To the Editor:
Recently, many gene alterations have been identified as causes of leukemia, most of which are gross rearrangements of transcription factors, receptors, and kinases derived from chromosomal translocations. In addition, mutations of tyrosine kinase receptors such as c-kit1 and FLT-32 have been reported in mastocytosis and myeloid leukemia, respectively. In particular, it is noticeable that duplication of the juxtamembrane region of FLT-3 is observed in 20% of patient leukemic cells. However, no cytokine receptors (type I cytokine receptor family) have been reported to be involved in human leukemia except that truncation of the C-terminal domain of the granulocyte colony-stimulating factor (G-CSF) receptor caused by various point mutations is implicated in a fraction of leukemic patients. Most of these leukemias are secondary acute myeloid leukemias (AMLs) developed from Kostmann syndrome, and the significance of the mutations in leukemogenesis is still controversial.3 4
MPL, thrombopoietin (TPO) receptor, is the only hemopoietin receptor (type I cytokine receptor family) identified as an oncogene.5,6 Thus, MPL was originally identified as a truncated form v-mpl that is an oncogene of a murine retrovirus MPLV, which causes myeloproliferative disorders in mice, and was later recognized as a receptor for TPO. Using a combined strategy including polymerase chain reaction (PCR)-driven random mutagenesis and retrovirus-mediated high-efficiency gene transfer, we have recently identified a constitutive active form of MPL.7 This point mutation causes a single amino acid substitution from Ser498 to Asn498 in its transmembrane domain. Expression of the mutant MPL in a mouse interleukin-3 (IL-3)–dependent pro-B cell line Ba/F3 resulted in constitutive activation of both the Ras-Raf-MAPK and the Jak-STAT pathways and IL-3–independent growth. Moreover, when the Ba/F3 transfectants expressing the mutant MPL were injected into syngeneic mice after sublethal irradiation, they developed severe infiltration of the Ba/F3 transfectants in liver and spleen, suggesting that the mutant form of MPL is highly oncogenic in vivo. We were interested in whether similar mutations can be found in patients' leukemic cells, and examined the sequence of the transmembrane portion of MPL in 43 patients, including 2 patients with essential thrombocytosis (ET), 6 with AML-M1, 6 with AML-M2, 1 with AML-M3, 3 with AML-M4, 2 with AML-M5, 9 with AML-M6, 12 with AML-M7 (megakaryoblastic leukemia), and 2 with myelodysplastic syndromes (MDS).
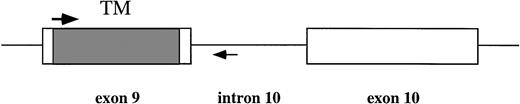
To sequence the corresponding part in the patients' sample and to avoid the contamination of the plasmid harboring the mutant MPL, a DNA fragment spanning the transmembrane portion of MPL (exon 9) and a part of intron 10 of the human MPL gene8 was amplified from high-molecular-weight DNA by PCR using a 5′ transmembrane primer (ATCTCCTTGGTGACC) and a primer in the 10th intron (AGATCTGGGGTCACACAGAG) (Fig 1). To avoid mutations during the recovery procedure as much as possible, we usedPfu polymerase for the reaction. PCR fragments were subcloned into the TA vector, and at least six subclones were sequenced for each patient. However, no mutations were found in the transmembrane portion of MPL. Our results indicate that the mutation in the transmembrane region of MPL is not a frequent cause of leukemogenesis.
PCR primers to amplify the transmembrane region of MPL from genomic DNA. TM (shadowed box), transmembrane region; arrows, PCR primers.
PCR primers to amplify the transmembrane region of MPL from genomic DNA. TM (shadowed box), transmembrane region; arrows, PCR primers.
There are two major transcripts for MPL, a full-length MPL-P and an alternative splicing form MPL-K.6 MPL-K is supposed to be translated from an alternative spliced mRNA harboring intron 10 after exon 9 encoding the transmembrane region (Fig 1). The function of MPL-K product was not known.6 During the course of our screening for MPL mutations in leukemic patients, we happened to find a sequence error in the sequence of intron 10 that had been published as a part of the MPL-K transcript (Fig 1). Thus, the sequence CG (1616-1617) was GGCC (1616-1619) in all patients tested as well as in a normal control, which will result in frame-shift and earlier termination in the MPL-K product (Fig 2). The predicted length of the intracellular domain of MPL-K should be 36 instead of 66 amino acids. To confirm this, it is required to molecularly clone cDNA for MPL-K and confirm the sequence of the corresponding part.
Structures of MPL-P and MPL-K. Amino acid sequences of the C-terminal half of the transmembrane region and the intracellular region are shown for MPL-P and MPL-K together with the putative correct MPL-K (MPL-Kc). The underlined sequence indicates the C-terminal half of the transmembrane domain. The amino acid sequences that are common between MPL-P and MPL-K and between MPL-K and MPL-Kc are indicated by vertical lines. The numbers on both sides are the amino acid number from the first methionine.
Structures of MPL-P and MPL-K. Amino acid sequences of the C-terminal half of the transmembrane region and the intracellular region are shown for MPL-P and MPL-K together with the putative correct MPL-K (MPL-Kc). The underlined sequence indicates the C-terminal half of the transmembrane domain. The amino acid sequences that are common between MPL-P and MPL-K and between MPL-K and MPL-Kc are indicated by vertical lines. The numbers on both sides are the amino acid number from the first methionine.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal