Abstract
We have previously identified a cellular population in murine bone marrow that facilitates engraftment of highly purified hematopoietic stem cells (HSC) across major histocompatibility complex (MHC) barriers without causing graft-versus-host disease. Here we investigated the effect of flt3 ligand (FL) and granulocyte colony-stimulating factor (G-CSF) on the mobilization of facilitating cells (FC) and HSC into peripheral blood (PB). Mice were injected with FL alone (day 1 to 10), G-CSF alone (day 4 to 10), or both in combination. The number of FC (CD8+/βTCR−/γδTCR−) and HSC (lineage−/Sca-1+/c-kit+) was assessed daily by flow cytometry. Lethally irradiated allogeneic mice were reconstituted with PB mononuclear cells (PBMC). FL and G-CSF showed a highly significant synergy on the mobilization of FC and HSC. The peak efficiency for mobilization of FC (21-fold increase) and HSC (200-fold increase) was reached on day 10. Our data further suggest that the proliferation of FC and HSC induced by FL in addition to the mobilizing effect mediated by G-CSF might be responsible for the observed synergy of both growth factors. Finally, the engraftment potential of PBMC mobilized with FL and G-CSF or FL alone was superior to PBMC obtained from animals treated with G-CSF alone. Experiments comparing the engraftment potential of day 7 and day 10 mobilized PBMC indicate that day 10, during which both FC and HSC reached their maximum, might be the ideal time point for the collection of both populations. © 1998 by The American Society of Hematology.
BONE MARROW transplantation (BMT) is the treatment of choice for numerous hematologic malignancies1as well as curative for several nonmalignant hematologic disorders, including hemoglobinopathies,2,3 aplastic anemia,4,5 platelet disorders,6 and deficiencies of soluble enzymes including chronic granulomatous disease and adenosine deaminase deficiency.7,8 In effect BMT represents a natural form of gene therapy.9 The replacement of the defective hematopoietic stem cell (HSC) and its derivatives by BMT is now also considered as a potential treatment option for the broad field of autoimmunity.10
In spite of prophylactic immunosuppressive therapy, graft-versus-host disease (GVHD) remains one of the major limitations in clinical BMT. Because the effector cells in GVHD are mainly T cells and natural killer (NK) cells,11,12 GVHD does not occur after transplantation of purified HSC.13-15 However, while purified HSC engraft in syngeneic recipients, HSC alone do not engraft in physiologic numbers across major histocompatibility complex (MHC) barriers.13-15 We have recently identified a cell population in murine bone marrow that facilitates engraftment of highly purified HSC in lethally irradiated MHC-disparate allogeneic recipient mice without causing GVHD.14 This facilitating cell (FC) population is phenotypically characterized by a unique combination of cell surface molecules, including CD8+/CD3+/CD45RB+/Thy1+/MHC class IIdim but αβTCR−/γδTCR−, and comprises 0.4% of murine bone marrow.14
There has recently been an increasing interest in the use of mobilized peripheral blood (PB) progenitor cells (PC) and PB HSC as an alternative to allogeneic bone marrow grafts. PC and HSC are collected from the donor in an outpatient setting without general anesthesia, and reduced engraftment times have been observed. Moreover, larger cell numbers are available. Another potential advantage reported from animal models is an enhanced graft-versus-leukemia (GVL) effect after transplantation of mobilized PB.16 The clinical trials using granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) mobilized PB have been encouraging.17-22
A number of hematopoietic growth factors and cytokines including G-CSF,23,24 GM-CSF,25,26 c-kit ligand,27,28 interleukin-7 (IL-7),29IL-8,30 and IL-1231 have shown to expand HSC and PC in vitro or cause their migration into the periphery in vivo. Another growth factor named flt3 ligand (FL) has now been identified.32-34 FL binds to its receptor, a member of the tyrosine kinase receptor family type III. This receptor appears to be selectively expressed on HSC and progenitor cells.35-37 FL has been shown to enhance the proliferation of PC and HSC in vitro32-40 and to mobilize HSC and PC in PB in vivo.41,42 Interestingly, when FL was used in combination with G-CSF or GM-CSF, a synergy for PC and HSC mobilization was observed and mobilized PB showed superior engraftment potential after transplantation into partially ablated syngeneic murine recipients.43-45
In the present study we evaluated the ability of FL alone, G-CSF alone, or the two in combination to mobilize cells of FC phenotype in the periphery and to study the kinetics of FC and HSC mobilization to define optimal timing for the collection of both populations. Both growth factors showed a highly significant synergy on the mobilization of FC and HSC. The kinetics for mobilization were similar for FC and HSC, with a peak occurring on day 10. G-CSF alone was not efficient at mobilizing FC. We further analyzed the distribution of FC and HSC in hematopoietic sites such as spleen and bone marrow of growth factor–treated mice at different time points. A dramatic expansion of both FC and HSC was observed in spleen of FL- and FL + G-CSF–treated animals, whereas no significant changes were detectable in spleen of mice injected with G-CSF alone. In bone marrow of animals treated with FL alone, the frequency of FC showed a fivefold increase. This phenomenon was not observed in animals that received G-CSF alone or in combination with FL. The engraftment potential of HSC and FC mobilized by FL and FL + G-CSF into fully ablated MHC-disparate recipients was superior to that for G-CSF treated donors alone.
MATERIALS AND METHODS
Animals.
Four- to 6-week-old male C57BL/10SnJ (B10, H-2b) and B10.BR.SgSnJ (B10.BR, H-2k) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Animals were housed in a barrier animal facility at the Institute for Cellular Therapeutics, Allegheny University of the Health Sciences, Philadelphia, PA, and cared for according to specific Allegheny University and National Institutes of Health animal care guidelines.
Growth factors.
Recombinant human FL was kindly provided by Immunex (Immunex Corp, Seattle, WA) and diluted in 0.1% mouse serum albumin (MSA; Sigma, St Louis, MO) at a concentration of 100 μg/mL. Recombinant human G-CSF was purchased from Amgen (Amgen Inc, Thousand Oaks, CA). Growth factors were diluted in saline before injections to a total volume of 500 μL, and B10.BR mice were injected once daily subcutaneously (SC). Mice received either 10 μg FL/d alone from day 1 to 10, 7.5 μg G-CSF/d alone from day 4 to 10, or a combination of FL and G-CSF.42-44 Control animals were injected with saline only.
Tissues.
PB was obtained daily from the tail vein of growth factor–treated animals. After individual count of peripheral blood mononuclear cells (PBMC) with a hemocytometer, cells were stained for flow cytometric analysis to study the kinetics of FC and HSC mobilization. In separate experiments PB was collected on days 0, 7, and 10 from growth factor–treated anesthetized animals via cardiac puncture into heparinized tubes and pooled for each group for reconstitution of allogeneic recipients. At the same time points, spleens and long bones were obtained and single-cell suspensions were prepared for flow cytometric analysis. Splenocytes were isolated by gently flushing the organ with media 199 (MEM; Life Technologies, Rockville, MD). Red blood cells (RBC) were lysed using ammonium chloride lysing buffer (ACK; prepared in our laboratory). Bone marrow was obtained from tibiae and femurs as described previously.46 Briefly, bones were flushed with MEM. Bone marrow cells were resuspended and filtered through a sterile nylon mesh. After centrifugation, cells were resuspended in MEM and counted.
Monoclonal antibodies.
Anti–H-2Kb–phycoerythrin (PE) (AF6-88.5); anti–H-2Kk–fluorescein isothiocyanate (FITC) and –Biotin (36-7-5); anti–GR1-FITC (RB6-8C5); anti–Mac-1 (CD11b)-FITC (M1/70); anti–CD8α-FITC and -APC (53-6.7); anti–CD11b-FITC (M1/70); anti–B220/CD45R-FITC (RA3-6B2); anti–αβTCR-FITC and -PE (H57-597); anti–γδTCR-FITC and -PE (GL3); anti–NK1.1-PE (PK 136); anti–Sca-1 (Ly-6A/E)-PE (D7); and anti–c-kit (CD117)-Biotin (2B8) were purchased from Pharmingen (San Diego, CA). Streptavidin-APC was purchased from Becton Dickinson (Mountain View, CA).
Detection of FC and HSC by flow cytometry.
The mobilization kinetics of FC and HSC in PB were analyzed daily for individual animals. Aliquots of 100 μL PB were incubated with monoclonal antibodies (MoAbs) for 30 minutes on ice. Cells were washed twice in fluorescence-activated cell sorter (FACS) medium (prepared in laboratory). Cells labeled with biotinylated MoAb were counterstained with streptavidin-APC for 15 minutes. RBC were lysed using ammonium chloride lysing buffer. PBMC were washed twice and fixed in 2% paraformaldehyde (Tousimis Research Corporation, Rockville, MD). Flow cytometric analysis was performed using a FACSCalibur (Becton Dickinson) as described previously.14 For analysis of FC and HSC, a minimum of 1 × 105 events were collected. FC were defined as cells residing in a wide lymphoid gate with a dim to intermediately positive expression of CD8 but negative for expression of αβTCR and γδTCR. For enumeration of HSC, cells positive for Sca-1 (Ly-6A/E) and negative for lineage markers (lin−) were gated. Gated cells were then analyzed for their expression of c-kit (CD117). Lin−/Sca-1+/c-kit+ cells were defined as HSC. Statistical analysis of flow data was performed using CELL Quest Software, Version 3.0.1 (Becton Dickinson). The percentage of FC and HSC of total PBMC was determined and the absolute number of FC and HSC per microliter blood was calculated based on individual PBMC counts. In addition, the percentage of FC and HSC in spleen and bone marrow was determined at different time points under treatment with FL and/or G-CSF.
Reconstitution of allogeneic recipients with mobilized PB.
To investigate the repopulating potential of FC and HSC in mobilized PB, allogeneic B10 mice were lethally irradiated with a single dose of 950 cGy total body irradiation (TBI; 117.18 cGy/min) from a cesium source (Nordion, Ontario, Canada). On day 7 or 10 of mobilization, PB was obtained from B10.BR mice, pooled for each treatment group, and counted. Three to 5 hours following irradiation, animals were reconstituted with mobilized whole blood containing 1 × 106, 2.5 × 106, or 5 × 106 PBMC diluted in MEM to a total volume of 1 mL via the lateral tail vein. Radiation controls as well as control animals reconstituted with equal numbers of PBMC from unmobilized PB were prepared. Reconstituted animals were monitored daily to detect failure of engraftment as indicated by excessive body weight loss, and survival was calculated based on the life-table method. In addition, PBMC counts were performed 10, 20, and 30 days following reconstitution.
Characterization of chimeras by flow cytometry.
Thirty days and 6 months after reconstitution, recipients were analyzed for evidence of donor cell engraftment by flow cytometry to detect the percentage of PBMC bearing H-2Kb (recipient) and H-2Kk (donor) markers. PB was collected from the tail vein into heparinized vials. After thoroughly mixing, 100 μL PB was incubated with H-2Kb–PE and H-2Kk–FITC MoAb for 30 minutes on ice. RBC were lysed using ammonium chloride lysing buffer. PBMC were washed twice and fixed in 2% paraformaldehyde (Tousimis Research Corporation). Lymphocytes, granulocytes, and monocytes were gated based on forward and side scatter and analyzed for anti–H-2Kb or anti–H-2Kk expression. PB from unmanipulated B10 and B10.BR mice served as controls.
To confirm HSC engraftment, the presence of multilineage chimerism was assessed using three-color flow cytometry 6 months after reconstitution. PB was obtained and stained with FITC- and PE-labeled lineage MoAbs and biotinylated H-2Kk MoAb, counterstained with streptavidin-APC, as described above.
Statistical analysis.
Statistical analyses were performed using unpaired two-tailed Student’s t-test, and P values < .05 were considered as significant. The 6-month survival of transplanted animals was assessed using Kaplan-Meier estimates. The 30-day and 6-month survival of different groups was compared using the Wilcoxon test, and Pvalues < .05 were considered as significant.
RESULTS
Kinetics of mobilization of FC and HSC in PB.
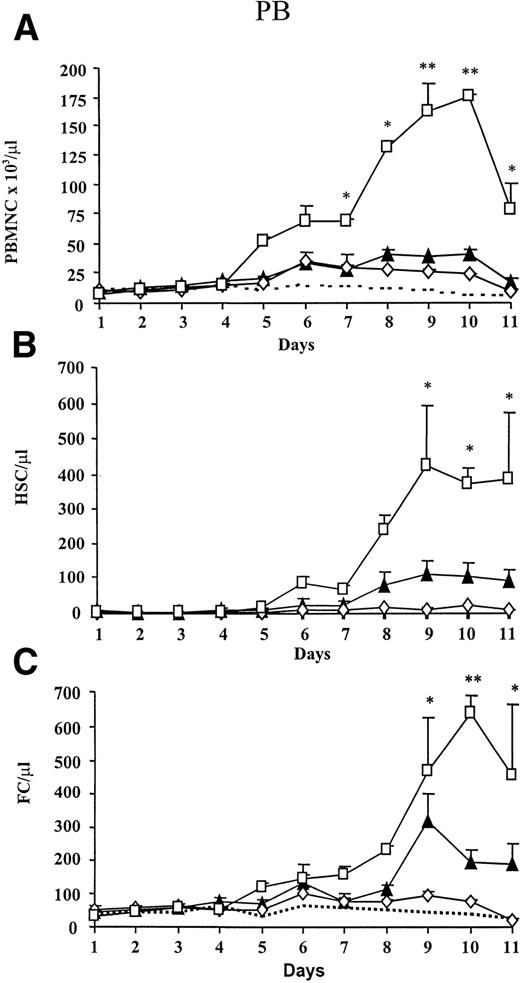
We evaluated the effect on the total number of PBMC in PB after administration of FL alone (day 1 to 10), G-CSF alone (day 4 to 10), or a combination of both growth factors. Animals treated with G-CSF and FL alone showed a threefold and fourfold increase of PBMC, respectively (Fig 1A). Combined administration of both growth factors showed a synergistic effect, because PBMC increased significantly and a maximum (22-fold increase) was observed on day 10. PBMC of animals injected with carrier only remained at baseline levels.
Kinetics of mobilization of PBMC, HSC, and FC under treatment with FL alone (▴), G-CSF alone (◊), FL + G-CSF (□), or carrier (—-). FL (10 μg/mouse) was injected SC for 10 days and G-CSF (7.5 μg/mouse) from day 4 to 10. (A) PB was obtained daily and PBMC were counted. The percentage of HSC (lineage−/Sca-1+/c-kit+) and FC (CD8+/βTCR−/γδTCR−) was analyzed by flow cytometry, and absolute numbers of (B) HSC and (C) FC were calculated based on individual PBMC counts. Results represent the mean (SEM) of two different experiments (n = 5 per group). PBMC or absolute numbers of FC and HSC that differed significantly from controls are marked (* P < .005 or ** P < .0005).
Kinetics of mobilization of PBMC, HSC, and FC under treatment with FL alone (▴), G-CSF alone (◊), FL + G-CSF (□), or carrier (—-). FL (10 μg/mouse) was injected SC for 10 days and G-CSF (7.5 μg/mouse) from day 4 to 10. (A) PB was obtained daily and PBMC were counted. The percentage of HSC (lineage−/Sca-1+/c-kit+) and FC (CD8+/βTCR−/γδTCR−) was analyzed by flow cytometry, and absolute numbers of (B) HSC and (C) FC were calculated based on individual PBMC counts. Results represent the mean (SEM) of two different experiments (n = 5 per group). PBMC or absolute numbers of FC and HSC that differed significantly from controls are marked (* P < .005 or ** P < .0005).
To assess the potential of growth factor administration on mobilization of FC and HSC, the absolute number of FC and HSC under treatment with FL alone, G-CSF alone, and a combination of FL and G-CSF was determined (Fig 1B and C). Although G-CSF alone resulted in a 17-fold increase of primitive HSC, only a modest effect on the mobilization of FC was noted. In contrast, FL as a single agent caused a 7-fold increase of FC and 36-fold increase of HSC, respectively, and peak levels for both populations occurred on day 9. A maximal elevation of both FC and primitive HSC was detectable when both growth factors were combined. An increase of HSC was detectable on day 6, and a plateau reflecting a more than 200-fold increase or an absolute number of approximately 400 HSC/μL PB was reached from day 9 to 11. The number of cells of FC phenotype increased on day 5 and a peak level representing a 21-fold increase was observed on day 10. In contrast to HSC, the number of FC declined rapidly after day 10.
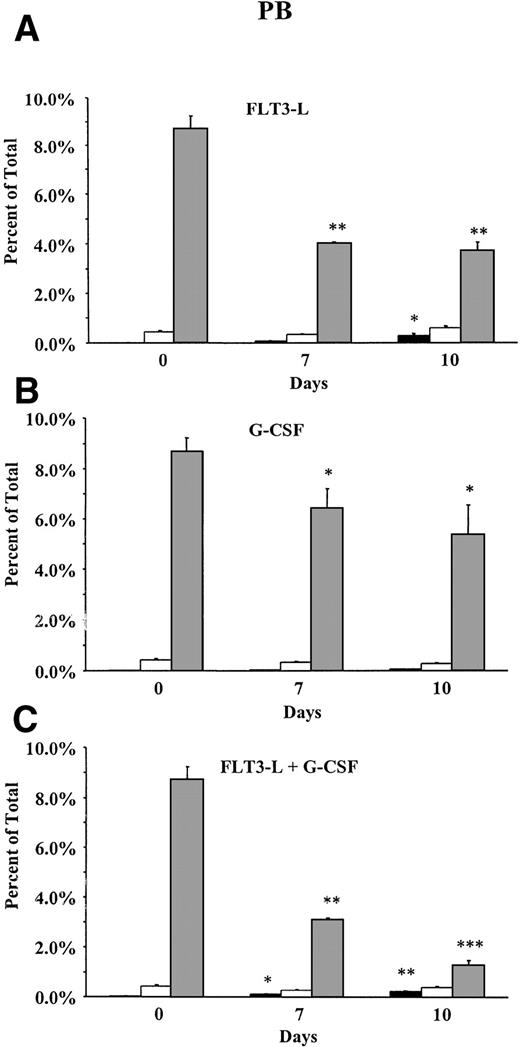
Because the majority of cells in PB after FL + G-CSF treatment were neutrophilic granulocytes, the relative percentage of CD8+T cells decreased significantly from 8.7% on day 0 to 1.3% on day 10 (Fig 2). In striking contrast, the percentage of FC remained at a constant level, whereas an increase in the percentage of HSC from 0.01% on day 0 to 0.36% on day 10 was observed. A similar observation was made when mice were treated with FL alone. CD8+ T cells in G-CSF–treated animals showed only a slight decrease (8.7% to 5.4%), and no significant changes in the percentage of FC and HSC were observed.
Percentage of HSC, FC, and CD8+ T cells in PB during treatment with (A) FL alone (n = 5), (B) G-CSF alone (n = 5), or (C) FL + G-CSF (n = 5). FL (10 μg/mouse) was injected SC from day 1 to 10 and G-CSF (7.5 μg/mouse) from day 4 to 10. PB was stained for HSC (▪) (lineage−/Sca-1+/c-kit+), FC (□) (CD8+/βTCR−/γδTCR−), and CD8+ T cells (▧) (CD8+/βTCR+). Results show the mean (SEM) percentage before and on day 7 and day 10 of growth factor administration. Percentages of HSC, FC, or CD8+ T cells that differed significantly from day 0 values are marked (* P< .05; ** P < .005; or *** P < .0005).
Percentage of HSC, FC, and CD8+ T cells in PB during treatment with (A) FL alone (n = 5), (B) G-CSF alone (n = 5), or (C) FL + G-CSF (n = 5). FL (10 μg/mouse) was injected SC from day 1 to 10 and G-CSF (7.5 μg/mouse) from day 4 to 10. PB was stained for HSC (▪) (lineage−/Sca-1+/c-kit+), FC (□) (CD8+/βTCR−/γδTCR−), and CD8+ T cells (▧) (CD8+/βTCR+). Results show the mean (SEM) percentage before and on day 7 and day 10 of growth factor administration. Percentages of HSC, FC, or CD8+ T cells that differed significantly from day 0 values are marked (* P< .05; ** P < .005; or *** P < .0005).
Distribution of FC and HSC in spleen and bone marrow of mice treated with growth factors.
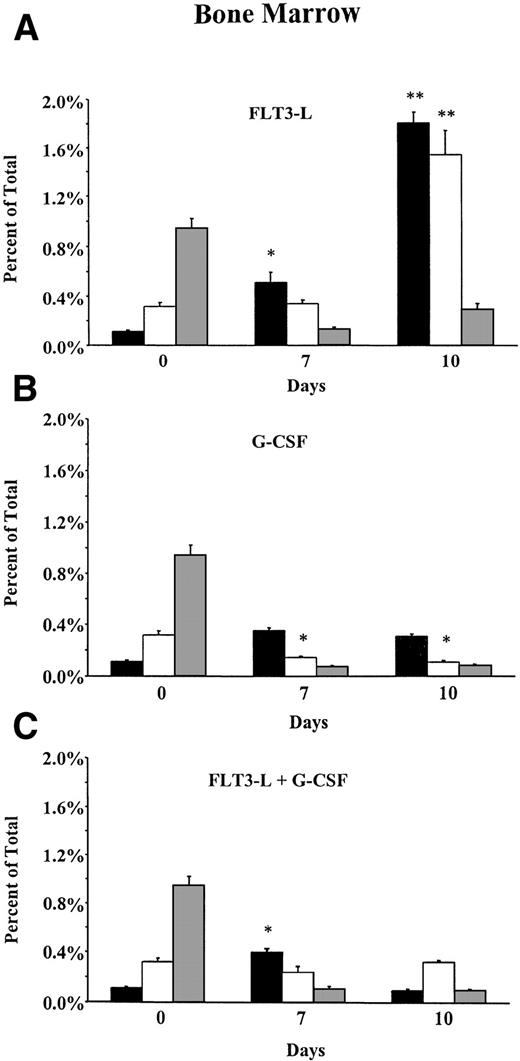
To address whether the observed increase in absolute numbers of FC and HSC in PB was due to mobilization of preexisting cells or due to de novo hematopoiesis, splenocytes and bone marrow cells from FL-, G-CSF–, and FL + G-CSF–treated mice were analyzed by flow cytometry, and percentages of FC and HSC were determined. Mobilization of mature cells from the bone marrow into the periphery occurred in all growth factor–treated animals as indicated by a significant reduction of the percentage of CD8+ T cells (Fig3A, B, and C). In the bone marrow of animals that received G-CSF alone, only a marginal increase in the percentage of HSC was present, whereas the frequency of FC decreased significantly during mobilization. In animals treated with FL and G-CSF, a significant increase in the percentage of HSC was observed on day 7. However, on day 10 of mobilization, the frequency of HSC in bone marrow decreased to baseline levels. Interestingly, in mice treated with FL alone an 18-fold and 5-fold increase in the percentage of HSC and FC was detected, respectively, indicating proliferation and/or lack of mobilization of FC and HSC in the absence of G-CSF.
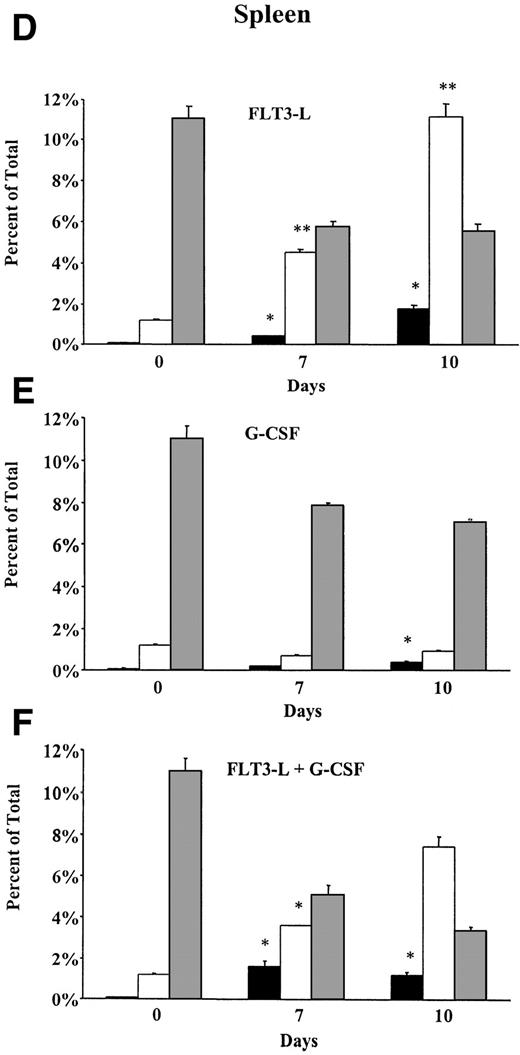
Distribution of HSC, FC, and CD8+ T cells in (A through C) bone marrow and (D through F) spleen of B10.BR mice treated with FL alone (10 μg/mouse; day 1 to 10); G-CSF alone (7.5 μg/mouse; day 4 to 10); or FL + G-CSF. Animals (n = 6 per group) were euthanized before, on day 7, or day 10 of growth factor administration. Long bones and spleens were obtained and processed for each individual animal. Bone marrow cells and splenocytes were analyzed for the percentage of HSC (▪) (lineage−/Sca-1+/c-kit+), FC (□) (CD8+/βTCR−/γδTCR−), and CD8+ T cells (▧) (CD8+/βTCR+) by flow cytometry. Results represent the mean (SEM) percentage on total bone marrow and total splenocytes. Percentages of HSC and FC that differed significantly from day 0 values are marked (* P < .05 or ** P < .005).
Distribution of HSC, FC, and CD8+ T cells in (A through C) bone marrow and (D through F) spleen of B10.BR mice treated with FL alone (10 μg/mouse; day 1 to 10); G-CSF alone (7.5 μg/mouse; day 4 to 10); or FL + G-CSF. Animals (n = 6 per group) were euthanized before, on day 7, or day 10 of growth factor administration. Long bones and spleens were obtained and processed for each individual animal. Bone marrow cells and splenocytes were analyzed for the percentage of HSC (▪) (lineage−/Sca-1+/c-kit+), FC (□) (CD8+/βTCR−/γδTCR−), and CD8+ T cells (▧) (CD8+/βTCR+) by flow cytometry. Results represent the mean (SEM) percentage on total bone marrow and total splenocytes. Percentages of HSC and FC that differed significantly from day 0 values are marked (* P < .05 or ** P < .005).
In spleen, the frequency of both FC and HSC increased over time under treatment with FL alone or FL in combination with G-CSF (Fig 3D and F). Cells of FC phenotype increased significantly from 1% on day 0 to 11% on day 10 in animals treated with FL alone and from 1% on day 0 to 7% on day 10 in animals that received a combination of both growth factors. The percentage of primitive HSC in spleen of FL- and FL + G-CSF–treated mice showed a 20-fold (0.09% on day 0 to 1.79% on day 10) and an 18-fold increase (0.09% on day 0 to 1.62% on day 7), respectively. In striking contrast, under treatment with G-CSF alone, the percentage of FC remained unchanged over time, whereas the frequency of HSC was slightly elevated (Fig 3E).
Short-term engraftment potential of mobilized PBMC in allogeneic recipients.
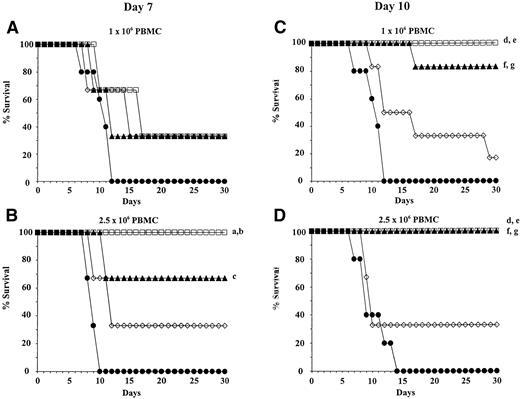
To determine the short-term engraftment potential of HSC and FC mobilized by treatment with FL, G-CSF, or FL + G-CSF, allogeneic B10 mice were lethally irradiated and reconstituted with whole PB containing varying numbers of PBMC. Recipients were transplanted with either 1 × 106, 2.5 × 106, or 5 × 106 PBMC from donors treated with growth factors for 7 or 10 days. The cell number of mobilized HSC and FC per kilogram body weight of recipients is shown in Table 1. Control animals received equal amounts of PBMC from untreated B10.BR mice. The 30-day survival of transplanted animals as a function of PBMC dose and time-point of collection of PB is shown in Fig 4. Animals reconstituted with 1 × 106 PBMC collected on day 7 from donors treated with FL, G-CSF, or FL + G-CSF showed a 33% survival at day 30. Control animals injected with 1 × 106 PBMC from untreated donors died within 12 days from irradiation-induced aplasia (Fig 4A). At a cell dose of 2.5 × 106 PBMC, the 30-day survival rate increased to 67% after treatment with FL alone and 100% after treatment with FL + G-CSF. No significant difference between both cell doses was observed for the G-CSF treatment group and control group (Fig 4B). The 30-day survival of irradiated recipients was superior when PB from FL- or FL + G-CSF–treated animals was collected after 10 days of growth factor administration. As few as 1 × 106 PBMC mobilized with FL alone rescued more than 80% of recipients, whereas with FL + G-CSF, 100% of transplanted animals survived (Fig 4C). At a PBMC dose of 2.5 × 106, 100% of animals transplanted with FL- and FL + G-CSF–mobilized PB were alive after 30 days (Fig 4D). When 1 × 106 and 2.5 × 106 PBMC mobilized with G-CSF as a single agent were given, the 30-day survival rate was only 20% and 33%, respectively. All control animals injected with PB from untreated donors died within 14 days from TBI-induced aplasia. Irradiation controls that received 950 cGy TBI without PBMC injection died within 10 days (data not shown). When the dose of PBMC collected on day 7 or day 10 was further increased to 5 × 106, no further improvement of the 30-day survival rate was observed for all treatment groups (data not shown).
HSC and FC Dose Per Kilogram Body Weight Injected Into Lethally Irradiated B10 Mice
| Donor Treatment . | PBMC Dose/Recipient . | Day 7 . | Day 10 . | ||
|---|---|---|---|---|---|
| HSC Dose/kg [×105] . | FC Dose/kg [×105] . | HSC Dose/kg [×105] . | FC Dose/kg [×105] . | ||
| FL | 1 × 106 | 0.06 ± 0.00 | 0.96 ± 0.07 | 0.63 ± 0.02-150 | 2.10 ± 0.07-150 |
| 2.5 × 106 | 0.17 ± 0.00 | 2.50 ± 0.00 | 1.59 ± 0.24-150 | 5.28 ± 0.79-150 | |
| 5 × 106 | 0.34 ± 0.02 | 5.12 ± 0.26 | 2.84 ± 0.25-150 | 9.41 ± 0.83-150 | |
| G-CSF | 1 × 106 | 0.06 ± 0.00 | 1.06 ± 0.07 | 0.18 ± 0.01 | 0.93 ± 0.07 |
| 2.5 × 106 | 0.17 ± 0.01 | 2.75 ± 0.09 | 0.43 ± 0.02 | 2.22 ± 0.09 | |
| 5 × 106 | 0.33 ± 0.04 | 5.43 ± 0.64 | 0.92 ± 0.24 | 4.77 ± 1.23 | |
| FL + G-CSF | 1 × 106 | 0.29 ± 0.01 | 0.88 ± 0.02 | 0.64 ± 0.03-150 | 1.21 ± 0.06-150 |
| 2.5 × 106 | 0.65 ± 0.04-150 | 1.96 ± 0.13-150 | 1.71 ± 0.07-150 | 3.24 ± 0.13-150 | |
| 5 × 106 | 1.27 ± 0.06-150 | 3.80 ± 0.19-150 | 3.38 ± 0.18-150 | 6.42 ± 0.34-150 | |
| Donor Treatment . | PBMC Dose/Recipient . | Day 7 . | Day 10 . | ||
|---|---|---|---|---|---|
| HSC Dose/kg [×105] . | FC Dose/kg [×105] . | HSC Dose/kg [×105] . | FC Dose/kg [×105] . | ||
| FL | 1 × 106 | 0.06 ± 0.00 | 0.96 ± 0.07 | 0.63 ± 0.02-150 | 2.10 ± 0.07-150 |
| 2.5 × 106 | 0.17 ± 0.00 | 2.50 ± 0.00 | 1.59 ± 0.24-150 | 5.28 ± 0.79-150 | |
| 5 × 106 | 0.34 ± 0.02 | 5.12 ± 0.26 | 2.84 ± 0.25-150 | 9.41 ± 0.83-150 | |
| G-CSF | 1 × 106 | 0.06 ± 0.00 | 1.06 ± 0.07 | 0.18 ± 0.01 | 0.93 ± 0.07 |
| 2.5 × 106 | 0.17 ± 0.01 | 2.75 ± 0.09 | 0.43 ± 0.02 | 2.22 ± 0.09 | |
| 5 × 106 | 0.33 ± 0.04 | 5.43 ± 0.64 | 0.92 ± 0.24 | 4.77 ± 1.23 | |
| FL + G-CSF | 1 × 106 | 0.29 ± 0.01 | 0.88 ± 0.02 | 0.64 ± 0.03-150 | 1.21 ± 0.06-150 |
| 2.5 × 106 | 0.65 ± 0.04-150 | 1.96 ± 0.13-150 | 1.71 ± 0.07-150 | 3.24 ± 0.13-150 | |
| 5 × 106 | 1.27 ± 0.06-150 | 3.80 ± 0.19-150 | 3.38 ± 0.18-150 | 6.42 ± 0.34-150 | |
B10.BR donors were treated with FL alone, G-CSF alone, or FL + G-CSF. PB was collected on day 7 or day 10, pooled for each group, and recipients were reconstituted with either 1 × 106, 2.5 × 106, or 5 × 106 PBMC (4 to 7 mice per group). The dose of HSC (lineage−/Sca-1+/c-kit+) and FC (CD8+/αβTCR−/γδTCR−) per kilogram body weight of recipients was calculated based on flow cytometric analysis and is expressed as the mean ± SD.
Doses at which the 30-day survival reached >80%.
Survival (30 days) of lethally irradiated recipients (B10) transplanted with mobilized PB from donor mice (B10.BR). Donors were treated once daily with FL alone (▴), G-CSF alone (◊), FL + G-CSF (□), or carrier only (•). PBMC were obtained from donors after 7 days (A and B) or 10 days (C and D) of growth factor administration and pooled for each group. Recipients were injected with 1 × 106 or 2.5 × 106 PBMC 3 to 5 hours after irradiation (4 to 7 mice per group). There was a significantly greater survival of mice reconstituted with PBMC from FL- and FL + G-CSF–treated donors when compared with unmobilized or G-CSF–mobilized PBMC. Differences between groups that reached statistical significance are marked (a: FL + G-CSF v control,P <0.05; b: FL + G-CSF v G-CSF, P < .05; c: FL v control, P < .05; d: FL + G-CSF vcontrol P < .005; e: FL + G-CSF v G-CSF, P<0.05; f: FL v control, P < .005; g: FL vG-CSF, P < .05).
Survival (30 days) of lethally irradiated recipients (B10) transplanted with mobilized PB from donor mice (B10.BR). Donors were treated once daily with FL alone (▴), G-CSF alone (◊), FL + G-CSF (□), or carrier only (•). PBMC were obtained from donors after 7 days (A and B) or 10 days (C and D) of growth factor administration and pooled for each group. Recipients were injected with 1 × 106 or 2.5 × 106 PBMC 3 to 5 hours after irradiation (4 to 7 mice per group). There was a significantly greater survival of mice reconstituted with PBMC from FL- and FL + G-CSF–treated donors when compared with unmobilized or G-CSF–mobilized PBMC. Differences between groups that reached statistical significance are marked (a: FL + G-CSF v control,P <0.05; b: FL + G-CSF v G-CSF, P < .05; c: FL v control, P < .05; d: FL + G-CSF vcontrol P < .005; e: FL + G-CSF v G-CSF, P<0.05; f: FL v control, P < .005; g: FL vG-CSF, P < .05).
To study the time course of HSC and PC engraftment, PBMC from individual animals transplanted with mobilized PB collected on day 10 were counted 10, 20, and 30 days following reconstitution. Engraftment, defined as a PBMC count of ≥ 500 PBMC/μL, was observed in ≥ 67% of recipients 20 days after reconstitution with 1 × 106and 2.5 × 106 PMBC from FL- and FL + G-CSF–treated donors (Table 2). After 30 days, 100% of these animals had a PBMC count of ≥ 500 PBMC/μL. When 5 × 106 PBMC from FL- and FL + G-CSF–treated animals were injected, engraftment occurred as early as on day 10 in 1 out of 3 and 3 out of 3 recipients, respectively. In striking contrast, animals reconstituted with equal amounts of PBMC from G-CSF–treated or unmanipulated donors did not present PBMC counts of ≥ 500 PBMC/μL at any of the time points tested (data not shown).
Time to Engraftment of Mobilized PBMC From B10.BR Mice Into Lethally Irradiated B10 Mice
| PBMC Dose . | Donor Treatment . | Time to Engraftment: Number of Animals With ≥500 PBMC/μL . | Median PBMC Count (range) [PBMC/μL] . | ||||
|---|---|---|---|---|---|---|---|
| Day 10 . | Day 20 . | Day 30 . | Day 10 . | Day 20 . | Day 30 . | ||
| 1 × 106 | FL | 0/3 | 2/3 | 3/3 | <100 | 560 (200-1,300) | 1,520 (750-4,800) |
| FL + G-CSF | 0/3 | 3/3 | 3/3 | <100 | 850 (620-1,080) | 750 (550-1,350) | |
| 2.5 × 106 | FL | 0/3 | 3/3 | 3/3 | <100 | 920 (520-4,480) | 3,500 (1,100-5,000) |
| FL + G-CSF | 0/3 | 2/3 | 3/3 | <100 | 740 (420-1,460) | 1,900 (1,500-5,600) | |
| 5 × 106 | FL | 1/3 | 2/3 | 3/3 | 220 (180-540) | 620 (460-1,480) | 4,000 (2,300-4,960) |
| FL + G-CSF | 3/3 | 3/3 | 3/3 | 900 (800-920) | 1,360 (1,240-3,960) | 5,800 (5,200-7,100) | |
| PBMC Dose . | Donor Treatment . | Time to Engraftment: Number of Animals With ≥500 PBMC/μL . | Median PBMC Count (range) [PBMC/μL] . | ||||
|---|---|---|---|---|---|---|---|
| Day 10 . | Day 20 . | Day 30 . | Day 10 . | Day 20 . | Day 30 . | ||
| 1 × 106 | FL | 0/3 | 2/3 | 3/3 | <100 | 560 (200-1,300) | 1,520 (750-4,800) |
| FL + G-CSF | 0/3 | 3/3 | 3/3 | <100 | 850 (620-1,080) | 750 (550-1,350) | |
| 2.5 × 106 | FL | 0/3 | 3/3 | 3/3 | <100 | 920 (520-4,480) | 3,500 (1,100-5,000) |
| FL + G-CSF | 0/3 | 2/3 | 3/3 | <100 | 740 (420-1,460) | 1,900 (1,500-5,600) | |
| 5 × 106 | FL | 1/3 | 2/3 | 3/3 | 220 (180-540) | 620 (460-1,480) | 4,000 (2,300-4,960) |
| FL + G-CSF | 3/3 | 3/3 | 3/3 | 900 (800-920) | 1,360 (1,240-3,960) | 5,800 (5,200-7,100) | |
PB was obtained from FL- or FL + G-CSF–treated donors on day 10. Recipients were reconstituted with 1 × 106, 2.5 × 106, or 5 × 106 PBMC and individual PBMC counts were performed 10, 20, and 30 days following PBMC infusion (n = 3 per group). The day at which ≥500 PBMC/μL were detectable was defined as time point of engraftment.
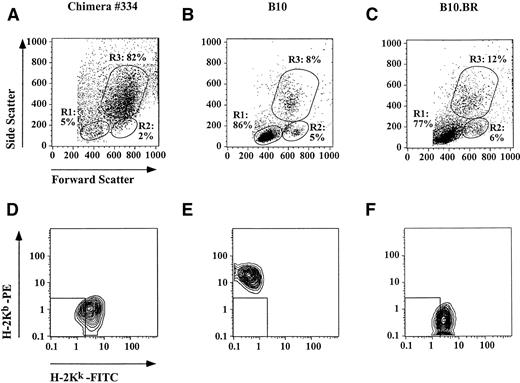
Flow cytometric analysis of PB obtained from transplanted animals 30 days following reconstitution was performed, and the lineage derivation of PBMC was determined based on cell size and granularity. To exclude contamination with radio-resistant or repopulating cells of host origin, PB was stained with MoAbs specific for host (H-2Kb) and donor (H-2Kk) MHC class I antigen. In engrafted recipients 91.2% ± 4.0% of PBMC were located in the granulocyte gate, whereas 5.6% ± 3.1% and 0.5% ± 0.1% of PBMC resided in the lymphocyte or monocyte gate, respectively (Fig 5). More than 95% of PBMC were of donor origin.
Flow cytometric analysis of PB obtained from a representative chimera 30 days after reconstitution with mobilized PB. B10 mice (H-2Kb) were lethally irradiated and transplanted with varying numbers of PBMC from growth factor–treated B10.BR donors (H-2Kk; 4 to 7 mice per group). PB from unmanipulated B10 and B10.BR mice served as controls. Lineage derivation of PBMC was analyzed based on forward and side scatter, and the percentage of cells residing in a lymphocyte (R1), monocyte (R2), or granulocyte gate (R3) was calculated. (A) The majority of PBMC in engrafted recipients were located in the granulocyte gate, (B and C) while most of PBMC from untreated controls resided in the lymphocyte gate. (D through F) In addition PB was stained with MoAb specific for recipient (H-2Kb) and donor (H-2Kk) MHC class I, and gated populations were analyzed by two-color flow cytometry. (D) Gated lymphocytes from engrafted recipients expressed exclusively donor MHC class I. Positive staining for donor but negative staining for recipient MHC class I was also observed when gated granulocytes and monocytes were analyzed (data not shown).
Flow cytometric analysis of PB obtained from a representative chimera 30 days after reconstitution with mobilized PB. B10 mice (H-2Kb) were lethally irradiated and transplanted with varying numbers of PBMC from growth factor–treated B10.BR donors (H-2Kk; 4 to 7 mice per group). PB from unmanipulated B10 and B10.BR mice served as controls. Lineage derivation of PBMC was analyzed based on forward and side scatter, and the percentage of cells residing in a lymphocyte (R1), monocyte (R2), or granulocyte gate (R3) was calculated. (A) The majority of PBMC in engrafted recipients were located in the granulocyte gate, (B and C) while most of PBMC from untreated controls resided in the lymphocyte gate. (D through F) In addition PB was stained with MoAb specific for recipient (H-2Kb) and donor (H-2Kk) MHC class I, and gated populations were analyzed by two-color flow cytometry. (D) Gated lymphocytes from engrafted recipients expressed exclusively donor MHC class I. Positive staining for donor but negative staining for recipient MHC class I was also observed when gated granulocytes and monocytes were analyzed (data not shown).
Long-term engraftment of mobilized HSC in allogeneic recipients.
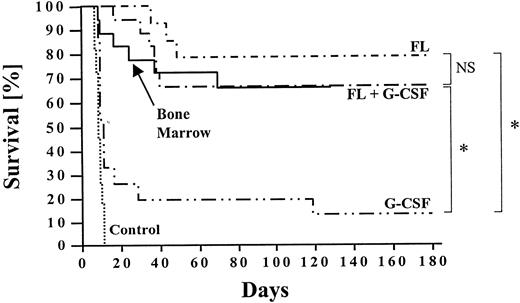
Long-term survival (>6 months) was 79% and 67% in animals transplanted with PBMC from FL- and FL + G-CSF–treated donors, respectively (Fig 6). This survival rate was comparable to that of recipients (n = 25) reconstituted with 1 × 106 untreated bone marrow cells from naı̈ve B10.BR donors. The majority of recipients reconstituted with mobilized PB developed clinical signs of acute GVHD within 30 to 60 days after transplantation as indicated by diarrhea and loss of body weight. However, GVHD was self-limiting in most of these animals. In striking contrast, long-term survival of animals transplanted with PBMC mobilized with G-CSF alone was significantly lower, and the estimated survival after 6 months was only 13%. None of the recipients transplanted with PBMC from carrier-treated B10.BR donors survived for more than 14 days.
Long-term survival (>6 months) of lethally irradiated and transplanted recipients was calculated using Kaplan-Meier estimates. B10 mice received 1 × 106 to 5 × 106 PBMC from B10.BR donors treated with FL alone, G-CSF alone, or FL + G-CSF (n ≥ 6 per group). Controls were transplanted with similar numbers of PBMC from untreated donors or 1 × 106 bone marrow cells. Survival between different groups was compared using the Wilcoxon test, and significant differences are marked (* P < .0001). The follow-up ranged from 3 to 6 months.
Long-term survival (>6 months) of lethally irradiated and transplanted recipients was calculated using Kaplan-Meier estimates. B10 mice received 1 × 106 to 5 × 106 PBMC from B10.BR donors treated with FL alone, G-CSF alone, or FL + G-CSF (n ≥ 6 per group). Controls were transplanted with similar numbers of PBMC from untreated donors or 1 × 106 bone marrow cells. Survival between different groups was compared using the Wilcoxon test, and significant differences are marked (* P < .0001). The follow-up ranged from 3 to 6 months.
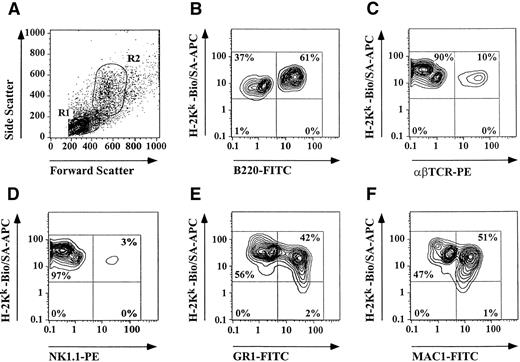
Representative animals transplanted with PB from donors treated with growth factors for 10 days were analyzed after 6 months, and the level of donor chimerism as well as the presence of multiple hematopoietic lineages was assessed by flow cytometry using lineage-specific MoAbs. All long-term surviving animals tested showed >99% donor chimerism and multiple lineages including B-cells, αβTCR+ T cells, γδTCR+ T cells, NK cells, macrophages, and granulocytes of donor origin were present in these animals (Table 3 and Fig 7). Percentages of analyzed donor-derived cell lines were comparable to those of naive B10.BR mice.
Characterization of Long-Term Surviving Animals (B10) Reconstituted With Mobilized PB From B10.BR Donors Treated With FL Alone, G-CSF Alone, or FL + G-CSF
| Donor Treatment . | n . | Donor Chimerism* . | B-Cells (%)† . | αβTCR+ T Cells (%)† . | γδTCR+ T Cells (%)† . | NK Cells (%)† . | Granulocytes (%)‡ . | Macrophages (%)‡ . |
|---|---|---|---|---|---|---|---|---|
| FL | 4 | >99% | 57.5 ± 14.6 | 22.1 ± 8.0 | 0.6 ± 0.1 | 2.3 ± 0.7 | 40.4 ± 5.4 | 35.2 ± 7.5 |
| G-CSF | 4 | >99% | 71.3 ± 0.8 | 15.0 ± 7.4 | 0.5 ± 0.1 | 2.0 ± 0.8 | 41.3 ± 10.5 | 61.9 ± 0.8 |
| FL + G-CSF | 6 | >99% | 48.7 ± 5.4 | 24.5 ± 7.8 | 0.7 ± 0.2 | 2.3 ± 0.4 | 24.4 ± 4.8 | 33.4 ± 8.4 |
| Donor Treatment . | n . | Donor Chimerism* . | B-Cells (%)† . | αβTCR+ T Cells (%)† . | γδTCR+ T Cells (%)† . | NK Cells (%)† . | Granulocytes (%)‡ . | Macrophages (%)‡ . |
|---|---|---|---|---|---|---|---|---|
| FL | 4 | >99% | 57.5 ± 14.6 | 22.1 ± 8.0 | 0.6 ± 0.1 | 2.3 ± 0.7 | 40.4 ± 5.4 | 35.2 ± 7.5 |
| G-CSF | 4 | >99% | 71.3 ± 0.8 | 15.0 ± 7.4 | 0.5 ± 0.1 | 2.0 ± 0.8 | 41.3 ± 10.5 | 61.9 ± 0.8 |
| FL + G-CSF | 6 | >99% | 48.7 ± 5.4 | 24.5 ± 7.8 | 0.7 ± 0.2 | 2.3 ± 0.4 | 24.4 ± 4.8 | 33.4 ± 8.4 |
PB was obtained from representative recipients 6 months after reconstitution. PBMC were stained with lineage- and donor-specific MoAbs for three-color flow cytometric analysis.
Level of donor chimerism was calculated on gated lymphocytes with positive staining for donor (H-2Kk) but negative staining for recipient MHC class I (H-2Kb).
The mean percentage (±SD) of PBMC with positive staining for lineage- and donor-specific MoAbs on gated lymphocytes.
The mean percentage (±SD) of PMBC with positive staining for lineage- and donor-specific MoAbs on gated granulocytes.
Assessment of long-term engraftment of mobilized HSC and FC by three-color flow cytometry. PB was obtained from lethally irradiated B10 mice (H-2Kb) 6 months after reconstitution with PBMC from growth factor–treated B10.BR mice (H-2Kk) and stained with lineage- and donor-specific MoAbs. Unmanipulated B10 and B10.BR mice served as controls (data not shown). Figure shows results of a representative long-term surviving chimera. (A) Lymphocytes (R1) and granulocytes/macrophages (R2) were gated based on forward and side scatter. Engraftment of multiple donor derived cell lines including (B) B-cells, (C) T cells, (D) NK cells, (E) granulocytes, and (F) macrophages were detectable 6 months after transplantation indicating HSC engraftment.
Assessment of long-term engraftment of mobilized HSC and FC by three-color flow cytometry. PB was obtained from lethally irradiated B10 mice (H-2Kb) 6 months after reconstitution with PBMC from growth factor–treated B10.BR mice (H-2Kk) and stained with lineage- and donor-specific MoAbs. Unmanipulated B10 and B10.BR mice served as controls (data not shown). Figure shows results of a representative long-term surviving chimera. (A) Lymphocytes (R1) and granulocytes/macrophages (R2) were gated based on forward and side scatter. Engraftment of multiple donor derived cell lines including (B) B-cells, (C) T cells, (D) NK cells, (E) granulocytes, and (F) macrophages were detectable 6 months after transplantation indicating HSC engraftment.
DISCUSSION
It has been previously shown that FL mobilizes large numbers of PBMC into the circulation of mice.41,42 A maximum effect was observed when FL was injected at a daily dose of 10 μg subcutaneously for 10 days, resulting in an increase of PBMC to 4 × 104/μL. In subsequent experiments a synergistic effect was observed when FL was used in combination with G-CSF.44,45 In these studies both FL and G-CSF were initiated on day 1, and a peak of PBMC as high as 1 × 105/μL was observed on day 8. FL in combination with GM-CSF was much less effective.44 Because it was previously reported that PBMC mobilized by G-CSF alone peaked on day 5 to 6,24 we initiated G-CSF treatment on day 4 to allow for maximal synergy of both growth factors. In fact, a peak of PBMC under treatment with FL + G-CSF was detected on day 10 and an average of 1.75 × 105 PBMC/μL was counted. This peak represents an almost twofold increase in PBMC numbers when compared with the experiments performed by Brasel et al.44 Thus, we show in this study that optimized timing of growth factor administration can further enhance the synergy between G-CSF and FL.
However, the peak of PBMC does not necessarily reflect the ideal time point for the collection of the desired cellular population from mobilized PB. Therefore, we were mainly interested in the kinetics of mobilization of FC and HSC. FC have been previously shown to be critical in engraftment of murine allogeneic HSC across MHC barriers.14,47,48 Although in our previous studies 1,000 HSC (lineage−/Sca-1+/c-kit+) purified from bone marrow engrafted routinely in lethally irradiated syngeneic mice, even a 10-fold increase in HSC failed to rescue allogeneic recipients from irradiation-induced aplasia. When as few as 30,000 FC (CD8+/TCR−/CD3+) positively selected by cell sorting were added to 10,000 purified HSC, 100% of allogeneic recipients engrafted and none of these animals developed GVHD.14
When G-CSF alone was injected, the number of cells with facilitating phenotype (CD8+/αβTCR−/γδTCR−) in PB of those animals was not increased significantly compared with carrier-treated controls. In contrast, FL as a single agent elevated (sevenfold) the absolute numbers of FC over time, and a peak occurred on day 9. Mobilization of FC by FL + G-CSF resulted in a highly significant synergy. Beginning on day 5, a continuous increase of FC was observed and a maximum (21-fold over controls) was reached on day 10. Interestingly, a similar pattern was observed for mobilization of HSC. When both factors were used in combination, a more than 200-fold increase of HSC occurred from day 9 to 11. G-CSF alone and FL alone were less effective (17-and 36-fold). Previous work has also shown that certain inbred mouse strains, including C57BL mice, show a limited elevation of progenitor cells in PB after mobilization with G-CSF alone.49 Our results in terms of HSC mobilization are in accordance with data presented by others identifying HSC/progenitor cells based on in vitro colony assays.43-45 In these studies a synergy of FL and G-CSF on the mobilization of burst-forming unit–erythroid (BFU-E), colony-forming unit–granulocyte-macrophage (CFU-GM), colony-forming unit–granulocyte, erythroid, monocyte, megakaryocyte (CFU-GEMM), and colony-forming unit–spleen (CFU-S) into PB was observed.43-45 However, different doses and timing of growth factor administration as well as different methods to assess the frequency of HSC make a direct comparison difficult.
Preliminary data from our laboratory suggests that FC may provide a tropic effect to maintain the HSC in a primitive state. Although HSC alone undergo apoptosis in vitro, the addition of FC maintains the HSC in G0 (manuscript in preparation). The fact that the kinetics for mobilization of FC and HSC are similar may suggest that the two are in close proximity in the hematopoietic microenvironment.
To understand the mechanism for the observed synergy of both growth factors on the mobilization of HSC and FC, we assessed the frequency of HSC (lineage−/Sca-1+/c-kit+) and FC (CD8+/TCR−) in spleen and bone marrow under treatment with both growth factors alone or in combination. As shown previously, G-CSF mobilized both mature and progenitor cells into the periphery as indicated by declining cellularity in bone marrow.24 The latter might be responsible for the slightly increased frequency of HSC in bone marrow observed in our G-CSF–treated animals rather than proliferation of those cells. Interestingly, when FL was used as a single agent, a highly significant increase in HSC (18-fold) and FC (5-fold) in bone marrow was observed on day 10. This is in accordance with results from Brasel et al,42 who reported 8.2-fold higher numbers of low-density lineage−/Sca-1+/c-kit+ HSC in bone marrow after treatment with 10 μg FL for 10 days when compared with untreated controls.42 In striking contrast, when we injected FL and G-CSF simultaneously, the percentage of HSC showed a fourfold expansion on day 7, but dropped to pretreatment levels on day 10, whereas the frequency of FC in bone marrow remained unchanged. This suggests that the proliferation of HSC and FC caused by FL in combination with the mobilizing effect of G-CSF might be responsible for the potent synergy of both growth factors to elevate HSC and FC numbers in PB.
In addition to the growth factor–mediated effects in bone marrow, there was a significant increase in cellularity and frequency of HSC/PC reported in spleens of FL-treated mice.42,45,50 We observed in our study a highly significant expansion of cells with FC phenotype and HSC in spleens of FL- and FL + G-CSF–treated mice. The percentage of FC in the spleen of mice injected with FL alone increased from less than 1% on day 0 to 11% on day 10. This increase seems to be specific for certain cell types, such as FC and HSC, because the frequency of CD8+ T cells declined after FL administration. A similar observation was made by Brasel et al,42 who showed that an increase of CD8+/Thy-1− cells in spleens of FL treated mice occurred.42 The same group reported later that a dramatic increase of dendritic cells was present in spleen under treatment with FL.50 When splenocytes from FL-treated mice were depleted of T cells, B cells, NK cells, and cells of erythroid lineage using MoAbs and complement, these cells could be divided into five groups based on their expression of the dendritic cell markers CD11c and CD11b. Interestingly, more than 50% of cells of population D (CD11cbright/CD11bdim) and E (CD11cbright/CD11b−) coexpressed CD8.50,51 Whether these two populations mediate a graft-facilitating effect is currently under investigation in our laboratory. However, El-Badri et al52 showed recently that dendritic cells isolated from murine bone marrow did not facilitate engraftment of purified HSC across MHC barriers.52Nevertheless, our preliminary data indicate that CD8+/TCR− cells from PB and spleens of FL + G-CSF–treated animals highly purified by cell sorting facilitate engraftment of allogeneic bone marrow across MHC barriers (unpublished observation).
This observation is further confirmed by the superior engraftment potential of PB from B10.BR donors mobilized with FL and FL + G-CSF in fully ablated allogeneic B10 mice. One hundred percent and 83% of animals transplanted with 1 × 106 FL + G-CSF– and FL-mobilized PBMC obtained on day 10 were rescued from irradiation-induced aplasia, respectively. The transplanted PBMC contained 0.63 or 0.64 × 105 HSC and 2.10 or 1.21 × 105 FC after treatment with FL + G-CSF and FL alone, respectively. In contrast, similar amounts of HSC and FC present in G-CSF-mobilized PB rescued only 33% of lethally irradiated animals, indicating a qualitative disadvantage of these cells after treatment with G-CSF alone. When PBMC were obtained on day 7 of growth factor administration, the engraftment potential was less efficient. Therefore, the collection of PB on day 10 at which the highest numbers of HSC and FC in PB were detectable seems to be favorable. Moreover, the long-term repopulating potential of HSC and FC from FL- and FL + G-CSF–treated allogeneic donors was superior when compared with donors treated with G-CSF alone. However, the development of GVHD as a result of high numbers of T and NK cells in whole PB has limited the long-term survival in those animals. Additional functional studies need to be performed in the future to determine whether mobilized facilitating cells defined by phenotypic criteria will function as effectively as those purified from naı̈ve bone marrow in previous studies.
In summary, treatment of mice with FL results in proliferation of cells with FC phenotype in bone marrow and spleen. In animals treated with a combination of FL and G-CSF, both FC and HSC were most efficiently mobilized into PB and peak levels for both populations were detected on day 10. This strategy might be useful in the clinical setting especially when HLA disparities between donor and recipient exist and FC are needed to achieve HSC engraftment. However, improved collection and processing of mobilized PB to contain mainly FC and HSC yet reduce the amount of contaminating T cells and NK cells might be necessary to avoid GVHD and enhance the engraftment potential.
Supported by Deutsche Forschungsgemeinschaft (M.N.) (Ne 620/1-2; Ex 11/1-1), and National Institutes of Health Grant Nos. DK 52294 and 2R01 DK52294-06AZ (S.T.I.).
Address reprint requests to Suzanne T. Ildstad, MD, Director, Institute for Cellular Therapeutics, Professor of Surgery, Allegheny University of the Health Sciences, Broad & Vine Streets, Mail Stop 490, Philadelphia, PA 19102.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal