Abstract
Paroxysmal nocturnal hemoglobinuria (PNH) is a clonal stem cell disorder characterized by complement-mediated hemolysis and deficient hematopoiesis. The development of PNH involves an acquired mutation in the X-linked PIG-A gene, which leads to incomplete bioassembly of glycosylphosphatidylinositol (GPI) anchors and absent or reduced surface expression of GPI-linked proteins. The origin and mechanisms by which the PNH clone becomes dominant are not well understood, but recently resistance to apoptosis has been postulated. To test the hypothesis that the PIG-A mutation and absence of GPI-linked surface proteins directly confer resistance to apoptosis, we isolated peripheral granulocytes from 26 patients with PNH and 20 normal controls and measured apoptosis induced by serum starvation. Granulocytes from patients with PNH were relatively resistant to apoptosis (38.8% ± 14.1%) as compared with granulocytes from controls (55.0% ± 12.0%, P < .001). However, this resistance to apoptosis was not related to the dominance of the PNH clone because patients with a low percentage of GPI-deficient granulocytes had a similar rate of apoptosis as those with a high percentage of GPI-deficient granulocytes. Similarly, the resistance to granulocyte apoptosis was not influenced by the degree of neutropenia or a prior history of aplastic anemia. To investigate formally the importance of GPI-linked surface proteins in apoptosis, we introduced the PIG-A cDNA sequence into the JY5 GPI-negative B-lymphoblastoid cell line using two different methods: (1) stable transfection of a plasmid containing PIG-A, and (2) stable transduction of a retroviral vector containing PIG-A. We then measured rates of apoptosis induced either by Fas antibody, serum starvation, or γ-irradiation. With each stimulus, apoptosis of JY5 with stable surface expression of GPI-linked proteins was not statistically different from the parent JY5 cell line or the JY25 (GPI-positive) cell line. Our data confirm that granulocytes from patients with PNH have a relative resistance to apoptosis as compared with normal granulocytes. However, this resistance does not vary with the level of expression of GPI-linked proteins, and stable introduction of PIG-A cDNA with correction of GPI-linked surface expression does not change the rate of apoptosis. Taken together, our data do not support the hypothesis that the PIG-A mutation and absence of GPI-linked surface proteins directly confer resistance to apoptosis in PNH. We conclude that the resistance to apoptosis in PNH is not related to the PIG-A mutation, indicating that other factors must be important in the origin of this phenomenon and the clonal dominance observed in PNH.
PAROXYSMAL NOCTURNAL hemoglobinuria (PNH) is an acquired clonal hematologic disorder that is characterized by a wide variety of clinical manifestations, including episodic hemolysis, venous thrombosis, deficient hematopoiesis, and occasionally, leukemia.1,2 The biochemical defect in PNH involves the defective synthesis of glycosylphosphatidylinositol (GPI) anchors that are used by certain surface proteins on hematopoietic cells.3-6 As a result of this defect, affected cells are missing all surface proteins that use GPI linkage, including proteins involved in complement regulation, immunologic receptors, enzymes, and several with unknown function.7,8 All blood cells are affected, including erythrocytes, granulocytes, monocytes, platelets, and lymphocytes.9-11 The molecular defect found in PNH is one or more acquired (nongermline) mutations in PIG-A, an X-linked gene involved in the first step of GPI anchor biosynthesis.12-16Patient mutations are predominantly of the frameshift type, and PIG-A mutations leading to partial or complete loss of PIG-A protein function have been identifed in all patients with PNH reported to date.17
Despite the elucidation of the biochemical and molecular defects in PNH, several unanswered questions remain. The association between aplastic anemia and PNH is poorly understood,18,19especially the evolution of aplastic anemia into PNH after treatment with immunomodulatory agents such as antithymocyte globulin or cyclosporine A.20-22 Equally puzzling is the observation that abnormal PNH cells typically achieve clonal dominance within the bone marrow and peripheral blood. In many patients with PNH, greater than 80% of their circulating granulocytes and erythrocytes are GPI-deficient,11,23 suggesting that the abnormal PNH clone has a distinct growth advantage over normal hematopoietic progenitors. Bessler et al24 have suggested that in patients with aplastic anemia, an acquired PIG-A mutation in a totipotent stem cell provides a growth advantage that allows marrow recovery from the aplasia. The nature of this growth advantage, ie, a proliferative versus a survival advantage, has not been elucidated to date. Brodsky et al25 recently reported that granulocytes from four patients with PNH had a relative resistance to apoptosis. Further, they reported that replacement of the PIG-A sequence into a deficient B-cell line reversed this resistance to apoptosis. The authors concluded that the PIG-A mutation and GPI-linked surface proteins are critical for the regulation of apoptosis, and that resistance to apoptosis in PNH leads to clonal dominance and possibly transformation into leukemia.25
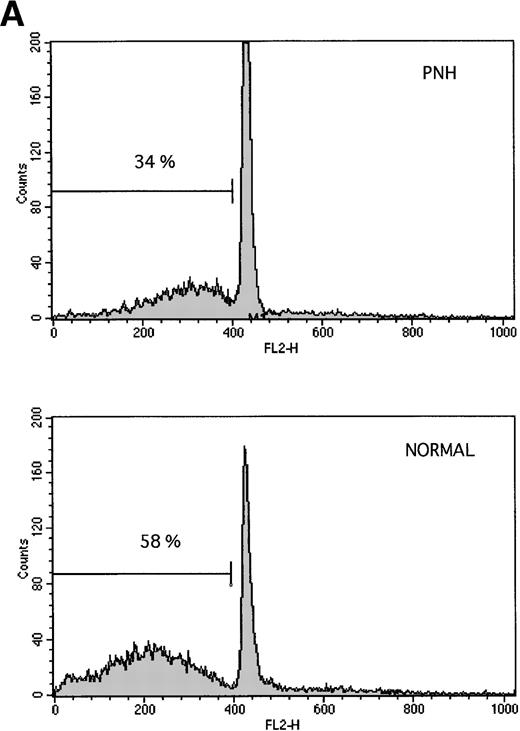
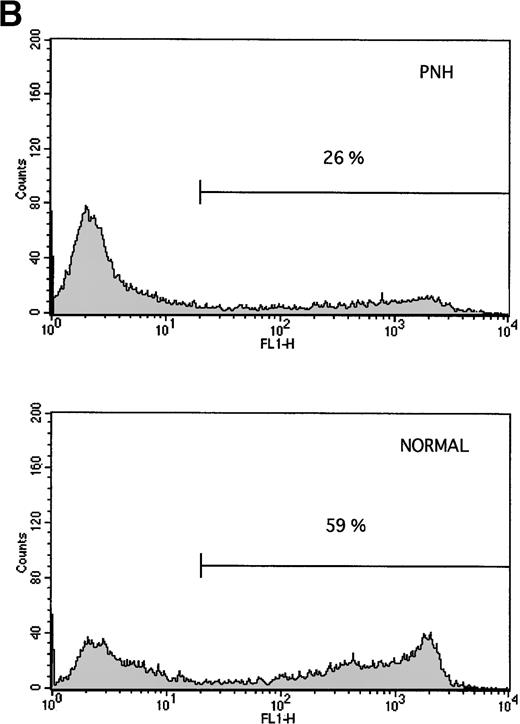
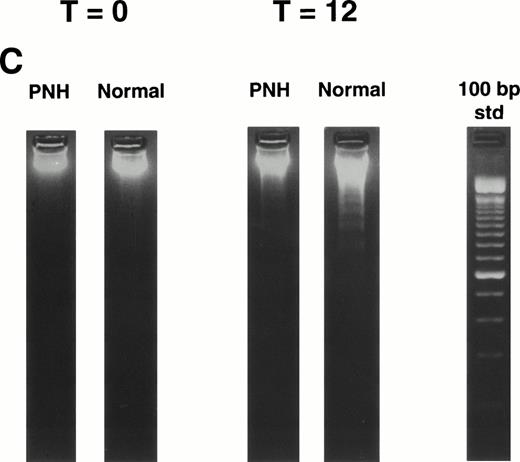
Granulocytes from PNH patients resist apoptosis as compared with normal granulocytes. Freshly purified granulocytes were serum starved and then analyzed for evidence of apoptosis. (A) PNH granulocytes at 12 hours were stained with propidium iodide and analyzed by flow cytometry. The narrow, tall peak on each histogram represents cells with a normal (2n) amount of DNA, whereas the broader peak represents apoptotic cells with a sub-2n amount of DNA. The granulocytes from a PNH patient have less apoptosis (34% sub-2n PI staining) than those from a normal control (58%). (B) Cells at 6 hours were stained with annexin V-FITC and PI to identify apoptotic cells that are positive for annexin binding but exclude PI. The granulocytes from a PNH patient have less apoptosis (26% annexin V-FITC staining) than those from a normal control (59%). (C) Genomic DNA was analyzed for fragmentation. At time zero no laddering was observed, but after 12 hours of serum starvation, DNA from PNH granulocytes had less laddering than the DNA from the granulocytes of a normal control.
Granulocytes from PNH patients resist apoptosis as compared with normal granulocytes. Freshly purified granulocytes were serum starved and then analyzed for evidence of apoptosis. (A) PNH granulocytes at 12 hours were stained with propidium iodide and analyzed by flow cytometry. The narrow, tall peak on each histogram represents cells with a normal (2n) amount of DNA, whereas the broader peak represents apoptotic cells with a sub-2n amount of DNA. The granulocytes from a PNH patient have less apoptosis (34% sub-2n PI staining) than those from a normal control (58%). (B) Cells at 6 hours were stained with annexin V-FITC and PI to identify apoptotic cells that are positive for annexin binding but exclude PI. The granulocytes from a PNH patient have less apoptosis (26% annexin V-FITC staining) than those from a normal control (59%). (C) Genomic DNA was analyzed for fragmentation. At time zero no laddering was observed, but after 12 hours of serum starvation, DNA from PNH granulocytes had less laddering than the DNA from the granulocytes of a normal control.
To test the hypothesis that the PIG-A mutation and absence of GPI-linked surface proteins directly confer resistance to apoptosis, we analyzed GPI-deficient cells for the ability to resist apoptosis after stimulation with apoptotic signals. Peripheral granulocytes from 26 patients with PNH had significantly less apoptosis after serum starvation than granulocytes from normal controls. However, stable introduction of PIG-A cDNA into the GPI-deficient JY5 cell line with correction of surface expression of GPI-linked proteins did not change the rate of apoptosis. Our data provide direct evidence that resistance to apoptosis in PNH is not related to the underlying PIG-A mutation or the lack of GPI-linked surface protein expression. Because the resistance to apoptosis in PNH is not directly related to the PIG-A mutation, other factors are necessary to explain this resistance to apoptosis and the clonal dominance observed in patients with PNH.
MATERIALS AND METHODS
Cells.
Venous blood was collected in EDTA from patients with PNH after informed consent using an institutional review board–approved protocol, and granulocytes were purified as previously described.6 JY5, a GPI-negative Epstein-Barr virus–transformed B lymphoblastoid cell line, and its normal GPI-positive counterpart JY25 (gifts from Dr Timothy Springer, Harvard) were maintained in RPMI 1640 with 10% fetal calf serum (FCS; GIBCO, Gaithersburg, MD). The T-cell line Jurkat, also grown in RPMI with 10% FCS, was used as a positive control for apoptosis.
Antibodies and reagents.
Monoclonal antibodies (MoAbs) included anti-Fas.3 (IgG1) for the detection of CD95 (Fas) antigen (Calbiochem, Cambridge, MA), anti-Fas CH-11 (IgM) for the induction of apoptosis (Upstate Biotech Inc, Lake Placid, NY), P3 as a negative isotype control (gift from Dr Barton Haynes, Duke), CD55 ascites and CD59 ascites for detection of GPI-deficient cells,23 and goat-anti-mouse-fluorescein isothiocyanate (FITC) (Kierkegaard, Gaithersburg, MD) for secondary amplification in immunophenotype assays. MoAbs CD59-FITC (Pharmingen, San Diego, CA) and NGFR-PE (Chromoprobe, Mountain View, CA) were used to document transduction of cells with retroviral vectors. Hygromycin-B (GIBCO) was used to select transfected cells. Propidium iodide (PI; Boehringer Mannheim, Indianapolis, IN) was used to assess cell viability and identify apoptotic cells. Annexin V-FITC (Caltag, Burlingame, CA) also was used to identify apoptotic cells.
Plasmids.
The pEB plasmid (mock control) and the plasmid containing the PIG-A cDNA sequence (pEBPIG-A) were generously provided by Dr Taroh Kinoshita (Osaka University, Osaka, Japan).12,26 These plasmids were used to stably transfect the GPI-deficient JY5 cell line by electroporation and allow hygromycin-B selection (400 μg/mL) as previously described.12 26
Retroviral vectors.
The retroviral vectors used for stable transduction of the JY5 cell line included a negative (mock) vector called MN27-30 and a vector containing the PIG-A cDNA sequence termed MPIN.31The MPIN vector was constructed using the MN vector and adding the PIG-A cDNA sequence and an internal ribosome entry site sequence.32 The MN and MPIN amphotropic vector packaging lines were generated by transfection of the ecotropic retroviral vector producer line E86, followed by infection of the amphotropic producer AM12.30,31 33
For retroviral vector gene transfer, JY5 cells (2 × 106) were centrifuged with 1 mL of freshly thawed MN or MPIN supernatant and 8 μg/mL of polybrene (Sigma, St Louis, MO) at 2,500 rpm for 90 minutes at room temperature (RT), and then incubated overnight at 37°C. The transduction procedure was repeated two additional times, and cells were resuspended in fresh media.31 After transduction with MN supernatant, NGFR-expressing JY5 cells (termed JY5/MN) were isolated by one round of fluorescence-activated cell sorting (FACS), and stable expression (>95%) of surface NGFR was confirmed. After transduction with MPIN supernatant, NGFR- and CD59-expressing JY5 cells (termed JY5/MPIN) were isolated by two rounds of FACS sorting, and stable expression (>98%) of both surface markers was confirmed.31
Induction of apoptosis.
All apoptosis experiments using freshly isolated peripheral granulocytes were performed with triplicate data points, using serum starvation for exactly 12 hours as the apoptotic stimulus.34 After the granulocytes were purified, aliquots of 1.0 × 106 cells in 1 mL of RPMI were placed in 12 × 75–mm polypropylene tubes (Becton Dickinson Labware, Lincoln Park, NJ) and incubated in 5% CO2 at 37°C. All apoptosis experiments using cell lines also were performed using triplicate data points, and six to eight independent experiments were performed for each apoptotic stimulus. Cell lines were grown to log phase in RPMI 10% FCS at 37°C with 5% CO2 before induction of apoptosis. Aliquots of 1.0 × 106 cells in 0.5 mL of media were placed into 12 × 75–mm polystyrene tubes (Becton Dickinson) with various apoptotic signals and incubated for 24 hours (Fas MoAb CH-11 at 500 ng/mL) or 96 hours (serum starvation or 4,000 cGy γ-irradiation from a 62Cs source) in 5% CO2 at 37°C.
Measurement of apoptosis.
Cells were obtained at the specified time points and analyzed for apoptosis using flow cytometry. Cell membranes were permeabilized with 50% ethanol and incubated with PI to stain DNA35 and then analyzed on a FACSCAN using LYSIS II software (Becton Dickinson, San Jose, CA). Cells with a hypodiploid amount of DNA (<2n, pre-G0G1) were considered to be apoptotic due to loss of fragmented DNA.36 Alternatively, cells were stained with annexin V-FITC and PI and analyzed by flow cytometry.37 38 Genomic DNA was extracted from 2.0 × 106 cells using a commercial kit (Gentra Systems, Minneapolis, MN) and resolved using a 2.5% agarose gel with ethidium bromide staining to identify the characteristic “laddering” of DNA fragments indicative of apoptosis.
Statistics.
The statistical analysis was performed using the Primer of Biostatistics (McGraw-Hill, New York, NY). Statistical difference between two groups, eg, PNH patients versus controls, was performed using the Student's t-test. Differences among more than two groups, eg, degree of clonal dominance, was performed using analysis of variance.
RESULTS
Patient characteristics.
A total of 26 patients with PNH were studied, including 17 women and 9 men, with a median age of 33 years (range, 17 to 66). The percentage of GPI-deficient circulating granulocytes, as measured by surface CD55 expression, ranged from 3.1% to 98.9%, with a mean of 72.8% and a median of 91.1%. Eight patients had an absolute neutrophil count (ANC) of ≤2,000/μL, 10 had an ANC between 2,000 and 4,000/μL, and 8 had an ANC of ≥4,000/μL. Nine of the patients had a documented prior history of aplastic anemia. A total of 20 normal adult controls were also studied, with a median age of 32 years (range, 17 to 55).
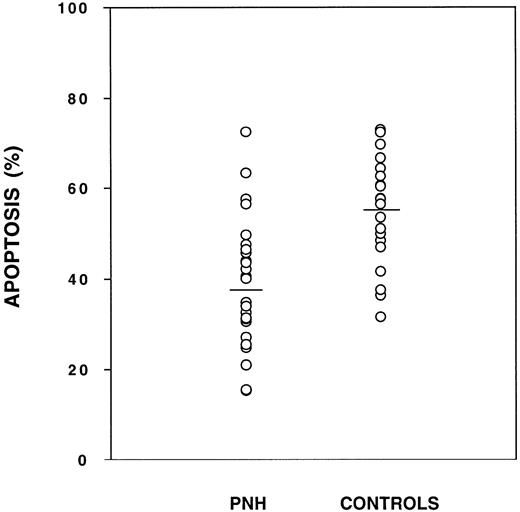
Granulocytes from PNH patients are relatively resistant to apoptosis as compared with granulocytes from normal controls. Cells were tested in triplicate and the mean is plotted for each patient (○). The mean for each group is shown as a horizontal line. The amount of apoptosis as measured by flow cytometry was significantly less for PNH granulocytes (38.8% ± 14.1%, n = 26) as compared with normal granulocytes (55.0% ± 12.0%, n = 20), P < .001. There was some overlap for individuals within each group.
Granulocytes from PNH patients are relatively resistant to apoptosis as compared with granulocytes from normal controls. Cells were tested in triplicate and the mean is plotted for each patient (○). The mean for each group is shown as a horizontal line. The amount of apoptosis as measured by flow cytometry was significantly less for PNH granulocytes (38.8% ± 14.1%, n = 26) as compared with normal granulocytes (55.0% ± 12.0%, n = 20), P < .001. There was some overlap for individuals within each group.
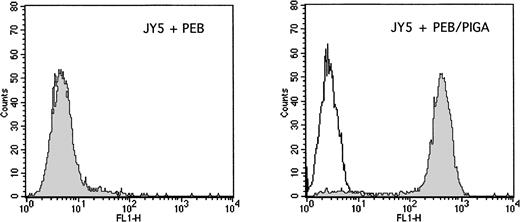
Stable transfection with the PIG-A cDNA sequence. The GPI-negative JY5 cell line was transfected by electroporation with either the PEB (mock control) plasmid or the PEB/PIGA plasmid. After hygromycin selection, cells were analyzed for the surface expression of CD59, which depends on a functioning PIG-A gene for complete assembly of GPI anchors. The left panel shows results after transfection with PEB, with negative staining observed with both the control antibody (white) and CD59 antibody (grey). The right panel shows results after transfection with PEB/PIGA, with high levels of surface CD59 observed as compared with the control antibody.
Stable transfection with the PIG-A cDNA sequence. The GPI-negative JY5 cell line was transfected by electroporation with either the PEB (mock control) plasmid or the PEB/PIGA plasmid. After hygromycin selection, cells were analyzed for the surface expression of CD59, which depends on a functioning PIG-A gene for complete assembly of GPI anchors. The left panel shows results after transfection with PEB, with negative staining observed with both the control antibody (white) and CD59 antibody (grey). The right panel shows results after transfection with PEB/PIGA, with high levels of surface CD59 observed as compared with the control antibody.
Granulocyte Apoptosis After 12 Hours of Serum Starvation in Patients With PNH and Controls
| Source of Granulocytes . | No. of Samples . | % Apoptosis (mean ± 1 SD) . | P Value . |
|---|---|---|---|
| Normal controls | 20 | 55.0 ± 12.0 | |
| PNH patients | 26 | 38.8 ± 14.1 | <.001 |
| Aplastic anemia | |||
| Yes | 9 | 37.6 ± 17.5 | |
| No | 17 | 39.2 ± 12.5 | .79 |
| Neutrophil count | |||
| ≤2,000/μL | 8 | 40.7 ± 17.9 | |
| 2,000-4,000/μL | 10 | 38.9 ± 11.5 | .92 |
| ≥4,000/μL | 8 | 37.7 ± 15.3 | |
| % GPI deficiency | |||
| ≤40% | 5 | 43.8 ± 18.6 | |
| 40%-90% | 6 | 33.4 ± 18.2 | .49 |
| ≥90% | 12 | 37.2 ± 9.3 |
| Source of Granulocytes . | No. of Samples . | % Apoptosis (mean ± 1 SD) . | P Value . |
|---|---|---|---|
| Normal controls | 20 | 55.0 ± 12.0 | |
| PNH patients | 26 | 38.8 ± 14.1 | <.001 |
| Aplastic anemia | |||
| Yes | 9 | 37.6 ± 17.5 | |
| No | 17 | 39.2 ± 12.5 | .79 |
| Neutrophil count | |||
| ≤2,000/μL | 8 | 40.7 ± 17.9 | |
| 2,000-4,000/μL | 10 | 38.9 ± 11.5 | .92 |
| ≥4,000/μL | 8 | 37.7 ± 15.3 | |
| % GPI deficiency | |||
| ≤40% | 5 | 43.8 ± 18.6 | |
| 40%-90% | 6 | 33.4 ± 18.2 | .49 |
| ≥90% | 12 | 37.2 ± 9.3 |
Stratification of PNH patients according to prior aplastic anemia, degree of neutropenia, or clonal dominance showed no statistical differences in rates of apoptosis. Three patients did not have simultaneous quantitation of GPI-deficient granulocytes.
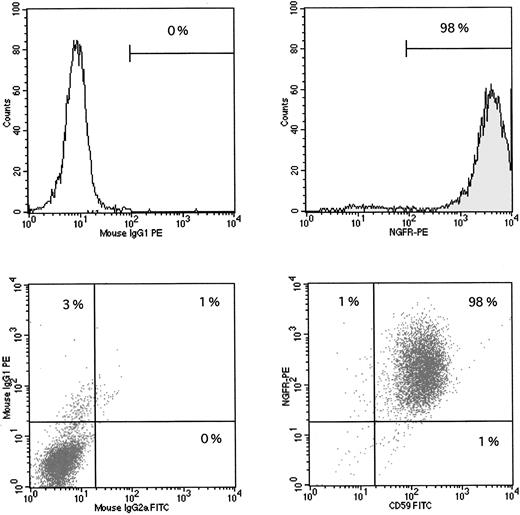
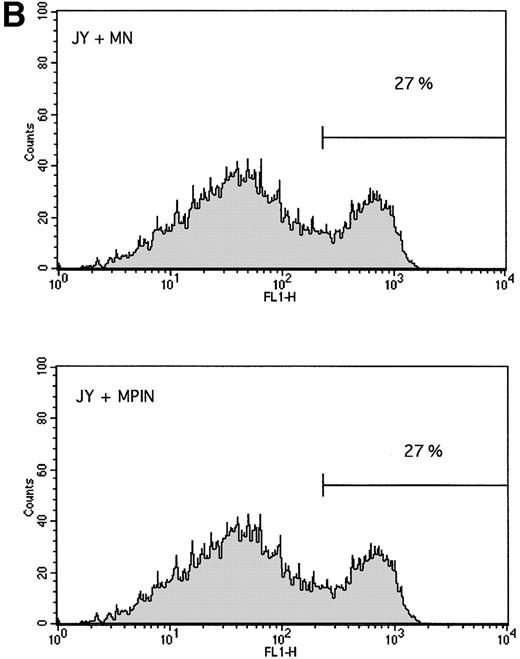
Stable transduction with the PIG-A cDNA sequence. The GPI-negative JY5 cell line was transduced with supernatants from either the MN (mock control) vector or the MPIN vector. After sorting, cells were analyzed for the surface expression of NGFR, which indicates successful transduction of the retroviral sequence, and CD59, which indicates a functioning PIG-A sequence. Upper left panel: JY5 with MN vector, control antibody. Upper right panel: JY5 with MN vector, NGFR-PE antibody, showing 98% surface expression of NGFR. Lower left panel: JY5 with MPIN vector, control antibodies. Lower right panel: JY% with MPIN vector, CD59-FITC and NGFR-PE antibodies, showing 98% surface expression of both NGFR and CD59, indicating successful transduction of the vector and a functioning PIG-A sequence.
Stable transduction with the PIG-A cDNA sequence. The GPI-negative JY5 cell line was transduced with supernatants from either the MN (mock control) vector or the MPIN vector. After sorting, cells were analyzed for the surface expression of NGFR, which indicates successful transduction of the retroviral sequence, and CD59, which indicates a functioning PIG-A sequence. Upper left panel: JY5 with MN vector, control antibody. Upper right panel: JY5 with MN vector, NGFR-PE antibody, showing 98% surface expression of NGFR. Lower left panel: JY5 with MPIN vector, control antibodies. Lower right panel: JY% with MPIN vector, CD59-FITC and NGFR-PE antibodies, showing 98% surface expression of both NGFR and CD59, indicating successful transduction of the vector and a functioning PIG-A sequence.
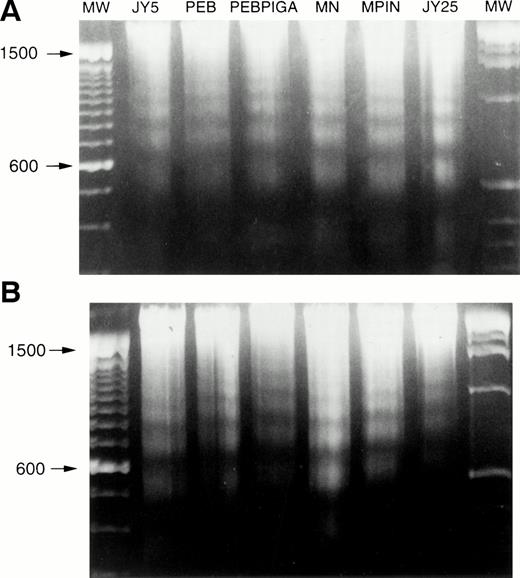
Apoptosis of cell lines. Cells were grown to log phase and subjected to apoptotic stimuli, and then genomic DNA was extracted and analyzed for DNA fragmentation. (A) Fas antibody, showing DNA laddering in all cell lines. (B) γ-irradiation, showing DNA laddering in all cell lines. MW, molecular weight.
Apoptosis of cell lines. Cells were grown to log phase and subjected to apoptotic stimuli, and then genomic DNA was extracted and analyzed for DNA fragmentation. (A) Fas antibody, showing DNA laddering in all cell lines. (B) γ-irradiation, showing DNA laddering in all cell lines. MW, molecular weight.
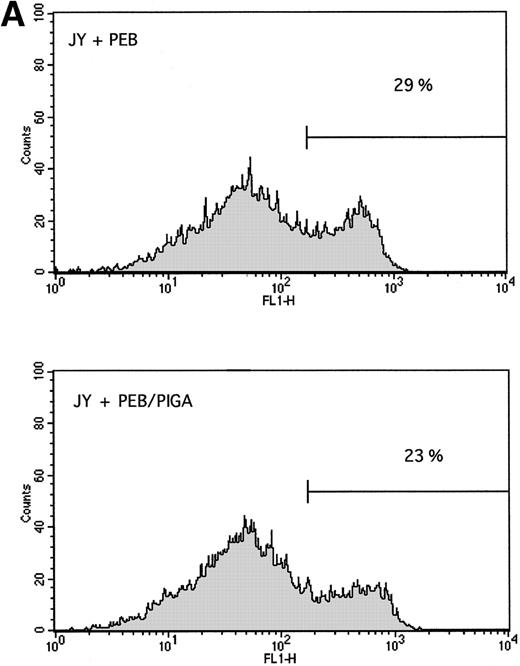
Stable expression of GPI-linked surface proteins does not affect the rate of apoptosis. The PIG-A cDNA sequence was stably introduced as described in Materials and Methods, and apoptosis was measured by annexin V-FITC binding. There was no difference in the rate of apoptosis 24 hours after γ-irradiation between (A) cells stably transfected with PEB versus PEB/PIGA, or (B) cells stably transduced with MN versus MPIN. Background levels of annexin V-FITC binding were higher for JY5 than for granulocytes.
Stable expression of GPI-linked surface proteins does not affect the rate of apoptosis. The PIG-A cDNA sequence was stably introduced as described in Materials and Methods, and apoptosis was measured by annexin V-FITC binding. There was no difference in the rate of apoptosis 24 hours after γ-irradiation between (A) cells stably transfected with PEB versus PEB/PIGA, or (B) cells stably transduced with MN versus MPIN. Background levels of annexin V-FITC binding were higher for JY5 than for granulocytes.
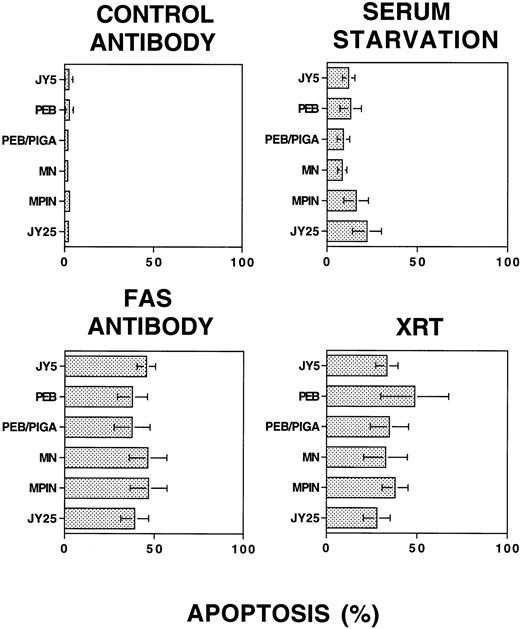
Apoptosis of cell lines does not depend on a functioning PIG-A gene sequence or levels of GPI-linked surface expression. Cells were subjected to stimuli and analyzed for apoptosis by PI staining and flow cytometry. All data points were performed in triplicate for each experiment, and the displayed results represent six to eight independent experiments (mean ± 1 SD) for each stimulus. Upper left panel: Control antibody, with minimal apoptosis detected. Lower left panel: Fas antibody, with apoptosis ranging from 37.5% ± 10.1% to 46.8% ± 10.3%. Upper right panel: Serum starvation, with apoptosis ranging from 8.5% ± 2.6% to 22.2% ± 8.0%. Lower right panel: γ-irradiation, with apoptosis ranging from 27.9% ± 7.4% to 48.8% ± 18.8%. There were no statistically significant differences among the various cell lines, according to the presence of a functioning PIG-A gene or surface expression of GPI-linked proteins.
Apoptosis of cell lines does not depend on a functioning PIG-A gene sequence or levels of GPI-linked surface expression. Cells were subjected to stimuli and analyzed for apoptosis by PI staining and flow cytometry. All data points were performed in triplicate for each experiment, and the displayed results represent six to eight independent experiments (mean ± 1 SD) for each stimulus. Upper left panel: Control antibody, with minimal apoptosis detected. Lower left panel: Fas antibody, with apoptosis ranging from 37.5% ± 10.1% to 46.8% ± 10.3%. Upper right panel: Serum starvation, with apoptosis ranging from 8.5% ± 2.6% to 22.2% ± 8.0%. Lower right panel: γ-irradiation, with apoptosis ranging from 27.9% ± 7.4% to 48.8% ± 18.8%. There were no statistically significant differences among the various cell lines, according to the presence of a functioning PIG-A gene or surface expression of GPI-linked proteins.
Apoptosis of circulating granulocytes.
Freshly purified granulocytes had less that 2% apoptosis at time zero as detected by propidium iodide staining (data not shown). After serum starvation, apoptosis was induced in both PNH and normal granulocytes (Fig 1). Using two different methods for measuring apoptosis (Fig 1A and B), granulocytes from patients with PNH had less apoptosis than granulocytes from normal controls. This difference in apoptosis was confirmed by analysis of DNA fragmentation (Fig 1C). When analyzed as a group ( Fig 2), the rate of apoptosis after serum starvation (mean ± 1 standard deviation) was significantly less for PNH granulocytes (38.8% ± 14.1%) as compared with normal granulocytes (55.0% ± 12.0%), P < .001. This resistance to apoptosis was not due to differences in the surface expression of the CD95 (Fas) antigen on the granulocytes at time zero or after serum starvation (data not shown).
We then analyzed our data according to several clinical and laboratory parameters ( Table 1). Patients with a prior history of aplastic anemia had a similar rate of apoptosis (37.6% ± 17.5%) as those with no history of aplasia (39.2% ± 12.5%),P = not significant (NS). Similarly, the resistance to apoptosis did not depend on the degree of neutropenia, because patients with an ANC ≤ 2,000/μL had a similar rate (40.7% ± 17.9%) as those with an ANC ≥ 4,000/μL (37.7% ± 15.3%),P = NS. Finally, the dominance of the PNH clone did not influence the resistance to apoptosis, because patients with ≥90% CD55-negative granulocytes had a similar rate (37.2% ± 9.3%) as those with ≤40% CD55-negative granulocytes (43.8% ± 18.6%),P = NS.
Apoptosis of cell lines.
To determine the importance of normal PIG-A gene function and surface expression of GPI-linked proteins on the rate of apoptosis, we introduced the PIG-A cDNA sequence into the GPI-deficient JY5 cell line using two different experimental strategies. As shown in Fig 3, stable transfection of JY5 with the PEB/PIGA plasmid documented greater than 98% surface expression of CD59, whereas cells transfected with the PEB (mock control) plasmid had no expression of CD59. Similarly, stable transduction of JY5 with the MPIN retroviral vector documented greater than 98% surface expression of both NGFR and CD59, whereas cells transduced with the MN (mock control) vector had only expression of NGFR (Fig 4).
Each of the cell lines, including the GPI-positive JY25 cell line, was then subjected to a variety of stimuli and analyzed for evidence of apoptosis. DNA laddering was observed in all cell lines after incubation with Fas antibody (Fig 5A) and surface expression of the Fas antigen was similar for all cell lines (data not shown). Similarly, DNA laddering was observed in all cell lines after exposure to γ-irradiation (Fig 5B) or serum starvation (data not shown). When analyzed by annexin V-FITC binding, there were no differences in the rate of apoptosis between cells transfected with PEB vs PEB/PIGA (Fig 6A), or cells transduced with MN versus MPIN (Fig 6B). These results were confirmed by PI staining, with no statistically significant differences identified among the cell lines with any of these apoptotic stimuli ( Fig 7).
DISCUSSION
An abundance of experimental data recently has emerged regarding the cellular, biochemical, and molecular defects in PNH. It is now generally accepted that in the majority of cases of PNH, an acquired PIG-A gene mutation occurs within a self-renewing totipotent stem cell and all of its progeny harbor this same PIG-A mutation. The absence of a functioning PIG-A gene leads to incomplete bioassembly of glycosylphosphatidylinositol anchors and reduced or absent surface expression of GPI-linked surface proteins. The lack of these surface proteins presumably leads to all of the clinical manifestations of PNH.17
In contrast to the abundance of information regarding the defects in PNH, the events (or series of events) that lead to the pathogenesis of PNH are poorly understood. The etiology of the acquired PIG-A gene mutation is unknown, although in a minority of cases an overall genetic instability has been postulated.39 The clinical observation that PNH can evolve from aplastic anemia,18 particularly after treatment with immunosuppressive therapy,20-22 has suggested that the absence of GPI-linked surface proteins may be advantageous for marrow recovery,24 possibly by evasion of endogenous immune suppression.19 This hypothesis is supported by the reports of patients with more than one independent PIG-A mutation,40 41 which suggests that at least some patients have a strong in vivo selection pressure to acquire PIG-A mutations.
The presence of a PIG-A mutation does not seem sufficient to explain all of the findings that occur in PNH. For example, in most patients with PNH the abnormal cells become clonally dominant, with greater than 80% of circulating erythrocytes and granulocytes commonly affected.11,23 This observation supports the hypothesis that the PIG-A mutant stem cell has an intrinsic proliferative advantage and can generate hematopoietic cells faster than normal stem cells within the marrow.42 However, several pieces of experimental data do not support this hypothesis. Our in vitro studies with PNH lymphocytes showed no difference in the proliferative capacity between purified GPI-deficient and normal peripheral T lymphocytes in response to mitogenic or antigenic stimulation.10 Studies using marrow progenitor cells from PNH patients have shown decreased in vitro colony growth as compared with that of normal volunteers.43-46 Finally, chimeric mice generated using PIG-A–disrupted embryonic stem cells did not develop an increase in the number of GPI-deficient blood cells,47 48 suggesting that the PIG-A mutation is not sufficient for clonal proliferation and dominance.
Brodsky et al25 recently reported that PNH is characterized by a resistance to apoptosis, and that the expression of GPI-linked proteins influences the rate of apoptosis. They measured apoptosis induced by serum starvation in granulocytes from 4 patients with PNH and found significantly less apoptosis than granulocytes from 5 controls. Although our experimental design was slightly different, our data for 26 patients and 20 controls are in agreement with these findings (Figs 1 and 2). Our average rate of apoptosis for PNH granulocytes as a group was significantly less than that for normal granulocytes, although there was some overlap for individuals in both groups (Fig 2). Importantly, we found no difference in the rate of apoptosis for granulocytes depending on the dominance of the PNH clone (Table 1). If the surface expression of GPI-linked surface proteins were important for resistance to apoptosis, then patients with a dominant PNH clone would be expected to have less apoptosis than those with a smaller clone. However, our data showed that patients with a dominant clone (≥90% GPI-negative granulocytes) had a similar rate of apoptosis as those with a smaller clone (≤40% GPI-negative granulocytes). These data are supported by the recent report of resistance to apoptosis in a variety of bone marrow failure syndromes including PNH, myelodysplasia, and aplastic anemia.49
To investigate formally the contribution of a functioning PIG-A gene and surface expression of GPI-linked surface proteins to the rate of apoptosis, we stably introduced the PIG-A cDNA sequence into the GPI-negative JY5 cell line by two different independent vectors using appropriate mock controls. We first electroporated the PEB/PIGA plasmid into JY5, selected with hygromycin, and established long-term transfectants that had high levels of surface CD59 expression (Fig 3). We also transduced the MPIN retroviral vector into JY5 with resulting high levels of surface CD59 expression (Fig 4). When apoptosis was induced either by Fas antibody, serum starvation, or γ-irradiation, there were no differences in the rates of apoptosis according to the presence of a functioning PIG-A gene sequence. The expression of GPI-linked surface proteins did not influence apoptosis as measured by DNA laddering (Fig 5), annexin V binding (Fig 6), or PI staining (Fig7). These results do not support the hypothesis that the PIG-A mutation and absence of GPI-linked surface proteins directly confer resistance to apoptosis in PNH.
In summary, our data confirm that granulocytes from PNH patients have reduced rates of apoptosis as compared with normal granulocytes. Our observations that the dominance of the PNH clone does not affect granulocyte apoptosis, coupled with the failure of a functioning PIG-A gene sequence to affect apoptosis of the JY5 cell line, provide evidence that this resistance to apoptosis is not due to the PIG-A mutation and the absence of GPI-linked protein expression. Although it is possible that the PIG-A mutation directly influences the apoptosis of hematopoietic progenitor cells within the bone marrow microenvironment, we hypothesize that other events influence the rate of granulocyte apoptosis in PNH, such as circulating levels of cytokines and other soluble factors,50-53 abnormal intercellular interactions within the bone marrow microenvironment,54,55 expression of bcl-2 family members,56,57 or other changes in gene and protein expression.58-60 Further studies on granulocyte apoptosis in PNH and other bone marrow disorders including aplastic anemia, inherited bone marrow failure syndromes, and myelodysplasia49,61 62 will help to explain the cause and significance of the resistance to apoptosis observed in PNH.
ACKNOWLEDGMENT
The authors thank Sharon Hall for her assistance with blood samples.
Supported by Grant No. HL-205691 from the National Heart, Lung, and Blood Institute, National Institutes of Health (R.E.W.).
Address correspondence to Russell E. Ware, MD, PhD, PO Box 2916, DUMC, Durham, NC 27710; e-mail: ware0005@mc.duke.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal