Abstract
We have examined the cytotoxic effects of cyclic adenosine-3′,5′-monophosphate (cAMP) derivatives on multiple myeloma cells lines and determined that the 8-Chloro substituted derivative (8Cl-cAMP) is one of the most potent. We report here that 8Cl-cAMP is cytotoxic to both steroid sensitive and insensitive myeloma cells with a half maximal concentration of approximately 3 μmol/L. 8Cl-cAMP toxicity in myeloma cells is dependent on phosphodiesterase activity in the serum of cell culture medium. A metabolite of 8Cl-cAMP, 8-Chloro-adenosine (8Cl-AD), kills myeloma cells as effectively as 8Cl-cAMP. Adenosine deaminase (ADA) converts 8Cl-AD into 8Cl-inosine and abrogates the cytotoxic effects of 8Cl-cAMP, 8Cl-AMP, and 8Cl-AD, as does 5-(p-Nitrobenzyl)-6-Thio-Inosine (NBTI), an inhibitor of nucleoside uptake. These data suggest that 8Cl-cAMP must be converted to 8Cl-AD and that 8Cl-AD is the compound that enters the cell. Contrary to glucocorticoid-mediated cell death in myeloma cells, the pathway of 8Cl-AD–mediated cell death appears to be independent of interleukin-6 (IL-6) actions. Although the exact mode of action for this agent is currently unknown, its ability to kill steroid sensitive and insensitive multiple myeloma cells in an IL-6 independent fashion may offer exciting new therapeutic options.
© 1998 by The American Society of Hematology.
MULTIPLE MYELOMA IS a neoplasia of antibody-secreting plasma cells and represents approximately 10% of all hematologic malignancies. The median survival time of all myeloma patients is 2.5 to 3 years. This median survival time has not changed substantially in over 3 decades.1 Marrow ablation followed by bone marrow or stem cell infusion may produce a prolonged remission, but this approach has been limited to young patients and the potential for a cure is not proven.2 One of the best therapeutic options for multiple myeloma is treatment with glucocorticoids, however, some patients do not initially respond to glucocorticoids, and those that do will inevitably become resistant to such therapy. To investigate the mechanism of hormone resistance, we have previously isolated and characterized a multiple myeloma cell line, MM.1,3 which was further selected into glucocorticoid sensitive and glucocorticoid resistant subtypes. The MM.1S cell line contains a normal compliment of functional glucocorticoid receptors and is killed by submicromolar concentrations of the synthetic glucocorticoid dexamethasone, whereas the MM.1RL cell line has no measurable expression of glucocorticoid receptors and is highly resistant to dexamethasone.4 5
Membrane permeable cyclic adenosine-monophosphate derivatives have been shown to have a wide variety of effects in multiple cell types and may share some common distal events with the glucocorticoid receptor-mediated pathway. For example, selection of a WEHI thymoma cell line for dexamethasone resistance resulted in one clone that was also resistant to cyclic adenosine-3′,5′-monophosphate (cAMP)-induced cell death.6 To investigate the possibility that cAMP analogs could be cytotoxic to both steroid sensitive and resistant MM.1 cells, we examined the effect of dibutyryl-cAMP and found that it causes apoptosis in both the MM.1S and MM.1R sublines.7 Because the cytotoxic concentration of Bu2-cAMP in vitro is too high to be used clinically, several other cAMP analogs were tested.
The 8-Chloro derivative of cAMP (8Cl-cAMP) is a very potent, site-selective cAMP analog that is under intensive investigation as a potential chemotherapeutic agent. Phase I clinical studies have been performed against solid tumors,8 demonstrating that it can be given safely, with evidence for clinical response. The mechanism of action of 8Cl-cAMP has been the subject of some debate. One potential mode of action is through modulation of protein kinase A (PKA) activity. Another theoretical pathway requires the conversion of 8Cl-cAMP by serum enzymes to 8Cl-adenosine. Here we report that 8Cl-cAMP kills both steroid sensitive and insensitive multiple myeloma cells in vitro; that this killing is dependent on its conversion to 8Cl-AD; and that this phenomenon cannot be reversed by the addition of exogenous interleukin-6 (IL-6). This represents the first report of cytotoxicity in multiple myeloma cells mediated by 8Cl-cAMP, a therapeutic agent with activity against both glucocorticoid sensitive and resistant disease.
MATERIALS AND METHODS
Cell culture.
MM.1 cells were previously established from the peripheral blood of a multiple myeloma patient.3 These cells were cultured in RPMI-1640 (GIBCO BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (GIBCO BRL), 2 mmol/L glutamine, 100 U/mL of penicillin, 100 μg/mL streptomycin, and 2.5 μg/mL fungizone. Cells were grown in a 37°C incubator with 5% CO2.
Chemicals.
IL-6 was purchased from R & D Systems (Minneapolis, MN), 8-chloro-cAMP, 8-chloro-AMP, and 8-chloro-adenosine were purchased from BioLog (La Jolla, CA), and dibutyryl-cAMP, 5-(p-Nitrobenzyl)-6-Thio-Inosine (NBTI), ADA, 3-isobutyl-1-methylxanthine (IBMX), camptothecin, and dexamethasone were purchased from Sigma (St Louis, MO).
Cell proliferation assay.
A total of 25,000 cells per well was plated into 96-well dishes in a final volume of 0.1 mL RPMI-1640 growth media containing drugs as described in the figure legends. The plates were removed after 1, 3, and 5 days of incubation for assay. Cell proliferation was determined by the Cell Titer Aqueous assay of Promega (Madison, WI), which measures the conversion of a novel tetrazolium compound into formazan by mitochondrial dehydrogenase enzymes in live cells. The amount of formazan was measured spectophotometrically at 490 nm and in the range observed is linear with the number of live cells in the assay. Each data point is the average of four independent determinations, and error bars represent standard deviation. The data are expressed as a percentage of the formazan produced by cells treated with control medium in the same assay. Each assay is representative of a minimum of three independent experiments.
DNA isolation.
Cells were grown in 25 cm2 flasks with 5 μmol/L 8Cl-adenosine. The DNA was isolated as described by Gong et al.9 Briefly, the cells were treated for the indicated times, harvested, and counted by trypan blue exclusion. A total of 5 × 106 cells were collected and fixed with 70% ethanol at −20°C overnight. The ethanol was removed and the cells resuspended in 40 μL of phosphate-citrate buffer (0.192 mol/L Na2HPO4, 4 mmol/L citric acid, pH 7.8) and incubated at room temperature for 30 minutes. The cells were pelleted by centrifugation, the supernatant removed to a fresh tube, and concentrated to approximately 30 μL by 15 minutes of vacuum drying. In this fashion, only the low molecular weight DNA is recovered because the cells were permeablized and not lysed. The majority of the high molecular weight DNA remains associated with the cell pellet. The isolated DNA was treated with RNase (0.1 mg/mL) for 30 minutes at 37°C in the presence of 0.025% sodium dodecyl sulfate (SDS), followed by a 30-minute 37°C incubation with 0.1 mg/mL proteinase K. The isolated DNA was then fractionated on a 1% agarose gel containing ethidium bromide for 4 hours at 2 V/cm. One million HL-60 cells, treated with 0.15 μmol/L camptothecin for 3 hours, served as a positive control.9
Flow cytometric analysis.
A total of 5 × 106 cells were treated with the indicated concentration of drug for either 24 or 48 hours, then washed with cold phosphate-buffered saline (PBS) and fixed in 40% cold ethanol at 4°C overnight. Cells were then washed with PBS, resuspended in 50 μg/mL RNase A in PBS for 30 minutes at 37°C, and then resuspended in 15 μg/mL propidium iodide in 38 mmol/L sodium citrate buffer. Flow cytometry was performed on a FACsort instrument and data analyzed using the CellQuest software package (Becton Dickinson, Bedford, MA). Data are presented as an average of three independent determinations.
RESULTS
8Cl-cAMP and 8Cl-AD concentration dependence curves.
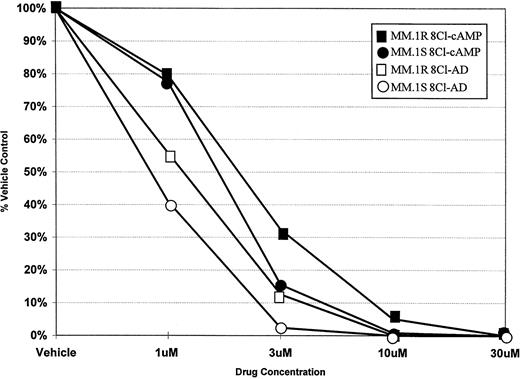
To determine the ability of 8Cl-cAMP to act as a cytotoxic agent in multiple myeloma cells, concentration dependence curves were generated for both 8Cl-cAMP and a potential metabolite of 8Cl-cAMP, 8Cl-AD (Fig 1). 8Cl-cAMP is effective against both MM.1S and MM.1R cell lines, with a concentration of 10 μmol/L causing a greater than 90% reduction in cell number. Interestingly, the 8Cl-cAMP metabolite, 8Cl-AD, is more potent than the parent compound. Both steroid sensitive and insensitive cell lines are growth inhibited, but the concentration required for 90% inhibition is approximately 3 μmol/L. MM.1R cells are only slightly less sensitive to both agents than their MM.1S counterparts. Additional myeloma cell lines were examined, including IM-9 and U266, and these also demonstrated similar dose-dependent decreases in proliferation upon treatment with either 8Cl-cAMP or 8Cl-AD (data not shown). The data presented are for 5 days of continuous treatment with these drugs; however, the antiproliferative effects are evident after shorter treatments and are readily apparent at 3 days.
Concentration dependence of 8Cl-cAMP- and 8Cl-AD–mediated growth inhibition in MM.1S and MM.1R cells. Cells are treated with the indicated concentration of drug for 5 days and then cell number is assayed and presented as described in Materials and Methods. MM.1S treated with 8Cl-cAMP (•); MM.1S treated with 8Cl-AD (○); MM.1R treated with 8Cl-cAMP (▪); MM.1R treated with 8Cl-AD (□).
Concentration dependence of 8Cl-cAMP- and 8Cl-AD–mediated growth inhibition in MM.1S and MM.1R cells. Cells are treated with the indicated concentration of drug for 5 days and then cell number is assayed and presented as described in Materials and Methods. MM.1S treated with 8Cl-cAMP (•); MM.1S treated with 8Cl-AD (○); MM.1R treated with 8Cl-cAMP (▪); MM.1R treated with 8Cl-AD (□).
Metabolic processing of 8Cl-cAMP.
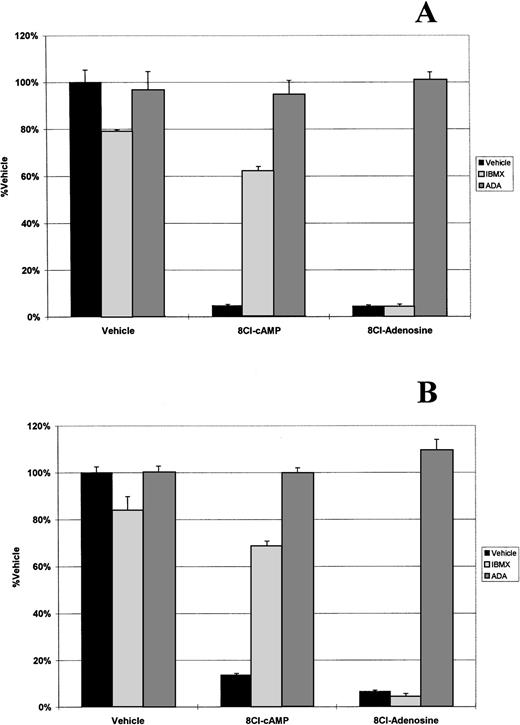
Because 8Cl-AD was more potent than 8Cl-cAMP and could be a metabolite of that drug, we analyzed the possibility that 8Cl-cAMP exerts its effects through an 8Cl-AD metabolite by examining key steps in the metabolic pathway. The first step examined was the activity of phosphodiesterase (PDE), which is present in serum and converts cAMP into AMP. IBMX is a potent inhibitor of PDE activity. When 8Cl-cAMP is given concurrently with 50 μmol/L IBMX to myeloma cells, the antiproliferative effect is greatly reduced (Fig 2). IBMX had no effect on the activity of 8Cl-AD. Therefore, extracellular PDE activity is required for activation of 8Cl-cAMP. This is further evidenced by the fact that 8Cl-cAMP activity in serum-free medium is greatly reduced (Fig 3).
8Cl-cAMP and 8Cl-AD activity in the presence of IBMX or ADA. MM.1S (A) or MM.1R (B) cells are treated with either control medium, medium containing 3 μmol/L 8Cl-cAMP, or medium containing 3 μmol/L 8Cl-AD for 5 days and cell number is assayed and presented as described in Materials and Methods. Where indicated, IBMX is added concurrently with treatment medium at a concentration of 50 μmol/L. Where indicated, ADA is added concurrently with treatment medium at a concentration of 2 U/mL.
8Cl-cAMP and 8Cl-AD activity in the presence of IBMX or ADA. MM.1S (A) or MM.1R (B) cells are treated with either control medium, medium containing 3 μmol/L 8Cl-cAMP, or medium containing 3 μmol/L 8Cl-AD for 5 days and cell number is assayed and presented as described in Materials and Methods. Where indicated, IBMX is added concurrently with treatment medium at a concentration of 50 μmol/L. Where indicated, ADA is added concurrently with treatment medium at a concentration of 2 U/mL.
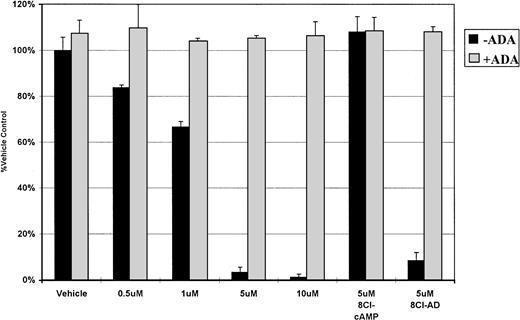
ADA inhibits 8Cl-AMP activity. MM.1S cells are treated with 8Cl-AMP for 5 days at the indicated concentrations in the presence (light bars) or absence (dark bars) of 2 U/mL of ADA. The assay is done in normal growth medium that does not contain serum. 8Cl-cAMP (5 μmol/L) and 8Cl-AD (5 μmol/L) are included as controls. Cell number is assayed and presented as described in Materials and Methods.
ADA inhibits 8Cl-AMP activity. MM.1S cells are treated with 8Cl-AMP for 5 days at the indicated concentrations in the presence (light bars) or absence (dark bars) of 2 U/mL of ADA. The assay is done in normal growth medium that does not contain serum. 8Cl-cAMP (5 μmol/L) and 8Cl-AD (5 μmol/L) are included as controls. Cell number is assayed and presented as described in Materials and Methods.
The activity of PDE on 8Cl-cAMP results in the formation of 8Cl-adenosine monophosphate, which is subsequently converted into 8Cl-AD by 5′-nucleotidase. The reverse reaction (8Cl-AD to 8Cl-AMP) can also occur. To investigate the possibility that 8Cl-AMP is the cytotoxic metabolite, we examined the ability of 8Cl-AMP to kill myeloma cells. Concentration dependence curves for this agent against MM.1 cells were generated and demonstrate that 8Cl-AMP inhibits myeloma cells in a dose-dependent fashion similar to 8Cl-cAMP (data not shown). We next addressed the possibility that 8Cl-AMP is active without further metabolic processing. To examine this, we treated cells concurrently with 8Cl-AMP and 2 U/mL ADA in serum-free conditions. Serum-free medium was used to eliminate the influence of serum nucleotidases (Fig 3). 8Cl-AD–mediated growth inhibition is greatly reduced by the addition of ADA, as is 8Cl-AMP–mediated growth (Figs 2and 3). This indicates that 8Cl-AMP must be converted to 8Cl-AD to exert its effect, as AMP is not a substrate for ADA. 8Cl-inosine, the product of ADA activity on 8Cl-AD, is inactive in this system. Furthermore, because the experiment was performed in serum-free conditions, the MM.1 cells must be producing 5′-nucleotidase. This is not unexpected, as other cells of B-cell lineage have been shown to express ecto-5′-nucleotidase (CD73), which has been implicated as having a role in mediating lymphocyte binding to endothelial and follicular dendritic cells.10 Because myeloma cells are antibody-producing plasma cells, it is reasonable to expect that they would also express this marker.
Taken together, these data strongly suggest that 8Cl-cAMP must be converted to 8Cl-AMP by serum phosphodiesterases, which is then converted to 8Cl-AD by removal of the phosphate. 8Cl-AD is the ultimate extracellular metabolite, as further metabolic products are not active.
8Cl-AD entry into the cell is required for activity.
NBTI, an inhibitor of nucleoside uptake,11 12 was used to determine if the drug must enter into the cell to exert its cytotoxic effect. In the presence of this inhibitor, the activity of both 8Cl-cAMP and 8Cl-AD were greatly reduced (Fig 4), indicating that nucleoside uptake is required for cytotoxic activity. Unsubstituted adenosine is not cytotoxic to MM.1 cell lines in concentrations up to 1 mmol/L (data not shown). It is expected that adenosine would be able to activate surface adenosine receptors, thereby activating adenylate cyclase and producing intracellular cAMP. However, because adenosine has no effect on MM.1 cells, we believe that such signaling is not involved in 8Cl-AD–mediated toxicity. Together, these observations would indicate that signaling through surface adenosine receptors is not the mechanism by which 8Cl-AD acts, and that the drug must enter the cell to be effective. Its fate after it enters the cell is as yet undetermined.
NBTI inhibits 8Cl-cAMP and 8Cl-AD activity. MM.1S cells are treated with 8Cl-cAMP for 5 days at the indicated concentrations in the presence (light bars) or absence (dark bars) of 50 μmol/L NBTI. MM.1S cells treated with 3 μmol/L 8Cl-AD are also included. Cell number is assayed and presented as described in Materials and Methods.
NBTI inhibits 8Cl-cAMP and 8Cl-AD activity. MM.1S cells are treated with 8Cl-cAMP for 5 days at the indicated concentrations in the presence (light bars) or absence (dark bars) of 50 μmol/L NBTI. MM.1S cells treated with 3 μmol/L 8Cl-AD are also included. Cell number is assayed and presented as described in Materials and Methods.
IL-6 does not inhibit 8Cl-cAMP or 8Cl-AD.
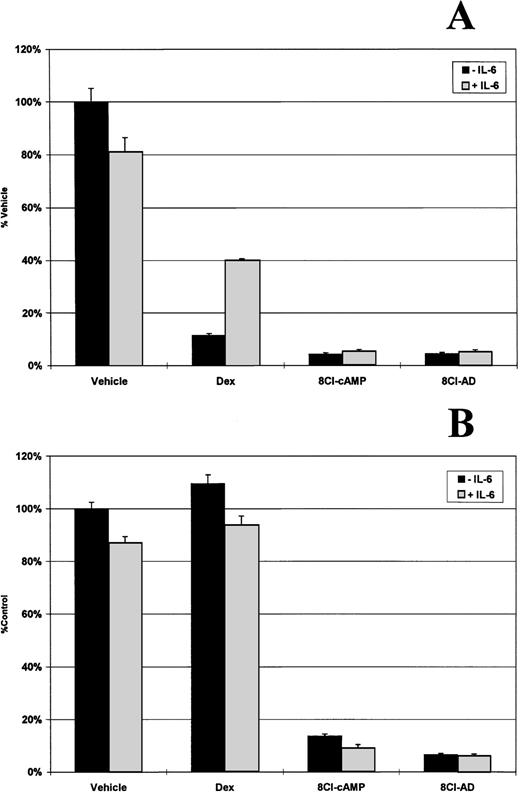
IL-6 is a potent growth factor for multiple myeloma, and it has been reported that glucocorticoids exert a large part of their antiproliferative effect through downregulation of both the cytokine and its receptor.13 Adding exogenous IL-6 to dexamethasone-treated MM.1S cells can partially inhibit glucocorticoid-induced cell death (Fig 5A). Dexamethasone treatment reduces cell number by approximately 90%, but in the presence of 500 pg/mL IL-6, there is only a 60% decrease . MM.1R cells are not affected by dexamethasone treatment (Fig 5B). In contrast, cells treated with 3 μmol/L 8Cl-cAMP or 8Cl-AD show a greater than 90% reduction in cell number that is not rescued by the addition of exogenous IL-6, indicating that it is unlikely that downregulation of this cytokine is the mechanism of action of 8Cl-AD–mediated cytotoxicity.
IL-6 does not inhibit 8Cl-cAMP or 8Cl-AD activity. MM.1S (A) and MM.1R (B) cells are treated for 5 days with control medium only, dexamethasone (1 μmol/L), 3 μmol/L 8Cl-cAMP, or 3 μmol/L 8Cl-AD in the presence (light bars) or absence (dark bars) of 500 pg/mL IL-6. Cell number is assayed and presented as described in Materials and Methods.
IL-6 does not inhibit 8Cl-cAMP or 8Cl-AD activity. MM.1S (A) and MM.1R (B) cells are treated for 5 days with control medium only, dexamethasone (1 μmol/L), 3 μmol/L 8Cl-cAMP, or 3 μmol/L 8Cl-AD in the presence (light bars) or absence (dark bars) of 500 pg/mL IL-6. Cell number is assayed and presented as described in Materials and Methods.
8Cl-AD causes apoptosis in MM.1S and MM.1R cell lines.
To determine if the antiproliferative effects of 8Cl-AD were due to its ability to induce apoptosis, DNA was isolated from MM.1S and MM.1R cells after treatment with 8Cl-AD and examined for the appearance of a DNA chromatin “ladder pattern” associated with apoptotic cell death.14 A very faint ladder is detected after 16 hours of treatment, which increases in intensity with increasing duration of drug incubation. A very intense ladder pattern is seen after 48 hours of treatment (Fig 6). The viability of these cells (assayed by trypan blue exclusion) decreases in parallel with DNA ladder formation (data not shown). While chromatin ladder formation is not the sole defining characteristic of apoptosis,15 it is a good marker for this process and certainly indicates that 8Cl-AD is acting as a cytotoxic, not a cytostatic, agent.
8Cl-AD induces chromatin ladder formation. Five million MM.1S cells are treated for the indicated times with 3 μmol/L 8Cl-AD and low molecular weight DNA is obtained as described in Materials and Methods. Markers are the 123-bp DNA ladder (GIBCO BRL). DNA is fractionated through 1% agarose. HL-60 cells treated with 0.15 μmol/L camptothecin serve as a positive control for the DNA isolation protocol.
8Cl-AD induces chromatin ladder formation. Five million MM.1S cells are treated for the indicated times with 3 μmol/L 8Cl-AD and low molecular weight DNA is obtained as described in Materials and Methods. Markers are the 123-bp DNA ladder (GIBCO BRL). DNA is fractionated through 1% agarose. HL-60 cells treated with 0.15 μmol/L camptothecin serve as a positive control for the DNA isolation protocol.
To further confirm that 8Cl-AD is acting through an apoptotic mechanism, flow cytometric analysis was performed with propidium iodide (Table 1). Apoptotic cells were defined as those with subdiploid DNA content and are presented as the percentage of total counted cells in the assay. Dexamethasone, used as a positive control for apoptosis, shows a dramatic increase in apoptotic cells by 24 hours. The apparent decrease in apoptotic cells by 48 hours of dexamethasone treatment may be due to the fact that most of the cells have already completed the apoptotic program. After 24 hours of 8Cl-AD treatment, approximately 30% of the cells are apoptotic, as compared with approximately 5% in the control. As further evidence that 8Cl-cAMP needs metabolic activation, only 13% of cells are apoptotic when treated with this drug for 24 hours, yet after 48 hours, the activity 8Cl-cAMP approaches that of 8Cl-AD at 24 hours. Also, 1 U/mL of ADA completely abrogates the affect of 8Cl-AD at both 24 and 48 hours, further demonstrating that this is indeed the active metabolite. Interestingly, ADA does not completely inhibit the activity of 8Cl-cAMP at 48 hours, which may indicate that there is a small proportion of cell death mediated by the native 8Cl-cAMP molecule.
Percentage of Apoptotic Cells as a Function of Drug Treatment and Time
| . | Vehicle . | DEX . | 8Cl-AD . | 8Cl-AD + ADA . | 8Cl-cAMP . | 8Cl-cAMP + ADA . | ADA . |
|---|---|---|---|---|---|---|---|
| 24 hours | 5.26 ± 0.22 | 39.81 ± 1.67 | 30.23 ± 0.34 | 6.25 ± 0.15 | 12.93 ± 1.26 | 10.9 ± 1.09 | 5.02 ± 0.48 |
| 48 hours | 6.77 ± 0.24 | 17.38 ± 1.89 | 30.12 ± 0.37 | 9.45 ± 0.66 | 28.12 ± 1.02 | 16.2 ± 0.03 | 7.74 ± 0.36 |
| . | Vehicle . | DEX . | 8Cl-AD . | 8Cl-AD + ADA . | 8Cl-cAMP . | 8Cl-cAMP + ADA . | ADA . |
|---|---|---|---|---|---|---|---|
| 24 hours | 5.26 ± 0.22 | 39.81 ± 1.67 | 30.23 ± 0.34 | 6.25 ± 0.15 | 12.93 ± 1.26 | 10.9 ± 1.09 | 5.02 ± 0.48 |
| 48 hours | 6.77 ± 0.24 | 17.38 ± 1.89 | 30.12 ± 0.37 | 9.45 ± 0.66 | 28.12 ± 1.02 | 16.2 ± 0.03 | 7.74 ± 0.36 |
MM.1S cells were treated with either 8Cl-cAMP (5 μmol/L) or 8Cl-AD (5 μmol/L) for the indicated times and then assayed using a flow cytometric assay for apoptosis (see Materials and Methods). Dexamethasone (1 μmol/L) was included as a positive control. Where indicated, cells were treated in the presence of 1 U/mL ADA. Data are presented as the average of three independent assays with standard deviation.
DISCUSSION
8Cl-cAMP–mediated cytotoxicity in multiple myeloma cell lines is dependent on its extracellular conversion to 8Cl-AD. This conversion requires the activity of serum PDE and 5′-nucleotidase. If PDE is inhibited, or if the conversion of 8Cl-AD to 8Cl-inosine is enhanced, 8Cl-cAMP is no longer effective, indicating that 8Cl-AD is the active agent. This finding was surprising, as we have previously demonstrated that agents, which are known to signal through PKA, including dibutyryl cAMP and forskolin, can be effective at inducing cell death in a dose-dependent fashion.7 The inability of ADA to completely inhibit 8Cl-cAMP–induced apoptosis, as demonstrated in the flow cytometric assay, may be due to its ability to mediate a small portion of its activity through PKA. This is consistent with our previous observations.7 However, the predominant cytolytic actions of 8Cl-cAMP are through the 8Cl-AD metabolite. This is especially apparent over longer time periods, as demonstrated by the proliferation assay data. 8Cl-AD is not causing cell death by stimulating surface adenosine receptors, thereby causing activation of adenylate cyclase and subsequent production of cAMP, as adenosine is unable to achieve the same effect. Furthermore, a nucleoside uptake inhibitor blocks 8Cl-AD action, indicating that the agent must be taken up into the cell for activity.
8Cl-cAMP inhibits the growth of several cell types in vivo, possibly due to its ability to influence the ratio of PKA isozymes in the cell.16,17 Two basic isoforms of the regulatory subunit have been identified (RI and RII), leading to formation of two types of the PKA holoenzyme, PKAI and PKAII. 8Cl-cAMP binds with high efficiency to one of the two sites of the RII type subunit, which is insufficient to cause release of the catalytic subunits. However, 8Cl-cAMP binds with similar efficiency to both sites of the RI subunit, leading to the release of the catalytic subunit and subsequent downregulation of the PKAI holoenzyme. The ratio of PKAI to PKAII has been shown to correlate with the differentiation state of several cell types. When PKAI is high in relation to PKAII, the cells tend to be in a proliferative state, while increased PKAII levels is associated with a differentiated or growth arrested state (reviewed in Cho-Chung18). Consequently, the selective degradation of PKAI by 8Cl-cAMP could be responsible for the toxicity of this agent.
8Cl-cAMP has also been reported to act through conversion to its 8Cl-AD metabolite in several systems, including mouse lung epithelial cell lines,19 human glioma,17 human mammary carcinoma,20 and human colon tumor cell lines.21 The exact mechanism of 8Cl-AD–mediated cell death is not well understood at this time. It is possible that 8Cl-AD inhibits DNA or RNA polymerase directly. It has been shown in human glioma cell lines that both 8Cl-cAMP and 8Cl-AD partially inhibit DNA and RNA synthesis, possibly through incorporation into these macromolecules.22 However, Lange-Carter et al19demonstrate that while 8Cl-cAMP conversion to 8Cl-AD is required for effectiveness, 8Cl-AD treatment results in a decrease of the levels of the RI subunit of PKA. It is possible that while the drug must be converted to the adenosine metabolite for entry into the cell, it may be reconverted into 8Cl-cAMP after entry by the cellular machinery. We are currently investigating all of these potential mechanisms of action in multiple myeloma cells.
A potential benefit to using 8Cl-cAMP (or 8Cl-AD) as an agent against multiple myeloma is its ability to act independently of IL-6. Hardin et al13 have shown that glucocorticoids can downregulate both this cytokine and its receptor in myeloma cells. Furthermore, glucocorticoid cytotoxicity to myeloma cells in vitro can be inhibited by addition of exogenous IL-6.13 A possible mechanism for this effect is through modulation of NF-κB activity. Glucocorticoids have been shown to regulate the production of the inhibitor of NF-κB, IκBα, thus inhibiting the ability of NF-κB to translocate to the nucleus and stimulate the production of cytokines, such as IL-6.23,24 IL-6 is abundant in the tumor millieu and is overproduced in approximately one third of multiple myeloma patients. This overproduction is correlated with a poor prognosis.25We have demonstrated that addition of exogenous IL-6 cannot inhibit the cytotoxic activity of 8Cl-cAMP or 8Cl-AD. The ability of 8Cl-cAMP to kill myeloma cells in an IL-6 independent fashion may have significant therapeutic implications.
The requirement for 8Cl-AD production may also have clinical significance. Relative to fetal bovine serum, human serum is low in PDE activity, thus the effectiveness of 8Cl-cAMP as a chemotherapeutic agent may be variable dependent on the PDE activity of the individual patient. 8Cl-cAMP added to tissue culture medium containing 20% human serum was unable to promote killing of myeloma cells, whereas 8Cl-AD remained effective (data not shown). Consequently 8Cl-cAMP, as a prodrug, may not be converted to the active metabolite in sufficient concentrations to be maximally effective in vivo. Studies of the pharmacokinetics of 8Cl-cAMP in both human breast cancer patients and nude mice bearing colon tumor xenografts indicate that the 8Cl-AD metabolite, while in low concentration in the plasma, was in a significantly higher concentration in tumor biopsies.26Therefore, while the effectiveness of 8Cl-cAMP may be dependent on PDE activity in the serum or tumor cell density, administration of 8Cl-AD directly can circumvent this problem and may enhance the efficacy of this therapeutic approach. We are currently pursuing the development of 8Cl-AD as a therapeutic alternative.
Supported by the Northwestern Memorial Foundation to N.L.K., by Grant No. CA 60061 to S.T.R. and N.L.K., and Grant No. P30 CA60553 to the Robert H. Lurie Cancer Center from the National Cancer Institute.
Address reprint requests to Robert G. Halgren, Northwestern University Medical Center, Lurie Comprehensive Cancer Center, 8-340 Olson Pavilion, 303 E Chicago Ave, Chicago, IL 60611; e-mail: r-halgren@nwu.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal