Abstract
Human neutrophils possess a very short half-life because they constitutively undergo apoptosis. Cytokines, such as granulocyte-macrophage colony-stimulating factor (GM-CSF), and other agents can rescue neutrophils from apoptosis but the molecular mechanisms involved in this rescue are undefined. Here, we show by Western blotting that human neutrophils do not express Bcl-2 or Bcl-X but constitutively express Bax. However, cellular levels of these proteins are unaffected by agents which either accelerate or delay neutrophil apoptosis. In contrast, neutrophils express the antiapoptotic protein Mcl-1 and levels of this protein correlate with neutrophil survival. Thus, cellular levels of Mcl-1 decline as neutrophils undergo apoptosis and are enhanced by agents (eg, GM-CSF, interleukin-1β, sodium butyrate, and lipopolysaccharide) that promote neutrophil survival. Neutrophils only possess few, small mitochondria, and much of the Mcl-1 protein seems to be located in nuclear fractions. These observations provide the first evidence implicating a Bcl-2 family member in the regulation of neutrophil survival. Moreover, this work also provides a potential mechanism whereby cytokine-regulated gene expression regulates the functional lifespan of neutrophils and hence their ability to function for extended time periods during acute inflammation.
THE HUMAN NEUTROPHIL constitutively undergoes apoptosis both in vivo and in vitro. The lifespan of the mature circulating neutrophil is estimated to be between 8 and 20 hours, but this can be increased to a few days if recruited into the tissues. The lifespan in vitro can also be extended by agents such as butyrate,1 granulocyte-macrophage colony-stimulating factor (GM-CSF),2-4 G-CSF,2 interleukin-1 (IL-1),2 IL-2,5 IL-4,6IL-6,7 IL-15,8 lipopolysaccharide (LPS),4,9 and glucocorticoids,10,11 all of which prolong neutrophil survival in vitro by delaying apoptosis. The mechanism by which apoptosis is delayed in neutrophils by these agents is undefined, although the involvement of Lyn kinase has been shown to be critical in the delay of apoptosis by GM-CSF.12 Most agents that can delay neutrophil apoptosis also act to increase gene expression in the neutrophil, whereas blocking protein synthesis in the neutrophil accelerates apoptosis.1 These findings suggest that the neutrophil requires active protein synthesis to avoid apoptosis, and this observation may indicate that a protein with rapid turnover is required to prevent neutrophil apoptosis.
Apoptosis can be regulated by the Bcl-2 family of proteins, with certain members of this family acting to protect from apoptosis (eg, Bcl-2, Bcl-XL, and Mcl-1), whereas others act to promote apoptosis (eg, Bax, Bcl-XS, and Bad).13 These proteins are found in the mitochondrial, endoplasmic, and nuclear membranes in other cell types, with recent evidence suggesting they act at the mitochondria to prevent apoptosis.13 The mechanism of action of these proteins in neutrophils may be predicted to be different because the mature neutrophil has few or no mitochondria.14-16 There are conflicting reports on the expression of Bcl-2 in mature neutrophils. On the one hand, the presence of Bcl-2 has been reported to be essential for neutrophils to be rescued from apoptosis17,18 and to be upregulated by LPS.19 In contrast, other reports indicate no expression of Bcl-2 in mature neutrophils.12,20-23 The Bcl-X protein is not expressed in neutrophils,22-24 no Mcl-1 was detected in protein blots of postnuclear extracts of neutrophils,22 and this protein only stained weakly by immunohistochemistry in bone marrow neutrophils.25 However, the apoptosis-promoting protein Bax is strongly expressed in neutrophils, which is suggested to explain why the neutrophil undergoes apoptosis so rapidly.22
In the course of screening a subtractive cDNA library enriched in GM-CSF–regulated clones, we isolated a clone encoding Mcl-1 (J.A.Q. and S.W.E., unpublished observation, July 1997). The Mcl-1 protein is known to be rapidly inducible in other cell types and has a high rate of turnover possibly due to its PEST motifs (proline glutamate, serine and threonine residues) targeting it for rapid proteolysis.26 27 This protein would therefore potentially fulfill the role of an apoptosis-delaying protein in neutrophils, because these cells are subject to acute regulation of apoptosis. In this study we have examined the expression of the Bcl-2 family members Bcl-2, Bcl-X, Mcl-1, and Bax in freshly isolated human neutrophils and in neutrophils treated to delay apoptosis, in an attempt to clarify the role of these proteins in neutrophil apoptosis. Our results indicate that neither Bcl-2 nor Bcl-X are expressed in mature neutrophils, but that Bax and Mcl-1 are both expressed at levels comparable with those expressed in other cell types. The expression of Mcl-1 is predominately localized to the nucleus in these cells that contain few mitochondria, whereas Bax is found in nonnuclear membranes. The expression of Mcl-1 is induced by all factors tested that delay neutrophil apoptosis (GM-CSF, butyrate, IL-1β, and LPS), with the level of expression of Mcl-1 correlating with the degree to which each agent is able to protect from apoptosis.
MATERIALS AND METHODS
Reagents.
Neutrophil isolation medium (NIM) was purchased from Cardinal Associates (Sante Fe, NM), and RPMI 1640 was from ICN Biomedicals Ltd (Thame, Oxfordshire, UK). Fetal calf serum (FCS) and L-Glutamine were from GIBCO-BRL, (Paisley, UK), rhGM-CSF was from Glaxo (Greenford, UK), and rhIL-1β from NIBSC (Potters Bar, UK). Anti–Bcl-2 monoclonal antibody (MoAb) was from Calbiochem-Novabiochem (UK) Ltd (Nottingham, UK), Bcl-X antisera from Santa Cruz Biotechnology Inc (Santa Cruz, CA), and Bax and Mcl-1 antisera from Pharmingen (San Diego, CA). Anti-rabbit IgG–horseradish peroxidase (HRP) secondary antibody, nitrocellulose membrane, electrochemiluminescence (ECL) detection reagent, and Hyperfilm ECL were all purchased from Amersham (Slough, UK). A 30% solution of acrylamide/bis-acrylamide was from Severn Biotech Ltd (Kidderminster, UK). All other specialist materials were purchased from Sigma (Poole, UK), with all reagents used being of the highest purity available.
Preparation of neutrophils.
Neutrophils were isolated from heparinized venous blood from healthy volunteers by one-step centrifugation through NIM (Cardinal Associates) as described in the manufacturer's instructions.28 After hypotonic lysis to remove contaminating erythrocytes,29neutrophils were resuspended in RPMI 1640 medium supplemented with 5% FCS and 2 mmol/L L-glutamine and counted using a Fuchs-Rosenthal hemocytometer slide. In all cases purity was greater than 97%, with viability of neutrophils as assessed by trypan blue exclusion greater than 95% immediately after purification.
Preparation of peripheral blood mononuclear cells (PBMNC).
Heparinized venous blood from healthy volunteers used for the preparation of neutrophils was spun through NIM as above, with PBMNC recovered at the upper layer.28 PBMNC were then counted as above and resuspended in RPMI 1640 medium supplemented with 5% FCS and 2 mmol/L L-glutamine.
Neutrophil incubations.
Neutrophils were resuspended at 5 × 106 cells/mL in RPMI 1640 medium supplemented with 5% FCS and 2 mmol/L L-Glutamine and incubated in polypropylene conical tubes at 37°C with gentle agitation in the absence (control) or presence of rhGM-CSF (100 U/mL), sodium butyrate (0.4 mmol/L), rhIL-1β (500 U/mL), or LPS (10 ng/mL). In some experiments cells were cultured for 6 hours and then rhGM-CSF added for the final 6 hours of culture. At various times, as indicated, cells were removed and processed as described below.
Morphological assessment of apoptosis.
Cytocentrifuge preparations of 105 cells, made up to a volume of 200 μL with sterile phosphate-buffered saline (PBS; 10 mmol/L potassium phosphate, 0.9 % NaCl, pH 7.4), were prepared at timed intervals during incubation, using a Shandon cytospin centrifuge (Shandon, Runcorn, UK). Cells were then stained using May-Grünwald-Giemsa. Cells were assessed for morphological changes characteristic of apoptosis (nuclear condensation, vacuolation), with the use of a × 40 objective. At least 500 cells per slide were counted. This method has been previously shown to correlate closely with other parameters of apoptosis.30
Western blotting.
After the isolation and culture of PBMNC and neutrophils, whole cell extracts were prepared by boiling cells in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) reducing sample buffer for 5 minutes. Samples were then frozen at −80°C until use. Postnuclear extracts were prepared exactly as described.22 Briefly, cells were lysed in 10 mmol/L Tris-HCl (pH 7.6), 150 mmol/L NaCl, 1% Triton X-100 (Sigma), 5 mmol/L EDTA, and 2 mmol/L phenylmethyl sulfonyl fluoride (PMSF) for 40 minutes on ice, then nuclei were removed by centrifugation at 15,000g for 10 minutes. Supernatants were then mixed with 2× SDS-PAGE reducing sample buffer, boiled, and frozen as above. An alternative method to prepare nuclear extracts was used to allow crude fractionation of cells into nuclear and nonnuclear fractions. Cells were lysed in 10 mmol/L Tris-HCl (pH 7.6); 10 mmol/L MgCl2; 250 mmol/L sucrose; 1% Triton X-100; 1 mmol/L dithiothreitol; 2 mmol/L PMSF; 3 μg/mL each of aprotinin, chymotrypsin, pepstatin A, leupeptin, and antipain for 10 minutes on ice; and then centrifuged at 2,000g for 5 minutes at 4°C to pellet the nuclei. The resulting supernatants were mixed with an equal volume of 2× SDS-PAGE reducing sample buffer and the pelleted nuclei resuspended in 1× SDS-PAGE reducing sample buffer, boiled, and frozen as above. Total lysates (ie, samples not subject to centrifugation to pellet nuclei) were mixed with an equal volume of 2× SDS-PAGE reducing sample buffer.
Protein extracts were separated by SDS-PAGE using the appropriate percentage acrylamide gel, with 5 × 105 cell equivalents loaded per lane. After electrophoresis proteins were transferred to nitrocellulose membranes using the BioRad mini protean II transfer apparatus (Bio-Rad, Hemel Hempstead, UK). Membranes were then Ponceau S stained and images captured using a Hamamatsu XC-77CE CCD camera (Improvision, Coventry, UK) and Image 1.44 VDM software (NIH, Bethesda, MD). These Ponceau S–stained membranes were used to compare the levels of actin in each lane to ensure equal protein loading levels per track. Membranes were then washed free of Ponceau S in PBS, blocked with a buffer containing 5% (wt/vol) dried skimmed milk, Tris-buffered saline (TBS) (150 mmol/L NaCl; 10 mmol/L Tris-HCl, pH 8.0), and 0.05% (vol/vol) Tween 20 for 1 hour at room temperature. After two brief washes in TBS (pH 8.0) and 0.05% Tween 20 (wash buffer), membranes were incubated with primary antibodies to either Bcl-2 (1:50), Bcl-X (1:1,000), Bax (1:2,000), or Mcl-1 (1:2,000) diluted in 0.5% (wt/vol) dried skimmed milk, TBS (pH 8.0), and 0.05% (vol/vol) Tween 20 (Ab buffer) overnight at room temperature. Membranes were then washed 2 × 30 seconds and 3 × 5 minutes in wash buffer before incubation with HRP-linked secondary antibody (1:50,000) diluted in Ab buffer for 1 hour at room temperature. After washes, antisera were detected using Amersham's ECL detection reagents and preflashed film. Care was taken not to overexpose film to allow comparison of expression of Bcl-2 family proteins between samples, using a Hamamatsu XC-77CE CCD camera and Image 1.44 VDM software. Efficiency of nuclear and nonnuclear fractionation was analyzed by visualizing histones 2A, 2B, and 3 in fixed Coomassie blue–stained gels after separation by SDS-PAGE of 5 × 105 cell equivalents.
In all experiments, neutrophil extracts were analyzed alongside cell extracts that have previously been shown to express the protein under investigation, as positive controls. These were Bcl-2 (PBMNC, KLC22); Bcl-XL (PBMNC, Jurkat, Raji); Bax (PBMNC, Raji); and Mcl-1 (phorbol 12-myristate 13-acetate [PMA]-treated U-937).
Statistical analysis.
The Student's t-test was used to evaluate the significance of differences between the sample means. Statistical significance was defined at P = .05 or P = .01.
RESULTS
Delay of neutrophil apoptosis by GM-CSF, butyrate, IL-1β, and LPS.
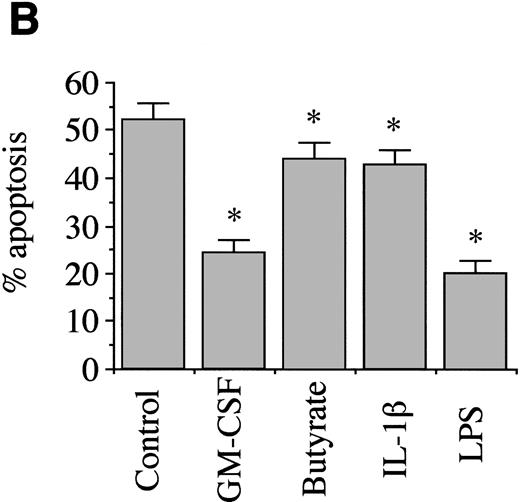
Freshly isolated neutrophils have a distinct morphology that is easily distinguishable from apoptotic neutrophils which show loss of total cell volume, compaction, of chromatin with loss of the multilobed nuclear structure and vacuolation (Fig 1A). Control incubations, incubated in the absence of any exogenous additions, showed 52.0% (±6.3) apoptosis by morphology after 12 hours in culture, showing the constitutive apoptosis that cultured neutrophils will undergo. Apoptosis was significantly delayed, compared with controls, by all other treatments used (Fig 1B), using the paired Student's t-test (P = .01). GM-CSF treatment showed 24.3% apoptosis (±5.2%), butyrate showed 44.0% apoptosis (±6.6%), IL-1β showed 42.9% apoptosis (±6.1%), and LPS showed 19.0% apoptosis (±5.7%). These results were obtained from at least three separate experiments.
Morphological assessment of neutrophil apoptosis after 12-hour culture. (A) shows cytospins, prepared as described in Materials and Methods. Nonapoptotic neutrophils with multilobed nuclei are clearly distinguishable from apoptotic neutrophils which have smaller, denser nuclei and decreased cell volume. (B) shows summary data of apoptosis by morphology, and the effects of various agents added to neutrophil suspensions, as described in Materials and Methods. * Indicates values that are significantly different from controls (P = .01) after at least three separate experiments.
Morphological assessment of neutrophil apoptosis after 12-hour culture. (A) shows cytospins, prepared as described in Materials and Methods. Nonapoptotic neutrophils with multilobed nuclei are clearly distinguishable from apoptotic neutrophils which have smaller, denser nuclei and decreased cell volume. (B) shows summary data of apoptosis by morphology, and the effects of various agents added to neutrophil suspensions, as described in Materials and Methods. * Indicates values that are significantly different from controls (P = .01) after at least three separate experiments.
Expression of Bcl-2, Bcl-X, Bax, and Mcl-1 in freshly isolated neutrophils.
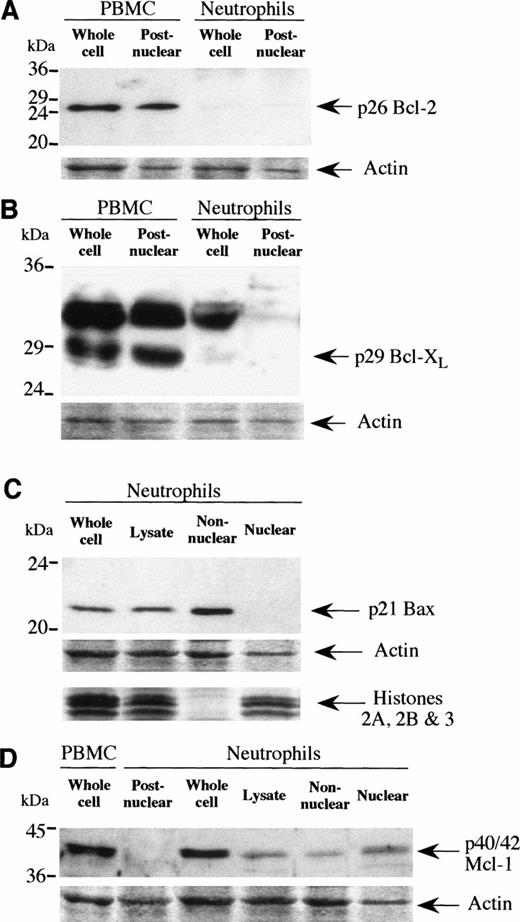
The expression of these proteins was examined by Western blotting using antibodies specific for each of the proteins to be tested. Figure 2A shows that neutrophils essentially express no Bcl-2, whereas this protein was abundant in PBMNC and KLC22 cells run as positive controls (data not shown). A faint p26 Bcl-2 band (at 26 kD) was only detected in neutrophil lysates after overexposure of blots to film for 10 minutes (not shown), whereas the film shown in Fig 2A was exposed for only 30 seconds. This level of detection of Bcl-2 in neutrophil preparations is at approximately one-fortieth the level observed in PBMNC, which is the level of PBMNC contamination usually found in our neutrophil preparations. Similarly, Bcl-XL (Fig 2B) is not expressed in neutrophils, but was abundant in Jurkat and Raji cells (not shown) and PBMNC. The p29 Bcl-XL doublet could be visualized after much longer exposure of film from neutrophil extracts, again indicating a contribution only from PBMNC contamination. Bcl-XS was also detected in PBMNC at much lower levels than that found for Bcl-XL (not shown), but no Bcl-XS was found in neutrophils (not shown). The strong band at approximately 33 kD is believed to result from nonspecific binding of the primary antibody, because this band is not seen in other published works using different Bcl-X antibodies. The Bax protein is strongly expressed in neutrophils (Fig 2C), and in Raji and PBMNC run as positive controls (data not shown). Only a single band at 21 kD was observed in these blots, whereas no bands were detected in extracts of Jurkat cells which were run as a negative control. Bax is not found in the nuclear fraction of freshly isolated neutrophils, but is strongly recovered in the nonnuclear fraction. The Mcl-1 protein is not found in the postnuclear fraction of neutrophils (Fig 2D), in agreement with the work of Ohta et al.22 However, Mcl-1 is found in neutrophil whole cell extracts at a level comparable with that seen in PBMNC. When run as a positive control, extracts from 6-hour PMA-stimulated U-937 cells showed only a doublet of 40 and 42 kD (not shown). Preparation of nuclear and nonnuclear neutrophil extracts using an alternative method in which proteolysis is minimized shows that Mcl-1 can be detected in neutrophil lysates and that Mcl-1 protein is predominately found in the nuclear fraction. The histones 2A, 2B, and 3 are shown from the fractionated samples, and show that fractionation successfully separated nuclear material from the rest of the cellular proteins. All further blots used whole cell lysates.
Expression of Bcl-2 family proteins in freshly isolated neutrophils. Expression of Bcl-2 (A), Bcl-XL (B), Bax (C), and Mcl-1 (D) were determined by Western blotting as described in Materials and Methods with PBMNC shown as a positive control for Bcl-2, Bcl-XL, and Mcl-1. Blots were stained with Ponceau S and the actin-stained band is shown for each blot to indicate equal protein loading per lane. Histones from cell fractionation are shown in (C) with identical samples used in (D). Each blot is representative of at least two separate experiments.
Expression of Bcl-2 family proteins in freshly isolated neutrophils. Expression of Bcl-2 (A), Bcl-XL (B), Bax (C), and Mcl-1 (D) were determined by Western blotting as described in Materials and Methods with PBMNC shown as a positive control for Bcl-2, Bcl-XL, and Mcl-1. Blots were stained with Ponceau S and the actin-stained band is shown for each blot to indicate equal protein loading per lane. Histones from cell fractionation are shown in (C) with identical samples used in (D). Each blot is representative of at least two separate experiments.
Expression of Bcl-2, Bcl-X, and Bax in neutrophils treated to delay apoptosis.
The expression of Bcl-2, Bcl-XL, and Bax remain unchanged in neutrophil suspensions treated to delay apoptosis with GM-CSF, butyrate, IL-β, or LPS, as shown in Fig3A, B, and C, respectively. The Bcl-2 and Bcl-X proteins were not induced after either 6-hour (not shown), 12-hour (Fig 3), or 24-hour (not shown) incubations of neutrophils. The Bax protein remained at essentially constant levels after both 6-hour (not shown) and 12-hour incubations and was expressed at levels similar to those found in PBMNC. Treatment of neutrophils with the cytokines IL-6, IL-2, and interferon-γ (all which delay neutrophil apoptosis), or tumor necrosis factor–α (which accelerates neutrophil apoptosis) also showed no induction of Bcl-2 or Bcl-X, and no change in the levels of the Bax protein (results not shown).
Expression of Bcl-2, Bcl-XL, and Bax in neutrophils treated to delay apoptosis. Expression of Bcl-2 (A), Bcl-XL (B), and Bax (C) were determined by Western blotting as described in Materials and Methods with PBMNC shown as a positive control. Ponceau S–stained actin is shown for comparison of protein loading per lane.
Expression of Bcl-2, Bcl-XL, and Bax in neutrophils treated to delay apoptosis. Expression of Bcl-2 (A), Bcl-XL (B), and Bax (C) were determined by Western blotting as described in Materials and Methods with PBMNC shown as a positive control. Ponceau S–stained actin is shown for comparison of protein loading per lane.
Induction of Mcl-1 protein by agents that delay neutrophil apoptosis.
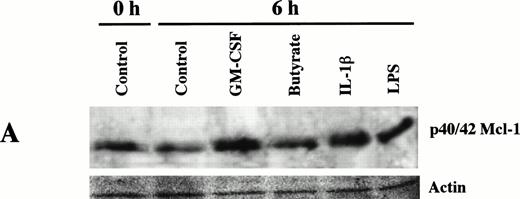
Mcl-1 protein expression was increased relative to untreated neutrophils by the apoptosis-delaying agents GM-CSF, butyrate, IL-1β, and LPS. This is shown in Fig 4A after 6-hour incubations and Fig 4B after 12-hour incubations. Table 1 shows the results of densitometric analysis of blots prepared after incubation of cells for 12 hours. Compared with control incubations, levels of Mcl-1 protein were increased as follows: GM-CSF, 2.87-fold (±0.69); butyrate, 1.46-fold (±0.23); IL-1β, 2.41-fold (±1.29); LPS, 2.70-fold (±0.94). These values were significantly different from control levels of Mcl-1 protein assessed by the paired Student'st-test, with GM-CSF significant at P = .01, whereas butyrate, IL-1β, and LPS were significant at P = .05.
Expression of Mcl-1 in neutrophils treated to delay apoptosis. Expression of Mcl-1 in freshly isolated neutrophils and neutrophils cultured for 6 hours (A) and 12 hours (B) in the conditions indicated, were determined by Western blotting as described in Materials and Methods. Ponceau S–stained actin is shown for comparison of protein loading per lane. Each treatment shown is representative of at least two separate experiments.
Expression of Mcl-1 in neutrophils treated to delay apoptosis. Expression of Mcl-1 in freshly isolated neutrophils and neutrophils cultured for 6 hours (A) and 12 hours (B) in the conditions indicated, were determined by Western blotting as described in Materials and Methods. Ponceau S–stained actin is shown for comparison of protein loading per lane. Each treatment shown is representative of at least two separate experiments.
Relative Levels of Expression of Mcl-1 in Neutrophils After 12-Hour Incubation in Culture
| . | Relative Mcl-1 Expression (12-h incubation) . |
|---|---|
| Control | 1.00 |
| (n = 6) | |
| GM-CSF | 2.87-150 |
| (±0.69, n = 6) | |
| Butyrate | 1.46-151 |
| (±0.23, n = 3) | |
| IL-1β | 2.41-151 |
| (±1.29, n = 3) | |
| LPS | 2.70-151 |
| (±0.94, n = 4) |
| . | Relative Mcl-1 Expression (12-h incubation) . |
|---|---|
| Control | 1.00 |
| (n = 6) | |
| GM-CSF | 2.87-150 |
| (±0.69, n = 6) | |
| Butyrate | 1.46-151 |
| (±0.23, n = 3) | |
| IL-1β | 2.41-151 |
| (±1.29, n = 3) | |
| LPS | 2.70-151 |
| (±0.94, n = 4) |
Values were obtained by densitometric analysis of Western blots, as described in Materials and Methods.
P ≤ .01.
P ≤ .05.
Mcl-1 expression in cells aged for 6 hours before the addition of GM-CSF.
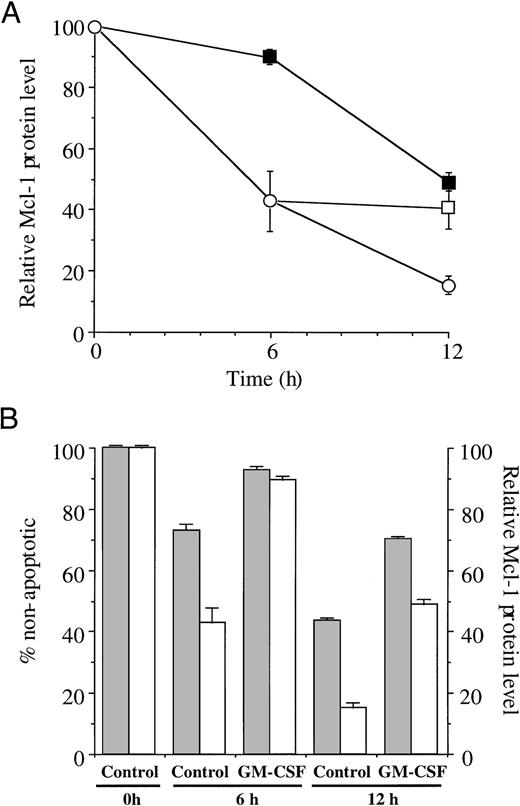
To determine if the level of Mcl-1 protein in neutrophils can be maintained after it has been allowed to decline for 6 hours as neutrophils progress into constitutive apoptosis, we cultured cells for 6 hours before the addition of GM-CSF. Figure 5A shows the means of two separate experiments, in which Mcl-1 protein levels were determined in freshly isolated cells, cells cultured for 6 and 12 hours with and without GM-CSF, and cells cultured for 6 hours before the addition of GM-CSF. Control incubations showed a steady decline in levels of Mcl-1 protein detected to 42.9% (±9.8) and 15.4% (±2.9) at 6 and 12 hours, respectively, of the time zero value. GM-CSF greatly decreased the loss of Mcl-1 protein levels with 89.3% (±2.1) and 49.1% (±3.3) remaining after 6 and 12 hours in culture. The addition of GM-CSF to 6-hour–aged cells also greatly diminished the loss of Mcl-1, with Mcl-1 protein levels only falling a further 1.5% to 40.4% (±6.5) after a total of 12-hour culture. This addition of GM-CSF also decreased the number of cells becoming apoptotic for the final 6 hours of culture. Without GM-CSF apoptosis increased from 26.7% (±4.1) at 6 hours to 56.2% (±1.9) after 12 hours. The addition of GM-CSF for the final 6 hours resulted in apoptosis increasing to only 30.5% (±7.2) (results not shown).
Maintenance of Mcl-1 expression by GM-CSF and correlation with delayed apoptosis. In (A), Mcl-1 expression was determined by Western blotting and densitometric analysis as described in Materials and Methods after 2-, 4-, 6-, and 12-hour culture in medium alone (control, ○), with GM-CSF (▪), and with GM-CSF added to control incubations after 6-hour incubation of control suspensions (□). (B) shows the relationship between Mcl-1 expression (□) and nonapoptotic neutrophils (▧). Apoptosis was assessed by morphology as described in Materials and Methods, with Mcl-1 expression measured as in (A).
Maintenance of Mcl-1 expression by GM-CSF and correlation with delayed apoptosis. In (A), Mcl-1 expression was determined by Western blotting and densitometric analysis as described in Materials and Methods after 2-, 4-, 6-, and 12-hour culture in medium alone (control, ○), with GM-CSF (▪), and with GM-CSF added to control incubations after 6-hour incubation of control suspensions (□). (B) shows the relationship between Mcl-1 expression (□) and nonapoptotic neutrophils (▧). Apoptosis was assessed by morphology as described in Materials and Methods, with Mcl-1 expression measured as in (A).
The proportion of nonapoptotic neutrophils in a population correlates with the abundance of Mcl-1 protein.
There is a clear relationship between Mcl-1 protein levels in a population of neutrophils and the number of neutrophils which have not become apoptotic. This is shown in Fig 5B in which the level of Mcl-1 expression (shown in Fig 5A) is plotted with the number of nonapoptotic neutrophils present after 6 and 12 hours in culture. The levels of Mcl-1 protein shown are the same as in Fig 5A, with the percentage of nonapoptotic cells at 73.3% (±4.5) and 43.8% (± 1.9) at 6 and 12 hours, respectively, in control incubations, with GM-CSF treatment showing 92.8% (±2.5) and 70.4% (±1.7) at 6 and 12 hours.
DISCUSSION
The identification of the Bcl-2 family of proteins, which are conserved throughout many species, has greatly enhanced our understanding of the control of apoptosis within many cells and tissues. A second family of proteins called the caspases, which are also conserved throughout many species, are proteases which cleave key proteins within the apoptotic cell.31 This proteolytic cleavage of targets by the caspases leads to the typical features of apoptosis, and is essential for the completion of the apoptotic program.32
Neutrophils constitutively undergo apoptosis and express relatively high levels of the proapoptotic protein Bax,20 and also strongly express caspase-3 (CPP-32).33 The expression of these two molecules which are involved in cell death is consistent with the short lifespan of the mature neutrophil. However, this short neutrophil lifespan can be significantly increased during exposure to agents such as cytokines, but no obvious explanation for this rescue from death has previously been shown. Expression of the Bcl-2 family member proteins in neutrophils has not been conclusively defined, due in part to the fact that members of this family are increasing as more are discovered, but mainly due to the different methodologies used by researchers to detect these proteins. Bcl-2 has been reported to be expressed in neutrophils and needed for rescue from apoptosis.17-19 Other reports indicate no detectable Bcl-2 in mature neutrophils.12 20-23 In this report we show by Western blotting that neutrophils essentially express no Bcl-2, even if rescued from apoptosis by a variety of agents. The conflicting reports on Bcl-2 expression in neutrophils may be explained by the different methodologies used. Flow cytometric or immunohistochemical analysis of Bcl-2 expression in neutrophils may give false results due to nonspecific binding of the antibodies to other neutrophilic proteins. Indeed, in our Western blot experiments using a MoAb raised against synthetic peptides corresponding to residues 44-52 of Bcl-2, we found strong binding of the primary antibody in neutrophil extracts to proteins of approximately 40 kD and approximately 80 kD (not shown), which were not observed in PBMNC or KLC 22 cells. These bands have not been identified but will clearly give false positives in fluorescence-activated cell sorting assays or after immunohistochemical staining. Such proteins may represent nonspecific binding of the antibodies or may indeed represent novel Bcl-2 family members expressed in neutrophils. Further work needs to be performed to identify these proteins.
Bcl-X is also shown in this paper to be absent from mature neutrophils, in agreement with the work of other groups.22-24 We have also shown that protection from apoptosis does not result in induction of Bcl-X to levels detectable by Western blot. Previous work in our laboratory identified a partial cDNA clone of Mcl-1 in a cDNA library enriched in GM-CSF regulated clones (J.A.Q. and S.W.E., unpublished observation, July 1997). This strongly suggested to us that Mcl-1 protein would be expressed in neutrophils, and that it may be upregulated by GM-CSF. However, the work of Ohta et al22showed no expression of the Mcl-1 protein in mature neutrophils. Further, although Mcl-1 protein expression was detected in neutrophil precursors by immunocytochemistry, relatively mature bone marrow neutrophils showed little and variable Mcl-1 expression.25We repeated the work of Ohta et al22 and, following their protocol, also found no expression of Mcl-1 in neutrophil postnuclear extracts. However, we found abundant Mcl-1 in cell extracts solubilized directly into boiling sample buffer. This is, to our knowledge, the first clear demonstration of the presence of an antiapoptotic Bcl-2 family protein in mature human neutrophils. In view of the low expression of Mcl-1 in bone marrow neutrophils, it is possible that expression of this protein is stimulated (albeit transiently) as the bone marrow neutrophils are signaled to leave the marrow and enter the circulation.
An alternative method to separate nuclei from the rest of the neutrophil organelles and cytoplasm, which minimizes protein degradation, showed that Mcl-1 was expressed primarily in the neutrophil nuclear fraction, but was also expressed in the nonnuclear fraction, albeit at lower levels. Even with this alternative method of crude cell fractionation we saw considerable degradation of Mcl-1 protein (Fig 2D comparing signals obtained with neutrophil whole cell and lysate samples). This degradation was not seen with PBMNC lysates (not shown), and may be explained by the release of the many proteases found in neutrophil granules rapidly degrading the labile Mcl-1 protein.
The expression of Bax in neutrophils is confirmed in this report, with crude fractionation indicating that Bax is not found in the nuclear fraction of freshly isolated neutrophils. We also show that the level of Bax protein remains essentially constant in neutrophils treated to delay apoptosis with a variety of agents. The presence of both Bax and Mcl-1 in mature neutrophils suggests an explanation for the constitutive apoptosis of mature neutrophils and the ability of cytokines to extend the neutrophil lifespan. Because Mcl-1 is a labile protein its cellular levels would be expected to decrease rapidly if expression of Mcl-1 was stopped or diminished, allowing the endogenously expressed Bax to dominate and lead to apoptosis. Induction of Mcl-1 expression would maintain the levels of Mcl-1 protein in the neutrophil, thereby preventing Bax from exerting its apoptotic effects. This simple model requires the constant production of new Mcl-1 protein to replace expressed Mcl-1 that would be expected to be degraded constantly and rapidly. Further, this system would allow for neutrophil death and survival to be quite tightly regulated and rapidly modulated, as the lability of the Mcl-1 protein would allow Bax levels to predominate after suspension of Mcl-1 expression.
This model for the control of entry into apoptosis in mature neutrophils is strengthened by our findings that Mcl-1 levels are significantly higher in cultured neutrophils that have been protected from entry into apoptosis by a variety of agents (Fig 4A and B, Table1). The level of Mcl-1 expression can also be maintained by the addition of GM-CSF to neutrophils that have already been aged for 6 hours without GM-CSF (Fig 5A); this addition of GM-CSF also protected these aged cells from further entry into apoptosis. This suggests that induction of Mcl-1 expression before the endogenous level of Mcl-1 has fallen below the threshold level neccessary to counteract Bax is sufficient to protect the cells from entry into apoptosis.
The level of Mcl-1 expression in differently treated neutrophil populations also correlates well with the proportion of the population that have not become apoptotic. This is evident by comparing the relative levels of Mcl-1 in cultured cells (Table 1) with the percentage of cells which are apoptotic after each treatment for 12 hours (Fig 1B). The more effective the treatment is at delaying apoptosis, the higher the level of Mcl-1 protein found in the aged cells. This correlation is further shown in Fig 5B, where both Mcl-1 protein levels and apoptosis were determined in parallel from the same cultures after both 6 and 12 hours of culture. It is clear that Mcl-1 protein levels decline more rapidly in cells not protected from apoptosis. These results also show that the decrease in Mcl-1 protein from each neutrophil population is more rapid than the rate of entry into apoptosis as assessed by morphology. Therefore, it is proposed that Mcl-1 protein loss from neutrophils leads to the initiation of the apoptotic program. This theory will be further tested by assessing the abundance of Mcl-1 protein in individual neutrophils while simultaneously measuring apoptosis in these cells.
Recent work has shown that Bcl-2 and Bcl-XL are able to prevent caspase activation (and therefore the initiation of apoptosis) by preventing the release of cytochrome c from mitochondria.34,35 The localization of Bax dimers to mitochondria has also been shown to be essential for Bax to exert its cytotoxic effects in both yeast and mammalian cells.36Neutrophils possess only few, very small mitochondria whose function within these cells is not clearly defined.14-16 Our crude fractionation experiments indicate that Mcl-1 protein is primarily located in nuclear extracts (that may additionally contain structures such as endoplasmic reticulum), whereas Bax does not colocalize with Mcl-1 but is located in nonnuclear extracts. Such observations indicate that Mcl-1 may perform protective functions in neutrophils in nonmitochondrial locations. In the nematode Caenorhabditis elegans, Ced-9 (a Bcl-2 homologue) binds to Ced-4 (of which functional homologues exist in mammals37,38), pulling Ced-4 out of the cytosol. This prevents Ced-4 from activating Ced-3 (a caspase), thereby preventing apoptosis. These events, to our knowledge, have not been demonstrated as yet in mammalian cells, but Bcl-XL has been shown to pull Ced-4 out of the cytosol.39 Overexpression of proapoptotic Bcl-2 family members prevents Ced-4 binding by Ced-9 (or Bcl-XL),40 which would allow Ced-4 to activate the caspases found in the cytosol. Similar events may take place in the neutrophil, with Mcl-1 located on nuclear (and perhaps endoplasmic) membranes, perhaps without any requirement for mitochondria or mitochondrial proteins. Indeed, Bcl-2 is able to block entry into apoptosis without localization to the mitochondria in certain circumstances.41 Further studies into neutrophil apoptosis should provide some useful insights into the mechanisms involved in the control of entry into apoptosis, and should include studies at the molecular level of interactions between Bax and Mcl-1, and caspases present in the neutrophil. It would also be interesting to determine if the cellular location of Bax and Mcl-1 is altered in neutophils undergoing apoptosis. By virtue of neutrophils having few functional mitochondria, these cells would seem to provide an attractive experimental model to study nonmitochondrial proapoptotic and antiapoptotic events.
Supported by North West Cancer Research Fund (UK).
Address reprint requests to Steven W. Edwards, PhD, School of Biological Sciences, Life Sciences Building, University of Liverpool, Liverpool L69 7ZB, UK; e-mail:sbir12@liverpool.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal