Abstract
As a mediator of neurogenic inflammation and pain, we hypothesized that levels of the neuropeptide Substance P (SP) would be elevated in patients with sickle cell disease (SCD) with vaso-occlusive pain crisis. SP is a known stimulator of tumor necrosis factor- (TNF-) release and a promoter of interleukin-8 (IL-8), which are reported to be increased in SCD. These cytokines enhance adhesion of leukocytes to endothelium and may play a role in vaso-occlusive events. Serum levels of IL-8, TNF, and SP were studied in three groups of children aged 2 to 18 years: 30 well children with SCD, 21 with SCD in pain crisis, and 20 healthy age-matched controls. Serum levels of SP were elevated in all SCD patients and were highest in patients in pain crisis. The percentage of sera with detectable levels of IL-8 (>5.0 pmol/L) was increased in SCD patients as compared with the control group. IL-8 levels were similar for well SCD patients and those with pain. TNF levels were not significantly different among the three groups. In three children with SCD, SP was measured at baseline and again during pain crisis. In each case, serum levels during pain crisis were higher than they were when the patient was well. We conclude that levels of SP are high in patients with SCD and increase during pain crisis. These results imply that SP plays a prominent role in the pain and inflammation of SCD and may be a measurable laboratory marker of vaso-occlusive crisis. We speculate that neurokinin receptor antagonists may have a therapeutic potential in the treatment of crisis pain.
© 1998 by The American Society of Hematology.
ALTHOUGH SICKLE CELL disease (SCD) was the first inherited disease to be understood at the molecular level and is one of the most common genetic diseases, the mechanisms underlying its complications remain poorly understood. It remains a disease of significant morbidity and mortality. The complications of SCD have often been attributed to vascular occlusion,1 2 however, there is growing evidence that inflammatory stresses within the microvasculature may play a significant role. Further understanding of the immune mediators involved may contribute to developing future treatments and help identify patients at risk of severe complications before they occur.
Vaso-occlusive pain crisis is one of the most common complications of SCD3 with clinical manifestations similar to those seen in other inflammatory disorders or with infection.4 Pain is frequently severe enough to warrant the use of parenteral narcotics. The affected area may be tender, accompanied by localized edema, erythema, and warmth, and the patient may have systemic signs of inflammation such as leukocytosis and fever.5 Consistent with these observations, the acute phase reactants C-reactive protein,6 α1-glycoprotein, and transferrin7are significantly elevated during painful crisis. High circulating levels of the inflammatory cytokines tumor necrosis factor-α (TNF-α), interleukin-1α (IL-1α), IL-6, histamine, and leukotriene B4 have also been described.8-12
The neuropeptide Substance P (SP) is an attractive candidate to explain both the clinical findings and the cytokine elevations present during vaso-occlusive pain crisis. SP is considered a major mediator of neurogenic pain and inflammation.13,14 It has been demonstrated to induce vasodilatation and plasma extravasation.15 SP induces release of histamine from mast cells,16 promotes secretion of several cytokines including IL-1, IL-6, IL-8, and TNF-α,17-20 and is itself a potent chemotactic factor.21 Each of these functions suggests that SP levels might be elevated during vaso-occlusive pain crisis. TNF-α and IL-8 are of further interest because their effects may impair blood flow and impede recovery from ischemic episodes by increasing adhesion of sickle red blood cells to endothelium22 and modulate neutrophil:endothelial cell interactions.23
MATERIALS AND METHODS
Patients were recruited for this study from the outpatient Hematology Clinic and the Emergency Department of the Children’s Hospital of Philadelphia, which is the site of a federal- and state-funded Comprehensive Sickle Cell Center. Patients were asked to participate if their hemoglobin genotype, as documented by hemoglobin electrophoresis was SS, SC, or S-β° thalassemia. Patients were excluded if they had received transfusion within the previous 6 months, if they were receiving hydroxyurea or medications other than penicillin or folate, or if they had a second chronic disease or inflammatory disorder. Well children with SCD were seen during routine clinic visits and asked to participate if there was no history of fever, pain, or infection for at least 4 weeks before the day of study. In the Emergency Department, children with SCD were recruited during evaluation for pain crisis. In the majority of cases, patients had been treated with oral acetaminophen and/or codeine at home. Patients were enrolled in the study if the pain was not controlled by these medications and was considered severe enough to require parental narcotics and, in most cases, hospital admission. Control subjects consisted of healthy, age-matched, hematologically normal children who were having blood drawn for other purposes. All aspects of this study were approved by the Committee for Protection of Human Subjects Institutional Review Board of the Children’s Hospital of Philadelphia.
After written consent was obtained from the patient, or their parent, venous whole-blood samples were obtained by a trained phlebotomist. Blood from patients in pain crisis was drawn before initiation of treatment. For cytokine assays, 5 mL of blood was drawn into a serum isolation tube and immediately placed on ice. After 1 hour, the chilled specimens were centrifuged at 2,500g for 15 minutes at 4°C. The serum was carefully withdrawn using a plastic pipette, aliquotted to fresh tubes, labeled, and frozen at −70°C until use.
Measurement of IL-8 and TNF-α.
Samples were thawed at room temperature immediately before assay. Thawed samples were not refrozen or reused. Each sample was tested and results confirmed in duplicate. Serum TNF-α and IL-8 concentrations were measured by sandwich enzyme-linked immunoassay using commercially available kits (T Cell Diagnostics, Inc, Cambridge, MA for IL-8, and Endogen, Inc, Cambridge, MA for TNF-α). The kits were used according to the manufacturer’s procedure. Results were determined by measuring the optic density of each well using a microtiter plate reader set at an absorbance of 450 nm with a LABREPCO Elx 800 microplate reader (Bio-Tek Instrument Inc, Horsham, PA). The detection limit of the TNF-α test kit is 1.5 pg/mL, and that for IL-8 is 2 pg/mL.
Measurement of SP.
Each freshly thawed sample was diluted with 4 vol of 4% acetic acid and passed through a C-18 reverse phase column (Varian Sample Preparation Products, Harbor City, CA) by gravity. The column was washed five times with the 4% acetic acid solution to remove all unbound material. SP was then eluted by the addition of buffer containing 60% acetonitrile and 1% trifluoroacetic acid. Eluted samples were dried with liquid nitrogen then reconstituted in distilled water. In control experiments, overall recovery of SP was greater than 90%.24 The SP was quantified by the use of an antigen competition, enzyme-linked immunoassay (Cayman Chemical Company, Ann Arbor, MI). Each sample was run and results confirmed in duplicate. Optical density was read at an absorbance of 405 nm. The limit of detection of this assay is 5 pg/mL.
Statistical analysis.
For cytokine measurements, values had skewed distributions. Therefore, all analyses were done either on the natural logarithm of the raw scale (t tests, Pearson correlation coefficient) or using nonparametric statistics (Wilcoxon rank-sum test). Statistical significance was declared if the P value was <.05. Calculations were performed using Stata Statistical Software, Release 4.0 (Stata Corp, College Station, TX).
RESULTS
A total of 51 samples was obtained on 20 male and 28 female patients with SCD, aged 2 to 18 years. Of the 51 samples, 30 were obtained during a routine visit for comprehensive sickle cell care and 21 samples from children in pain crisis. Three children were studied both during a routine evaluation and again before admission for pain crisis. One sickle cell patient was white, the remainder were black. Control subjects consisted of 20 healthy children aged 2 to 19 years. Of these subjects, 16 were white and 4 were black.
Possible associations among serum levels of IL-8, SP, and TNF-α were examined, as well as relationship to demographics. Data which could not be confirmed in duplicate because of an insufficient volume of sample was excluded from analysis. No correlation was found for either age or sex with any of the measured cytokine levels. To control for differences in race between the control subjects and the patients with SCD, comparisons were repeated and confirmed after excluding all white subjects from data analysis. High SP levels correlated with high levels of IL-8 (P < .001); however, no such correlation was found for SP and TNF-α, or TNF-α and IL-8.
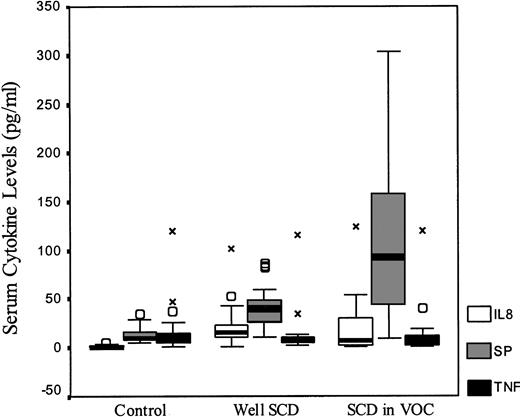
Figure 1 compares the data for controls and patients with SCD. There was no significant difference in TNF-α levels across all groups. IL-8 levels were higher for all SCD patients when compared with controls (P < .001), but no difference was seen between well SCD patients and those in pain crisis (P = .37). Levels of SP were elevated in both groups with SCD when compared with normal controls. Well SCD patients had higher levels than controls (P < .001) and levels were higher in SCD patients in crisis than in those who were well (P = .003).
Serum cytokine levels of IL-8, SP, and TNF in healthy controls and patients with SCD. The measured cytokine levels box-plots are shown by SCD status. (○) Indicate outliers within each serum cytokine; X, extremes.
Serum cytokine levels of IL-8, SP, and TNF in healthy controls and patients with SCD. The measured cytokine levels box-plots are shown by SCD status. (○) Indicate outliers within each serum cytokine; X, extremes.
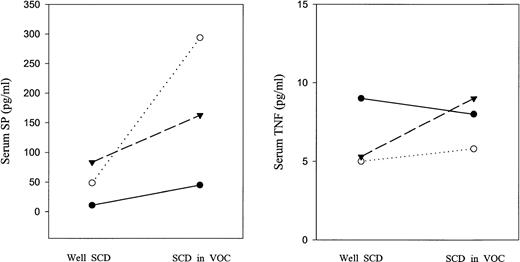
In the three children on whom serum samples were measured at baseline and again during pain crisis, in each case, the serum levels of SP during pain crisis were higher than they were when the patient was well. IL-8 levels increased in two patients, but fell in one. TNF-α did not markedly change in any of the three patients. These data are shown in Fig 2.
Serum cytokine levels of SP and TNF in three patients with SCD when well and in vaso-occlusive crisis. Dotted line indicates patient 1; dashes, patient 2; solid line, patient 3.
Serum cytokine levels of SP and TNF in three patients with SCD when well and in vaso-occlusive crisis. Dotted line indicates patient 1; dashes, patient 2; solid line, patient 3.
DISCUSSION
SP is the most extensively studied and characterized tachykinin, a family of bioactive neuropeptides defined by their common pharmacologic properties and conserved carboxyl-terminal sequences.25Released by sensory nerves and by a variety of nonneuronal sources, such as endothelial cells, macrophages, and eosinophils,18SP has an important function in the modulation of nociception.15 Furthermore, SP and the other tachykinins mediate the events of neurogenic inflammation, hyperemia, plasma extravasation, and leukocyte adhesion to the vascular endothelium.26 SP also induces bronchoconstriction and mucus secretion.27 Because of these effects, neurokinin receptor antagonists have been actively investigated as analgesics28 and for the treatment of asthma.29
Sickle cell crisis pain is typically nociceptive in nature, and it is likely that SP plays a significant role. The sequence of events in SCD that lead to the sensation of pain are not understood, but are thought to develop secondary to vascular occlusion. Sickle erythrocytes adhere strongly to endothelium,30,31 suggesting that endothelial cell injury and subsequent neutrophil activation play a role. The resulting mediators of inflammation, such as histamine, bradykinin, and prostaglandins activate or sensitize nociceptive afferent nerve fibers.32 SP is released by afferent nerves after appropriate stimulation and enhances the inflammatory response by exerting direct effects on polymorphonuclear neutrophils and monocytes and by promoting the release of other inflammatory cytokines.
Increased serum concentrations of SP during a painful event is an expected finding, but the high baseline levels are not as easily explained. This finding is consistent with data from prior studies, which have concluded that there is a persistent inflammatory process in patients with SCD.33 It is also likely that chronic asymptomatic endothelial injury is a contributing factor. Vascular intimal hyperplasia is frequent in these patients and is accepted as the most important cause of stroke in SCD.34 Another possible interpretation of this finding is the role of SP in the control of hematopoiesis. SP receptors are present on hematopoietic cells and SP enhances myeloid and erythroid colony formation via induction of IL-1, IL-3, IL-6, and granulocyte-macrophage colony-stimulating factor (GM-CSF).18,35 In SCD, patients with low levels of fetal hemoglobin (HbF) (< 9%) have high levels of GM-CSF and IL-3,11 and an inverse relationship was observed between HbF and serum values of TNF-α.9 Such observations suggest that SP may be produced in response to hematopoietic stress.18 35
Although SP promotes secretion of TNF-α, by monocytes in vitro,20 we were unable to reproduce the results of previous studies, which reported elevated levels of TNF-α in patients with SCD.9,10 Several factors may account for this discrepancy. In one of these studies,9 TNF-α was measured in plasma, whereas we used serum. We do not believe that this is a contributing factor, as results in our laboratory showed nearly equivalent levels of this cytokine in both plasma and serum. Assay sensitivity may differ between our enzyme-linked immunosorbent assay (ELISA) and those used in the other two studies, however, our assay was sufficiently sensitive to detect TNF-α in nearly all of the patients and healthy controls studied. Our results are consistent with other reports, which did not find significantly detectable levels of TNF-α in patients with the acute complications of SCD.4,36Another possible confounding factor is that the age of our study population was significantly younger than those in the previous studies.9,10 Although our data does not demonstrate a correlation between cytokine measurements and age in children under 20 years, there is evidence that cytokine levels do differ with age. Abboud et al37 demonstrated high concentrations of IL-8 and TNF-α in adults with SCD, but neither was detected in serum from children with this disease. We did detect increased levels of IL-8 in our SCD patients as compared with healthy control subjects in whom IL-8 was not measurable.
Our results support the hypothesis that SP is an important mediator of both the pain and the inflammation observed in sickle cell vaso-occlusive crisis and demonstrates that measurement of serum SP level is a useful laboratory marker of ongoing sickle cell crisis. Furthermore, these findings suggest that neurokinin receptor antagonists may have a therapeutic potential in the treatment of SCD patients in pain crisis.
Supported by Grant No. MH49981 from the National Institutes of Mental Health, National Institutes of Health, Bethesda, MD (to S.D.D.).
Address reprint requests to Steven D. Douglas, MD, Children’s Hospital of Philadelphia, 34th and Civic Center Blvd, Room 1211 Abramson Research Building, Philadelphia, PA 19194; e-mail:douglas@email.chop.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal