance of two mice who received CIK cells at week 4 compared with two mice who received no CIK cells. Clear evidence of macroscopic disease is evident in the control animals, which was not observed in the mice that received the autologous CIK cells. In 5 of 10 mice who had received CIK cells at week 4, there was no macroscopic evidence of tumor (P = .03 compared to control animals). Three of these mice showed no rearrangement of the JH region and were negative for EBV by DNA PCR. Of the five mice who did develop tumors, these tended to be smaller (<1.5 cm). By 8 weeks, when some of the mice already had macroscopic evidence of disease, treatment with CIK cells was not effective. In this group of mice, 7 had large tumors and 3 of the 10 mice had small tumors, similar to control mice.
EBV-associated lymphoma in control versus CIK cell–treated mice. SCID mice were injected with low-density CML cells from patient M.R. in matrigel. Groups of mice were treated with autologous CIK cells at 4 and 8 weeks following the injection of the CML cells. At 12 weeks the majority of the mice developed lymphomas that were found to be EBV associated. Two control mice that did not receive CIK cells are shown in the left hand panels. The large arrows indicate the lymphomas. On the right hand panel two mice who received CIK cells at week 4 are shown. No tumors are visible, but the remains of the matrigel can be seen (small arrows).
EBV-associated lymphoma in control versus CIK cell–treated mice. SCID mice were injected with low-density CML cells from patient M.R. in matrigel. Groups of mice were treated with autologous CIK cells at 4 and 8 weeks following the injection of the CML cells. At 12 weeks the majority of the mice developed lymphomas that were found to be EBV associated. Two control mice that did not receive CIK cells are shown in the left hand panels. The large arrows indicate the lymphomas. On the right hand panel two mice who received CIK cells at week 4 are shown. No tumors are visible, but the remains of the matrigel can be seen (small arrows).
In a third group of mice injected with blast crisis cells, all mice developed subcutaneous tumors of varying sizes, and the percentage of human CD43+ cells in the BM or spleen ranged from 1% to 95%. Cohorts of mice were treated with HLA-matched PBL or expanded CIK cells; however, all animals had subcutaneous tumors and detectable disease in the BM and spleen, using CML blast crisis cells from this patient with aggressive disease.
DISCUSSION
Enhanced understanding of the phenotype and biological activity of effector cell populations with antitumor cell activity as well as the cytokines that activate these cells has led to novel treatment strategies. One such population of expanded effector T cells, termed CIK cells, containing substantial numbers of CD3+CD56+ cells have been shown to have both in vitro and in vivo activity against human lymphoma cell lines in SCID models of human disease.19 In this study we have expanded CIK cells from patients with CML and tested their in vitro and in vivo activity in an autologous CML disease model using SCID mice. Ph-negative CIK cells containing substantial numbers of CD3+CD56+ cells could be generated from most CML patients. The fold expansion of CD3+CD56+cells was variable between patients, ranging from 2- to 525-fold but was reproducible in individual patients (data not shown). The expansion of CIK cells from CML patients was less than in our previous work using normal donors19 but similar to the expansion seen in CIK cultures from lymphoma patients (CD3+expansion 17- to 90-fold, CD3+CD56+ expansion 23- to 241-fold). The two cultures started from frozen cells showed exceptionally poor expansion. Similar variability in expansion of NK cells on M210B4 feeder layers has been documented between patients but with reproducibility in individual patients, suggesting the variability in expansion is inherent to the patient cells and not due to variations in the culture conditions.17 The fold expansion of CD3+CD56+ cells did not appear to correlate with the starting numbers of CD3+ or CD3+CD56+ cells, the time from diagnosis, or previous treatment. Remarkably we were able to generate Ph-negative CIK cells from patients with high white blood cell counts or blast crisis.
The starting population for the CIK cultures was PB that contained a variable number of T cells; however, after approximately 14 days in culture virtually all of the cells present were CD3+ T cells. Under these culture conditions, which favor T-cell expansion, the non–T-cell populations likely die off. Importantly, these T cells are not derived from the malignant clone and are Ph chromosome negative.
CIK cells are Ph negative by cytogenetic analysis; however, care should be taken in interpreting cytogenetics of cultured cells as culture conditions dictate which cells are likely to be dividing. Several of the cultures were Ph negative at day 7 under CIK conditions but were Ph positive when recultured under myeloid conditions at this early time point. However, after 21 days in culture, myeloid cells were no longer detectable in all but one individual. Scheffeld32 reported that CD3+CD56+ cells were bcr-abl negative at day 56 of culture but the CD56− cells were bcr-abl positive. All of our bulk cultures were bcr-abl positive assayed by RT-PCR using nested primers at an earlier time point (day 28). The significance of this finding is unclear because the CIK cultures do not sustain significant numbers of clonogenic Ph-positive cells. At day 21 to 28 of culture, only a minority of patients grew a few small colonies (<100 cells). Only one patient, who was newly diagnosed and most likely to have circulating normal progenitors, grew large multilineage (including erythroid) colonies that were found to be Ph negative by cytogenetics. These results suggest that Ph-positive malignant cells likely die off under CIK conditions. It has been shown that Ph-positive progenitors are selectively purged under optimal myeloid conditions,10 and others have cultured BM from patients with AML in first remission with IL-2 to purge leukemic progenitors.33 Thus, under CIK culture conditions the Ph-positive cells are effectively purged, whereas the cytolytic CD3+CD56+ and CD3+CD56− T cells expand significantly.
CIK cells derived from all patients had shown cytotoxicity against the tumor cell lines K562 and OCI-Ly8, whereas fresh ficolled PBMNCs showed no cytotoxicity. Cytotoxicity was observed from day 14 on and generally peaked around day 28 as did the percentage of CD3+CD56+ cells in the culture, which increased to a plateau (data not shown). The degree of cytotoxicity between patient cell cultures was not absolutely related to the percentage of CD3+CD56+ cells. However, there was a trend toward better killing with an increasing percentage of CD3+CD56+ cells. Outgrowth of NK cells did not account for the cytotoxicity as only one culture had significant numbers (7%) of CD3−CD16+CD56+ cells. This was associated with superior cytotoxicity against K562, an NK-sensitive cell line. Removal of CD3+CD56+ cells from the bulk cultures significantly reduced cytotoxicity (data not shown), which is similar to expanded cells from normal individuals.19
Cytotoxicity using both autologous and allogeneic CIK cells against CML targets labeled with Cr51 was inconsistent, but the targets were a heterogenous population of cells. Four patients (including two blast crisis patients) were further analyzed by colony growth assays, and significant inhibition of colony growth was observed following incubation of the CML cells with autologous expanded CIK cells. In contrast CIK cells showed little inhibition of normal myeloid colony growth but some inhibition of erythroid colony growth, which was not blocked by antibodies to HLA class 1, CD2, CD18 or reproduced by incubation targets with supernatant alone. Inhibition of BFU-E colony growth may be due to production of IFN-γ, which has recently been reported to induce apoptosis of erythroid progenitors via the Fas receptor.34 Allogeneic CIK cells were able to suppress the colony growth of CML but not normal progenitor cells. This observation is consistent with the hypothesis that the CML cells have lost the ability to inhibit NK and NK-like populations of cells possibly due to downregulation of HLA class I or an alteration in the peptides that normally bind to killer inhibitory receptors (KIRs) or other inhibitory molecules and suppress NK cell activity.35CD3+CD56+ effector cells in CIK cultures do express killer inhibitory receptors (data not shown), which may be important in regulating killing and is being further investigated. An alternative hypothesis is that the CML progenitor cells are more sensitive to cytokines such as IFN-γ. The fact that Ph-negative colonies could be grown after 28 days in CIK culture conditions from patient L.C., with untreated CML, suggests that CIK cells do not inhibit normal long-term culture initiating cells (LTCICs).
To test the in vivo efficacy of CIK cells, we engrafted CML into SCID mice using matrigel. CP-CML has been difficult to engraft in SCID and NOD/SCID mice despite the use of large numbers of cells36,37; however, recently reliable engraftment of CML in NOD/SCID mice has been achieved.38 The reason for this is unclear, but we postulated it may be related to the requirement for human cytokines or an appropriate microenvironment for the cells to proliferate. Matrigel is a soluble extract of basement membranes that is liquid below 22°C and gels rapidly at 37°C, which may allow the human cells to grow in a localized more favorable environment before migrating to the mouse BM and spleen. Matrigel contains laminin, collagen type IV, proteoglycans, a variety of growth factors, and is used in many types of tumor invasion assays. Following subcutaneous injection of CP-CML cells suspended in matrigel into SCID mice, we have detected bcr-abl sequences for up to 35 weeks in the BM or SP.30 This long-term engraftment indicates that an immature hematopoietic cell engrafted in the mice. Bcr-abl positive colonies could also be detected from cultured murine BM, indicating that the hematopoietic progenitor cells were able to migrate from the matrigel to the murine bone marrow.
Using this system we tested the in vivo activity of expanded CIK cells against autologous tumor cell targets. In these experiments, CP-CML cells were admixed with matrigel and injected subcutaneously into SCID mice. After 4 weeks expanded autologous CIK cells were injected intravenously into some mice. Mice that received the CIK cells derived from either peripheral blood or bone marrow were significantly less likely to be bcr-abl positive as assayed by RT-PCR of RNA from the bone marrow or spleen than control mice that did not receive the effector CIK cells. In one experiment the mice developed large tumors following the injection of the CML cells, which were found to be an EBV-transformed human B-cell lymphoma that is occasionally observed in SCID mice that receive human cell grafts that are replete with B cells. In those mice that received autologous CIK cells 4 weeks after the injection of the CML cells, half of the mice had no macroscopic evidence of disease, and in the remainder of the mice the size of the tumors were significantly smaller than either the control mice or mice that received CIK cells after 8 weeks. In addition, some of the mice without macroscopic disease were also PCR-negative for EBV sequences. This animal model shows the effectiveness of autologous T cells as opposed to allogeneic effector cells against EBV lymphoma. In other studies, allogeneic EBV-specific CTLs were required to achieve significant tumor regression in SCID models of EBV lymphomas.39
The exact mechanism of the in vivo effect is not known. The expanded CIK cells clearly have cytotoxic activity against target cell lines and also suppress autologous CML myeloid progenitor cell growth. In addition, CIK cells may function through the production of cytokines, such as IFN-γ or TNF. Current studies in our laboratory have shown that CIK cells express fas ligand (data not shown), and this may also be an important mechanism of killing by CIK cells that would not be demonstrable using a standard 4-hour Cr51 release assay.
These studies further document the in vitro and in vivo efficacy of CIK cells against these autologous tumors, especially when present in a minimal disease state. It is important to recognize that these in vivo studies were performed with a single injection of autologous CIK cells without the exogenous administration of IL-2, which is similar to our previous studies with lymphoma targets using allogeneic CIK cells and tumor cell lines.19 The enhanced in vivo activity observed in the absence of exogenous IL-2 may prove to be a distinct advantage of this population of cells as compared to IL-2–activated NK cells, which require exogenous IL-2 for in vivo activity. Clinically, IL-2 administration has been associated with significant toxicity.
In summary, we have shown that CIK cells can be expanded from PBMNCs from most patients with CML. These cells are derived from CD3+ T cells, and the cultures contain primarily CD3+CD56− and CD3+CD56+ T cells that expand dramatically under these culture conditions. The CD3+CD56+ T cells have been shown to have significant cytolytic activity against a broad array of tumor cell targets. The CIK cells derived from CML patients are Ph chromosome negative; produce cytokines such as IL-2, IFN, and TNF; and have in vitro and in vivo cytotoxicity. These cells may prove to be an effective form of immunotherapy, for example, following a bone marrow or peripheral blood transplant.
ACKNOWLEDGMENT
Statistical analysis was performed by Ruby Wong PhD. The helpful advice of Dr Karl Blume was greatly appreciated.
Supported in part by a grant from The Jose Carreras para la Lucha contra la Leucemia (FIJC-92/INT-2) and by USA Public Health Service Grant (CA-49605).
Address reprint requests to Robert S. Negrin, MD, Room H1353, Bone Marrow Transplant Division, Stanford University Hospital, Stanford, CA 94305; e-mail: Negrs@leland.Stanford.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal