Abstract
Increased levels of endothelin-1 (Et-1), a potent vasoconstrictor, have been correlated with hypertension and neuronal damage in ischemic/reperfusion injury. The presence of polymorphonuclear cells (PMNs) in the brain has been shown to be directly responsible for this observed pathology. To address the question of whether Et-1 plays a role in this process, human brain-derived endothelial cells (CNS-ECs) were cultured with Et-1. The results demonstrate that Et-1 induces production of the neutrophil chemoattractant interleukin-8 (IL-8) twofold to threefold after 72 hours; mRNA was maximal after 1 hour of stimulation. Conditioned culture medium derived from Et-1–stimulated CNS-ECs induced a chemotactic response in the PMN migration assay. The inflammatory cytokines tumor necrosis factor- (TNF) and IL-1β functioned additively with Et-1 in increasing IL-8 production. In contrast, transforming growth factor-β (TGF-β), but not IL-10, completely abolished the effect of Et-1 on IL-8 production. However, Et-1 did not modulate intercellular adhesion molecule-1 (ICAM-1) expression. These data demonstrate that Et-1 may be a risk factor in ischemic/reperfusion injury by inducing increased levels of the neutrophil chemoattractant IL-8.
© 1998 by The American Society of Hematology.
ENDOTHELIN-1 (Et-1), a 21–amino acid peptide originally isolated from endothelial cells, was shown to mediate vasoconstriction.1 Cerebral microvessels show a marked sensitivity to Et-1 and are able to produce this peptide.2,3 Three forms of the peptide have been characterized and designated Et-1, -2, and -3. Both Et-1 and Et-3 are present in the brain. Endothelial cells produce only Et-1, the most potent known vasoconstrictor.4-6 In addition to vasoconstriction,1 Et-1 peptides have neuroregulatory and physiologic functions.7-9 Increased levels of Et-1 in plasma and cerebrospinal fluid of patients with hypertension, ischemic stroke, and subarachnoidal hemorrhage have implicated Et-1 as a possible mediator of cerebrovascular responses in these disorders.10-13 The elevated levels of Et-1 in ischemic stroke correlate significantly with the severity of neurologic deficits.14 However, it is not clear whether increased levels of Et-1 are the cause or the result of disease processes, and there is considerable evidence for either situation. Et-1 has been shown to induce pathology in a variety of experimental protocols. Tissue levels of Et-1 in rats subjected to either permanent or transient focal ischemia were shown to be significantly increased.15 In humans, increased Et-1 tissue levels were observed during the reperfusion period.16 Elevated levels of Et-1 are clearly associated with central nervous system (CNS) pathology in stroke; however, the mechanism of its action has not been elucidated.
One possible mechanism of Et-1 activity may be involved in affecting polymorphonuclear cell (PMN) activity during initiation or progression of ischemia/reperfusion damage. Studies have shown that ischemic brain injury involves an initial influx or neutrophils followed by infiltration of monocytes/macrophages into CNS tissue.17Activated neutrophils are involved in tissue repair, but may also cause tissue damage through the release of oxygen free radicals, proteinases, and neurotoxins.18 The significance of PMN accumulation in ischemia/reperfusion is evident by the impact of PMN depletion on neuronal damage. Neutropenia induced by antibodies to PMNs decreased the damage in ischemia19 and improved the neuronal recovery after complete20 or incomplete21 cerebral ischemia. Antibodies to neutrophil adhesion molecules CD11/CD18, the ligand for intercellular adhesion molecule-1 (ICAM-1), decreased neutrophil binding and infiltration,22,23 leading to significant reduction in ischemic damage.17 The critical importance of PMNs to reperfusion injury was further confirmed by reducing disease with anti–ICAM-1 antibodies or in ischemia induced in ICAM-1 knockout mice.24 25
The question is then raised as to the mechanism by which the accumulation of neutrophils in ischemic tissue is achieved. The neutrophil chemotactic factor interleukin-8 (IL-8) was examined, because it is extremely potent in mediating neutrophil migration and transmigration.26 IL-8 is an α-chemokine that activates motility and directional migration, upregulates integrin expression, and increases respiratory burst activity of PMNs.27,28 IL-8 has been shown to be induced by the inflammatory cytokines, tumor necrosis factor-α (TNF) and IL-1β, and endotoxin.29
The function of IL-8 in the initiation of CNS disease is only now being examined. A recent study shows that IL-8 is produced during hypoxia,26 suggesting a possible role for this chemokine in ischemia/reperfusion damage at the endothelial cell surface. In the present study, the effect of Et-1 on IL-8 production by CNS endothelial cells (CNS-ECs) was investigated. The results show that Et-1 upregulates the production of IL-8 by CNS-ECs, and this regulation occurs at the transcriptional level. Furthermore, TNF and IL-1β enhance this effect, whereas transforming growth factor-β (TGF-β) downregulates Et-1–induced IL-8 production.
MATERIALS AND METHODS
Reagents.
The following reagents were purchased: Et-1 (Peninsula Laboratories, Belmont, CA), TNF (Boehringer Mannheim, San Diego, CA), IL-1β (Genzyme Corp, Cambridge, MA), TGF-β1 (Genzyme), IL-10 (R&D Systems, Minneapolis, MN), goat polyclonal and mouse monoclonal anti-human IL-8 antibodies (R&D Systems), mouse monoclonal anti–ICAM-1 (Immunotech, Marseille, France), FITC-goat anti-mouse serum (Becton Dickinson, San Jose, CA), normal goat serum (Vector Laboratories, Burlingame, CA), and goat anti–IL-1β and anti–IL-1α (R&D Systems).
Cell culture.
CNS-ECs were derived from human brain as previously described in detail.30 Cells were cultured in RPMI 1640 medium (GIBCO Laboratories, Grand Island, NY) supplemented with 100 ng/mL endothelial cell growth factor (Endogro; Vectec, Albany, NY), 2 mmol/Ll-glutamine, 10 mmol/L HEPES, 24 mmol/L sodium bicarbonate, 300 U heparin USP, 1% penicillin/streptomycin, and 10% fetal calf serum (FCS). Endogro-free medium was used 24 hours before the experiment. The purity of CNS-ECs (95%) was confirmed by immunocytochemical staining for von Willebrand factor (vWF), glial fibrillary acidic protein (GFAP) for astrocytes, and CD11b for macrophages as previously described.30 The cells were used until passage four to five only, because it was found that with an increasing passage number (> seven to 10), the intensity of vWF and γ-glutamyl transpeptidase decreased. Cells were routinely examined for possible contaminating populations before each experiment using CD11b and GFAP.
IL-8 assay.
IL-8 production was evaluated using the commercially available enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems). Briefly, CNS-ECs were grown in culture to confluence in 10% FCS in 60-mm dishes in a volume of 3 mL; 24 hours before initiation of the experiment, this medium was replaced by medium containing 2% FCS. The culture supernatant (100 μL) was removed after 72 hours, unless otherwise stated, and evaluated for IL-8 content using the ELISA kit. The experimental groups were set up in triplicate, and ELISA samples were evaluated in quadruplicate. The data are expressed as nanograms per 106 cells. Cell counts were determined using trypan blue exclusion. Cell viability was routinely greater than 95%.
Immunocytochemistry.
Cells were treated as already described. At the termination of the experiment, cell cultures were rinsed with phosphate-buffered saline (PBS), prepared in suspension, and cytocentrifuged (5 × 104 cells per slide). Air-dried slides were fixed in acetone for 5 minutes, again allowed to dry, and then subjected to the staining procedure.30 Briefly, cell preparations were treated with the primary monoclonal mouse antibody (18 hours) and subsequently washed with PBS twice (10 minutes), followed by incubation with the biotin-labeled secondary antibody, horse anti-mouse Ig (Vector Laboratories) (30 minutes). The slides were then incubated with avidin-biotin-horseradish peroxidase complex (Vector Laboratories) (30 minutes) followed by treatment with aminoethylcarbasole solution (10 minutes), and counterstained with Mayer’s hematoxylin (1 minute). Irrelevant isotype-matched antibody was used in place of the primary antibody as the negative control.
RNase protection analysis.
Radioactively labeled RNA antisense probes were prepared following the manufacturer’s protocol. Using the In Vitro Transcription Kit (Pharmingen, San Diego, CA), 10 μL 32P-UTP (3,000 Ci/mmol, 10 mCi/mL; NEN Research, Wilmington, DE) and 1 μL GACU pool were added to the RNase protection assay (RPA) template set (HCK-5), which is a human chemokine multiprobe set including IL-8 and the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Pharmingen). Also included were T7 polymerase, DTT, RNAsin, and transcription buffer as suggested by the manufacturer. GAPDH expression may vary somewhat, but the expression level in this culture system appeared consistent independent of the stimuli used in these experiments. The reaction was terminated by adding 2 μL DNase for 30 minutes at 37°C. The probe was then extracted using Tris-saturated phenol: chloroform:isoamylalcohol (25:24:1; GIBCO-BRL) and chloroform:isoamylalcohol sequentially, and then ethanol-precipitated. The radiolabeled RNA pellet was air-dried and solubilized with hybridization buffer.
RNA from 2 to 3 × 106 cultured cells per experimental group was isolated and prepared according to a modification of the acid phenol method using Trizol reagent (Life Technologies, Gaithersburg, MD) as specified by the manufacturer. Total RNA (10 μg) together with 6 × 105 cpm of probe was heat-denatured at 90°C and then hybridized overnight at 56°C. Subsequently, the samples were treated with the RNase cocktail followed by proteinase K cocktails, and then precipitated using ammonium acetate and ethanol. Air-dried samples were solubilized in 1× loading buffer, denatured at 90°C for 3 minutes, and then placed on ice. The protected fragments were resolved in 5% acrylamide/8-mol/L urea gel (24 cm length); the gel was dried and exposed to Hyper film (Amersham Life Science, Arlington Heights, IL) at −70°C. The protected bands were observed for IL-8 (181 bp) and GAPDH (96 bp). Data are presented as the ratio of the spectrophotometric density of IL-8/GAPDH bands. Differences among the groups were calculated as the ratio of the band densities of the experimental/control groups. The manufacturer-recommended yeast tRNA negative control and a positive control were included in every RPA experiment.
Flow cytometry.
ICAM-1 expression on CNS-ECs was determined using flow cytometry. Briefly, cells were incubated overnight in 2% FCS and stimulated with Et-1 or TNF for 18 hours. Cells were then detached using trypsin (0.1%) in PBS-EDTA (0.5 μmol/L) and washed with PBS. The primary antibody was added for 1 hour (ICAM-1, 1:500 dilution) and washed twice with PBS before application of the secondary FITC-conjugated goat anti-mouse antibody (1:100; Becton Dickinson) for 30 minutes. Cells were again washed twice and submitted to flow analysis on a FACstar (Becton Dickinson) using 5,000 cells per count. ICAM-1 expression is presented as the percent positive cells. The data are representative of one of three experiments.
Neutrophil migration assay.
PMNs were isolated from whole blood of healthy human subjects using a mixture of sodium metrizoate and Ficoll gradient centrifugation (1-step Polymorphs; Accurate Chemical & Scientific Corp, Westbury, NY). Approximately 5 mL whole blood was drawn into a syringe containing 0.5 mL 3.8% sodium citrate as an anticoagulant. The blood was then layered over 3.5 mL Polymorphprep in a 12-mL tube and centrifuged at 500g for 35 minutes at 20°C. After centrifugation, two leukocyte bands were visible, and the lower band of PMNs was resuspended in RPMI supplemented with 0.05% FCS and 0.1% bovine serum albumin (BSA). PMNs were then washed twice for 10 minutes at 400 ×g. Cell purity determined by morphology following hematoxylin staining was 94% to 98%, and viability was greater than 95% as determined by trypan blue dye exclusion.
Migration of PMNs was performed in cell culture inserts (Becton Dickinson, Frankin Lakes, NJ) using a 3-μm pore PET track-etched membrane and 24-well format. After 72 hours, media from the differently treated CNS-EC groups cultured in RPMI with 0.05% FCS/0.1% BSA were added to the lower compartment. Media that were not exposed to cells were used as the control. In the upper compartment, 3 × 105 PMNs in culture media were added. The chambers were incubated for 30 minutes at 37°C in 5% CO2. At the conclusion of this period, all liquid in the lower compartments was individually collected and centrifuged, and the cells were counted on a hemocytometer. The results are presented as the mean ± SD of three separate experiments, and are expressed as the increase in the percent of cells migrating in the experimental groups divided by the number of cells migrating when exposed to control media multiplied by 100.
Statistics.
Values are presented as the mean ± SEM, unless otherwise stated. Statistical significance was evaluated using Student’st-test for paired comparison; P < .05 was considered significant.
RESULTS
Et-1 upregulates IL-8 production in CNS-ECs.
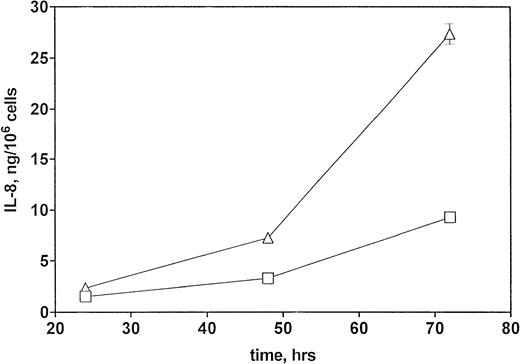
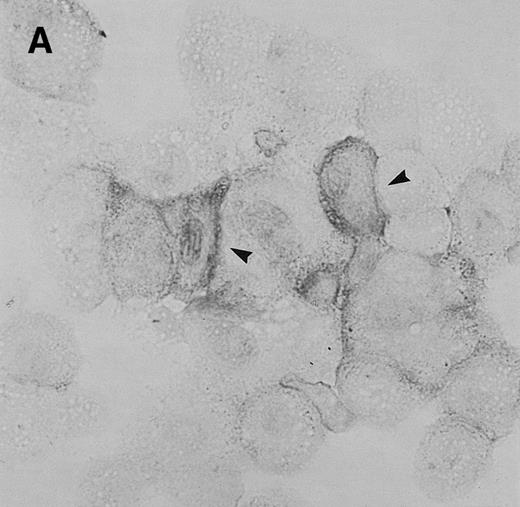
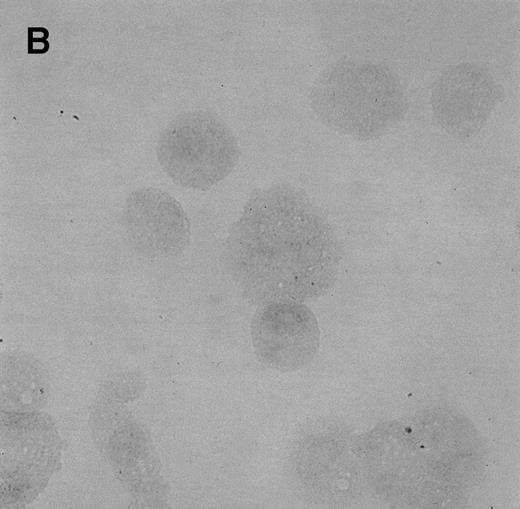
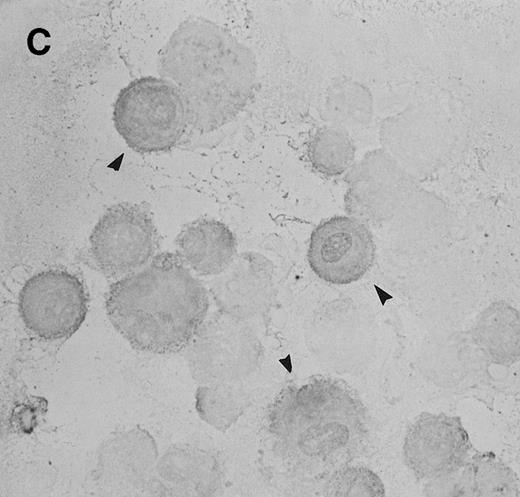
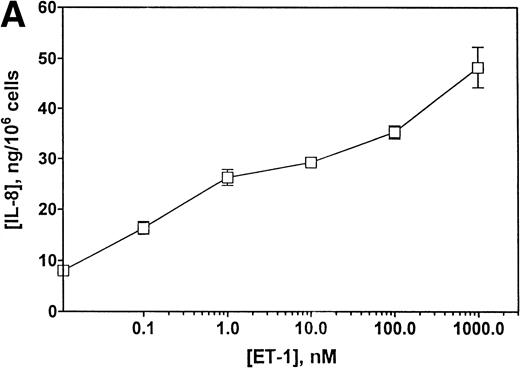
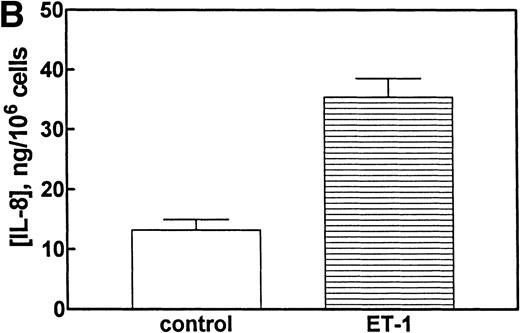
To determine whether CNS-ECs respond to Et-1 by producing IL-8, the cultures were first grown to confluency and then repeatedly treated with Et-1 at 24-hour intervals for the required experimental period. It was noted that subconfluent cultures were generally less responsive to Et-1; therefore, cultures used in this study were greater than 90% confluent. Furthermore, the addition of Et-1 only at initiation of the experiment resulted in a reduced effect. The results using the ELISA technique on culture supernatants showed that 24-hour samples expressed little increased IL-8 production compared with control levels (Fig 1). Each point represents quadruplicate samples; the SEM was usually less than 5%. The concentration of IL-8 in the culture supernatant of 48- and 72-hour cultures increased twofold and threefold, respectively. To determine whether IL-8 protein was synthesized before 48 hours, the kinetics of IL-8 production were analyzed on a single cell level. CNS-ECs were treated with Et-1 for 6, 24, and 48 hours and then examined using immunocytochemistry. Results in Fig2A demonstrate that as early as 6 hours, 20% of the cells were IL-8–positive. After 24 hours, 50% to 70% of the cells stained for IL-8 (Fig 2C). The 48-hour cell preparations appeared similar to the 24-hour cultures (data not shown). Control untreated cultures exhibited less than 3% positivity (Fig 2B and D). Irrelevant isotype-matched monoclonal antibody did not show significant staining. To determine the optimal concentration of Et-1, a range of Et-1 concentrations were examined. Results in Fig3 show that at 10−8 to 10−6 mol/L Et-1, there was a significant increase of IL-8 production compared with controls; at Et-1 concentrations above 10−6 mol/L, cell morphology was altered, and adhesion decreased while IL-8 levels remained constant (data not shown). Based on these results, all experiments presented here used 10−7 mol/L Et-1 unless otherwise stated. To confirm that the observed twofold to threefold increase in IL-8 production with Et-1 stimulation is consistent and reproducible, 11 independent experiments were performed comparing the supernatants from control and Et-1–treated cells. Results in Fig 3B demonstrate that Et-1 significantly (P < .001) increased IL-8 production in CNS-ECs.
Kinetics of IL-8 production by Et-1–stimulated CNS-ECs. Confluent CNS-ECs were cultured in the absence (□) or presence (▵) of Et-1 (10−7 mol/L). At 24-, 48-, and 72-hour intervals, supernatants were examined for IL-8 production using the ELISA technique. Data are the mean ± SEM of quadruplicate samples. This is one of three representative experiments.
Kinetics of IL-8 production by Et-1–stimulated CNS-ECs. Confluent CNS-ECs were cultured in the absence (□) or presence (▵) of Et-1 (10−7 mol/L). At 24-, 48-, and 72-hour intervals, supernatants were examined for IL-8 production using the ELISA technique. Data are the mean ± SEM of quadruplicate samples. This is one of three representative experiments.
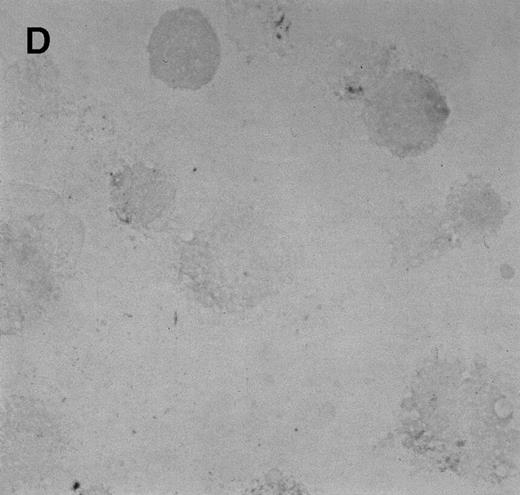
Immunocytochemistry of Et-1–treated CNS-ECs. Cell preparations of treated and untreated cultures were stained with monoclonal anti-human IL-8 after 6 hours (A, treated; B, control) or 24 hours (C, treated; D, control). Arrows indicate positive cells (original magnification × 400).
Immunocytochemistry of Et-1–treated CNS-ECs. Cell preparations of treated and untreated cultures were stained with monoclonal anti-human IL-8 after 6 hours (A, treated; B, control) or 24 hours (C, treated; D, control). Arrows indicate positive cells (original magnification × 400).
(A) Concentration-dependent increase in IL-8 production stimulated with Et-1. Confluent CNS-ECs were exposed to different concentrations of Et-1, as well as media alone. After 72 hours, supernatant samples were analyzed for IL-8 production using the IL-8 ELISA technique. Data are the mean ± SEM of triplicate samples. The first point is Et-1 at zero concentration. The results represent one of three experiments performed. (B) Eleven experiments using Et-1 (100 nmol/L)-treated and untreated cell (72 hours) supernatants were analyzed for IL-8 production. Results for the two groups are significantly different (P < .001).
(A) Concentration-dependent increase in IL-8 production stimulated with Et-1. Confluent CNS-ECs were exposed to different concentrations of Et-1, as well as media alone. After 72 hours, supernatant samples were analyzed for IL-8 production using the IL-8 ELISA technique. Data are the mean ± SEM of triplicate samples. The first point is Et-1 at zero concentration. The results represent one of three experiments performed. (B) Eleven experiments using Et-1 (100 nmol/L)-treated and untreated cell (72 hours) supernatants were analyzed for IL-8 production. Results for the two groups are significantly different (P < .001).
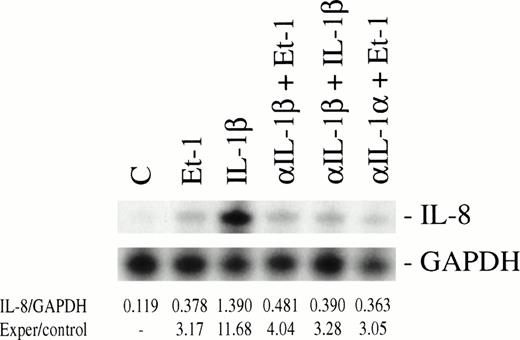
To determine whether Et-1 regulates IL-8 at the level of mRNA, the multiple RPA was performed, wherein IL-8 and GAPDH gene were present in the same template set. CNS-EC cultures were exposed to Et-1 for 0.5, 1, and 4 hours. The results showed that Et-1 induced IL-8 mRNA within 0.5 hours (52% increase), with the maximal level of transcription at 1 hour (2.4-fold increase) followed by a decrease in IL-8 mRNA at 4 hours compared with control levels (Fig 4). GAPDH levels did not appear to change with activation and therefore were used to monitor the amount of RNA applied to the gel as a standard. Because IL-1β has been shown to increase IL-8 production31 and endothelial cells produce IL-1β,32 we performed experiments to test whether Et-1 induced IL-8 production directly or via IL-1β production. In Fig 5, CNS-ECs were stimulated with Et-1 in the presence of anti–IL-1β, and then IL-8 mRNA was analyzed. The results showed that anti-IL-1β does not affect Et-1–induced IL-8 mRNA. The effect of IL-1α was also evaluated using anti–IL-1α antibody; the data demonstrate that anti-IL-1α antibody did not block Et-1–mediated IL-8 production (Fig5). As a control, anti–IL-1β antibody significantly blocked IL-1β–induced IL-8 mRNA. These data show that Et-1–induced IL-8 production was not mediated through IL-1β or IL-1α.
Kinetics of IL-8 mRNA expression with Et-1 treatment by RPA. Confluent CNS-EC cultures were treated with Et-1 (10−7 mol/L) for 0.5, 1, or 4 hours or left untreated. Subsequently, total RNA was isolated from the cells and probed with32P-labeled riboprobes for IL-8 or GAPDH. The results were visualized by autoradiography. The protected size corresponding to IL-8 (181 bp) and GAPDH (96 bp) is shown.
Kinetics of IL-8 mRNA expression with Et-1 treatment by RPA. Confluent CNS-EC cultures were treated with Et-1 (10−7 mol/L) for 0.5, 1, or 4 hours or left untreated. Subsequently, total RNA was isolated from the cells and probed with32P-labeled riboprobes for IL-8 or GAPDH. The results were visualized by autoradiography. The protected size corresponding to IL-8 (181 bp) and GAPDH (96 bp) is shown.
Et-1–induced IL-8 mRNA expression is independent of IL-1β mRNA synthesis. Confluent CNS-EC cultures were treated with either Et-1 (10−7 mol/L) or IL-1β (10 pg/mL) in the absence or presence of anti-IL-1β (200 μg/mL) or anti-IL-1 (200 μg/mL) for 1 hour. Control cultures included untreated CNS-ECs and cultures exposed only to anti–IL-1β. Total RNA was isolated from the different treatment groups and probed with 32P-labeled IL-8 or GAPDH riboprobes. Specific bands for IL-8 and GAPDH were visualized using autoradiography. The bands were identified by their appropriate size (IL-8, 181 bp; GAPDH, 96 bp). Results are calculated as the ratio of the spectrophotometric density measurement of the IL-8 band divided by the corresponding GAPDH band, followed by a comparison of experimental group and control values.
Et-1–induced IL-8 mRNA expression is independent of IL-1β mRNA synthesis. Confluent CNS-EC cultures were treated with either Et-1 (10−7 mol/L) or IL-1β (10 pg/mL) in the absence or presence of anti-IL-1β (200 μg/mL) or anti-IL-1 (200 μg/mL) for 1 hour. Control cultures included untreated CNS-ECs and cultures exposed only to anti–IL-1β. Total RNA was isolated from the different treatment groups and probed with 32P-labeled IL-8 or GAPDH riboprobes. Specific bands for IL-8 and GAPDH were visualized using autoradiography. The bands were identified by their appropriate size (IL-8, 181 bp; GAPDH, 96 bp). Results are calculated as the ratio of the spectrophotometric density measurement of the IL-8 band divided by the corresponding GAPDH band, followed by a comparison of experimental group and control values.
Cytokines modulate Et-1–induced IL-8 production.
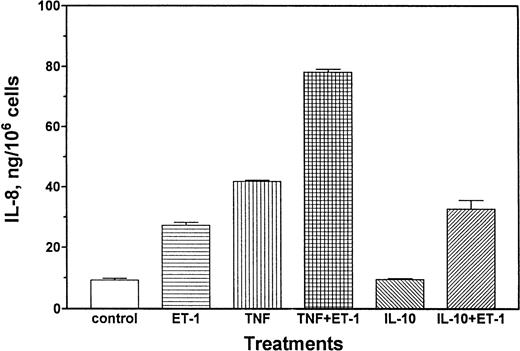
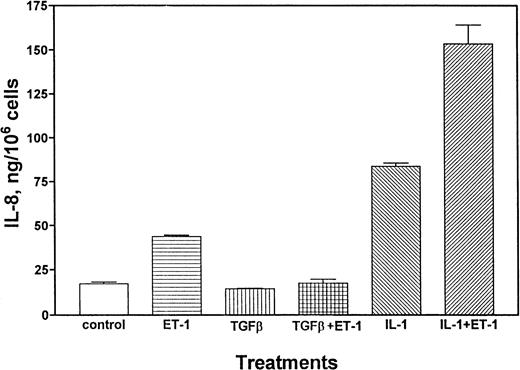
A series of experiments were performed to determine whether Et-1–induced production of IL-8 at the protein level can be modulated by proinflammatory or antiinflammatory cytokines. Results in Fig6 demonstrate that either Et-1 treatment alone or TNF (10 pg/mL) treatment alone increased IL-8 protein production (2.9-fold and 4.5-fold, respectively), and these reagents together had an additive (8.4-fold) effect. In contrast, IL-10 (10 ng/mL), an antiinflammatory cytokine known to downregulate immune function,33 did not affect Et-1–induced IL-8 protein production (Fig 6). IL-10 itself did not regulate IL-8 production. The effects of TGF-β on Et-1–induced IL-8 secretion were examined. TGF-β (10 ng/mL) did not affect IL-8 production by CNS-ECs, but abolished Et-1–induced IL-8 production (Fig7). IL-1β (1 pg/mL) was also a potent inducer of IL-8 (fivefold). IL-1β and Et-1 together had an additive effect on IL-8 production (Fig 7).
TNF modulates Et-1–induced IL-8 production. Confluent CNS-ECs were exposed to Et-1 (10−7 mol/L), TNF (10 pg/mL), or IL-10 (10 ng/mL) separately or in the combinations of TNF + Et-1 or IL-10 + Et-1. After 72 hours, the culture supernatant was examined for IL-8 production using the ELISA technique. Results are the mean ± SEM of triplicate cultures. The data represent one of three experiments.
TNF modulates Et-1–induced IL-8 production. Confluent CNS-ECs were exposed to Et-1 (10−7 mol/L), TNF (10 pg/mL), or IL-10 (10 ng/mL) separately or in the combinations of TNF + Et-1 or IL-10 + Et-1. After 72 hours, the culture supernatant was examined for IL-8 production using the ELISA technique. Results are the mean ± SEM of triplicate cultures. The data represent one of three experiments.
TGF-β and IL-1β regulate Et-1–induced IL-8 production. CNS-EC cultures were left untreated or treated with Et-1 (10−7 mol/L), TGF-β (10 ng/mL), or IL-1β (1 pg/mL) separately or in the combinations of TGF-β + Et-1 or IL-β + Et-1. After 72 hours of culture, the supernatants were analyzed for IL-8 production using the ELISA. Results are the mean ± SEM of triplicate cultures. This represents one of three experiments performed.
TGF-β and IL-1β regulate Et-1–induced IL-8 production. CNS-EC cultures were left untreated or treated with Et-1 (10−7 mol/L), TGF-β (10 ng/mL), or IL-1β (1 pg/mL) separately or in the combinations of TGF-β + Et-1 or IL-β + Et-1. After 72 hours of culture, the supernatants were analyzed for IL-8 production using the ELISA. Results are the mean ± SEM of triplicate cultures. This represents one of three experiments performed.
Et-1 produces a functional chemoattractant.
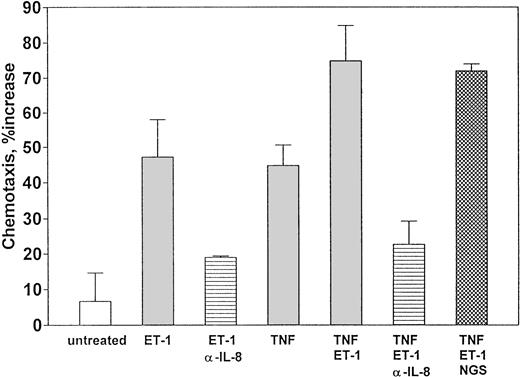
To determine whether Et-1 induced production of functional IL-8, CNS-ECs were treated with Et-1 as previously described. The supernatants from 72-hour cultures were added to the lower compartment as described in the methods. The results are expressed as the ratio of the number of cells migrating in the presence of culture supernatants from the different experimental groups to the number of cells present when medium alone was added. The results (Fig8) show that Et-1 increased PMN migration by sevenfold compared with untreated cell cultures. Supernatants from 24- and 48-hour cultures were less effective (data not shown). To determine whether the effect of Et-1 and TNF (1 ng/mL) together had a similar functional effect on PMNs, supernatants from cultures stimulated with both reagents were tested. The results show that supernatant from cultures exposed to Et-1 and TNF together induced greater migration (75%) compared with Et-1 (48%) or TNF (45%) alone. To determine whether IL-8 was responsible for this migration, polyclonal goat anti–IL-8 antibody (200 μg/mL) was added to the Et-1–induced supernatant for 1 hour before the migration assay was initiated. The results demonstrated that anti–IL-8 antibody blocked PMN migration by Et-1–treated culture supernatant by 60% compared with non–antibody-treated culture supernatants. Anti–IL-8 antibody also decreased the activity in Et-1 + TNF supernatants by 70% (Fig 8). Control goat antibody had no effect on cell migration using the different supernatants.
Et-1–treated CNS-ECs produce functional neutrophil chemotaxis factor. Confluent cultures of CNS-ECs were left untreated or treated with Et-1 (10−7 mol/L), TNF (1 ng/mL), or both Et-1 + TNF for 72 hours. Supernatants were then collected, and Et-1 or Et-1 + TNF supernatants were exposed to either goat anti–IL-8 antibody (200 μg/mL) or goat serum (200 μg/mL). The experimental supernatants were then placed in the lower compartment of the migration chamber. Freshly isolated PMNs were placed in the upper chamber. After 30 minutes, cells in the lower compartment were collected and counted using the trypan blue technique. The data are accumulated from 5 experiments and presented as the mean ± SEM. The data were calculated as the ratio of the number of cells migrating when exposed to experimental supernatants compared with media alone (media that had not been in contact with cells) times 100.
Et-1–treated CNS-ECs produce functional neutrophil chemotaxis factor. Confluent cultures of CNS-ECs were left untreated or treated with Et-1 (10−7 mol/L), TNF (1 ng/mL), or both Et-1 + TNF for 72 hours. Supernatants were then collected, and Et-1 or Et-1 + TNF supernatants were exposed to either goat anti–IL-8 antibody (200 μg/mL) or goat serum (200 μg/mL). The experimental supernatants were then placed in the lower compartment of the migration chamber. Freshly isolated PMNs were placed in the upper chamber. After 30 minutes, cells in the lower compartment were collected and counted using the trypan blue technique. The data are accumulated from 5 experiments and presented as the mean ± SEM. The data were calculated as the ratio of the number of cells migrating when exposed to experimental supernatants compared with media alone (media that had not been in contact with cells) times 100.
ET-1 does not affect ICAM-1 expression.
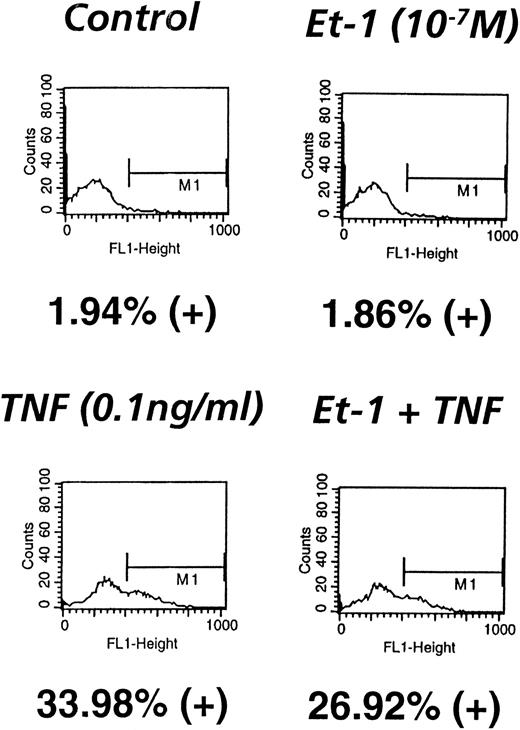
Because PMN binding to ECs is crucial in ischemic/reperfusion injury, the role of Et-1 in modulating ICAM-1 was examined. The results demonstrate that in one of three representative experiments, Et-1 did not modulate ICAM-1 expression after 18 hours of treatment (2% positive; Fig 9), whereas TNF (0.1 ng/mL) significantly upregulated this activity (34% positive). Et-1–treated CNS-EC cultures were also examined after 24, 28, and 72 hours, with no observed increase in ICAM-1 (data not shown). To determine whether Et-1 can modify TNF-induced expression of ICAM-1, both Et-1 and TNF together were used to treat CNS-ECs. The results show that TNF-induced ICAM-1 expression was not modified by Et-1 (27% positive; Fig 9). TNF at concentrations of 1 pg/mL to 1 ng/mL did not exhibit an additive effect with Et-1 (data not shown). These data show that Et-1 does not affect ICAM-1 expression and that endotoxin-like impurities are not responsible for the observed Et-1 activity.
ICAM-1 expression is not modulated by Et-1. CNS-EC cultures were exposed to Et-1 (10−7 mol/L) or TNF (0.1 ng/mL) or left untreated for 18 hours. The cells were subsequently resuspended and incubated with anti–ICAM-1 and FITC-conjugated anti-mouse antibody consecutively. Labeled cells were analyzed in a FACS sorter, and the number of FITC-positive cells was determined. Data are the number of positive cells divided by the total number of cells counted (5,000 cells per sample).
ICAM-1 expression is not modulated by Et-1. CNS-EC cultures were exposed to Et-1 (10−7 mol/L) or TNF (0.1 ng/mL) or left untreated for 18 hours. The cells were subsequently resuspended and incubated with anti–ICAM-1 and FITC-conjugated anti-mouse antibody consecutively. Labeled cells were analyzed in a FACS sorter, and the number of FITC-positive cells was determined. Data are the number of positive cells divided by the total number of cells counted (5,000 cells per sample).
DISCUSSION
Numerous studies have implicated neutrophils in the development of neuronal damage during and after ischemia in the brain.34-36 Therefore, it is important to understand by what mechanism PMNs are recruited to the damaged site in CNS tissue and to identify factors that regulate this activity. The present study has demonstrated that Et-1 stimulates CNS-ECs to produce IL-8, thereby linking hypertension with ischemia and reperfusion injury. The increase in protein production detected here is twofold to threefold at Et-1 concentrations of 10−9 to 10−7 mol/L, suggesting that low levels of Et-1 may have significant impact on the local microenvironment. Furthermore, these data demonstrate that increased Et-1 levels are likely associated with consistently higher basal levels of IL-8 at the tissue level.
Et-1 rapidly induced an increase in IL-8 mRNA within 30 minutes following stimulation of CNS-ECs, with a maximum value at 1 hour. By 4 hours, IL-8 mRNA was reduced to levels similar to the controls. This pattern of relatively short-lived mRNA may account for the need to continually restimulate the CNS-ECs with Et-1 every 24 hours for maximal IL-8 production. A similar kinetic pattern was observed in thrombin-induced IL-8 production in human umbilical vein endothelial cells.37 Based on immunocytochemistry, IL-8 protein was shown to be expressed within 6 hours of stimulation, with an increasing number of positive cells at 24 hours. The detection of IL-8 only after 48 hours using the ELISA is likely due to the lack of sensitivity of this technique at the high media volume to cell ratio in this culture system. The rapid increase in IL-8 production supports a physiologic role for this chemokine because in clinical observations PMN infiltration is already apparent at 4 hours postreperfusion, with monocytes detected at the ischemic site only after 18 to 24 hours postinjury. IL-8 production is also detected at the tissue level at this early time without being detected in the plasma.38
Endothelial cells are a source of IL-1β, and this cytokine has been shown to effectively upregulate IL-8 production.39,40 In the case of hypoxia, IL-1β mRNA is upregulated after 16 hours, late compared with IL-8, whereas other systems show IL-1β production early in the response.41 Our results support the notion that Et-1 acts directly on endothelial cells and IL-1β is not an intermediary. The present data show that anti-IL-1β antibody did not block Et-1–induced IL-8 mRNA transcription while blocking IL-1β–induced IL-8 mRNA synthesis.
We have found that Et-1 and IL-1β or TNF function in an additive manner. The additive effect of Et-1 with TNF or IL-1β on IL-8 production, particularly at low concentrations of either TNF or IL-1β (10 pg/mL), suggests these proinflammatory cytokines have significant impact on PMN recruitment. TNF, a cytokine produced by monocytes/macrophages and T cells during immune activation or infection, has been shown to upregulate IL-8 mRNA production within 1 hour, with maximum expression at 6 hours.27 IL-1β, produced by endothelial cells as well as leukocytes, also induces IL-8 production.32 During bacterial or viral infection, plasma levels of inflammatory cytokines increase, with pathology observed at high concentrations.42 The results presented here suggest that TNF and IL-1β at low concentrations causing relatively low levels of responsiveness can, in the presence of Et-1, produce significantly higher levels of IL-8, thereby increasing the possible disease progression.
Our results demonstrate that although Et-1 induced IL-8 production, it did not increase ICAM-1 expression on CNS-ECs. However, PMN damage in ischemic/reperfusion pathology involves both recruitment and adhesion of endothelial cells,17 with ICAM-1 being critical for PMN binding and subsequent transmigration into tissue.43Neutrophil activation is induced by the binding of CD11b/CD18 (MAC-1) integrins to ICAM-1, the ligand expressed on endothelial cells.17 IL-8 has been shown to upregulate PMN expression of the β2 integrins CD11b and CD11c.40 Thus, the first stage in CNS damage is PMN recruitment mediated by IL-8, and the second stage is PMN-EC adhesion mediated by upregulation of integrins on PMNs and upregulation of ICAM-1 adhesion molecules on endothelial cells. As we have shown here, Et-1 does not upregulate ICAM-1; therefore, further activation would likely require the presence of proinflammatory cytokines, particularly TNF or IL-1β. TNF and IL-1β are known to increase ICAM-1 expression on endothelial cells.44-46 Further interaction between IL-8 and TNF or IL-1β should occur during transmigration. The net effect of the simultaneous presence of Et-1 and TNF or IL-1β would be a significant enhancement of ischemia/reperfusion injury.
We have further investigated the role of Et-1 interaction with other regulatory cytokines by including TGF-β, which, in contrast to TNF or IL-1β, is often associated with downregulation of inflammatory processes.47-53 The in vivo relevance of TGF-β has been documented with its decline in experimental allergic encephalomyelitis49 and its upregulation in HIV infection.54 Both of these pathologic processes support the function of TGF-β as an immune suppressor in the CNS. Furthermore, there is a suggestion that TGF-β may be important in stroke, with evidence that serum levels of this growth factor are decreased in patients with ischemic stroke in the acute stage compared with age-matched normal controls.36 Our results show that TGF-β is a potent inhibitor of IL-8 production. Based on the results presented here, the mechanism by which TGF-β regulates IL-8 production in CNS-ECs may be, in part, at the level of transcription, but more likely at other processes. Thus, in ischemic stroke, TGF-β may function as an endogenous immune-suppressive agent and is therefore potentially useful therapeutically to inhibit IL-8 production by endothelial cells. In contrast to TGF-β, IL-10, also known to have immune-suppressive effects on endothelial cells, astrocytes, and lymphocytes,33,55 56 did not affect IL-8 production. These data emphasize the selective effect of TGF-β in downregulating IL-8 synthesis.
In conclusion, the present study demonstrates that CNS-ECs, upon stimulation with Et-1, produce functional IL-8. This Et-1–induced IL-8 synthesis can be upregulated by the proinflammatory cytokines TNF or IL-1β and downregulated by TGF-β. These data suggest that circulating Et-1, even at low levels, is likely a contributing factor to PMN recruitment. The presence of low levels of the inflammatory cytokines TNF and IL-1β together with Et-1 may play a significant role in the enhanced IL-8 production and subsequent neutrophil activation and eventual neuronal damage observed in ischemia/reperfusion injury.
ACKNOWLEDGMENT
The authors thank MoLi Chen and Donald Krasniak for expert technical assistance, and Myrna Cisneros for secretarial assistance.
Supported by National Institutes of Health Grant No. P01-NS31946.
Address reprint requests to F.M. Hofman, PhD, Department of Pathology, University of Southern California School of Medicine, 2011 Zonal Ave, HMR 312, Los Angeles, CA 90033.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal