Abstract
A live attenuated AroA− auxotrophic mutant ofSalmonella typhimurium (SL7207) has been used as carrier for the pCMVβ vector that contains the β-galactosidase (β-gal) gene under the control of the immediate early promoter ofCytomegalovirus (CMV). We tested whether orally administered bacterial carrier could enter and deliver the transgene to antigen-presenting cells (APCs) through the natural enteric route of infection and whether β-gal expression could generate a protective response against an aggressive murine fibrosarcoma transduced with the β-gal gene (F1.A11) that behaves operationally as a tumor-associated antigen. After three courses, at 15-day intervals, mice developed both cell-mediated and systemic humoral responses to β-gal. Mice vaccinated with the Salmonella harboring pCMVβ, but not with plasmid-less carrier, showed resistance to a challenge with F1.A11 cells. These experiments suggest that Salmonella-based DNA immunization allows us to specifically target antigen expression in vivo to APCs. To prove that the transgene is actually expressed by APCs as a function of an eukaryotic promoter, the green fluorescent protein (GFP) was placed under the control of either the eukariotic CMV or a prokaryotic promoter. Using cytofluorometric analysis, GFP was detected only in splenocytes of mice receiving a Salmonella carrier harboring GFP under the CMV promoter. These results indicate that transgene expression occurs because of a Salmonella-mediated gene transfer to eukaryotic cells. Finally, approximately 19% of the splenocytes expressed GFP. Among them, F4/80+ macrophages and CD11cbright dendritic cells (DCs) were scored as positive for GFP expression. Extensive work has been performed trying to optimize the way to transfect DCs, ex vivo, with genes coding for relevant antigens. We show here, for the first time, that DCs can be directly and specifically transduced in vivo such to induce DNA vaccination against tumors.
© 1998 by The American Society of Hematology.
CANCER VACCINES are proposed for management of patients with minimal residual disease or with high risk of tumor recurrence, an approach that could also be extended to the treatment of individuals with genetic predisposition to cancer.1 Compared with conventional therapies, oral vaccination is particularly suitable for this category of patients because of its noninvasive nature and minimal associated distress or side effects.2 The primary goal of a vaccine is to target the immunizing antigen(s) to appropriate bone marrow-derived antigen-presenting cells (APCs), especially dendritic cells (DCs), which are instrumental for the activation of virgin T lymphocytes.3
Naked DNA-based vaccines, administered by intradermal or intramuscular injection, have emerged as a promising approach to control infectious diseases and, to some extent, tumors.4 The mechanism inducing host immune response after DNA immunization is not yet fully elucidated. Several recent reports point to APCs, rather than to myocytes or other somatic cells, as responsible for antigen processing and presentation after DNA injection in vivo.5-7 Because of the low number of APCs present in muscle, especially in the absence of inflammatory stimuli, immune response induced by intramuscular injection of plasmid DNA seems to be limited to very potent antigens. This makes highly desirable the ability to deliver the antigen-encoding DNA specifically to APC at inductive sites of immune responses and possibly within a context able to signal the immune system with the presence of an existing danger.
Attenuated bacterial carrier strains have been recently used as a DNA delivery system in vitro8-10 and in vivo.11 We report here that, using Salmonella typhimurium as an oral carrier for genetic immunization, effective protection against tumor development can be achieved as a function of in vivo transduction of APCs, including DCs.
MATERIALS AND METHODS
Mice.
Female BALB/c (H-2d), 6 to 12 weeks of age, were purchased from Charles River (Calco, Italy) and maintained at the Istituto Nazionale Tumori under standard conditions according to Institutional Guidelines. This study was approved by the Institutional Ethic Committee for animal use in experimental research.
Cell lines and peptides.
The clone F1.A11 (H-2d) expressing β-galactosidase (β-gal) was obtained by transduction of spontaneously transformed BALB/c fibroblast cell line F1 with the LBSN retroviral vector.12 These cells express major histocompatibility complex-I (MHC-I) molecules but not MHC-II and are highly tumorigenic. P13.1 (H-2d) expressing β-gal and its parental P815 cell lines were used in the cytotoxicity measurement. The βGP1 peptide, encompassing the immunodominant H-2Ldrestricted β-gal epitope, was used for in vitro cytotoxic T lymphocytes (CTL) restimulation.13
Bacterial strains, plasmids, and media.
The auxotrophic S typhimurium AroA− strain SL7207 (S typhimurium 2337-65 derivative hisG46, DEL407 [aroA::Tn10{Tc-s}]) was kindly provided by B.A.D. Stocker (Stanford University, School of Medicine, Stanford, CA). Bacterial strains were routinely grown at 37°C in LB broth or agar (Sigma, St Louis, MO), supplemented with 100 μg/mL of ampicillin when required. The prokaryotic and eukaryotic green fluorescent protein (GFP) expression vectors pASV214 and pEGFP-C2 (Clontech, Palo Alto, CA) were used to characterize gene transfer in vivo, whereas the plasmid pCMVβ (Clontech) was used for genetic immunization in tumor protection studies. Listeria monocytogenes vaccine strain Δmpl2pGKV20 and its preparation have been previously described.12
Immunization protocols.
For vaccination, bacteria were grown overnight until they reached mid-log phase. They were then harvested by centrifugation (3,000g) and resuspended in 5% sodium bicarbonate buffer. Mice were immunized four times at 15-day intervals by gently feeding them with the bacterial suspension (5 × 108 colony-forming units [CFU]/mouse) in a volume of approximately 30 μL. Control mice received the plasmid-less SL7207 strain or buffer only as sham vaccine.
Measurement of antigen-specific antibodies and Ig isotype assay.
Sera samples were taken 8 days after the second immunization and tested for their reactivity to β-gal in an antigen capture enzyme-linked immunosorbent assay (ELISA) that can detect IgG levels down to 10 ng/mL, as previously described.12 Sera were also tested on ovalbumin coated plates (10 μg/mL) as negative control.
Analysis of cytotoxic T-cell activity.
At days 15, 30, and 45 after primary immunization, spleens were removed from 3 immunized mice and pooled in a single-cell suspension prepared by mechanical dissociation. Splenocytes were restimulated at 5 × 106 cells with the synthetic peptide βGP1 (1 μmol/L) in 1.5 mL medium final volume in 24-well plates. After 5 to 7 days, viable cells were harvested and tested in a51Cr-release assay for their ability to lyse the β-gal–expressing tumor cell line P13.1; the P815 parental cell line was used as a negative target.
Cytokines assay.
Cytokines were assayed in supernatants of nylon wool-purified T lymphocytes (1 × 104/well) cultured 24 to 48 hours in the presence of immobilized anti-CD3 antibody (clone 145-2C11; 20 μg/mL) in 96-well flat-bottomed plates. Cytokines were measured by sandwich ELISA using monoclonal antibody (MoAb) pairs BVDA-1D11/BVD6-24G2 and R4-6A2/XMG1.2 (Pharmingen, San Diego, CA) for interleukin-4 (IL-4) and interferon-γ (IFN-γ) determination, respectively.
In vivo protection studies.
Two weeks after the last immunization, animals received in the left rear flank a subcutaneous challenge of F1.A11 living cells (104 cells/mouse). Mice were inspected for tumor growth and size every second day. Tumor growth was measured using calipers and was recorded as the narrowest and longest surface length. Tumor size (in square millimeters) was calculated as the product of the mean of these two lengths per animal averaged over the total number of animals in the group. Statistical differences in tumor size were calculated using the Student’s t-test. The differences in tumor take were evaluated by χ2 test. Mice that were tumor free about 40 days after first challenge received a second contralateral challenge and were further inspected for tumor growth.
Characterization of in vivo gene transfer.
Mice were fed with SL7207 harboring either pASV2 or pEGFP-C2 vectors that contain the GFP gene under the control of prokaryotic and eukaryotic promoters, respectively, following the above-mentioned vaccination protocol. GFP-expressing cells in spleen suspensions were detected by flow cytometry. The phenotype of the GFP-positive cells (FL1+) was determined by double-fluorescence analysis after staining with biotinylated anti-CD11c (clone N418), anti-F4/80 (clone F4/80), anti-B220 (clone RA3), anti-Thy1.2 (clone B5.5), and appropriate isotype-matched negative controls (Pharmigen) in the presence of Fc-blocking reagent (clone 24G2; Pharmingen) and using phycoerythrin (PE)-Streptavidin (Pharmingen) as second-step reagent.
RESULTS
It has been recently shown that S typhimurium carrier strain harboring eukaryotic expression vectors can mediate gene transfer in vivo and trigger both cellular and humoral immune responses against the encoded antigens.11
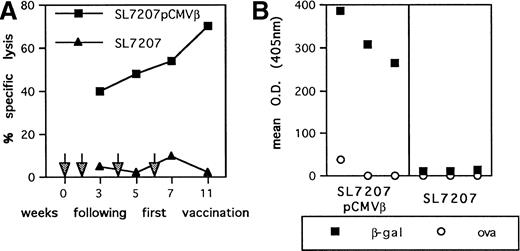
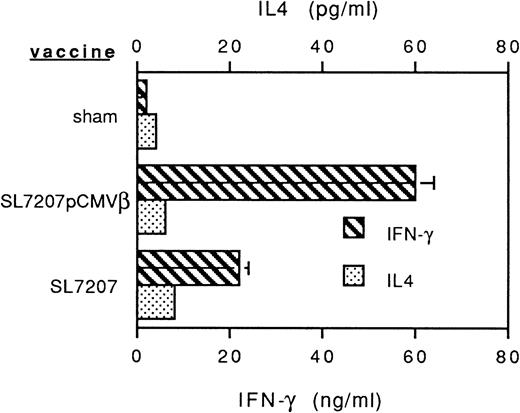
To assess the potential of oral genetic immunization to control tumor development, we had used a tumor model based on a highly aggressive murine fibrosarcoma transduced with the β-gal gene (F1.A11 cells), which has been shown to behave operationally as a tumor-associated antigen (TAA).13,15 The attenuated S typhimurium AroA− SL7207 strain harboring the pCMVβ vector that contains β-gal gene under the control of the immediate early promoter of Cytomegalovirus (CMV) has been used.11 After a primary immunization per oral, mice received three boosts at 15-day intervals via the same route. In vitro infection with this strain resulted in an efficient expression of β-gal by spleen, bone marrow, and peritoneal macrophages (not shown). In vivo vaccination elicited a tumor-specific T-cell–mediated response: a TAA-specific cytotoxic response was detectable within 3 weeks after the primary immunization and steadily increased during the course of the treatment (Fig 1A). Additionally, a systemic humoral response was measurable as the presence of specific anti-TAA antibodies in the sera of immunized mice (Fig 1B). To determine the subclass distribution of the anti–β-gal IgG, serum samples were analyzed for levels of IgG1, 2a, 2b, and 3, resulting in a predominant IgG2a isotype (not shown). In addition, cytokines secreted by T lymphocytes from vaccinated mice were measured by ELISA after stimulation with immobilized anti-CD3 antibody. Mice receiving SL7207 (pCMVβ) vaccine display a typical Th1 profile characterized by significant production of IFN-γ and negligible IL-4 release; interestingly, lymphocytes from mice receiving the plasmid-less carrier displayed the same cytokine profile, which most likely contributes to the explanation of the adjuvant effect of the carrier per se (Fig 2). These results confirm that DNA encoding the TAA has been delivered by the carrier to host cells and that it has been properly transcribed, translated, and presented to the elements of immune system, resulting in type-1 cellular and humoral immune responses.
Immune response elicited by S typhimurium-mediated DNA immunization. (A) CTL-mediated recognition of β-gal–expressing tumor target cells. Splenocyte cells from mice vaccinated with SL7207 (pCMVβ; ▪) or SL7207 (▴) at the indicated time points (arrows) were restimulated in vitro for 5 days in presence of βGP1 peptide. After culture, lymphocytes were tested in a51Cr-release assay at a 50:1 E/T ratio using P13.1 as target. Results are the mean of triplicates; the percentage of nonspecific lysis of P815− cells has been subtracted. (B) Serum IgG levels were measured in antigen-capture ELISA: (▪) β-gal–reactive IgG; (○) ovalbumin-reactive IgG. Results are from sera pools of 3 mice each.
Immune response elicited by S typhimurium-mediated DNA immunization. (A) CTL-mediated recognition of β-gal–expressing tumor target cells. Splenocyte cells from mice vaccinated with SL7207 (pCMVβ; ▪) or SL7207 (▴) at the indicated time points (arrows) were restimulated in vitro for 5 days in presence of βGP1 peptide. After culture, lymphocytes were tested in a51Cr-release assay at a 50:1 E/T ratio using P13.1 as target. Results are the mean of triplicates; the percentage of nonspecific lysis of P815− cells has been subtracted. (B) Serum IgG levels were measured in antigen-capture ELISA: (▪) β-gal–reactive IgG; (○) ovalbumin-reactive IgG. Results are from sera pools of 3 mice each.
Profiles of cytokines released by anti-CD3–stimulated T lymphocytes from mice receiving Salmonella-based vaccines. Spleen T lymphocytes were isolated and nylon-wool purified 45 days after primary vaccine administration and plated in the presence of immobilized anti-CD3 MoAb for 24 or 48 hours, respectively, to measure IFN-γ and IL-4 release. Cytokine levels were determined by sandwich ELISA.
Profiles of cytokines released by anti-CD3–stimulated T lymphocytes from mice receiving Salmonella-based vaccines. Spleen T lymphocytes were isolated and nylon-wool purified 45 days after primary vaccine administration and plated in the presence of immobilized anti-CD3 MoAb for 24 or 48 hours, respectively, to measure IFN-γ and IL-4 release. Cytokine levels were determined by sandwich ELISA.
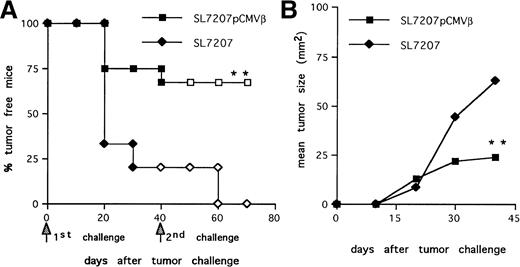
We analyzed the efficacy of the immune response elicited afterSalmonella-mediated DNA-transfer to prevent tumor take. Mice immunized with the strain SL7207 (pCMVβ) or plasmid-less carrier as control were challenged subcutaneously with F1.A11 cells (104/mouse). About 80% of the animals vaccinated with the plasmid-less carrier developed tumors (Fig3A), whereas tumor take was significantly reduced (P < .01) in the animals receiving the recombinant carrier. Within this group, the few animals that developed tumors had significantly reduced tumor size and prolonged survival compared with controls (Fig 3B).
Protective memory response against tumor challenge in mice receiving S typhimurium-mediated DNA oral immunization. (A) Tumor-take curves. After immunization with SL7207 (pCMVβ; squares) or SL7207 (triangles), mice received a first subcutaneous challenge with F1A11 tumor cells (solid symbols); after 40 days, tumor-free mice received a second contralateral challenge (open symbols). Tumor growth was inspected every other day by palpation. Each group included 15 mice. (B) Tumor size (in square millimeters) progression in immunized mice. Statistically significant differences are indicated: P < .01 (**).
Protective memory response against tumor challenge in mice receiving S typhimurium-mediated DNA oral immunization. (A) Tumor-take curves. After immunization with SL7207 (pCMVβ; squares) or SL7207 (triangles), mice received a first subcutaneous challenge with F1A11 tumor cells (solid symbols); after 40 days, tumor-free mice received a second contralateral challenge (open symbols). Tumor growth was inspected every other day by palpation. Each group included 15 mice. (B) Tumor size (in square millimeters) progression in immunized mice. Statistically significant differences are indicated: P < .01 (**).
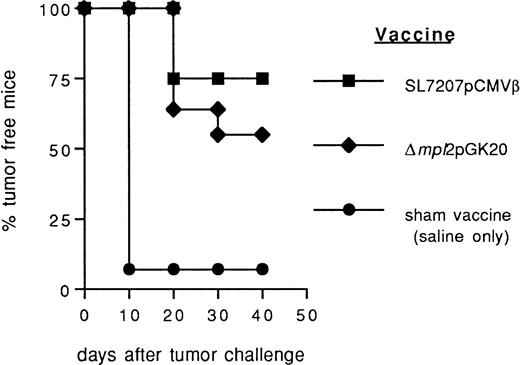
Also, recombinant Listeria carriers expressing the target antigen as a bacterial protein have been previously tested for oral vaccination in animal tumor models.12 16 We compared the efficacy of Salmonella-based DNA vaccine with that ofListeria-derived protein vaccine (Δmpl2pGK20) previously described. Salmonella-based vaccine seems more efficient, although moderately, than Listeria Δmpl2pGK20 in preventing the take of F1.A11 fibrosarcoma (Fig 4). However, more extensive comparison between DNA and protein-based oral vaccination mediated by the same bacterial carrier are necessary to raise a conclusion.
Comparison of the efficacy of Salmonella- andListeria-based vaccines in preventing the growth of murine fibrosarcoma F1.A11. After immunization with Salmonella SL7207 (pCMVβ; ▪), Listeria ▵mpl2pGK20 (⧫), and saline only (•) mice received a subcutaneous challenge with F1A11 tumor cells. Tumor growth was inspected every other day by palpation. Each group included 15 mice.
Comparison of the efficacy of Salmonella- andListeria-based vaccines in preventing the growth of murine fibrosarcoma F1.A11. After immunization with Salmonella SL7207 (pCMVβ; ▪), Listeria ▵mpl2pGK20 (⧫), and saline only (•) mice received a subcutaneous challenge with F1A11 tumor cells. Tumor growth was inspected every other day by palpation. Each group included 15 mice.
Long-lasting immune memory is a requisite for an effective antitumor response and metastases control.12 Therefore, the ability of Salmonella-based vaccine to trigger a persistent antitumor immunity was further evaluated. Mice that remained disease-free after the first challenge were subjected to a second contralateral challenge with F1.A11 cells. Animals immunized with SL7207 (pCMVβ) completely rejected the second challenge (Fig 3A, □). In contrast, the few mice (20%) that remained tumor free in the group receiving the plasmid-less carrier quickly developed tumors (Fig 3A, ◊), likely due to their lack of TAA-specific immune response (Fig 1A and B).
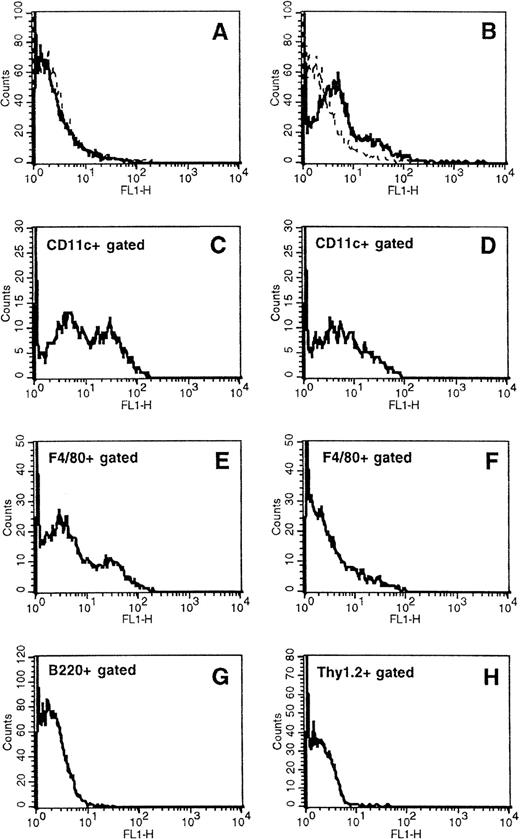
To determine whether APCs express the transgene delivered by the orally administered Salmonella carrier, mice were fed with the SL7207 strain harboring an expression vector encoding the GFP under the control of either an eukaryotic (pEGFP-C2) or a prokaryotic (pASV2) promoter. Using cytofluorometric analysis, GFP was detectable in spleen cells of mice treated with SL7207 (pEGFP-C2) starting on day 28 after the first vaccine administration (Fig 5B). In contrast, GFP was not detectable in spleen cells of animals receiving the Salmonella containing the constitutive expression system (pASV2), which directs GFP synthesis only within the carrier (Fig 5A, D, and F). GFP expression by pASV2 was undetectable even at earlier time points, immediately after vaccination (8 to 24 hours; data not shown), likely because of the rapid in vivo clearance of the bacterial carrier. These results confirmed that antigen can be expressed in vivo, at the systemic level, by eukaryotic cells afterSalmonella-mediated gene transfer. The phenotype of the splenocytes subpopulation(s) expressing GFP was characterized with MoAbs recognizing DC, macrophage, or lymphocyte subsets. Approximately 19% of the splenocytes expressed GFP (Fig 5B). Among them, about 50% of the CD11cbright DCs (Fig 5C) and 30% of F4/80+ macrophages (Fig 5E) were scored as positive for GFP expression. No B220+ or Thy1.2+ lymphocytes were scored positive for GFP expression (Fig 5G and H). These results confirm that Salmonella-mediated transgene expression occurs within APCs. This expression is long-lasting, being detectable up to 7 days after the last vaccine administration, and it is detectable within secondary lymphoid organs. GFP expression by DCs points to the potential of a vaccination approach that results in DC-mediated delivery of transfected antigens to the T-cell–dependent areas of secondary lymphoid organs.
Characterization of in vivo S typhimurium-mediated DNA gene transfer to APCs in orally vaccinated mice. Expression of GFP, naturally emitting green fluorescence, was determined by flow cytometry in total spleen cells from mice receiving the S typhimurium SL7207 strain carrying either the expression vector pASV2 (A, D, and F) or pEGFP-C2 (B, C, E, G, and H), which contain the GFP coding gene under the control of either a constitutive prokaryotic or eukaryotic promoter, respectively. Spleen cell subsets expressing GFP were identified by two-color fluorocytometric analysis after staining with biotinylated anti-CD11c, anti-F4/80, anti-B220, and anti-Thy1.2 followed by PE-Streptavidin. (C) and (D) display GFP expression by cells gated as positive for CD11c marker; (E) and (F) display GFP expression by cells gated as positive for F4/80 marker; (G) and (H) display GFP expression by cells gated as positive for B220 and Thy1.2, respectively. Control samples stained with appropriated biotin-conjugated isotype-matched negative control MoAbs followed by PE-Streptavidin were included for gate setting.
Characterization of in vivo S typhimurium-mediated DNA gene transfer to APCs in orally vaccinated mice. Expression of GFP, naturally emitting green fluorescence, was determined by flow cytometry in total spleen cells from mice receiving the S typhimurium SL7207 strain carrying either the expression vector pASV2 (A, D, and F) or pEGFP-C2 (B, C, E, G, and H), which contain the GFP coding gene under the control of either a constitutive prokaryotic or eukaryotic promoter, respectively. Spleen cell subsets expressing GFP were identified by two-color fluorocytometric analysis after staining with biotinylated anti-CD11c, anti-F4/80, anti-B220, and anti-Thy1.2 followed by PE-Streptavidin. (C) and (D) display GFP expression by cells gated as positive for CD11c marker; (E) and (F) display GFP expression by cells gated as positive for F4/80 marker; (G) and (H) display GFP expression by cells gated as positive for B220 and Thy1.2, respectively. Control samples stained with appropriated biotin-conjugated isotype-matched negative control MoAbs followed by PE-Streptavidin were included for gate setting.
DISCUSSION
We have shown that Salmonella-based DNA immunization allows us to specifically target antigen expression in vivo to APCs, thus inducing MHC-I– and MHC-II–restricted antitumor immune responses. Vaccine-mediated induction of TAA-specific immune response, although required, could be insufficient to reject a tumor in absence of appropriate inflammatory costimuli, because such costimulation may avoid tolerance or ignorance of the antigen.17 The bacterial carrier may function as a natural adjuvant, because bacteria are known to induce release of tumor necrosis factor-α (TNF-α), IFN-γ, IL-12, and other proinflammatory mediators18 that enhance early innate immunity and thereafter create an inflammatory context that likely favors DC maturation to antigen-presenting function.3 Therefore, Salmonella not only carries the eukaryotic expression cassette, but also may act as natural adjuvant.
After DNA immunization, muscle cells receiving the naked DNA injection express the antigen that is likely captured, processed, and presented by APCs as a consequence of cross-priming.7,19,20 Few APCs may also be directly transduced by injected DNA.21 Antigen presentation by somatic cells is generally inefficient because of the lack of either MHC-II or costimulatory molecules; even resting DCs are a priori poor APCs and require additional signals such as tissue injury or infections to complete their maturation and activate their functions. Therefore, the efficacy of naked DNA vaccination largely depends on the presence of either immunostimulatory motifs within plasmid DNA22 or coinjected DNA coding for inflammatory cytokines15,23 or on drug-mediated tissue injury,24 all of which induce local inflammation and recruitment of APCs. In mice vaccinated with Salmonella-based carriers, gene transfer would preferentially occur in phagocytic cells, which are the main target for bacterial infection in vivo.25 The protein(s) encoded by the transgene would then be released within the APCs and delivered to the antigen presentation pathways. Stimulation of APCs by bacterial compounds would further promote their functional maturation and migration towards inductive sites of lymphoid organs tuning up the immune response.17
Recombinant vaccines that involve the use of engineered bacteria have been approached. Listeriamonocytogenes displays characteristics that made it a promising cancer vaccine vector.12,16,26 Antigens have been expressed inListeria after different strategies to get intracellular protein, protein fused with listeriolisin O, or fusion protein to be retained on the bacterial surface or exported outside the carrier. However, the use of bacterial carrier for transfer of genetic material has never been approached before in vivo, despite evidence that indicates that bacteria can mediate DNA transfer to mammalian cells in vitro.8-10 The possibility of macromolecule delivery in vivo to specific tissues, cells, or cellular compartments remains one of the major challenges that biotechnology should face. Bacterial carriers might offer the possibility of targeting and expressing in vivo human proteins other than small viral or bacterial protein into phagocytic cells. In aiming at this goal, some factors should be considered: (1) the molecular size and the posttranscriptional modifications of the protein; (2) the possible toxicity of the protein for the carrier; and (3) the possibility that the competition between the synthesis of heterologous and bacterial proteins may influence the performance of the carrier by affecting its viability and invasiveness. Extensive work has been performed to transfect DCs in vitro with genes coding for relevant antigens for immunotherapy of tumors.27We demonstrate, for the first time to our knowledge, that S typhimurium, used as a DNA-delivery system, induces antigen expression in vivo not only in macrophages, but also in DCs. This finding underscores the possibility of loading DCs without need for ex vivo manipulations. We cannot exclude the possibility that cells other than APCs expressing the antigen may also contribute to the overall vaccine efficacy. Finally, S typhimurium-based carriers may offer the opportunity to gene-target monocyte/macrophages to correct their genetic defects.
ACKNOWLEDGMENT
The authors thank B.A.D. Stocker for kindly providing us with the SL7297 strain, Prof K.N. Timmis for valuable discussion, and Ralph Steinman, B. Perussia, and G. Parmiani for critical reading.
Supported by Telethon-Italy (Grant No. A102), AIRC, and CNR.
Address reprint requests to Mario P. Colombo, PhD, Division of Experimental Oncology D, Istituto Nazionale per Lo Studio e la Cura dei Tumori, Via Venezian 1, I-20133 Milano, Italy; e-mail:mcolombo@istitutotumori.mi.it.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal