Abstract
The inherent variability of conformational diseases is demonstrated by two families with different mutations of the same conserved aminoacid in antithrombin. Threonine 85 underlies the opening of the main β-sheet of the molecule and its replacement, by the polar lysine, in antithrombin Wobble, resulted in a plasma deficiency of antithrombin with an uncharacteristically severe onset of thrombosis at 10 years of age, whereas the replacement of the same residue by a nonpolar methionine, antithrombin Wibble, gave near-normal levels of plasma antithrombin and more typical adult thromboembolic disease. Isolated antithrombin Wibble had a decreased thermal stability (Tm 56.2, normal 57.6°C) but was fully stabilized by the heparin pentasaccharide (Tm 71.8, normal 71.0°C), indicating that the prime abnormality is a laxity in the transition of the main sheet of the molecule from the 5- to 6-stranded form, as was confirmed by the ready conversion of antithrombin Wibble to the 6-stranded latent form on incubation. That this transition can occur in vivo was shown by the finding of nearly 10% of the proband’s plasma antithrombin in the latent form and also, surprisingly, of small but definitive amounts of latent antithrombin in normal plasma. The latent transition will be predictably accelerated not only by gross mutations, as with antithrombin Wobble, to give severe episodic thrombosis, but also by milder mutations, as with antithrombin Wibble, to trigger thrombosis in the presence of other predisposing factors, including the conformational stress imposed by the raised body temperatures of fevers. Both antithrombin variants had an exceptional (25-fold) increase in heparin affinity and this, together with an increased inhibitory activity against factor Xa, provides evidence of the direct linkage of A-sheet opening to the conformational basis of heparin binding and activation.
© 1998 by The American Society of Hematology.
IT IS NOW BECOMING apparent that a variety of diseases, such as the prion encephalopathies,1the degenerative neuropathies,2 and Alzheimer’s disease,3 all arise from inappropriate changes in the conformation of an underlying protein. The risk of such changes particularly occurs with proteins whose physiological function involves an inherent alteration in their conformation.4 A prototypic example of this type of conformational change is provided by the serpin family of proteins that includes the principal protease inhibitors of human plasma such as α1-antitrypsin, C1-inhibitor, and antithrombin.5,6 The function of the serpins as protease inhibitors is dependent on their ability to undergo an extraordinary change in conformation that can be likened to the action of a mousetrap. This trap-like action of the serpins results from their having an exposed peptide loop that functions as a substrate bait for the target protease. Cleavage of this reactive centre bait triggers an overall change in the conformation of the serpin structure, with the opening of the large 5-stranded sheet that is the dominant feature of the molecule. The opening of the sheet allows the entry of the cleaved reactive loop to form a central sixth strand to the sheet. This spring-like movement of the reactive loop7 traps the protease that is covalently linked to it in a stable, virtually irreversible complex8 that is then catabolized from the circulation by the liver.
The triggering of the conformational trapping mechanism of the serpins is dependent on precise interactions that allow the opening of the 5-stranded sheet of the molecule by a sliding movement on the structures that underlie it.9 Mutations of amino acids within these underlying structures can result in a premature triggering of the 5- to 6-stranded conformational change to give dysfunction and other consequences that have been graphically described for the serpins as a whole as the syndrome of the hypersensitive mousetrap.4 Thus, the same homologous mutation in different serpins can give10 with antithrombin, thrombosis; with C1-inhibitor, angioedema; and with α1-antitrypsin, emphysema. The most evident presentation of the conformational changes in the syndrome as a whole has to date been through the formation of polymeric aggregates of the serpins, formed, it is believed, from the insertion of the reactive loop of one molecule into the prematurely opened A-sheet of the next, and so on.11 But there has long been the prediction12that the formation of these loop-sheet polymers should also be accompanied by the formation of the monomeric 6-stranded latent conformation, with the intact reactive loop prematurely inserted as the sixth strand to give a stable inert form. Although the abnormal transition to this latent conformation in antithrombin has been demonstrable in vitro,13 14 it has not, until now, been shown to occur in vivo.
We report here studies of two new variants of antithrombin associated with premature thrombosis that illustrate the characteristic phenotypic variability of conformational diseases. The two antithrombin variants, descriptively named Wibble and Wobble, both have amino acid substitutions of the same conserved residue, threonine-85, which directly underlies the opening of the 5-stranded sheet of the molecule. In antithrombin Wobble, threonine-85 is replaced by a positively charged amino acid, causing a predictable gross decrease in stability compatible with its presentation as an apparent type I antithrombin deficiency. The other variant, antithrombin Wibble, is of particular interest, as here the less severe substitution of the threonine, by a bulky but non-charged aminoacid, results in apparently normal secretion of the antithrombin. However, the variant is susceptible to spontaneous transition from the normal 5-stranded to the abnormal 6-stranded form. This is confirmed by the finding in the patient’s plasma of monomeric latent antithrombin.
A bonus from the thorough studies of mutants of proteins associated with disease is the unexpected clues that these natural mutants provide as to aspects of normal function. Recently, we have completed a series of crystallographic structures showing in detail the changes that take place to give the heparin activation of antithrombin.15However, a new observation in our study here, that antithrombin Wibble has a profound increase in heparin affinity, opens a unique and quite unexpected insight into the mechanism by which antithrombin binds and is activated by heparin.
MATERIALS AND METHODS
Materials
High-affinity heparin of approximately 7.8 kD molecular weight was prepared from crude heparin from porcine mucosa (Grampian Enzymes, Mirbister Harray, Orkney, UK) using a combination of gel filtration on Sephadex G-10016 and affinity chromatography on antithrombin-Sepharose, as described previously.17 Human factor Xa was from Boehringer Mannheim (Lewes, UK). Heparin pentasaccharide was a gift from Dr Maurice Petitou (Sanofi Research, Toulouse, France), the high-affinity form with an extra sulfate was used15 for determination of thermal stability and the natural pentasaccharide for binding affinity and kinetics.
Methods
Measurement of plasma antithrombin levels.
Plasma antithrombin antigen levels were measured by a sandwich enzyme-linked immunosorbent assay (ELISA) using a polyclonal antiserum to antithrombin (Dako, High Wycombe, UK) as a capture antibody and its peroxidase conjugate for detection. Thrombin inhibitory activity was measured in the presence of heparin using a BioMèrieux (Basingstoke, UK) kit on an ACL analyzer.
Antithrombin gene analysis.
Standard procedures were used to isolate high molecular weight genomic DNA from peripheral whole blood. Oligonucleotide primers, designed to amplify all coding regions of the antithrombin gene (exons 2 to 6) together with intron/exon boundaries,18 were synthesized on an Applied Biosystems (Foster City, CA) automated DNA synthesiser. With the exception of exons 3a and 3b, which were amplified as a single 1.3-kb fragment, all exons were amplified individually. Two hundred fifty micrograms of genomic DNA samples were diluted in a final volume of 50 μL containing 30 pmol of each primer, 200 μmol/L each dNTP, and 1 U Biotaq (Bioline, London, UK) in 50 mmol/L KCl, 1.5 mmol/L MgCl2, 0.1% Triton X-100, 10 mmol/L Tris-HCl, pH 8.8. Samples were denatured by heating for 7 minutes at 95°C and then subjected to 35 cycles of denaturation at 94°C, annealing at different temperatures depending on the exon, and extension at 72°C. All steps were for 1 minute, except where exons 3a and 3b were being amplified, when extension was for 2 minutes. In all cases, the final extension step was increased by 10 minutes. Amplified polymerase chain reaction (PCR) product was purified either directly using the Reagent Pack for PCR Product Pretreatment (Amersham, Little Chalfont, UK) or, after electrophoresis in 1% agarose, using the Qiaex Gel Extraction kit (Qiagen, Düsseldorf, Germany) and elution of the DNA into 40 μL of H2O. One to 9 μL of the purified PCR product was then sequenced using one of the amplification primers end-labeled with32P and the Thermo Sequenase Cycle Sequencing kit from Amersham.
Isolation of RNA and synthesis of cDNA.
Buffy coats were collected by centrifugation of peripheral blood for 10 minutes at 2,800g and total RNA then isolated using Trizol reagent (GIBCO BRL, Paisley, UK) and the procedure supplied by the manufacturer. To synthesize cDNA, 1 μg of RNA was diluted in 19 μL water, denatured at 65°C for 5 minutes, and then placed on ice. Three hundred units of Moloney murine leukaemia virus reverse transcriptase (GIBCO BRL) and 30 U of RNAsin (Promega, Southampton, UK), diluted in 21 μL of 153 mmol/L KCl, 6.1 mmol/L MgCl2, 2 mmol/L DTT, 2 mmol/L dNTPs, 0.214 mg/mL random hexamers, 102 mmol/L Tris-HCl, pH 8.3, was added and the sample was incubated at 37°C for 2 hours. The reverse transcriptase was denatured by heating at 65°C for 10 minutes and the cDNA was stored at −20°C until required.
Analysis of AT3 gene expression.
The two mutations identified at nucleotide 2718 create an additional recognition site for the restriction enzyme Sty I within exon 2, providing a marker with which to examine expression of mRNA derived from the normal and mutated alleles. Thus, the PCR was used to amplify a 447-bp fragment spanning exons 2 and 3a of the antithrombin gene from the randomly generated cDNA prepared as described above. PCRs were performed in a 50 μL volume containing 4 μL random cDNA, 100 μmol of each dNTP, 15 pmol of each of the primers AT24 and AT20, and 1 U Biotaq (Bioline) diluted in 16 mmol/L (NH4)2SO4, 0.01% Tween-20, 1.5 mmol/L MgCl2, 67 mmol/L Tris-HCl, pH 8.8. Samples were denatured for 7 minutes at 95°C and then subjected to 35 cycles of 1-minute steps of denaturation at 94°C, annealing at 56°C, and extension at 72°C. The final extension step was increased by 10 minutes. The amplified product was digested overnight at 37°C withSty I (New England Biolabs, Boston, MA) and then analyzed by electrophoresis in 8% polyacrylamide. Two fragments of 251 and 196 bp were observed where C was present at nucleotide position 2718, as in the normal allele, whereas the presence of either A or T at position 2718 gave rise to three fragments of 196, 155, and 96 bp.
Purification of antithrombin.
Variant antithrombin was purified from the patient plasma using precipitation of plasma with dextran sulfate and calcium chloride,19 after which the supernatant was diluted with an equal volume of equilibration buffer (50 mmol/L Tris-HCl, 10 mmol/L sodium citrate, 5 mmol/L sodium EDTA, 150 mmol/L NaCl, pH 7.4). This mixture was applied to a heparin-Sepharose affinity chromatography column previously equilibrated with the same buffer and the column was washed with equilibration buffer, followed by the same buffer containing 0.4 mol/L NaCl. The antithrombin was eluted using a gradient from 0.4 to 2 mol/L NaCl in the equilibration buffer. Antithrombin peaks were further purified by anion exchange chromatography on Q-Sepharose fast flow, as described previously.13
Preparation of latent antithrombin and thermal stability assessment.
Latent antithrombin was prepared as described.20 Fifty milligrams of pure α-antithrombin was diluted to 1 mg/mL in 20 mmol/L Tris-HCl, 250 mmol/L sodium citrate, pH 7.4, and incubated overnight at 60°C (16 hours). The sample was exchanged into 20 mmol/L Tris-HCl, 10 mmol/L sodium citrate, pH 7.4, by ultrafiltration on Amicon concentrator using a YM30 membrane, after which it was purified on heparin-Sepharose and ion-exchange chromatography as described above, except that the gradient on heparin-Sepharose was from 0 to 2 mol/L NaCl. The latent antithrombin eluted at approximately 0.4 mol/L NaCl on the gradient. Thermal stability of samples was assessed21by incubating either pure preparations (0.2 mg/mL in 20 mmol/L Tris-HCl, pH 7.4) or plasma for 2 hours at the temperatures indicated in the figure legends. The incubation was stopped by the addition of polyacrylamide gel electrophoresis (PAGE) gel loading buffer and snap freezing in liquid nitrogen. The samples were later analyzed as described below. Changes of protein secondary structure with temperature were also measured by monitoring the CD signal at 222 nm using a JASCO J-720 (Tokyo, Japan) spectrapolarimeter. Samples in 50 mmol/L phosphate buffer (pH 7.4) containing minimal amounts of chloride ions were assessed in a 0.05 mg/mL. The temperature within the cuvette was maintained by a computer-controlled water bath connected to a water jacket integral to the cuvette holder and monitored by a sensor directly located in the holder. Thermal unfolding experiments were performed using a heating rate of 60°C per hour. The resulting data were then fitted to a two-state protein unfolding model as described.22
Electrophoresis methods.
The purity of antithrombin preparations was analyzed by PAGE in a Tris-Glycine gel system with a stacking gel pH of 6.8 and running gel pH of 8.8.23 The analysis of the formation of polymers in antithrombin preparations was performed using PAGE as described,24 with a stacking gel pH of 6.9 and a running gel pH of 8.9. Samples of plasma that were analyzed were applied to the PAGE system, after which they were blotted to nitrocellulose25 and probed using rabbit anti-antithrombin antibodies (Dako, Ely, UK), followed by a goat antirabbit IgG-horseradish peroxidase conjugate, with detection via an ECL kit (Amersham). Transverse urea gradient (TUG) gels containing a gradient of 0 to 8 mol/L urea were prepared in the PAGE system24according to methods described.26 Rocket immunoelectrophoresis was performed according to the method of Laurell.27 N-terminal sequencing was performed on proteins that had been electrophoresed in a Tris-Tricine gel system28 and blotted to polyvinylidene difluoride membrane.29
Determination of heparin binding affinity for antithrombin.
Equilibrium binding constants for the interaction between antithrombin and heparin pentasaccharide or high-affinity heparin were determined using titrations of the heparin species (0 to 500 μmol/L) into antithrombin solutions (25 nmol/L) in which the increase of fluorescence emission at 340 nm, with excitation at 280 nm, was followed, as described.17 Buffers used were 20 mmol/L Tris-HCl, 0.1% PEG 8000, 0.1 mmol/L EDTA, pH 7.4, containing 100 mmol/L NaCl (I0.15) or 250 mmol/L NaCl (I0.3).
Rapid kinetics of heparin binding to antithrombin variants.
The kinetics of heparin pentasaccharide binding to antithrombin variants were determined using stopped-flow spectrofluorimetry essentially as described.30 The antithrombin molecules (α-antithrombin, antithrombin Wibble, and antithrombin Rouen VI) in the buffers described above were at 100 nmol/L, and the increase in fluorescence above 300 nm (excitation at 280 nm) on the addition of 0.5 to 20 μmol/L heparin pentasaccharide was monitored in a stopped flow fluorimeter (Applied Photophysics SX-17, Leatherhead, UK). The increase in fluorescence was fitted to a single exponential function to yield the kobs for each interaction. At least 16 sets of data were summed and each experiment was repeated to obtain kobs and error values for each heparin concentration, which were then analyzed by nonlinear regression of plots against the heparin concentration to obtain the individual rate constants.
Association rate constant with factor Xa.
The association rate constant for the interaction between antithrombin and factor Xa was analyzed under pseudo-first order conditions using a discontinuous assay system essentially as described.17
RESULTS
Variant Identification and Family Histories
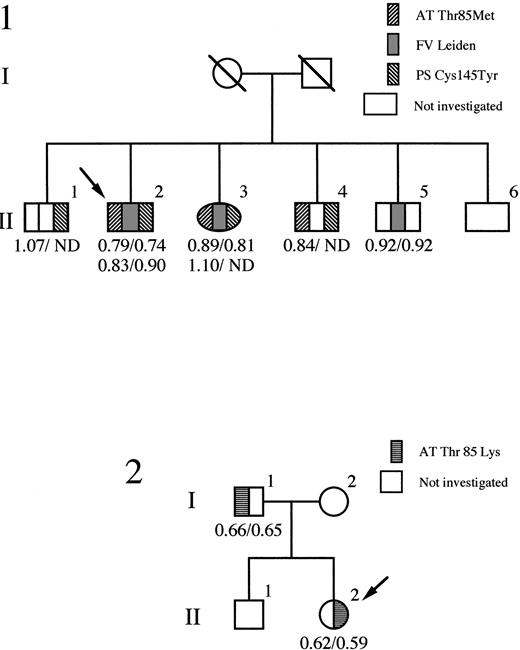
The histories of the two families are summarized in Fig1. Identification of the mutation in the antithrombin of each family was performed on genomic DNA from whole blood, with direct sequencing of all exons (except exon 1) as well as all intron-exon boundaries. The only abnormalities were present in exon 2, where in the proband of each family a different heterozygous nucleotide substitution was present at position 2718. For family 1 (antithrombin Wibble), a C to T transition codes for the replacement of the threonine in position 85 of antithrombin by methionine, and in family 2 (antithrombin Wobble) a transversion of C to A codes for the replacement of the same threonine by lysine. The two mutations at nucleotide 2718 create a novel recognition site for the restriction enzyme Sty I allowing rapid confirmation of the mutation and also screening of relatives of the probands within each family, as in Fig 1. Reverse transcription and PCR of antithrombin mRNA, present ectopically in peripheral blood lymphocytes, confirmed in each case the presence of stably expressed mRNA species derived from both the mutated alleles.
Pedigrees of family 1 (antithrombin Wibble) and family 2 (antithrombin Wobble) with probands identified as arrowed. Antithrombin activity/antigen levels are indicated. ND, not determined. The segregation of the protein S variant (PS Cys145Tyr) and factor V Leiden is shown in family 1.
Pedigrees of family 1 (antithrombin Wibble) and family 2 (antithrombin Wobble) with probands identified as arrowed. Antithrombin activity/antigen levels are indicated. ND, not determined. The segregation of the protein S variant (PS Cys145Tyr) and factor V Leiden is shown in family 1.
Antithrombin Wibble (Thr85Met) was identified in a 47-year-old man who was on long-term warfarin therapy having suffered three episodes of deep vein thrombosis, the first occurring, with a pulmonary embolus, at 24 years of age. His mother had died of a pulmonary embolus at 63 years of age and a sister, with the Thr85Met mutation, has recently had a deep vein thrombosis. The proband’s risk of thrombosis was complicated by the additional presence of the heterozygous factor V Leiden abnormality, together with a heterozygous protein S deficiency (Cys145Tyr), as previously reported.31
Antithrombin Wobble (Thr85Lys) was identified in a female first admitted at 10 years of age with a Mycoplasma pneumoniae chest infection that had caused her to be bed-ridden for 12 days. Details related to her treatment have been previously reported,32but, briefly, on admission, she had signs of a chest infection as well as a swollen tender left leg and groin that had first become painful 36 hours earlier. A femoral vein thrombosis was confirmed by venogram and she was anticoagulated with an infusion of heparin, to which she was initially resistant. Subsequently, she has been on oral warfarin and has remained well. Family studies showed that the proband’s father was also a heterozygote for the Thr85Lys antithrombin variant, although there was no history of thrombosis.
Plasma Findings
Antithrombin activity and antigenic concentrations (both expressed as proportions of an average normal of 1) are shown in Fig 1. Antithrombin Wobble carriers in family 2 have equivalent activity/antigen levels of antithrombin of nearly 0.6, close to the 0.5 level of each expected in a nonsecreted type I deficiency.33 However, the level at 0.6 suggested the possibility of an inefficiently expressed, rather than nonexpressed, variant. This was supported by heparin-Sepharose chromatography of the patient’s plasma that showed as well as the peak of normal antithrombin, a small well-defined peak of antithrombin with greatly increased heparin affinity, eluting at 1.6 mol/L NaCl. This contained only one tenth the amount of antithrombin present in the normal peak and, consequently, there was insufficient material for further characterization.
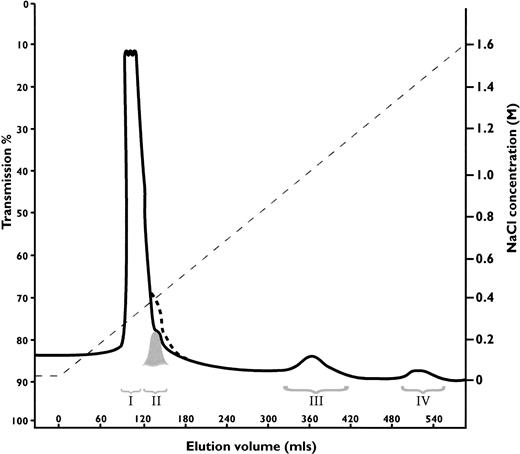
Antithrombin Wibble, in family 1, was associated with plasma antithrombin activities and antigenic concentrations usually close to normal. The presence of adequate amounts of the abnormal antithrombin for further studies was confirmed by heparin-Sepharose affinity chromatography of plasma from carriers of antithrombin Wibble in family 1. This showed (Fig 2) the presence of an extra peak of high-affinity antithrombin eluting at 1.5 mol/L NaCl as compared with the normal component at 1.0 mol/L NaCl. Monitoring of thrombin inhibitory activity (not shown) indicated equivalence of activity in both the normal and the new high-affinity peaks. The yields of the abnormal component (0.5 mg from 50 mL of plasma) were sufficient to allow studies of its function.
Heparin-Sepharose affinity chromatography of plasma from a carrier of antithrombin Wibble, with peak III being the normal component (-antithrombin) and peak IV the abnormal antithrombin Wibble. Latent antithrombin was shown in a control procedure to give a shoulder on the first peak indicated by dashed line. The corresponding peak II from the plasma sample was almost completely latent-antithrombin Wibble (see Fig 3D).
Heparin-Sepharose affinity chromatography of plasma from a carrier of antithrombin Wibble, with peak III being the normal component (-antithrombin) and peak IV the abnormal antithrombin Wibble. Latent antithrombin was shown in a control procedure to give a shoulder on the first peak indicated by dashed line. The corresponding peak II from the plasma sample was almost completely latent-antithrombin Wibble (see Fig 3D).
Thermal Stability Tests
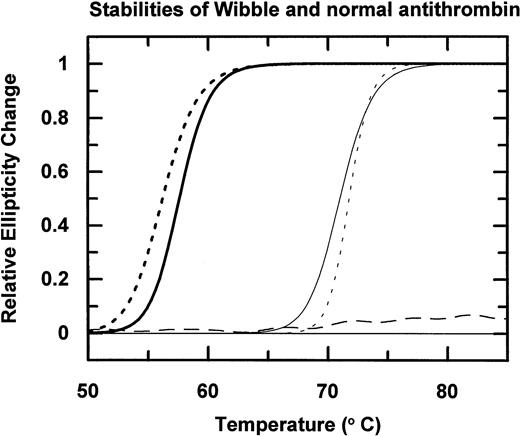
Incubation of the isolated antithrombin over a range of temperatures followed by electrophoresis (Fig 3A) shows that antithrombin Wibble polymerises on incubation at 50°C as compared with the normal antithrombin component, which is stable at 50°C but polymerizes at 60°C. The conformational instability of antithrombin Wibble was more definitively assessed by measurement of changes of circular dichroic ellipticity over a range of temperatures shown in diagrammatic form in Fig 4, with defined melting point temperatures as in Table1. These confirm a significant instability in the Wibble variant but also show how this instability is corrected on addition of excess high-affinity heparin pentasaccharide, which fully returns its thermal stability to equivalence with that of the normal antithrombin/pentasaccharide complex.
(A) Nondenaturing PAGE electrophoresis of isolated antithrombins Wibble and normal control incubated for 2 hours at a range of temperatures as indicated. Because of limited material staining was by immuno- (Western) blotting. The gels show the formation of polymers, commencing at 50°C with antithrombin Wibble and 60°C with the normal control. (B) Electrophoresis in nondenaturing PAGE of antithrombin from the abnormal (Wibble) peak IV (see Fig 2) and the normal peak III. The two samples were incubated at 50°C for 24 hours with removal of aliquots at times denoted on the figure. The appearance of the faster latent band begins at 6 hours and is almost complete at 24 hours. Note the latent form just appearing at 24 hours in the normal control. Traces of polymers are present in the peak IV sample after 6 hours of incubation. (Staining is with Coomassie blue, as compared with the more sensitive immuno-staining of [A]). (C) Nondenaturing PAGE electrophoresis of antithrombin Wibble (peak IV, Fig 2) before (W) and after (W41)° 24 hours of incubation13 at 41°C, pH 8.0; standards for latent (L) and normal () antithrombin are shown; and also β-antithrombin (β) to exclude contamination of peak IV. The gel is sensitively silver-stained; note the absence of polymers in the W41° band, which shows almost complete conversion to the latent form. (D) Electrophoresis in nondenaturing PAGE of samples (at two different concentrations) from peak II and peak IV of the heparin-Sepharose chromatography of a carrier of antithrombin Wibble (Fig 2). On left is a control (normal ) antithrombin from peak III, and on the right is a control latent antithrombin.
(A) Nondenaturing PAGE electrophoresis of isolated antithrombins Wibble and normal control incubated for 2 hours at a range of temperatures as indicated. Because of limited material staining was by immuno- (Western) blotting. The gels show the formation of polymers, commencing at 50°C with antithrombin Wibble and 60°C with the normal control. (B) Electrophoresis in nondenaturing PAGE of antithrombin from the abnormal (Wibble) peak IV (see Fig 2) and the normal peak III. The two samples were incubated at 50°C for 24 hours with removal of aliquots at times denoted on the figure. The appearance of the faster latent band begins at 6 hours and is almost complete at 24 hours. Note the latent form just appearing at 24 hours in the normal control. Traces of polymers are present in the peak IV sample after 6 hours of incubation. (Staining is with Coomassie blue, as compared with the more sensitive immuno-staining of [A]). (C) Nondenaturing PAGE electrophoresis of antithrombin Wibble (peak IV, Fig 2) before (W) and after (W41)° 24 hours of incubation13 at 41°C, pH 8.0; standards for latent (L) and normal () antithrombin are shown; and also β-antithrombin (β) to exclude contamination of peak IV. The gel is sensitively silver-stained; note the absence of polymers in the W41° band, which shows almost complete conversion to the latent form. (D) Electrophoresis in nondenaturing PAGE of samples (at two different concentrations) from peak II and peak IV of the heparin-Sepharose chromatography of a carrier of antithrombin Wibble (Fig 2). On left is a control (normal ) antithrombin from peak III, and on the right is a control latent antithrombin.
Thermal stability of antithrombin Wibble (dashed line) and a normal control (continuous line) measured by changes in circular dichroic ellipticity at 222 nm. Addition of the high-affinity heparin pentasaccharide remarkably stabilizes both antithrombins (curves on right), with the even greater stabilization and sharper melting transition of antithrombin Wibble, probably reflecting its increased heparin affinity. The stable horizontal dashed line is latent antithrombin Wibble from peak II in Fig 2.
Thermal stability of antithrombin Wibble (dashed line) and a normal control (continuous line) measured by changes in circular dichroic ellipticity at 222 nm. Addition of the high-affinity heparin pentasaccharide remarkably stabilizes both antithrombins (curves on right), with the even greater stabilization and sharper melting transition of antithrombin Wibble, probably reflecting its increased heparin affinity. The stable horizontal dashed line is latent antithrombin Wibble from peak II in Fig 2.
Antithrombin Thermal Stability Tm
| Antithrombin . | Without Bound Pentasaccharide . | With Bound Pentasaccharide . | ||
|---|---|---|---|---|
| Tm (°C) . | Standard Error . | Tm(°C) . | Standard Error . | |
| Control | 57.6 | 0.05 | 71.0 | 0.06 |
| Wibble | 56.2 | 0.09 | 71.8 | 0.06 |
| Rouen-VI | 56.1 | 0.06 | ND | ND |
| Antithrombin . | Without Bound Pentasaccharide . | With Bound Pentasaccharide . | ||
|---|---|---|---|---|
| Tm (°C) . | Standard Error . | Tm(°C) . | Standard Error . | |
| Control | 57.6 | 0.05 | 71.0 | 0.06 |
| Wibble | 56.2 | 0.09 | 71.8 | 0.06 |
| Rouen-VI | 56.1 | 0.06 | ND | ND |
Abbreviation: ND, not determined.
Latent Antithrombin
The formation of latent antithrombin by the abnormal antithrombin became apparent on incubation for 24 hours at both 41°C and 50°C of the isolated antithrombin Wibble (peak IV in Fig 2) together with a matching normal antithrombin control (peak III) fractionated from the same plasma sample (Fig 3B). The characteristic change in electrophoretic mobility of latent antithrombin13 appeared after 12 hours at 41°C in the Wibble antithrombin and was nearly complete by 24 hours (Fig 3C), but there was no formation of the latent band in the normal component. The transition was accelerated, as shown in Fig 3B, by incubation at 50°C with, interestingly, the commencement of the transition to the latent form evident in the normal component after 24 hours of incubation at 50°C. The occurrence of spontaneous conversion of antithrombin Wibble to the latent form was subsequently confirmed by TUG-gel electrophoresis of antithrombin from peak IV that had been stored for 4 weeks at 4°C.
The discrepancy between the amount of normal as compared with Wibble antithrombin on heparin-Sepharose chromatography (Fig 2) suggested that conversion of the abnormal component to latent antithrombin might have occurred in vivo within the circulation. To check this, the elution peak at 0.3 mol/L NaCl, which covers the affinity of 6-stranded antithrombin, was divided into two, as indicated in Fig 2, and each fraction was then separately assessed. The first large peak (I) on nondenaturing gel electrophoresis with Western (immuno-) blotting was seen to contain only a minor proportion of antithrombin, but the second smaller peak (II) was found to be almost wholly formed of antithrombin, which had the characteristic electrophoretic mobility of the latent form (Fig 3D). Quantitation by rocket immunoelectrophoresis showed peak III (normal antithrombin) contained 1.3 mg of antithrombin as compared with peak IV (Wibble) 0.5 mg. The quantity of the latent component in peak II was estimated from the peak area together with its electrophoretic density at 0.3 mg. Confirmation that peak II contained latent antithrombin was evidenced by a stability curve identical to that of a latent control (Fig 4) together with sodium dodecyl sulfate-PAGE (SDS-PAGE) analysis, which confirmed that the component had the characteristic mobility of antithrombin with an intact, noncleaved, reactive center loop.
Latent Antithrombin in Normal Plasma
To check the finding of latent antithrombin in the plasma of the affected individuals in family 1, pre-prepared latent antithrombin was added to a normal control plasma sample to show that it eluted in a peak position identical to that of peak II in Fig 2 at an NaCl concentration of 0.36 mol/L. This was confirmed, but in doing so it was noticed that a similar, although smaller shoulder at peak II was present with normal plasma even in the absence of added latent antithrombin. Recovery of the material from this peak from normal plasma showed it to have the characteristic stability of latent or cleaved antithrombin. SDS-PAGE electrophoresis and amino-terminal sequencing confirmed that the shoulder contained, as expected, some cleaved antithrombin, but also a greater amount of uncleaved, latent antithrombin.
Heparin Affinity
The heparin affinity-chromatography results indicated that antithrombin Wibble and Wobble both had an extraordinary and equivalent increase in heparin affinity. This was quantifiable for the isolated antithrombin Wibble as in Table 2, which compares the affinity constants (Kd) for native α-antithrombin, the Wibble variant, and another conformational variant Rouen-VI.13 The increase in affinity for the core pentasaccharide is 25-fold with the Wibble variant, versus threefold with the Rouen-VI variant.
Functional Properties of Variants
| . | Kd for Heparin Pentasaccharide* (nmol/L) . | Rate of Reaction Xa (mmol/L−1 s−1) . |
|---|---|---|
| α-Antithrombin | 550 ± 40 | 1.5 × 103 |
| Wibble (Thr85Met) | 22 ± 2 | 4 × 103 |
| Rouen-VI (Asn187Asp) | 170 ± 25 | ND |
| . | Kd for Heparin Pentasaccharide* (nmol/L) . | Rate of Reaction Xa (mmol/L−1 s−1) . |
|---|---|---|
| α-Antithrombin | 550 ± 40 | 1.5 × 103 |
| Wibble (Thr85Met) | 22 ± 2 | 4 × 103 |
| Rouen-VI (Asn187Asp) | 170 ± 25 | ND |
Abbreviation: ND, not determined.
I 0.3.
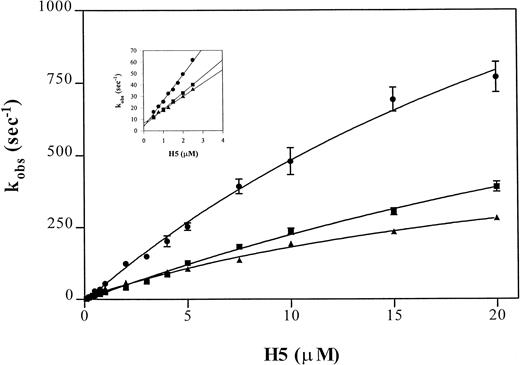
The binding of heparin to antithrombin can be assessed by rapid kinetics as a two-step process.30 The dissociation and overall association rate constant are estimated using a linear regression of rate values determined at low heparin concentrations and, because estimates of the reverse rate constants are inaccurate at physiological ionic strength at I 0.15, this is best performed at I 0.3. The rapid kinetic analysis of pentasaccharide binding to antithrombin and to the Wibble and Rouen VI variants, using the above-described protocol, yielded the curves shown in Fig5. Analysis of the individual rate constants (Table 3) shows that the high affinity of both of the variant antithrombins is due to a shift, particularly marked with antithrombin Wibble, towards a stable high-affinity complex with the heparin pentasaccharide, shown by the markedly decreased koff value. The results also indicate that the conformation of the higher affinity variants changes much faster on binding heparin, as shown by the k2 constant for the conformational change. This is more marked for the Wibble variant than for Rouen VI, in accord with their relative heparin binding affinities.
Comparison of observed pseudo-first order rate constants (kobs) for pentasaccharide (H5) binding to antithrombin variants. Average kobs values of fluorescence enhancement were measured after mixing pentasaccharide with antithrombin in a stopped flow fluorimeter using pentasaccharide concentrations at least fivefold higher than the antithrombin concentration (0.1 μmol/L) in buffers of I0.15 (main graph) or I0.3 (inset). The monitored fluorescence enhancements were fitted by a single exponential non-linear regression to yield the kobs values. Each point represents the average of duplicate determinations of eight measurements per concentration. The lines are shown for (•) antithrombin Wibble, (▪) antithrombin Rouen VI, and (▴) -antithrombin.
Comparison of observed pseudo-first order rate constants (kobs) for pentasaccharide (H5) binding to antithrombin variants. Average kobs values of fluorescence enhancement were measured after mixing pentasaccharide with antithrombin in a stopped flow fluorimeter using pentasaccharide concentrations at least fivefold higher than the antithrombin concentration (0.1 μmol/L) in buffers of I0.15 (main graph) or I0.3 (inset). The monitored fluorescence enhancements were fitted by a single exponential non-linear regression to yield the kobs values. Each point represents the average of duplicate determinations of eight measurements per concentration. The lines are shown for (•) antithrombin Wibble, (▪) antithrombin Rouen VI, and (▴) -antithrombin.
Rate Constants for Heparin Pentasaccharide Binding to Antithrombin Variants
| . | Ionic Strength . | K1 (μmol/L) . | k2 (s−1) . | kon(μmol/L−1 · s−1) . | koff (k−2) (s−1) . |
|---|---|---|---|---|---|
| α-Antithrombin | 0.15 | 20.8 ± 2.1 | 563 ± 35.4 | 38.7 ± 1.6 | 2.1* |
| 0.3 | 11.6 ± 0.4 | 6.6 ± 0.5 | |||
| Thr85Met | 0.15 | 34.2 ± 3.5 | 2172 ± 213 | 64.3 ± 3.6 | 0.06* |
| 0.3 | 22.6 ± 0.8 | 3.7 ± 0.6 | |||
| Rouen VI | 0.15 | 54.9 ± 4.96 | 1464 ± 145 | 48.2 ± 4.1 | 0.62* |
| 0.3 | 14.96 ± 0.22 | 3.64 ± 0.46 |
| . | Ionic Strength . | K1 (μmol/L) . | k2 (s−1) . | kon(μmol/L−1 · s−1) . | koff (k−2) (s−1) . |
|---|---|---|---|---|---|
| α-Antithrombin | 0.15 | 20.8 ± 2.1 | 563 ± 35.4 | 38.7 ± 1.6 | 2.1* |
| 0.3 | 11.6 ± 0.4 | 6.6 ± 0.5 | |||
| Thr85Met | 0.15 | 34.2 ± 3.5 | 2172 ± 213 | 64.3 ± 3.6 | 0.06* |
| 0.3 | 22.6 ± 0.8 | 3.7 ± 0.6 | |||
| Rouen VI | 0.15 | 54.9 ± 4.96 | 1464 ± 145 | 48.2 ± 4.1 | 0.62* |
| 0.3 | 14.96 ± 0.22 | 3.64 ± 0.46 |
*Calculated from Kd and konvalues.
Association Rate Constant With Factor Xa
It has been shown that the interaction between antithrombin and factor Xa is sensitive to the conformation of the reactive site loop of antithrombin.34 The second order rate constant for the interaction between the Wibble (Thr85Met) variant and factor Xa was determined using discontinuous rate assays and was found to be 4 ± 0.2 × 103 (mol/L−1 · s−1) compared with that of 1.5 ± 0.15 × 103(mol/L−1 · s−1) for the interaction with α-antithrombin (summarized in Table 2).
DISCUSSION
We record here a unique experiment of nature in which separate mutations in two families provide both an archetypal example of a severe episodic conformational disease and also a matching forme fruste version that gives a model, effectively in slow motion, of the molecular mechanism involved. A particular advantage of this natural experiment is that it allows the study of the mutant antithrombin in its authentically glycosylated and functional form in a way that could not, at present, be reproduced in the laboratory by recombinant expression.
Conformational Disease and Thrombosis
The serpin protease inhibitors of human plasma provide the best characterised example of a newly recognized clinical entity—the conformational diseases.4 The first example of a conformational disease to be studied in the serpins was the common genetic deficiency of α1-antitrypsin resulting from a conformational change in a variant α1-antitrypsin35 at its site of synthesis in the liver. A consequence of this conformational change is the aggregation of polymers of the variant in the liver,11 with resultant risk of cirrhosis and, because of the associated plasma deficiency of the inhibitor, the slow onset of emphysematous lung degeneration.
By comparison to this slow onset of disease associated with abnormalities of α1-antitrypsin, the same molecular lesions in the closely homologous serpin antithrombin10give the acute and clearly defined consequence of venous thrombosis. An illustration of how this can arise from either a sudden or cumulative loss of antithrombin is provided here with members of family 2 who are heterozygotes for the conformationally unstable variant—antithrombin Wobble. The cumulative effects of conformational changes in this family are seen as an overall plasma deficiency of antithrombin, but there is also the additional risk of a rapidly induced conformational change to give severe episodic thrombosis. This acute and severe onset of thrombosis occurred in the proband of family 2 with the development of an ileo-femoral thrombosis at 10 years of age, a much earlier age than expected for the typical thromboses occurring with a direct type I deficiency of antithrombin, which rarely results in thrombosis before 16 years of age. In particular, the association of the onset of the thrombosis in the antithrombin Wobble proband with a preceding pyrexial respiratory infection is typical of the episodic presentation previously described with another conformational variant, antithrombin Rouen-VI.13 This originally presented in a 20-year-old with a vena caval thrombosis, associated with a preceding pneumonic fever.
Further examples of such episodic thrombosis have been described in families with other molecular abnormalities of antithrombin likely to cause conformational instability.10,33 However, these families have been difficult to investigate due to an almost complete deficiency from their plasma of the abnormal antithrombin, leading to their mistaken classification as type I, nil-synthesis deficiencies. This labeling would indeed have occurred with family 2 here had it not, by chance, been accompanied by the identification in family 1 of a much less severe mutation (antithrombin Wibble) at precisely the same molecular site on the antithrombin molecule. The consequence of the less drastic mutation in family 1 is a much milder molecular instability, with near normal levels of the mutant antithrombin in the plasma but with a clearly demonstrable susceptibility to conformational change and loss of inhibitory function. Clinically, the abnormality by itself probably has only borderline effects, although it is likely to be a critical factor in the triggering of the series of thromboembolic events in family 1. Nevertheless, it is probable that these have only occurred due to the concomitant presence in the family (Fig 1) of two additional predispositions to thrombosis—heterozygous protein S deficiency and the factor V Leiden abnormality.31
Molecular Lesions and Instability
Interest in the detailed structural pathology of these two new variants was aroused by the recognition that the mutations had occurred at a site on the molecule known to have a critical function in controlling the conformational stability of antithrombin.9 A previous study of the structural pathology of the serpins10 had identified a vulnerable zone of the molecule that underlies the opening of its main β-pleated sheet, the A-sheet (Fig 6A and B). In particular, the studies of Yu et al36 had shown that even a minor steric decrease at the site of the conserved threonine 85 (59 in α1-antitrypsin) resulted in a significant increase in the stability of the molecule. They replaced this threonine by a smaller alanine and showed that this resulted in a tightening of the closed 5-stranded A-sheet present in the active form of the molecule (Fig6A-I). As compared with this stable recombinant mutant, the two mutations identified in this study both involve the substitutions of bulkier sidechains at position 85 (Fig 6B) and hence will predictably result in a decreased conformational stability. As expected, the replacement of threonine 85 by the large and highly polar lysine in antithrombin Wobble gives an instability of such severity that the variant is substantially (but not completely) lost from the plasma. However, replacement of threonine 85 by the bulky but nonpolar methionine in antithrombin Wibble results in a small, but demonstrable, decrease in thermal stability, as shown in Fig 4. Strong evidence that this instability results from a more ready opening of the A-sheet is provided by the reversion of the variant to full stability (Fig 4) on the bolstering of the 5-stranded conformation by the addition of the high-affinity heparin pentasaccharide (Fig 6C-II).
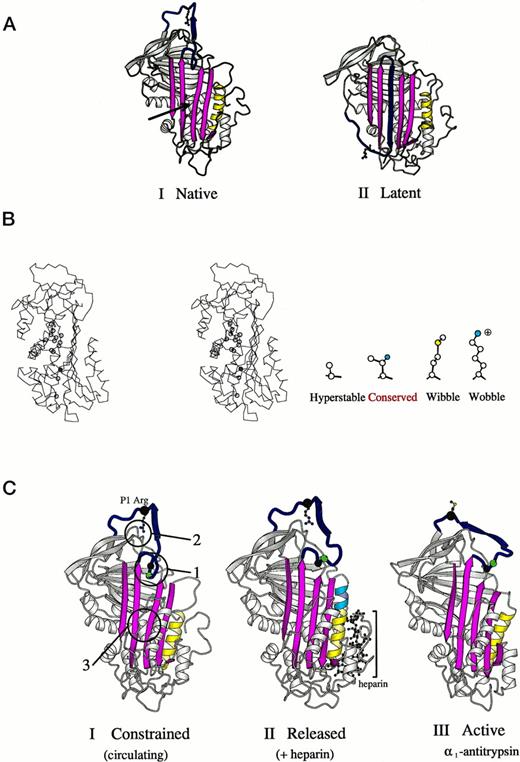
(A) Crystallographic structures15,39,42 of antithrombin (I) and its latent form (II) in a schematic ribbon depiction illustrating the mousetrap-like mechanism of inhibition. The reactive center loop of the native molecule (blue with Arg393 as ball and stick model) can spontaneously insert as shown here as the middle strand of the A-sheet (magenta) to give the inactive but stable latent conformation. The region critical to sheet opening is indicated by the arrow in I. (B) Stereo outline showing a side view of the region underlying the opening of the A-sheet of antithrombin as arrowed in (A-I). The position of amino acids known, from other mutants,10,36 to affect the stability of sheet movement are shown as circles. Filled in blue is threonine 85 (59 in α1-antitrypsin), the site of the Wibble and Wobble mutations. The nature of amino acid mutations at this site and their effect on stability are indicated in the side diagram. (C) Crystallographic depictions of the structure of antithrombin15 before (I) and after (II) activation by the heparin pentasaccharide, with (III) the fully activated archetypal conformation of 1-antitrypsin.43 The structures show the sequential rearrangements involved in activation with closure of the 5-stranded A-sheet and exposure of the side chain of the reactive center. Identified constraints that hold the molecule in its low-activity (circulating) form are numbered in I. 1, the insertion of the hinge of the loop into the A-sheet; 2, the hydrogen bonding of the reactive center arginine to the body of the molecule; and 3, the stability of the semi-opened A-sheet, (Adapted and reprinted with permission from Jin et al.15 Copyright 1997 National Academy of Sciences, U.S.A.)
(A) Crystallographic structures15,39,42 of antithrombin (I) and its latent form (II) in a schematic ribbon depiction illustrating the mousetrap-like mechanism of inhibition. The reactive center loop of the native molecule (blue with Arg393 as ball and stick model) can spontaneously insert as shown here as the middle strand of the A-sheet (magenta) to give the inactive but stable latent conformation. The region critical to sheet opening is indicated by the arrow in I. (B) Stereo outline showing a side view of the region underlying the opening of the A-sheet of antithrombin as arrowed in (A-I). The position of amino acids known, from other mutants,10,36 to affect the stability of sheet movement are shown as circles. Filled in blue is threonine 85 (59 in α1-antitrypsin), the site of the Wibble and Wobble mutations. The nature of amino acid mutations at this site and their effect on stability are indicated in the side diagram. (C) Crystallographic depictions of the structure of antithrombin15 before (I) and after (II) activation by the heparin pentasaccharide, with (III) the fully activated archetypal conformation of 1-antitrypsin.43 The structures show the sequential rearrangements involved in activation with closure of the 5-stranded A-sheet and exposure of the side chain of the reactive center. Identified constraints that hold the molecule in its low-activity (circulating) form are numbered in I. 1, the insertion of the hinge of the loop into the A-sheet; 2, the hydrogen bonding of the reactive center arginine to the body of the molecule; and 3, the stability of the semi-opened A-sheet, (Adapted and reprinted with permission from Jin et al.15 Copyright 1997 National Academy of Sciences, U.S.A.)
The initial demonstration of the effects of the instability of antithrombin Wibble was performed by the standard procedure21 of incubation for 2 hours at a range of temperatures (Fig 3A). This showed, as a dominant feature, the premature formation of polymeric forms, as previously described with the loop-sheet polymers of α1-antitrypsin in which the reactive loop of one molecule inserts into the opened A-sheet of the next molecule and so on.11 However, a slower exposure of antithrombin Wibble to a range of temperatures, from 41°C to 50°C for 24 hours, showed the predominant conformational change to be monomeric rather than polymeric.
Latent Transition
It was proposed12 in 1991 that the intact reactive center loop of the serpins could become incorporated as the middle strand of the A-sheet and that this would not only explain the observed properties of latent plasminogen activator inhibitor (PAI-1), but also be inducible in other plasma serpins, notably in antithrombin. This has indeed proved to be so, with, as well as PAI-1,37 the formation of the latent conformation being inducible in α1-antitrypsin,21α1-antichymotrypsin,38 and, as has been crystallographically confirmed,15,39 in antithrombin (Fig6A-II). The occurrence of the latent transition of antithrombin has been shown to have biological implications in that it is a complicating product in the pasteurization of fractionated plasma antithrombin14 and it has also been shown to be formed by the in vitro incubation of the unstable Rouen-VI variant of antithrombin.13 However, until the investigation of family 1 with antithrombin Wibble, there had been no evidence that the latent transition could occur as a spontaneous in vivo phenomenon.
The results from the heparin-Sepharose chromatography of plasma from the Wibble heterozygote (Fig 2) indicated a substantial new antithrombin component (peak II) detected as a shoulder of the large peak eluting at 0.3 mol/L NaCl. Electrophoresis of this material showed it had the characteristic mobility of latent antithrombin (Fig 3D) on both native and SDS-PAGE, along with the equally characteristic hyperstability20 of the latent form both on TUG-gel electrophoresis (not shown) and on thermal exposure (Fig 4).
During the process of calibrating the chromatographic elution of latent antithrombin, the surprise finding was made in a normal plasma sample of a trace amount of latent antithrombin, estimated at near 1% of the total plasma antithrombin. This, together with a somewhat smaller amount of proteolytically cleaved antithrombin, indicates likely pathways contributing to the normal turnover and senescence of the protein.
Along with other structural evidence,4,10,15,36 these results fit well with the analogy, in the introduction to this report, between the conformation of the plasma serpins and the metastable state of a set mousetrap. The conformational state of antithrombin is similarly metastable and the observation of small amounts of latent antithrombin in normal plasma is compatible with the occurrence of occasional spontaneous conformational transitions during the lifetime of the antithrombin in the plasma. The results from families 1 and 2 support the conclusion that this slow rate of transition will be greatly accelerated both in vivo and in vitro by the mutations present in the critical zone underlying the opening of the A-sheet of the molecule. As shown with antithrombin Wibble, this results in a spontaneous transition to the latent form and the mutation present in antithrombin Wobble will predictably result in even greater instability. Thus, with this abnormality, the occurrence of even a small increase in body temperature, as in the proband of family 2 during a respiratory infection,32 could suffice to cause a large-scale transition with loss of inhibitory activity and consequent sudden and severe thrombosis.
Heparin Affinity and Activation
The bonus finding from the investigation of antithrombins Wibble and Wobble are the insights they provide as to the conformational contribution to the binding and activation of antithrombin by heparin. Both variant antithrombins showed an extraordinary increase in heparin affinity as evidenced in Fig 2, with the elution of antithrombin requiring 1.5 mol/L NaCl and that of the Wobble variant (not shown) requiring 1.6 mol/L NaCl. Quantitatively the Kd for binding of antithrombin Wibble and the heparin pentasaccharide (Table 2) is by far the highest affinity yet recorded for either natural or modified forms of antithrombin. Analysis of the kinetics of binding (Fig 5 and Table3) shows that the increase primarily results from the greater stability of the complex formed between the pentasaccharide and heparin. The large increase in the constant defining the conformational change upon heparin pentasaccharide binding, k2, indicates that the rate of this change is increased significantly in antithrombin Wibble, both as compared with normal antithrombin and also with the already increased rate in antithrombin Rouen VI. The higher rate order of interaction of antithrombin Wibble with factor Xa (Table 2) is also an indication of an increased release of its reactive loop into the active inhibitory configuration. Both of these results fit with the predicted likelihood that the Wibble and Wobble variants will have an increased flexibility in the opening and shutting of the A-sheet, giving a more ready release of the reactive loop and also a more ready transition to the closed form of the sheet on the addition of heparin (Fig 6C).
Taken together, the results from this study with other recent work15,40,41 strongly support the concept that antithrombin is held in its relatively inactive and low heparin-affinity form by a summation of constraints that are collectively released by complex interactions initiated on the binding of heparin (Fig 6C). These include the release of the reactive loop from the A-sheet, a breaking of the hydrogen bonds formed by the reactive centre arginine with the body of the molecule, and a closing of the A-sheet (Fig 6C-I). There is now evidence that changes at any of these foci of constraint can by themselves result in a transition to the high heparin-affinity conformation.40 The additional understanding provided by this present study, in a particularly clear-cut way, is the dependence of the maintenance of the natural low heparin-affinity state on the stabilization of the A-sheet. Thus, useful though it may be to think of the effects of heparin in terms of a domino mechanism, with well-defined changes being sequentially propagated throughout the molecule from the binding site, the activation mechanism is likely to be much more complex than this, with the binding of heparin flipping the molecule from a constrained to an unconstrained form. Of course, this flipping of a molecular conformation will occur that much more readily if it already has a wibble or a wobble!
N.J.B. and R.N.P. contributed equally to this report.
Supported in part by the Wellcome Trust, the British Heart Foundation, and the MRC of Great Britain.
Address reprint requests to R.W. Carrell, FRCP, PhD, Department of Haematology, University of Cambridge, MRC Centre, Hills Road, Cambridge CB2 2QH, UK.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 3. (A) Nondenaturing PAGE electrophoresis of isolated antithrombins Wibble and normal control incubated for 2 hours at a range of temperatures as indicated. Because of limited material staining was by immuno- (Western) blotting. The gels show the formation of polymers, commencing at 50°C with antithrombin Wibble and 60°C with the normal control. (B) Electrophoresis in nondenaturing PAGE of antithrombin from the abnormal (Wibble) peak IV (see Fig 2) and the normal peak III. The two samples were incubated at 50°C for 24 hours with removal of aliquots at times denoted on the figure. The appearance of the faster latent band begins at 6 hours and is almost complete at 24 hours. Note the latent form just appearing at 24 hours in the normal control. Traces of polymers are present in the peak IV sample after 6 hours of incubation. (Staining is with Coomassie blue, as compared with the more sensitive immuno-staining of [A]). (C) Nondenaturing PAGE electrophoresis of antithrombin Wibble (peak IV, Fig 2) before (W) and after (W41)° 24 hours of incubation13 at 41°C, pH 8.0; standards for latent (L) and normal () antithrombin are shown; and also β-antithrombin (β) to exclude contamination of peak IV. The gel is sensitively silver-stained; note the absence of polymers in the W41° band, which shows almost complete conversion to the latent form. (D) Electrophoresis in nondenaturing PAGE of samples (at two different concentrations) from peak II and peak IV of the heparin-Sepharose chromatography of a carrier of antithrombin Wibble (Fig 2). On left is a control (normal ) antithrombin from peak III, and on the right is a control latent antithrombin.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/8/10.1182_blood.v92.8.2696/5/m_blod42043003w.jpeg?Expires=1769255603&Signature=PF1mGAXEbNnvxOuUB3NvOxvz3AjjSItEFc3xrZH7CVOJcwZlUusVcJORUWt7iusPJgj72CmUWwvZ7dRJw8oFBHO9G5Yjwdds6X8oi44gUdGC3TfWZcN1rVH1GQiNoHF5xQlMUwNyO7OgzmpL5UrIUW-OK2xYofOoKEJM-7Zl~euRdoEyqJW1FhIsIT4Myxs6yrqkDiGAL4Zds184lVmlo1lmI5N1~KYtL48g4UaF2FJbX71fEqd7RAMhaUdfErtsv0pl1~FzoiPS5w-dfaZ7DOTUfPqKoKrCklBZB7gPdII-I1gTwR9RbpmCPjovLj2Zfcst6V~uh1M~g-OEORIPOQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal