Abstract
Stable gene transfer to human pluripotent hematopoietic stem cells (PHSCs) is an attractive strategy for the curative treatment of many genetic hematologic disorders. In clinical trials, the levels of gene transfer to this cell population have generally been low, reflecting deficiencies in both the vector systems and transduction conditions. In this study, we have used a pseudotyped murine retroviral vector to transduce human CD34+ cells purified from bone marrow (BM) and umbilical cord blood (CB) under optimized conditions. After transduction, 71% to 97% of the hematopoietic cells were found to express a low-affinity nerve growth factor receptor (LNGFR) marker gene. Six weeks after transplantation into immunodeficient NOD/LtSz-scid/scid (NOD/SCID) mice, LNGFR expression was detected in 6% to 57% of CD45+ cells in eight of nine engrafted animals. Moreover, proviral DNA was detected in 8.3% to 45% of secondary colonies derived from BM cells of engrafted NOD/SCID mice. Our data show consistent transduction of SCID-repopulating cells (SRCs) and suggest that the efficiency of gene transfer to human hematopoietic repopulating cells can be improved using existing retroviral vector systems and carefully optimized transduction conditions.
© 1998 by The American Society of Hematology.
GENE TRANSFER INTO pluripotent hematopoietic stem cells (PHSCs) is one of the most promising alternatives for the curative treatment of a variety of inherited and acquired disorders of blood cells. In murine syngeneic bone marrow (BM) transplantation models, a significant proportion of cells participating in long-term engraftment of lethally irradiated mice can be reproducibly and stably transduced ex vivo by the current generation of retroviral vectors.1-3 However, transfer of this technology to humans, nonhuman primates, and other large outbred animals has been much less successful.4-13 The reasons for this discrepancy are uncertain, but probably reflect incomplete understanding of culture conditions required to maintain the integrity and functionality of the PHSC, an inability of the current generation of murine retroviral vectors to transduce quiescent cells,14,15 and a deficiency of receptors on the PHSC surface for the commonly used amphotropic retroviral envelope.16 17
In animal models and human trials, high levels of gene transfer to clonogenic progenitor cells and long-term culture-initiating cells (LTC-ICs) in vitro have not been predictive of long-term reconstitution. The development of efficient protocols for PHSC gene transfer has therefore been limited by the failure of in vitro surrogate progenitor assays to represent the repopulating cell fractions of the human hematopoietic system. To address this problem, alternative assay systems have been developed that test the ability of human hematopoietic cells to engraft immunodeficient mice.18-24 In one such model, which is based on the engraftment of severe combined immunodeficiency disease (SCID) and nonobese diabetic/SCID (NOD/SCID) mice, a novel population of human hematopoietic cells, defined as SCID-repopulating cells (SRCs), have been shown to be capable of extensive proliferation and multilineage (lymphoid and myeloid) differentiation in vivo.25Furthermore, this activity is highly enriched in CD34+CD38− fractions, and based on the kinetics of engraftment, represents a more primitive cell population than most LTC-ICs and CFCs.23,26-28 However, ex vivo manipulation of these cells has also been shown to be detrimental to their functionality in terms of repopulation,29 and the levels of gene transfer using murine retroviruses (amphotropic and gibbon ape leukemia virus (GALV) pseudotypes) have been consistently very low.23 Engraftment of pluripotent populations in the beige-nude–X-linked immunodeficiency (bg/nu/xid) model has produced similar findings.24 30 These studies reflect more closely the situation found in large animal studies and human gene therapy trials and indicate the use of this surrogate in vivo assay system for preclinical development of novel vector systems for stem cell gene transfer and optimization of clinically applicable transduction protocols.
In this study, we have evaluated the efficiency of gene transfer to primitive human hematopoietic cells using a GALV-pseudotyped murine retroviral vector, and optimized ex vivo transduction conditions. We show here that these cells retain their ability to repopulate NOD/SCID mice and can be transduced relatively efficiently.
MATERIALS AND METHODS
Recombinant human cytokines and growth factors.
Stem cell factor (SCF), interleukin (IL)-3, IL-6, Flt3-Ligand (Flt3-L), and anti-transforming growth factor (TGF)β1 antibodies were obtained from R & D Systems Inc (Minneapolis, MN). Recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF) and recombinant human granulocyte colony-stimulating factor (G-CSF) were from Amgen (Thousand Oaks, CA).
Purification of hematopoietic CD34+ cells.
Human BM was obtained under local anesthesia from the iliac crest of healthy adult volunteers after informed consent and ethical approval. Samples of umbilical cord blood (CB) were obtained from discarded placental and umbilical tissues by drainage of the blood into sterile collection bags. The BM and CB samples were diluted 1:3 in phosphate-buffered saline (PBS) and enriched for mononuclear cells by density gradient over Ficoll-Paque (1.077 g/mL; Seromed, Berlin, Germany). The BM-derived low density cell fraction was subjected to one cycle of plastic adherence (1 to 2 hours) before the isolation of CD34+ cells. CD34+ cells were isolated by superparamagnetic microbeads selection using the miniMACS system according to the manufacturer’s instructions (Miltenyi Biotec, Inc, Gladbach, Germany). The purity of the cell population ranged between 75% and 97% CD34+ cells as estimated by fluorescence-activated cell sorting (FACS) analysis using either a phycoerythrin (PE)- or a fluorescein isothiocyanate (FITC)-conjugated mouse monoclonal antibody against the human CD34 antigen (anti-hematopoietic-progenitor-cell-antigen-2[anti-HPCA-2], Becton Dickinson; San Jose, CA).
Transduction of human hematopoietic CD34+ cells.
For the transduction of human CD34+ cells, retroviral supernatant was harvested from 80% confluent PG-13 monolayers after 12 to 16 hours cultivation in serum-free X-VIVO10 medium (Boehringer Ingelheim; Heidelberg, Germany) supplemented with 1% bovine serum albumin (BSA; Stem Cell Technologies, Vancouver, Canada), 2 mmol/L L-glutamine and 1% penicillin/streptomycin, filtered (0.45 μm) and kept frozen at −80°C until use. The CD34+-enriched target cell population was prestimulated at a cell concentration of 1 × 105 cells/mL for 20 hours in serum-free X-VIVO10 supplemented with 1% BSA, 2 mmol/L L-glutamine and 1% penicillin/streptomycin in the presence of IL-3 (20 ng/mL), IL-6 (100 U/mL), SCF (50 ng/mL), Flt3-L (100 ng/mL), and anti-TGFβ1 (100 ng/mL). After prestimulation, transduction was performed for 3 consecutive days by replacing half of the cell culture medium with a GALV-pseudotyped LNSN retroviral supernatant (titer: 1 to 5 × 106 ) supplemented with the cytokine combination mentioned above and protamine sulfate to a final concentration of 4 μg/mL. Cells were spun at 2,500 rpm/minute at 32°C for 90 minutes31 before further incubation at 37°C, 5% CO2 for an additional 2.5 hours. Afterwards, half of the medium was replaced with X-VIVO10 containing IL-3, IL-6, SCF, TGFβ1, Flt3-L, and anti-TGFβ at the concentrations mentioned above. In some experiments, plates were precoated with the recombinant fibronectin-fragment CH296 (Boehringer Ingelheim). At day 5, cells were harvested, washed, counted, and analyzed for expression of the low-affinity nerve growth factor receptor (LNGFR) and CD34 by flow cytometry.
Flow cytometric analysis of transduced cells.
After transduction, cells were washed twice in PBS containing 1% heat inactivated fetal calf serum (FCS) and 0.1% sodium azide. To assess for LNGFR expression, cells were incubated with an unconjugated mouse antihuman LNGFR antibody (Boehringer Mannheim, Mannheim, Germany), which was detected with a goat antimouse F(ab)-fluorescein isothiocyanate (FITC) (Dianova; Hamburg, Germany). Alternatively, a biotinylated primary LNGFR antibody (kindly provided by Dr S. Seeber, Boehringer Mannheim, Penzberg, Germany) was used and subsequently detected with PE-conjugated streptavidin (Dianova). For the flow cytometric analysis of engrafted NOD/SCID mice, directly conjugated antibodies against human cell surface antigens were purchased from Becton Dickinson (Oxford, UK) (CD19-FITC, CD34-FITC, CD38-PE, CD45-PerCP) or DAKO, Ltd (High Wycombe, UK) (CD2-PE, CD3-FITC, CD13-PE). A total of 1 × 106 cells obtained from the BM of injected mice were incubated for 30 minutes on ice with saturating amounts of antibodies in staining buffer (PBS, 5% FCS, 0.01% sodium azide). A sample from each mouse was also stained with directly conjugated isotype-matched control antibodies (Becton Dickinson). After incubation, cells were washed three times and fixed in 1% paraformaldehyde. Flow cytometric analysis was performed on a FACScan or FACSCalibur using the CellQuest software package (Becton Dickinson). In all experiments, isotype controls were used to set the quadrant markers such that the quadrant defining negative PE and FITC fluorescence contained at least 97% of the isotype control cells. The engrafted human cells were detected by CD45 positivity and the expression of the lineage markers. LNGFR expression was determined on the CD45-gated population.
Progenitor cell assays.
For clonogenic assays, transduced cells were plated in 35-mm dishes containing 0.35% agar, 25% FCS (Hyclone; Erembodegem-Aalst, Belgium), 50 ng/mL SCF, 20 ng/mL IL 3, 10 ng/mL G-CSF, and 10 ng/mL GM-CSF in McCoy’s medium (Life Technologies; Gaithersburg, MD). Cultures were incubated at 37°C in a 5% CO2 humidified atmosphere and colonies were enumerated after 10 to 15 days. For LTC-IC assay, 500 or 1,000 cells were seeded on a preestablished monolayer of the murine FBMD-1 cell line32 (kindly provided by R.E. Ploemacher, Rotterdam, The Netherlands) in MyeloCult (Stem Cell Technologies) containing 20 ng/mL IL-3, 100 U/mL IL-6, and 50 ng/mL SCF. Cultures were incubated for 5 weeks at 37°C, 5% CO2 with weekly changes of half of the medium. At the end of the 5-week LTC-IC assay period, the nonadherent and adherent fractions were harvested and assayed for the content of hematopoietic progenitors by plating 100,000 hematopoietic cells in clonogenic assays, as described above and scored 14 days later. LNGFR-positive colonies were detected by immunostaining techniques (manuscript in preparation). Similarly, 2 × 105 cells derived from the BM of engrafted cells 6 weeks after injection were analyzed for the presence of human hematopoietic progenitors. Cells were plated in Methocult (Stem Cell Technologies), supplemented with Iscove’s modified Dulbecco’s medium (IMDM), 30% FCS, human growth factors (25 ng/mL SCF, 10 U/mL IL-3, 9 U/mL GM-CSF, 2 U/mL erythropoietin [Epo]; all R&D Systems), 2 mmol/L L-glutamine, and 50 μmol/L 2-mercapto-ethanol, resulting in 0.9% final concentration of methylcellulose. The cultures were incubated in a fully humidified atmosphere at 5% CO2.
NOD/SCID mouse reconstitution assay.
The NOD/LtSz-scid/scid (NOD/ SCID) mice (original stocks kindly provided by John E. Dick, Hospital for Sick Children, Toronto, Canada) were housed in sterile microisolator cages in a laminar flow caging system (Thoren, Hazleton, PA) and supplied with sterile food, acidified water, and bedding. All manipulations were conducted in a laminar flow hood. Transduced CD34+ cells were injected intravenously via the tail vein of 6- to 8-week-old mice, which had been sublethally irradiated with 325 cGy (137Cs source). Mice were killed by CO2 inhalation 6 weeks after injection and BM cells were harvested for flow cytometric analysis and growth of hematopoietic progenitors.
Polymerase chain reaction (PCR) for human LNGFR.
The presence of LNGFR provirus in secondary colonies was determined using the primers 5′-TGTGTGAGCCCTGCCTGGAC, beginning at position 300 in exon 2 and 5′-CGAGCCCTCTGGGGGTGTGG position 725 in exon 4 of the LNGFR gene. The amplification cycle was 30 seconds at 94°C, 1 minute at 66°C, and 1 minute at 72°C, with a final elongation step of 10 minutes at 72°C. After 30 cycles, the specific 425-bp product was detected by Southern blotting.
RESULTS
Optimized transduction of CD34+cells using LNGFR expression.
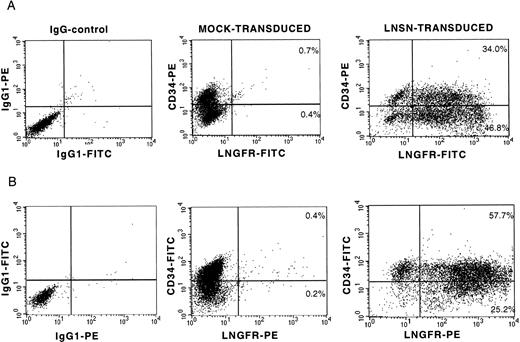
On the basis of previous studies suggesting that retroviral vectors generated on the PG13 packaging cell line may have advantages over amphotropic vectors for transduction of human hematopoietic cells,13,33-35 a GALV-pseudotyped LNSN retroviral vector was used in all experiments. The LNSN construct, which contains the full-length low-affinity receptor for human nerve growth factor (LNGFR) under the transcriptional control of the Moloney murine leukemia virus (Mo-MuLV) long terminal repeat (LTR),36 was selected for these studies because LNGFR expression allows rapid evaluation of gene transfer by flow cytometric analysis and immunocytochemistry.36-39 To optimize gene transfer to human CD34+ cell populations, a detailed study of several parameters that could improve efficiency was performed (manuscript in preparation). The optimized transduction protocol included prestimulation of the CD34+ cells for 20 hours in serum-free medium (X-VIVO10) supplemented with 1% BSA, 2 mmol/L L-glutamine, IL-3, IL-6, SCF, FLT3-L, and anti-TGFβ1. Thereafter, cells were transduced under identical conditions for 4 hours on 3 consecutive days and included a spinoculation step (2,500 rpm/min, 32°C, 90 minutes).31 At the end of the transduction period, a twofold to threefold expansion in total cell numbers was observed, with half of the cells retaining expression of the CD34 cell surface antigen (35% to 46% for BM cells and 57% to 72% for CB cells). Transduction efficiency was determined by flow cytometric analysis of LNGFR expression on day 5. Representative profiles of transduced BM and CB-derived CD34+ cells are shown (Fig 1). In a series of 10 independent experiments, transduction efficiencies of 70% or higher were achieved (Fig 2). Best transduction efficiency was achieved when plates coated with the recombinant human fibronectin fragment, CH-269, were used in conjuction with spinoculation (97.4% LNGFR positive cells; Table 1), confirming the observations of others.40-43 In addition, between 53% and 95% of the cells coexpressed CD34 and LNGFR (Fig 2). LNGFR expression on mock-transduced cells was negligible (Fig 1, central panels).
LNGFR expression on BM- and CB-derived CD34+ cells after gene transfer with a GALV-pseudotyped LNSN retroviral vector. Flow cytometric analysis of two representative transduction experiments into CD34+ cells derived from BM (A) and umbilical CB (B). The left panels show the isotype controls for nonspecific IgG1 staining. The CD34 and LNGFR expression on mock-transduced cells and LNSN-transduced cells is shown at the central and right panels, respectively.
LNGFR expression on BM- and CB-derived CD34+ cells after gene transfer with a GALV-pseudotyped LNSN retroviral vector. Flow cytometric analysis of two representative transduction experiments into CD34+ cells derived from BM (A) and umbilical CB (B). The left panels show the isotype controls for nonspecific IgG1 staining. The CD34 and LNGFR expression on mock-transduced cells and LNSN-transduced cells is shown at the central and right panels, respectively.
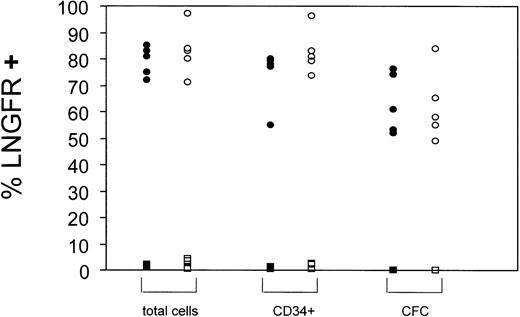
Summary of gene transfer efficiencies into BM- and CB-derived CD34+ cells. Gene transfer efficiencies into hematopoietic cells (total cells), CD34+ cells, or CFC were estimated by FACS (total cells and CD34+) or immunostaining techniques (CFC). Data from LNSN-transduced cells (circles) and mock transduced cells (squares) are presented. Black symbols: BM-derived CD34+ cells, open symbols: CB-derived CD34+ cells. Each symbol represents a single experiment.
Summary of gene transfer efficiencies into BM- and CB-derived CD34+ cells. Gene transfer efficiencies into hematopoietic cells (total cells), CD34+ cells, or CFC were estimated by FACS (total cells and CD34+) or immunostaining techniques (CFC). Data from LNSN-transduced cells (circles) and mock transduced cells (squares) are presented. Black symbols: BM-derived CD34+ cells, open symbols: CB-derived CD34+ cells. Each symbol represents a single experiment.
Efficiency of Gene Transfer Into NOD/SCID Repopulating Human Hematopoietic Cells
| Experiment . | Efficiency of Gene Transfer . | No. of Transplanted Cells . | Human Cell Engraftment (% CD45+ cells) . | Gene-Marked Cells Derived From the BM of NOD/SCID Mice (% of all CD45+ cells) . | LNGFR PCR Second Colonies (LNGFR+/total colonies) . | |
|---|---|---|---|---|---|---|
| (% LNGFR+ cells) . | (% CD34+LNGFR+/CD34+ cells) . | |||||
| BM 3 | 80.8 | 79.6 | 1.25 × 106 | 1.2 | 6.0 | |
| 2.5 × 106 | 0.3 | 0 | ||||
| 2.5 × 106 | 0 | 0 | ||||
| BM 5 | 83.1 | 78.6 | 3.0 × 106 | 5.1 | 5.6 | |
| 4.2 × 106 | 0 | 0 | ||||
| CB 1 | 70.8 | 74.2 | 1.25 × 106 | 9.5 | 16.9 | 2/10 |
| 1.25 × 106 | 12.8 | 16.7 | 1/13 | |||
| 1.25 × 106 | 9.0 | 25.3 | 1/13 | |||
| CB 3 | 83.3 | 81.3 | 1.3 × 106 | 0 | 0 | |
| 1.3 × 106 | 3.6 | 13.7 | 1/20 | |||
| CB 4 | 84.2 | 80.8 | 0.5 × 106 | 0 | 0 | |
| 0.5 × 106 | 0 | 0 | ||||
| CB 5-150 | 97.4 | 98.0 | 3.0 × 106 | 33.3 | 56.6 | 9/20 |
| 3.0 × 106 | 0.9 | 15.1 | ||||
| Experiment . | Efficiency of Gene Transfer . | No. of Transplanted Cells . | Human Cell Engraftment (% CD45+ cells) . | Gene-Marked Cells Derived From the BM of NOD/SCID Mice (% of all CD45+ cells) . | LNGFR PCR Second Colonies (LNGFR+/total colonies) . | |
|---|---|---|---|---|---|---|
| (% LNGFR+ cells) . | (% CD34+LNGFR+/CD34+ cells) . | |||||
| BM 3 | 80.8 | 79.6 | 1.25 × 106 | 1.2 | 6.0 | |
| 2.5 × 106 | 0.3 | 0 | ||||
| 2.5 × 106 | 0 | 0 | ||||
| BM 5 | 83.1 | 78.6 | 3.0 × 106 | 5.1 | 5.6 | |
| 4.2 × 106 | 0 | 0 | ||||
| CB 1 | 70.8 | 74.2 | 1.25 × 106 | 9.5 | 16.9 | 2/10 |
| 1.25 × 106 | 12.8 | 16.7 | 1/13 | |||
| 1.25 × 106 | 9.0 | 25.3 | 1/13 | |||
| CB 3 | 83.3 | 81.3 | 1.3 × 106 | 0 | 0 | |
| 1.3 × 106 | 3.6 | 13.7 | 1/20 | |||
| CB 4 | 84.2 | 80.8 | 0.5 × 106 | 0 | 0 | |
| 0.5 × 106 | 0 | 0 | ||||
| CB 5-150 | 97.4 | 98.0 | 3.0 × 106 | 33.3 | 56.6 | 9/20 |
| 3.0 × 106 | 0.9 | 15.1 | ||||
Abbreviations: BM, bone marrow; CB, umbilical cord blood.
Transduction was performed on CH-296–coated plate.
Gene transfer into CFCs and LTC-ICs.
Immunocytochemical detection of LNGFR expression in hematopoietic colonies indicated an efficiency of gene transfer to colony-forming progenitors (CFCs) of 63.4% ± 11.7% (range, 49.0% to 84.0%) (Fig 2). Absence of background staining in mock-transduced preparations confirmed the specificity of detection (not shown). No difference in the efficiency of CFC transduction was observed between CD34+ cells derived from BM (63.6% ± 10.1%) or from CB (63.1% ± 13.4%). To assess gene transfer into more primitive progenitors, transduced cells were maintained under long-term culture conditions on FBMD-1 cells.32 Of colonies derived from progenitor cells removed from the culture after 5 weeks, 23.6% ± 4.3% (range, 19.3 to 28.0%) expressed LNGFR by immunostaining.
Gene transfer into SRCs.
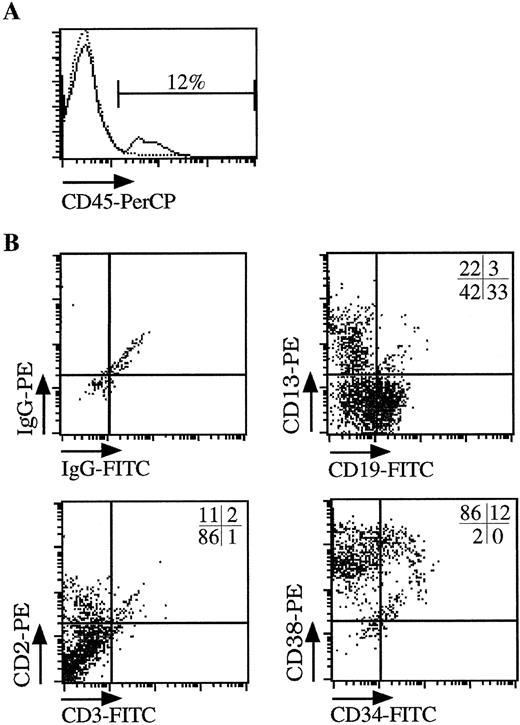
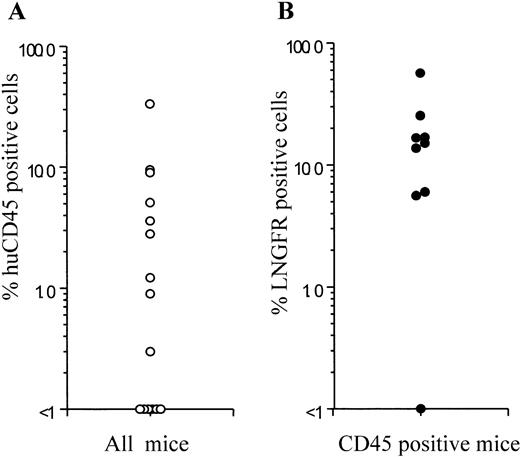
To test for transduction of primitive human cells with repopulating ability, hematopoietic cells (derived from BM or CB) were injected into the tail vein of sublethally irradiated NOD/SCID mice after transduction under the optimized conditions outlined above. Cells recovered from mouse femurs 6 weeks after engraftment were analyzed for the percentage of human cells (CD45 expression) and expression of the LNGFR marker gene by flow cytometry and for the level of gene transfer to CFCs by PCR. Results are summarized in Table 1. After transplantation of between 0.5 and 4.2 × 106 cells, nine of 14 animals showed detectable levels (0.3% to 33.3%) of human cell engraftment measured by flow cytometric detection of CD45 expression (Figs 3A and4). Multilineage engraftment determined by surface immunophenotype (CD19, CD13, CD2), and CFC profile (not shown) was observed in all nine animals (Fig 3B). In five animals, no CD45+ cells were detectable, although engraftment at levels below those measurable in these studies is possible.
Multilineage engraftment of human hematopoietic cells into NOD/SCID mice. Sublethally irradiated NOD/SCID mice were injected with CB-derived CD34+ cells transduced for 3 days under serum-free conditions (CB#1 in Fig 2). (A) FACS analysis of hematopoietic cells obtained from the femur of animals injected with human CB-derived CD34+ cells (solid line) or control animals (dotted line). The percentage of human cells was calculated from the number of CD45+ cells found in the femur of the animals after 6 weeks. (B) Multilineage engraftment of human cells. The CD45+ cells shown in (A) were further analyzed for the presence of myeloid cells (CD13), lymphoid cells (CD19, CD2), and immature progenitor cells (CD34, CD38). The percentage of each cell population is shown in the upper right corner of each quadrant.
Multilineage engraftment of human hematopoietic cells into NOD/SCID mice. Sublethally irradiated NOD/SCID mice were injected with CB-derived CD34+ cells transduced for 3 days under serum-free conditions (CB#1 in Fig 2). (A) FACS analysis of hematopoietic cells obtained from the femur of animals injected with human CB-derived CD34+ cells (solid line) or control animals (dotted line). The percentage of human cells was calculated from the number of CD45+ cells found in the femur of the animals after 6 weeks. (B) Multilineage engraftment of human cells. The CD45+ cells shown in (A) were further analyzed for the presence of myeloid cells (CD13), lymphoid cells (CD19, CD2), and immature progenitor cells (CD34, CD38). The percentage of each cell population is shown in the upper right corner of each quadrant.
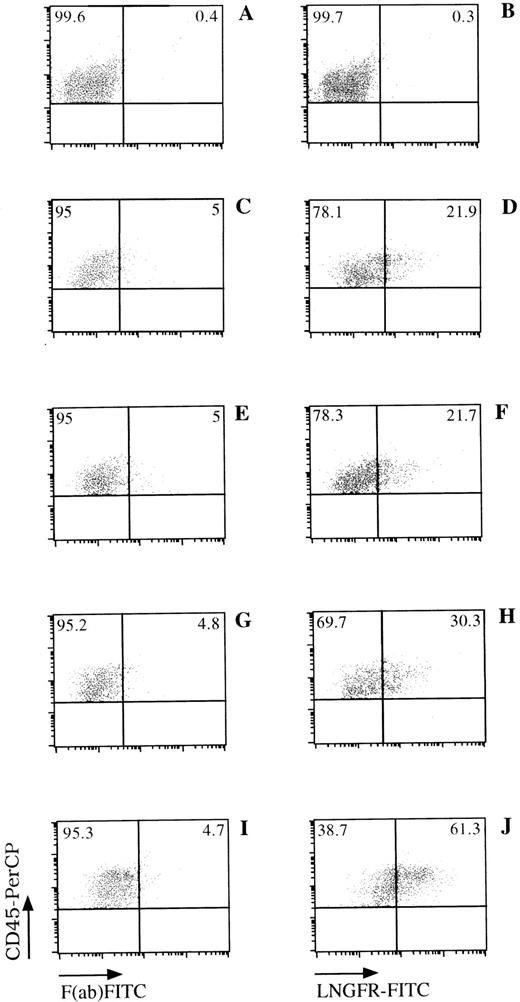
LNGFR expression on human CD45+ cells obtained from the BM of NOD/SCID mice. NOD/SCID were sublethally irradiated and injected with LNSN-transduced CB-derived CD34+ cells (panels C through J) or mock-transduced cells (panels A and B). Six weeks later, BM cells were obtained from the femur of these animals and analyzed for the presence of human cells (CD45+) and LNGFR expression by FACS. FACS data from mice engrafted with cells derived from CB#1 (panels C through H) or CB#5 (panels I and J) is shown. The left panels show the CD45+expression versus the F(ab)-FITC isotype control. The right panels show the CD45 and LNGFR expression on LNSN-transduced (D through J) or mock-transduced (B) cells. Fluorescence intensities are displayed in logarithmic scale.
LNGFR expression on human CD45+ cells obtained from the BM of NOD/SCID mice. NOD/SCID were sublethally irradiated and injected with LNSN-transduced CB-derived CD34+ cells (panels C through J) or mock-transduced cells (panels A and B). Six weeks later, BM cells were obtained from the femur of these animals and analyzed for the presence of human cells (CD45+) and LNGFR expression by FACS. FACS data from mice engrafted with cells derived from CB#1 (panels C through H) or CB#5 (panels I and J) is shown. The left panels show the CD45+expression versus the F(ab)-FITC isotype control. The right panels show the CD45 and LNGFR expression on LNSN-transduced (D through J) or mock-transduced (B) cells. Fluorescence intensities are displayed in logarithmic scale.
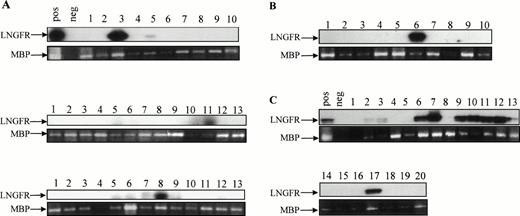
Efficiency of gene transfer to the input population of CD34+ cells ranged from 70.8% to 97.4%, as measured by flow cytometric detection of LNGFR expression. Of the CD34+cells remaining at this time after the transduction procedure, 74.2% to 98.0% also expressed the LNGFR marker gene (Table 1). Six weeks after transplantation, LNGFR+ cells were detected in the CD45+ cell population at levels ranging from 5.6% to 56.6% in eight of nine engrafted animals (Figs 4 and5). In those animals successfully engrafted with CB-derived cells (six of nine), 13.7% to 56.6% of CD45% cells were positive for LNGFR expression (Table 1). LNGFR+ cells were not detectable in animals engrafted with mock-transduced CB-derived CD34+ cells (Fig 4A and B), indicating that the positive signals obtained were derived from successfully transduced cells. Confirmation of gene transfer to SRCs was obtained by PCR amplification of a transgene-specific sequence in genomic DNA extracted from secondary colonies (colony-forming unit–granulocyte-macrophage [CFU-GM] and burst-forming unit erythroid [BFU-E]). In five of five samples, proviral DNA was detected in individual colonies at rates comparable to that predicted by LNGFR expression (Table 1, Fig 6).
Frequency of human cell engraftment and LNGFR expression in NOD/SCID mice injected with transduced BM- or CB-derived CD34+ cells. BM- or CB-derived CD34+ cells were transduced with a GALV-pseudotyped LNSN retroviral vector and injected into sublethally irradiated NOD/SCID mice. The left panel shows the percentage of human cell engraftment (CD45+cells) 6 weeks after injection. The right panel shows the LNGFR expression in CD45+ cells obtained from the BM of engrafted animals. Each symbol represents one mouse.
Frequency of human cell engraftment and LNGFR expression in NOD/SCID mice injected with transduced BM- or CB-derived CD34+ cells. BM- or CB-derived CD34+ cells were transduced with a GALV-pseudotyped LNSN retroviral vector and injected into sublethally irradiated NOD/SCID mice. The left panel shows the percentage of human cell engraftment (CD45+cells) 6 weeks after injection. The right panel shows the LNGFR expression in CD45+ cells obtained from the BM of engrafted animals. Each symbol represents one mouse.
Detection of proviral genome in secondary colonies derived from engrafted NOD/SCID mice. Secondary colonies were established with cells obtained from the BM of engrafted NOD/SCID animals 6 weeks after injection. The presence of LNSN proviral DNA in hematopoietic colonies derived from three mice injected with CB#1 (A), one mouse injected with CB#3 (B), and one mouse injected with CB#5 (C) was assessed by a sensitive LNGFR-specific PCR. The 425-bp–specific LNGFR product was detected by Southern blotting. Amplification of a sequence from the human mannose binding protein (MBP) gene was used to control for the presence of DNA.63
Detection of proviral genome in secondary colonies derived from engrafted NOD/SCID mice. Secondary colonies were established with cells obtained from the BM of engrafted NOD/SCID animals 6 weeks after injection. The presence of LNSN proviral DNA in hematopoietic colonies derived from three mice injected with CB#1 (A), one mouse injected with CB#3 (B), and one mouse injected with CB#5 (C) was assessed by a sensitive LNGFR-specific PCR. The 425-bp–specific LNGFR product was detected by Southern blotting. Amplification of a sequence from the human mannose binding protein (MBP) gene was used to control for the presence of DNA.63
DISCUSSION
Efficient gene transfer to human PHSCs has been limited by incomplete understanding of their biologic properties and by deficiencies of vector systems and ex vivo transduction conditions. These confounding factors are reflected in data from clinical trials and from studies in which transduced cells are engrafted in immunodeficient mice.4,6,9,10-12,23,24,44 The NOD/SCID model system has been shown to support the engraftment and retention of primitive human hematopoietic cells with the potential for extensive proliferation and multilineage differentiation.23,25-27,45,46 Unlike the majority of LTC-ICs, which are incapable of repopulation, SRCs are found exclusively in the CD34+CD38- cell fraction at a calculated frequency of approximately 1 in 600 in CB and BM and are therefore phenotypically and functionally distinct.26 Furthermore, kinetic experiments indicate that engraftment of SRCs is followed by a large expansion of LTC-ICs in vivo, suggesting that these are derived from a more primitive cell.27 Although both CFCs and LTC-ICs are readily transduced, the efficiency of gene transfer to SRCs has generally been very low, and the repopulating potential is markedly compromised by ex vivo culture.13,23,29 Similar findings have been reported for pluripotent cells engrafting bg/nu/xid mice, although there are qualitative and quantitative differences in repopulating cell engraftment patterns in this model compared with that of SRCs.24 30
Multiple factors probably contribute to the inefficiency of gene transfer to repopulating cells. The majority of CD34+CD38− cells are quiescent15 and therefore refractory to transduction by the current generation of murine retroviral vectors, which require breakdown of the nuclear membrane to achieve entry of the nucleoprotein complex into the nucleus before integration.14 Ram-1, the receptor for the amphotropic retroviral envelope, is expressed at very low levels on CD34+CD38− cells, and virus binding can only be detected after cytokine stimulation.16,17 In contrast, GALV-pseudotyped retroviruses have been shown to mediate higher levels of gene transfer to CD34+ and CD34+CD38−cells, although they have not been extensively evaluated in repopulating assay systems.13,33-35,43 Furthermore, current gene transfer protocols require the removal of PHSC from their natural microenvironmental niches and their manipulation ex vivo, conditions, which may alter the integrity and functionality of these cells.29 However, stem cells can also be protected in vitro by culture on feeder cells or stromal monolayers.47,48 At least partially, this function can be replaced by appropriate combinations of cytokines and growth factors. Flt3 ligand (FL), in particular, has been shown to act synergistically with a range of other cytokines to stimulate proliferation and amplification of very primitive (CD34+CD38−) hematopoietic cells both in vitro and in vivo.46,49-54 Moreover, FL enhances transduction efficiency of primitive progenitor cells and preserves the ability of transduced cells to repopulate bg/nu/xid mice after prolonged periods of in vitro culture.44
Ideally, ex vivo manipulation of PHSCs should preserve the intrinsic properties of these cells. On the basis that maintenance or even expansion of the PHSC can be achieved after cultivation of CD34+CD38− CB-derived cells in serum-free medium,46 55 one major objective of this study was to establish gene transfer into repopulating cells under serum-free conditions. In addition, the use of total CD34+ cells as the target population for gene transfer, rather than highly purified subfractions, is compatible with current clinical practice in stem cell transplantation and thus allows for a rapid transfer of the transduction protocol to clinical situations. The final protocol described in this study represents a major step towards the achievment of this goal.
Previous attempts to transduce CD34+ cells under serum-free conditions have shown gene transfer into CFCs at levels ranging from 1% to 29%.12,56 In an effort to improve gene transfer in the absence of serum, we examined the efficiency of gene transfer to CD34+ cells under varying culture conditions (manuscript in preparation). The combination of cytokines (IL-3, IL-6, SCF, and FL) together with anti-TGFβ1 antibodies was chosen to stimulate cell division in primitive populations and to retain repopulating potential over extended culture periods.57-62Under these conditions, high gene transfer rates into CD34+hematopoietic cells were reproducibly achieved, independently of the donor and source of material used (>70% LNGFR+ cells). Significant repopulating potential of transduced cells was also retained over the 5-day ex vivo culture period, and the majority of engrafted NOD/SCID mice also showed high levels of marker gene expression (6% to 57%) in total CD45+ (lymphoid and myeloid) populations. Comparable percentages of provirus-positive CFCs were detected in secondary colony-forming assays. These results are highly suggestive of successful gene transfer to primitive multilineage repopulating cells, although formal proof of this would require the demonstration of common integrants in lymphoid and myeloid lineages.
In this study, we have shown that efficient retrovirus-mediated gene transfer to cells with the capacity to repopulate sublethally irradiated NOD/SCID mice (SRCs) can be achieved by optimization of transduction conditions ex vivo and optimization of the vector. These are the first studies to show efficient gene transfer to the SRC population using GALV-pseudotyped viruses and support the further investigation of this envelope for transduction of human PHSCs. They also reinforce the use of surrogate repopulating assays for testing of novel vector systems and development of clinically applicable gene therapy protocols. Further improvements of cell culture systems and development of vectors that obviate the requirement for cell division are likely to further enhance transduction of human repopulating cell populations.
ACKNOWLEDGMENT
We are indebted to S. Seeber (Boehringer Mannheim, Penzberg) for the LNSN construct and LNGFR antibodies, R.E. Ploemacher (Erasmus University, Rotterdam, The Netherlands) for the FBMD-1 cell line, T. Tonn (Blood Bank, Frankfurt) for CB samples, and Mike Blundell for technical assistance.
Supported by grants from the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie to M.G. and O.G.O. (FKZ: 01KV9558), the Hermann J. Abs Program of the Deutsche Bank AG, the Primary Immunodeficiency Association, Chronic Granulomatous Disease Research Trust, the European Commission (to G.B.), and the Wellcome Trust (to A.J.T.). The Georg-Speyer-Haus is supported by the Bundesministerium für Gesundheit and the Hessisches Ministerium für Wissenschaft und Kunst.
A.J.S. and G.B. contributed equally to this work.
Address reprint requests to Manuel Grez, PhD, Georg-Speyer-Haus, Paul-Ehrlich-Strasse 42, 60596 Frankfurt, Germany; e-mail:grez@em.uni-frankfurt.de.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal