Abstract
Recently, a distinctive entity characterized by expression of the anaplastic lymphoma kinase (ALK) protein [most frequently due to the t(2;5)(p23;q35)-associated NPM-ALK fusion] has emerged within the heterogenous group of non-Hodgkin’s lymphomas (NHL) classified as anaplastic large-cell lymphoma (ALCL). Sporadic variant 2p23/ALK abnormalities identified in ALK-positive ALCL indicate that genes other than NPM may also be involved in the deregulation of ALK and lymphomagenesis. We report here three cases with an inv(2)(p23q35) detected by fluorescence in situ hybridization (FISH) in young male patients with ALK-positive ALCL. In contrast to ALCL cases with the classical t(2;5)(p23;q35) that usually show both cytoplasmic and nuclear or predominantly nuclear alone localization of the NPM-ALK chimeric product, in all three cases with an inv(2)(p23q35) the ALK protein accumulated in the cytoplasm only, supporting the previous assumption that the oncogenic potential of ALK may not be dependent on its nuclear localization. As the first step to identify theALK partner gene involved in the inv(2)(p23q35), we performed extensive FISH studies and demonstrated that the 2q35 breakpoint occurred within the 1,750-kb region contained within the 914E7 YAC. Moreover, a striking association of the inv(2)(p23q35) with a secondary chromosomal change, viz, ider(2)(q10)inv(2)(p23q35), carrying two additional copies of the putative ALK-related fusion gene, was found in all three patients, suggesting that, in contrast to the standard t(2;5)/NPM-ALK fusion, multiple copies of the putative 2q35-ALK chimeric gene may be required for efficient tumor development. In summary, we demonstrate that the inv(2)(p23q35), a variant of the t(2;5)(p23;q35), is a recurrent chromosomal abnormality in ALK-positive ALCL, the further characterization of which should provide new insight into the pathogenesis of these lymphomas.
© 1998 by The American Society of Hematology.
ANAPLASTIC LARGE-CELL lymphoma (ALCL), recognized as a distinctive subtype of non-Hodgkin’s lymphoma (NHL) in the recent REAL classification,1 comprises lymphomas characterized by large pleomorphic tumor cells, expression of CD30/Ki-1 antigen, a member of the TNF-receptor family,2 and a T or null immunophenotype. This particular subgroup appears to have a better overall survival than other large-cell lymphomas and occurs predominantly in children and adolescent patients.3-5 Until now, the only recurrent chromosomal abnormality identified in ALCL was the t(2;5)(p23;q35). As demonstrated by Morris et al,6 this translocation leads to the fusion of the nucleophosmin gene (NPM) on chromosome 5q35 to the 2p23 gene encoding the novel receptor tyrosine kinase ALK, which is highly related to leukocyte tyrosine kinase (LTK) and normally expressed only in the nervous system.7,8 The t(2;5)(p23;q35) has been observed in approximately 30% to 50% of ALCL cases documented by cytogenetics9 and in between 12% and 80% of ALCL cases analyzed by reverse transcription-polymerase chain reaction (RT-PCR) (reviewed in Sarris et al10). Immunostaining using polyclonal and monoclonal antibodies directed against the cytoplasmic portion of the ALK protein have proved to be a very efficient approach to identify ALCL with 2p23/ALK rearrangements.11-17Anti-ALK immunostaining combined with cytogenetics, fluorescence in situ hybridization (FISH), and/or molecular studies showed that the ALK gene can also be deregulated by mechanisms other than the t(2;5), such as t(1;2)(q25;p23),13 cryptic insertion of the ALKgene in the vicinity of NPM in a t(2;5)-negative condition,14 and inv(2)(p23q35).14 The latter abnormality, which is not recognizable by classical cytogenetics, was detected by us in 1 of 13 ALK-positive ALCL cases analyzed at random by two-color FISH with ALK and 5q35-specific probes. Our studies reported here demonstrate that the inv(2)(p23q35) results in the translocation of a portion of the ALK gene locus from 2p23 to 2q35. Moreover, we detected two additional copies of the rearrangedALK gene on the i(2)(q10) chromosome in all analyzed cells from this patient. By analogy with the t(2;5)/NPM-ALK, the 2q35 region presumably contains an as-yet-unidentified gene that, likeNPM, can deregulate ALK and be involved in the pathogenesis of ALCL. We have collected now three such cases, indicating that the inv(2)(p23q35) is a recurrent abnormality in ALK-positive ALCL, and have studied them with FISH to better characterize the 2q35 breakpoint.
MATERIALS AND METHODS
Patients.
Tumor samples from the patients were referred to the Centre for Human Genetics and Department of Pathology for analysis from the Department of Hematology, University of Leuven (Leuven, Belgium). The main clinical and hematological findings of the reported cases are summarized in Table 1.
Clinical Data
| Case No. . | Sex/ Age . | Time of Diagnosis . | Organ Involvement/ Stage-150 . | Hb (g/dL) . | WBC (×109/L) . | WBC Differential . | Plt (×109/L) . | LDH-151 (U/L) . | Treatment . | Present Status . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/37 | November 1993 | Axillary lymph nodes/IB | 14.5 | 6.5 | Normal | 233 | 265 | RT (40 Gy) | CR (May 1998) |
| 2 | M/28 | November 1997 | Cervical, supraclavicular, mediastinal, abdominal and inguinal lymph nodes, spleen, liver/IIIB | 11.3 | 7.7 | Normal | 356 | 269 | CHOP | LFU |
| 3 | M/23 | December 1997 | Axillary, mediastinal lymph nodes/II | 14.2 | 6.4 | Normal | 348 | 275 | CHOP | CR (May 1998) |
| Case No. . | Sex/ Age . | Time of Diagnosis . | Organ Involvement/ Stage-150 . | Hb (g/dL) . | WBC (×109/L) . | WBC Differential . | Plt (×109/L) . | LDH-151 (U/L) . | Treatment . | Present Status . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/37 | November 1993 | Axillary lymph nodes/IB | 14.5 | 6.5 | Normal | 233 | 265 | RT (40 Gy) | CR (May 1998) |
| 2 | M/28 | November 1997 | Cervical, supraclavicular, mediastinal, abdominal and inguinal lymph nodes, spleen, liver/IIIB | 11.3 | 7.7 | Normal | 356 | 269 | CHOP | LFU |
| 3 | M/23 | December 1997 | Axillary, mediastinal lymph nodes/II | 14.2 | 6.4 | Normal | 348 | 275 | CHOP | CR (May 1998) |
Abbreviations: RT, radiotherapy; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; CR, complete remission; LFU, lost to follow-up; WBC, white blood cell count; Plt, platelets; LDH, lactate dehydrogenase.
Clinical stage according to the Ann Arbor classification.
LDH normal range (240 to 480 U/L).
Histopathology and phenotyping.
A portion of the biopsy material from each of the three cases was snap-frozen and stored at −80°C until use. The remaining material was fixed in buffered formalin and/or B5 and embedded in paraffin. Immunophenotyping was performed on paraffin-embedded and/or frozen material with a panel of monoclonal antibodies to CD2 (OKT11), CD3 (Leu4), CD4 (Leu3a/OKT4), CD5 (Leu1), CD7 (Leu9), CD8 (OKT8), CD19 (Leu12), CD20 (L26), CD22 (Leu14), and CD30 (BerH2) using a streptavidin-biotin-peroxidase three-stage technique. Immunohistological detection of ALK protein was performed on both frozen and paraffin sections. The latter were subjected to microwaving (750 W for 3 cycles of 5′ each) using 1 mmol/L EDTA buffer, pH 8.0,18,19 as the antigen retrieval solution. After microwave heating, the sections were allowed to cool at room temperature for approximately 20 minutes and then washed in Tris-buffered saline and stained by the immunoalkaline phosphatase technique, as previously described.20 Briefly, sections were incubated with ALK113 and ALKc monoclonal antibodies17 (as undiluted supernatant), followed by rabbit antimouse Ig (Dako, Glostrup, Denmark) and APAAP complexes. To maximize the sensitivity of the method, the incubations with rabbit antimouse Ig and APAAP complexes were repeated once. All antibody steps were for 30 minutes with intervening 5-minute washes in 0.05 mol/L Tris-buffered saline, pH 7.6. Endogenous alkaline phosphatase was blocked with 1 mmol/L levamisole. Slides were then counterstained for 5 minutes in Gill’s hematoxylin and mounted in Kaiser’s glycerol gelatin (Merck, Darmstadt, Germany).
Cytogenetics.
One-day cultures of lymphoma cells were used for cytogenetic analysis in all cases. Ten to 24 G-banded karyotypes were analyzed and classified according to the International System for Human Cytogenetic Nomenclature.21
FISH analysis.
FISH was performed as previously described.22 Probes applied in FISH experiments are listed in Table2. The ALK and NPM loci were investigated using an ALK P1 clone (designatedALK-DMPC-HFF#1-1111H1) and three cosmid clones (13, 15-2, and 47C12) from the 5q35 region located immediately centromeric to theNPM locus.23 Rearrangement of ALK was further analyzed with the Vysis LSI ALK probe assay (Vysis, Inc, Downer’s Grove, IL) that contains two differently labeled probes located either 3′ telomeric (spanning 250 kb and labeled with SpectrumOrange) or 5′ centromeric (spanning 300 kb and labeled with SpectrumGreen) of the t(2;5) breakpoint of the ALK gene at 2p23. YACs assigned to 2q were selected from the STS-based map reported by Chumakov et al.24SHIP (SH2-containing inositol 5-phosphatase) gene probes (h4.2-A2/Hg4.1-A2) at 2q3725 and subtelomeric probes for 2p (86J13/PAC) and 2q (210E14/P1)26were gifts of L.R. Rohrschneider (Fred Hutchinson Cancer Research Center, Seattle, WA) and L. Kearney (Institute of Molecular Medicine, John Radcliffe Hospital, Oxford, UK), respectively. In case no. 2, which contained polyploid cells, chromosome 2 was identified by cohybridization with a digoxigenin-11-dUTP–labeled centromere-specific probe (D2Z; Oncor, Gaithersburg, MD) and G-banding using DAPI counterstaining. Between 5 and 12 abnormal metaphases were studied in each experiment. The FISH data were collected on a Leitz DMRB fluorescence microscope equipped with a cooled black and white CCD camera run by SmartCapture software (Vysis, Stuttgart, Germany).
Results of FISH Studies With Applied DNA Probes
| Probe . | Loci . | Localization . | FISH . | |||
|---|---|---|---|---|---|---|
| Chr. 2 . | inv(2) . | i(2)(q10) . | Chr. 5 . | |||
| PAC 86J13 | 2psubtel | 2p25 | p | q | q/q | — |
| P1ALK-DMPC-HFF#1-1111H1 | 3′ALK | 2p23 | p | q | q/q | — |
| LSI ALK | 5′ALK | 2p23 | p | p | — | — |
| 3′ALK | 2p23 | p | q | q/q | ||
| y947F4 | WI-9795, D2S115, D2S2215 | 2q35 | q | q | q/q | — |
| y884F10 | D2S143, CHLC.ATA21C10, D2S107, CHLC.GATA26D05, WI-4205, D2S128 | 2q35 | q | q | q/q | — |
| y914E7 | D2S137, WI-8542, WI-10304, WI-8999, D2S107 | 2q35 | q | p/q | q/q | — |
| y951B8 | AFMB015ZA5, CHLC.GAAT1B02, D2S126, D2S339, D2S2372, D2S2148, D2S344, D2S377 | 2q35 | q | p | — | — |
| y743C9 | D2S2323, WI-8028, WI-6726, D2S133, CHLC.GGAT2F12.1215, CHLC.GGAT2F12 | 2q35 | q | p | — | — |
| y749F2 | D2S2390, D2S2204 | 2q35 | q | p | — | — |
| y770F5 | D2S158, WI-9260, CHLC.ATA20H03 D2S159, D2S401 | 2q35 | q | p | — | — |
| y752E10 | D2S2176, WI-8964, D2S206, D2S331, D2S2344, WI-4508, WI-7254 | 2q37 | q | p | — | — |
| h4.2-2/Hg4.1-A2 cDNA | SHIP | 2q37 | q | p | — | — |
| P1 210E14 | 2qsubtel | 2q37.3 | q | p | — | — |
| cos 13, 15-2, 47C12 | 5q35/centr. to NPM | 5q35 | — | — | — | q |
| Probe . | Loci . | Localization . | FISH . | |||
|---|---|---|---|---|---|---|
| Chr. 2 . | inv(2) . | i(2)(q10) . | Chr. 5 . | |||
| PAC 86J13 | 2psubtel | 2p25 | p | q | q/q | — |
| P1ALK-DMPC-HFF#1-1111H1 | 3′ALK | 2p23 | p | q | q/q | — |
| LSI ALK | 5′ALK | 2p23 | p | p | — | — |
| 3′ALK | 2p23 | p | q | q/q | ||
| y947F4 | WI-9795, D2S115, D2S2215 | 2q35 | q | q | q/q | — |
| y884F10 | D2S143, CHLC.ATA21C10, D2S107, CHLC.GATA26D05, WI-4205, D2S128 | 2q35 | q | q | q/q | — |
| y914E7 | D2S137, WI-8542, WI-10304, WI-8999, D2S107 | 2q35 | q | p/q | q/q | — |
| y951B8 | AFMB015ZA5, CHLC.GAAT1B02, D2S126, D2S339, D2S2372, D2S2148, D2S344, D2S377 | 2q35 | q | p | — | — |
| y743C9 | D2S2323, WI-8028, WI-6726, D2S133, CHLC.GGAT2F12.1215, CHLC.GGAT2F12 | 2q35 | q | p | — | — |
| y749F2 | D2S2390, D2S2204 | 2q35 | q | p | — | — |
| y770F5 | D2S158, WI-9260, CHLC.ATA20H03 D2S159, D2S401 | 2q35 | q | p | — | — |
| y752E10 | D2S2176, WI-8964, D2S206, D2S331, D2S2344, WI-4508, WI-7254 | 2q37 | q | p | — | — |
| h4.2-2/Hg4.1-A2 cDNA | SHIP | 2q37 | q | p | — | — |
| P1 210E14 | 2qsubtel | 2q37.3 | q | p | — | — |
| cos 13, 15-2, 47C12 | 5q35/centr. to NPM | 5q35 | — | — | — | q |
RESULTS
Histopathology and phenotyping.
Hematoxylin-eosin–stained biopsy sections from all three cases showed essentially identical findings. The normal lymph node parenchyma was largely replaced by a dense, monotonous proliferation of atypical cells. These cells were characterized by a large, occasionally slightly indented nucleus with prominent nucleoli and an ample amount of eosinophilic cytoplasm.
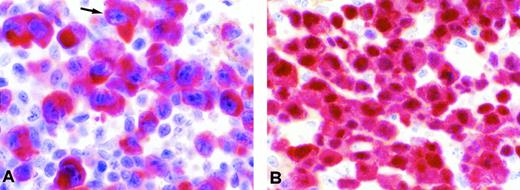
Immunophenotyping demonstrated that the neoplastic cells in all three cases failed to express either pan-B–cell or pan-T–cell markers but did express CD30 and ALK protein. The latter expression was mainly found in the cytoplasm, with no clearcut nuclear or nucleolar staining seen in frozen or in paraffin sections (Fig1A), in contrast to ALCL characterized by a t(2;5)(p23;q35) showing a cytoplasmic and nuclear anti-ALK immunostaining (Fig 1B). The diagnosis of ALK-positive ALCL was made based on these data.
Examples of anti-ALK immunostaining. (A) Large anaplastic cells from case no. 1 with the inv(2)(p23q35) showing strong diffuse ALK positivity confined to the cytoplasm. (B) In comparison, a case of CD30+ ALCL with the t(2;5)(p23;q35) showing expression of the NPM-ALK protein both in the cytoplasm and the nucleus is shown (A and B: lymph node formalin-fixed paraffin sections immunostained with the ALKc monoclonal antibody; APAAP technique; original magnification ×800).
Examples of anti-ALK immunostaining. (A) Large anaplastic cells from case no. 1 with the inv(2)(p23q35) showing strong diffuse ALK positivity confined to the cytoplasm. (B) In comparison, a case of CD30+ ALCL with the t(2;5)(p23;q35) showing expression of the NPM-ALK protein both in the cytoplasm and the nucleus is shown (A and B: lymph node formalin-fixed paraffin sections immunostained with the ALKc monoclonal antibody; APAAP technique; original magnification ×800).
Cytogenetics and FISH.
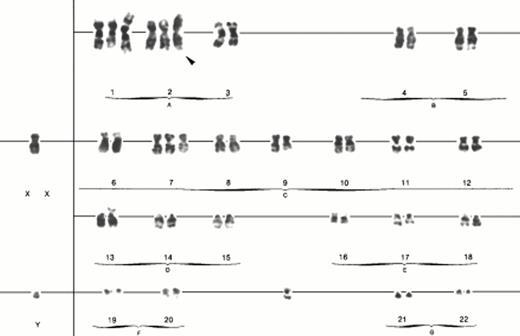
The results of cytogenetic analysis are summarized in Table3. The common karyotypic feature of all three cases was the presence of i(2)(q10). An example of the karyotype from case no. 3, illustrating the i(2)(q10), is shown in Fig2. The presence of inv(2)(p23q35) was initially detected in studies performed on case no. 1 after two-color FISH assay using an ALK P1 clone and 5q35 cocktail probes failed to identify the typical t(2;5)(p23;q35).17 Rather, this cytogenetically cryptic abnormality resulted in the relocation of this ALK P1 clone, which contains the 3′ portions of the locus that are typically incorporated into the NPM-ALK fusion gene from 2p23 to 2q35 instead of to the NPM locus at 5q35 (Fig3A). The observed pattern ofALK P1 clone hybridization also suggested that the associated i(2)(q10) chromosome in this case is composed of two long arms produced by the inv(2)(p23q35) [ider(2)(q10)inv(2)(p23q35), further referred to as simply i(2)(q10)]. These suspected chromosome 2 abnormalities were further confirmed by FISH using the 2p and 2q subtelomeric probes, 86J13 and 210E14, respectively. Clone 86J13 hybridized to the short (p) arm of normal chromosome 2, to the long (q) arm of the inv(2)(p23q35), and to both arms of the i(2)(q10) (Fig 3B), whereas clone 210E14 showed a hybridization signal on the q arm of normal chromosome 2 and the p arm of the inv(2)(p23q35) (data not shown).
Karyotypes According to Cytogenetic and FISH Analysis
| Case No. . | Cytogenetic Results . | Revised Karyotype After FISH Analysis . |
|---|---|---|
| 1 | 46,X,−Y,+i(2)(q10)[10] | 46,X,−Y,inv(2)(p23q35),+ider(2)(q10)inv(2)(p23q35) |
| 2 | Polypoid,i(2)(q10),+mar,inc[9]/46,XY[15] | polypoid,inv(2)(p23q35),+ider(2)(q10)inv(2)(p23q35),+mar,inc |
| 3 | 50,XY,+idic(1)(p11),+i(2)(q10),+7,add(13)(p11),+mar[10] | 49,XY,+idic(1)(p11),ider(2)(q10)inv(2)(p23q35),+7,add(13)(p11), +mar[21] 50,XY,+idic(1)(p11),+ider(2)(q10)inv(2)(p23q35),+7, add(13)(p11),+mar[19] 50,XY,+idic(1)(p11),inv(2)(p23q35),+ider(2)(q10)inv(2)(p23q35),+7,add(13)(p11),+mar[1] |
| Case No. . | Cytogenetic Results . | Revised Karyotype After FISH Analysis . |
|---|---|---|
| 1 | 46,X,−Y,+i(2)(q10)[10] | 46,X,−Y,inv(2)(p23q35),+ider(2)(q10)inv(2)(p23q35) |
| 2 | Polypoid,i(2)(q10),+mar,inc[9]/46,XY[15] | polypoid,inv(2)(p23q35),+ider(2)(q10)inv(2)(p23q35),+mar,inc |
| 3 | 50,XY,+idic(1)(p11),+i(2)(q10),+7,add(13)(p11),+mar[10] | 49,XY,+idic(1)(p11),ider(2)(q10)inv(2)(p23q35),+7,add(13)(p11), +mar[21] 50,XY,+idic(1)(p11),+ider(2)(q10)inv(2)(p23q35),+7, add(13)(p11),+mar[19] 50,XY,+idic(1)(p11),inv(2)(p23q35),+ider(2)(q10)inv(2)(p23q35),+7,add(13)(p11),+mar[1] |
Examples of FISH experiments performed on case no. 1 (A through D), case no. 2 (E), and case no. 3 (F). Applied probes included ALK P1 (red) and 5q35 cosmids (green) (A), 86J13 (2psubtel) (B), LSI ALK (5′ end of ALK [green]; 3′ end of ALK [red]) (C), ALK P1 (red) and 914E7 (green) (D through F), and D2Z (red) (E). Long arrows, short arrows, and arrowheads indicate the normal chromosomes 2, inv(2)(p23q35), and i(2)(q10), respectively.
Examples of FISH experiments performed on case no. 1 (A through D), case no. 2 (E), and case no. 3 (F). Applied probes included ALK P1 (red) and 5q35 cosmids (green) (A), 86J13 (2psubtel) (B), LSI ALK (5′ end of ALK [green]; 3′ end of ALK [red]) (C), ALK P1 (red) and 914E7 (green) (D through F), and D2Z (red) (E). Long arrows, short arrows, and arrowheads indicate the normal chromosomes 2, inv(2)(p23q35), and i(2)(q10), respectively.
Considering that a rearrangement of the ALK gene by inv(2)(p23q35) could not be unequivocally demonstrated using theALK P1 probe containing only the 3′ end of ALK, we applied the recently developed Vysis LSI ALK FISH assay that consists of two differently labeled probes for the centromeric (5′) and telomeric (3′) sides of the t(2;5)(p23;q35) breakpoint of theALK gene. Using this assay, ALK rearrangement was readily demonstrated by separation of the centromeric (green) and telomeric (red) ALK probes on the inv(2)(p23q35) chromosome (Fig 3C).
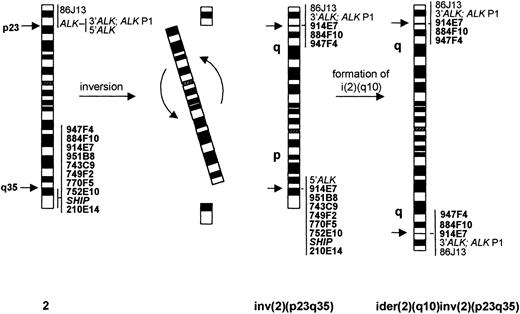
To determine the position of the 2q35 breakpoint of the inv(2)(p23q35), FISH with a panel of YACs previously mapped to 2q35-q37 and with probes from the SHIP gene (2q37) was performed using metaphases from case no. 1. The results are summarized in Table 2. Briefly, two YAC clones (947F4 and 884F10) showed signals on the long arms of the inv(2)(p23q35) and i(2)(q10), six 2q probes (951B8, 743C9, 749F2, 770F5, 752E10, h4.2-A2/Hg4.1-A2[SHIP]) hybridized to the short arm of the inv(2)(p23q35) and did not hybridize with the i(2)(q10), and the 914E7 YAC showed a split signal on the p and q arms of inv(2)(p23q35) and labeled both arms of the i(2)(q10), indicating that it contained the 2q35 breakpoint region (Fig 3D). The FISH results for case no. 1 are shown schematically in Fig4.
Schematic representation of the normal chromosome 2, inv(2)(p23q35), and ider(2)(q10) inv(2)(p23q35), together with the FISH pattern of applied probes.
Schematic representation of the normal chromosome 2, inv(2)(p23q35), and ider(2)(q10) inv(2)(p23q35), together with the FISH pattern of applied probes.
Cytogenetic analysis of case no. 2 showed nine polyploid metaphases with i(2)(q10), in addition to 15 normal cells. Despite the poor quality of chromosomes in the polyploid cells that precluded further karyotypic analysis, FISH experiments with the ALK P1 clone clearly showed hybridization signals at 2p23, at 2q35, and on both arms of the i(2)(q10) in all 7 analyzed polyploid cells (Fig 3E). Additional application of the LSI ALK FISH assay demonstrated separation of the 5′ end and 3′ end ALK probes on 1 or 2 copies of chromosome 2 per cell (whereas in other copies of chromosome 2 both probes hybridized together at 2p23), and the presence of the red signal corresponding to the 3′ end ALK probe on both arms of the i(2)(q10) (not shown). These findings are consistent with the presence of the inv(2)(p23q35) and ider(2)(q10)inv(2)(p23q35) in this case. The number of aberrant chromosomes 2 in the cells analyzed was variable, and either one inv(2)(p23q35) and two i(2)(q10), or two inv(2)(p23q35) and one, or two i(2)(q10) per cell could be detected. The 914E7 YAC clone previously found to span the 2q35 breakpoint in case no. 1 showed the same hybridization pattern in case no. 2 (Fig 3E).
All 10 karyotyped metaphase cells from case no. 3 showed the presence of the i(2)(q10) chromosome accompanied by additional karyotypic abnormalities (Fig 2). FISH with the ALK P1 clone showed hybridization signals on both long arms of the i(2)(q10). However, in contrast to the previous cases, both chromosomes 2, when present, seemed to be normal and yielded hybridization signals at 2p23 only. Only one metaphase of a total of 41 cells tested with the ALKP1, 86J13, and the 914E7 and 743C9 YAC probes showed the presence of both aberrant chromosomes, inv(2)(p23q35) and i(2)(q10) (not shown). Among the remaining analyzed metaphases, 21 had two normal chromosomes 2 and 19 cells showed the presence of only one normal chromosome 2, in addition to the i(2)(q10). For this reason, we could not demonstrate a split signal of the 914E7 YAC on the inv(2)(p23q35), because the abnormality was not present in the cells analyzed in the particular experiment from which images were captured for publication (Fig 3F). However, considering that the 914E7 YAC clone hybridized to the i(2)(q10), whereas the neighboring distal YAC 743C9 did not, we presume that the 2q35 breakpoint in case no. 3 occurs in the same region as the two previous cases.
DISCUSSION
Histopathological, cytogenetic, and FISH data presented here clearly indicate that the inv(2)(p23q35) is a recurrent chromosomal abnormality in ALK-positive ALCL. This variant rearrangement, in a manner similar to the classical t(2;5)(p23;q35), targets the ALK gene on chromosome 2 and leads to aberrant expression of its product, as demonstrated by positive immunostaining with anti-ALK antibody. By analogy to the t(2;5)/NPM-ALK rearrangement, it may be predicted that the 2q35 region to which the 3′ portion of theALK gene has been relocated by the inv(2)(p23q35) is the site of a gene involved in a fusion with ALK and, in consequence, that this gene drives the expression and kinase activation of the chimeric ALK protein. This partner gene has not yet been identified, but using FISH with a panel of DNA probes for 2q, we demonstrated that the breakpoint is encompassed within the 1,750-kb insert of the 914E7 YAC clone. No known expressed sequence tags (ESTs) have been reported in this region to date. Localization of the 2q35 breakpoint within the same YAC clone in all three cases included in this study indicates that the inv(2)(p23q35) is likely to result in the consistent generation of a specific ALK fusion gene and chimeric product.
Identification of the inv(2)(p23q35) by classical cytogenetics is difficult, because this rearrangement involves terminal bands on both arms of chromosome 2 that are of the same size and similar banding patterns. Given that the inv(2)(p23q35) was consistently associated with i(2)(q10) in all cases reported here, the presence of i(2)(q10) may serve as a useful cytogenetic indicator for this cryptic and indiscernible aberration. Of course, PCR approaches (either RNA- or DNA-based) designed to identify the classical NPM-ALK fusion would be falsely negative for the involvement of the ALK gene in the genesis of this molecular subtype of ALCL. Until the characterization of the 2q35 gene locus altered by this abnormality, the most efficient means to identify these lymphomas would seem to be the use of anti-ALK immunostaining to verify expression of the protein, followed by FISH analysis with ALK probes, as described here.
Our FISH analysis showed that the i(2)(q10) originates from the inv(2)(p23q35) [ider(2)(q10)inv(2)(p23q35)] and thus represents a secondary chromosomal abnormality resulting in tetrasomy of 2q and the occurrence of two additional copies of the putative chimeric gene involving ALK. However, in none of the cases studied, have we observed the original subclone containing exclusively the inv(2)(p23q35). These findings may be to some extent functionally analogous to the amplification of the Ph′/BCR-ABL observed during the development of some cases of blast crisis in chronic myelogenous leukemia (CML)27 and to the gain of an extra copy of the der(11)t(11;21)(q23;q11) during the transformation of myelodysplastic syndrome (MDS) cases with the t(11;21).28 However, in these two instances, the appearance of additional copies of an aberrant chromosome/gene is a phenomenon associated with transformation of an indolent condition to an aggressive one, whereas in the ALCL cases reported here, the i(2)(q10) occurred in all abnormal metaphases from the time of diagnosis. Whereas a single copy of the classical t(2;5)/NPM-ALK rearrangement seems to be sufficient for malignant transformation, and a causative role for this chimera in the pathogenesis of ALCL has been recently supported by demonstration of its oncogenic properties by in vitro transformation assays and in a mouse model,29-31 the above-described findings lead us to speculate that for unknown reasons the oncogenic potential of the putative 2q35-ALK–encoded fusion protein may be less pronounced or its expression level insufficient for efficient tumor development; therefore, an extra dosage of this chimeric gene could favor malignant transformation.
Immunohistopathology indicated that all three cases described here represent examples of the common type of ALCL. As expected from the monomorphic character of the neoplastic population,14 these lymphomas were shown to express the ALK protein using both the ALK1 (data not shown)13 and ALKc monoclonal antibodies.17 With both antibodies, a clear cytoplasmic staining was obtained, whereas the additional nuclear and/or nucleolar staining that seems to characterize t(2;5)/NPM-ALK–positive ALCL cases was not obviously present (Fig 1). This finding is in agreement with the very recently reported observations of Mason et al,32 who demonstrated that, in ALCL with the (1;2)(q25;p23), an altered ALK protein accumulates only within the cytoplasm. Moreover, these investigators observed a similar pattern of immunostaining with an engineered TPR-ALK hybrid protein, in which the NPM segment was replaced by the TPR homodimerization domain that is present in the transforming TPR-MET chimeric protein. The investigators concluded that the nuclear/nucleolar accumulation of NPM-ALK, which occurs apparently because of heterodimerization of the chimera with the normal NPM protein and resultant nucleo-cytoplasmic shuttling mediated by NPM, is not a prerequisite for an oncogenic ALK kinase activity. Rather, the NPM gene in t(2;5)/NPM-ALK–positive ALCL functions to provide a promoter that drives expression of the ALK gene in lymphoid cells (in which the gene is normally silent) and also to activate the ALK kinase domain by encoding a homodimerization motif to mimic ligand-induced receptor cross-linking. Our observation that the putative ALK fusion protein produced by the inv(2)(p23q35) is found within the cytoplasm only suggests that the 2q35 partner gene altered by the abnormality encodes a cytoplasmic protein with oligomerization capabilities. It is noteworthy that a significant minority (about 20%) of ALK-positive lymphomas reported in a recent study of 123 cases possessed anti-ALK immunoreactivity that was restricted to the cytoplasm16and, therefore, perhaps due to ALK rearrangements other thanNPM-ALK. Our identification of the inv(2)(p23q35) in 3 of 12 ALK-positive ALCL cases collected in our laboratory and documented by cytogenetic and/or by FISH (10 cases in Pittaluga et al14 and 2 additional cases reported here) suggests that this genetic abnormality could be responsible for the development of a considerable number of these lymphomas, an assumption that can now be tested with the FISH assays used here.
In conclusion, we have identified the inv(2)(p23q35) as a second recurrent chromosomal abnormality in ALK-positive ALCL. Like the classical t(2;5)(p23;q35), this variant rearrangement was detected in young adults and was shown to rearrange the ALK gene at 2p23, leading to aberrant ALK protein expression detectable by immunostaining with ALK-specific antibodies. The consistent association of the inv(2) with the secondary chromosomal aberration [ider(2)(q10)inv(2)(p23q35)], which results in extra copies of the rearranged ALK gene and accumulation of the aberrant ALK protein in malignant cells that is confined to the cytoplasm, further distinguishes this genetic subtype of ALCL from other ALK-positive lymphomas. These findings should be helpful in further understanding the mechanisms by which aberrant ALK activity contributes to the development of NHL.
ACKNOWLEDGMENT
The authors thank Magda Dehaen, Griet Hasevoets, and Xiaoli Cui for technical assistance and Rita Logist for help in preparation of the manuscript. We also thank Dr Karen Pulford (The Leukaemia Research Fund Immunodiagnostics Unit, University Department of Cellular Science, John Radcliffe Hospital, Oxford, UK) for supplying the ALK1 monoclonal antibody and Dr John Proffitt (Vysis, Inc) for supplying the VYSIS LSIALK assay probes for our use before their commercial release.
Supported in part by the Italian Association for Cancer Research (A.I.R.C.), National Cancer Institute (NCI) Grant No. CA 69129 (S.W.M.), NCI Cancer Center CORE Grant No. CA 21765, and the American Lebanese Syrian Associated Charities (ALSAC), St. Jude Children’s Research Hospital. Also supported by a grant from the project “Cryptic genomic changes in haematological malignancies” funded by the Flemish government as a contribution to the action “Kom op tegen Kanker/Vlaams Kanker Liga,” and by the Biomed Concerted Action, CT 94-1703.
This text present research results of the Belgian program on Interuniversity Poles of Attraction initiated by the Belgian State, Prime Minister’s Office, Science Policy Programming. The scientific responsibility is assumed by the authors.
Address reprint requests to Herman Van den Berghe, Center for Human Genetics, Herestraat 49, B-3000 Leuven, Belgium.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 3. Examples of FISH experiments performed on case no. 1 (A through D), case no. 2 (E), and case no. 3 (F). Applied probes included ALK P1 (red) and 5q35 cosmids (green) (A), 86J13 (2psubtel) (B), LSI ALK (5′ end of ALK [green]; 3′ end of ALK [red]) (C), ALK P1 (red) and 914E7 (green) (D through F), and D2Z (red) (E). Long arrows, short arrows, and arrowheads indicate the normal chromosomes 2, inv(2)(p23q35), and i(2)(q10), respectively.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/8/10.1182_blood.v92.8.2688/5/m_blod420wlo03z.jpeg?Expires=1769118866&Signature=yrVzt~tanqWqmBM1iShoZDcBAx-WlyGfa7KHRqz-4lVeCv5UqqXN6WmeWevGcGllyyrgEc23JH7D0GaRiNoGSVoMeWtI2LubbFjtlu4cVpdgArH6BOn8yMOlQ8QJjAT2QCI74daB~TeYqGD4RNiZyjCn7qrmhIpw-rvwkOOjqqigfFQJFGMeAjhCLe0PHQLLW3kmh6WB8XkRArVG~0dokHDUViRn76Mk76YLQJKY3oISOOHkZCdh~ZWyIBn4iBlZSds6hRPRViZ2xghi~phF-GYc7gZA7C5HN2jtEyQms3T0vQ08tmEEMYzdbYHqkjleyD3ckCNlDQmu8ZfxDJWekA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal