Abstract
SLP-76 and Cbl are complex adapter proteins that have the capacity to bind to smaller adapter proteins, such as Grb2, which subsequently binds the nucleotide exchange protein Sos in the transmission of intracellular signals. SLP-76, Cbl, Shc, and Grb2 have been implicated in immunoreceptor tyrosine-based activation motif (ITAM) signaling, leading to activation of Ras. However, their mechanism of action has not been determined. To date, there have been no reports of SLP-76 involvement in FcγRI-receptor signaling and no data exist for an interaction between Cbl, Shc, and SLP-76 in vivo. We provide evidence that SLP-76, Cbl, and Shc are tyrosine phosphorylated on FcγRI-receptor stimulation and are associated with the adapter protein Grb2 in γ-interferon–differentiated U937 cells (U937IF). The interactions between SLP-76 and Cbl and SLP-76 and Grb2 are present in resting U937IF cells. However, the interaction between SLP-76 and Grb2 becomes augmented twofold on FcγRI-receptor aggregation. Our results provide the first evidence for a phosphorylation-dependent interaction between SLP-76 and Shc, induced at least 10-fold on FcγRI receptor stimulation. Our data indicate that a significant portion of a multimolecular complex containing Cbl, SLP-76, Shc, and Grb2 is distinct from a trimolecular complex containing the Ras guanine nucleotide exchanger Sos, Shc, and Grb2. FcγRI-induced tyrosine phosphorylation of SLP-76, Cbl, Shc, and the highly induced SLP-76-Shc interaction provide the first evidence that SLP-76 and Cbl are involved in FcγRI signaling and suggest a functional significance for these interactions in FcγRI signal relay in the control of Ras in myeloid cells.
© 1998 by The American Society of Hematology.
ELUCIDATING THE SIGNAL transduction pathway through Fcγ receptors in monocytes and macrophages has significant clinical implications in understanding and manipulating the inflammatory process, autoimmune disorders, and myeloid immunity. The FcγRI receptor is a member of the immunoglobulin gene superfamily that also includes the T-cell receptor (TCR), B-cell receptor (BCR), and other Fc receptors.1,2 In contrast to growth factor receptors such as insulin, epidermal growth factor (EGF), and platelet-derived growth factor (PDGF), these receptors have no intrinsic kinase activity. Signaling through these receptors is mediated through a conserved stretch of amino acids consisting of paired tyrosines and leucines in the consensus sequence (D/E)XXYXXL(Y)6-8 YXXL, termed the immunoreceptor tyrosine-based activation motif (ITAM).3 FcγRI receptor crosslinking occurs with stimulation by IgG molecules whose Fc fragments interact with the FcγRIα subunit. This then leads to conformational activation of the FcγRIγ subunit that further activates src-family kinase (SRTK) such as Hck, Lyn, and Fgr.1,2,4,5 The activated SRTK then phosphorylates tyrosine residues within the ITAM, which recruits binding and subsequent activation of the Syk kinase.6-8 Activated nonreceptor protein kinases phosphorylate complex adapter proteins that induce protein-protein interactions leading to translocation, activation, and regulation of Ras at the cell membrane. The mechanism by which Ras is controlled in myeloid FcγRI signaling is unknown, but is likely to involve complex adapter proteins.
One adapter protein Shc, is thought to be involved in the activation of Ras.9-17 It is ubiquitously expressed and occurs in two isoforms of 46 kD and 52 kD in hematopoetic cells. The Shc protein contains an amino-terminal phosphotyrosine-binding (PTB) domain, a central collagen homology (CH) region, and a carboxyl-terminal SH2 domain, but no apparent catalytic domain.18-20 It is a substrate of multiple tyrosine kinases and can transform cells when overexpressed.18 Tyrosine-phosphorylated Shc associates with the SH2 domain of Grb2, an adapter protein composed solely of an SH2 domain flanked on either side by an SH3 domain and the guanine nucleotide exchange factor Sos, localizing the molecular complex to the plasma membrane in which it is believed that activation of Ras occurs.9 10
Data from a number of investigators suggest a role for Cbl in the regulation of Ras.21,22 The v-cbl oncogene was originally described as the transforming gene of the Cas NS-1 murine retrovirus that induces pre-B lymphomas and myeloid leukemias in mice.23,24 The c-cbl proto-oncogene product is a cytoplasmic protein that contains a nuclear localization domain along with a PTB domain in its amino terminus, a ring finger domain, a c-terminal proline-rich region, and leucine zipper domain.25 26
Cbl is ubiquitously expressed in mammalian cells. It contains the above mentioned domains of interest and exhibits no intrinsic enzymatic activity.27 It has been shown to be a substrate for tyrosine kinase activity activated by the EGF and colony-stimulating factor (CSF)-1 receptors21,28,29 as well as Fc-, T-, and B-cell receptors.30-35 Cbl is known to constitutively interact with SH3 domains of Grb2. Recently, we described a novel Grb2-associated protein SLP-76 (SH2 domain-containing leukocyte protein of 76 kD) that is found only in hematopoetic cells.36 This 533 amino acid protein contains several tyrosines in its amino-terminal end, a central proline-rich region that binds the SH3 domain of Grb2, and a single SH2 domain in the carboxy-terminal region.36It has been shown to be a tyrosine phosphorylation substrate of ZAP-70 and plays a role in potentiating TCR-mediated induction of the nuclear factor of activated T cells (NFAT) and interleukin-2 (IL-2) promoter activity.37-39 Mizuno et al40 have shown an association between SLP-76 and the phosphatase SHP-1, which may modulate signaling through the B-cell antigen receptor. SLP-76 has been shown to be associated with Grb2 and a 120 kD phosphoprotein in FcγRIIA signaling in platelets.41 Hendricks-Taylor et al42 have reported SLP-76 phosphorylation on FcεRI stimulation in rat basophilic leukemia cells.
To date there have been no reports showing a role for SLP-76 or Cbl in FcγRI signaling in myeloid cells. Herein, we report the constitutive association of SLP-76 with Cbl and Grb2 in the myeloid cell line U937. We also show that the interaction between SLP-76 and Grb2 is further induced on FcγRI stimulation. SLP-76 tyrosine phosphorylation is associated with binding to the Shc adapter protein and is induced by FcγRI receptor activation. The phosphorylation dependence of some of these associations is shown by treatment with potato acid phosphatase (PAP), which results in disruption of SLP-76-Shc and decreases SLP-76-Grb2, but has no effect on SLP-76-Cbl or Cbl-Grb2 interactions. From our experiments, we conclude that the Cbl-SLP-76-Grb2 complex is at least partly distinct from a Grb2 complex containing Sos. The association of SLP-76, Cbl, and Grb2 along with the inducible binding of SLP-76 to Shc provides the first evidence that a Cbl-SLP-76-Grb2-Shc complex may function in FcγRI signaling in myeloid cells.
MATERIALS AND METHODS
Reagents and chemicals.
The FcγR-specific antibodies (monoclonal antibody [MoAb] 32.2 is an F(ab′)2 fragment and 197 is whole-mouse IgG) were generously provided by Medarex, Inc (West Lebanon, NH). The presence of F(ab′)2 fragment only without the contaminating Fc portion of IgG is certified by Mederex based on immunoelectrophoresis and high-pressure liquid chromotography (HPLC) analysis. The cross-linking antibody was a rabbit antimouse F(ab′)2 fragment (RαM) purchased from Organon Teknika (Durham, NC). Polyclonal rabbit anti-Cbl and anti-Sos antibodies were obtained from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Anti–SLP-76 antibody produced in sheep has been described previously.37 Monoclonal antiphosphotyrosine and anti-Shc antibodies were purchased from UBI (Lake Placid, NY). Monoclonal anti-Grb2 antibody was obtained from Transduction Lab (Lexington, KY). Goat antirabbit and antimouse antibodies conjugated to alkaline phosphatase (AP) were purchased from Southern Biotechnology Associates, Inc (Birmingham, AL). Rabbit antigoat conjugated to AP was purchased from Sigma (St Louis, MO). PAP was purchased from Boehringer Mannheim (Mannheim, Germany).
Cells.
The U937 and THP-1 cell lines were obtained from ATCC (Rockville, MD) and cultured in RPMI 1640 with 10% fetal bovine serum (FBS). U937IF cells were prepared by culturing U937 cells in RPMI 1640 with 10% FBS and 250 U/mL human recombinant γ-interferon (IFN) for 4 to 5 days (Genentech Corp, San Francisco, CA). The U937IF cells were maintained at a concentration of 5 × 105 cells/mL and the medium was replenished with fresh medium containing IFN every 2 to 3 days.
Stimulation of U937IF cells.
U937IF or THP-1 cells were collected and washed once in cold Hank’s Balanced Salt Solution (HBSS). Monoclonal antibodies against the FcγRI receptor (MoAb 32.2, which is an F(ab′)2 fragment, were used in all immune-precipitation experiments, Fig 1, 2, 3, and 5; whereas MoAb 197, which is whole mouse IgG, was used in glutathione S-transferase (GST)-fusion protein-binding experiments, Fig 4, was added to 2 × 107 cells in 500 μL of HBSS and incubated on ice for 30 minutes. Cells were prewarmed to 37°C for 2 minutes. Secondary RαM antibody F(ab′)2 fragment was then added at a concentration of 10 ug/mL and the cells were incubated at 37°C for varying periods of time. The addition of the secondary antibody at 37°C was considered the start of stimulation. At the end of stimulation, cells were cooled rapidly by the addition of 800 uL of cold HBSS. The cells were then centrifuged at 1500g at 4°C for 5 minutes and the supernatant discarded. Then either immunoprecipitations or GST fusion protein-binding experiments were performed as described below.
Immunoprecipitation, electrophoresis, and immunoblotting.
Immunoprecipitations were designed to preserve noncovalent protein-protein interactions. Cells were lysed with an extraction buffer (EB buffer) and incubated at 0°C for 30 minutes. This EB buffer contained 1% Triton X-100, 10 mmol/L Tris pH 7.6, 50 mmol/L NaCl, 0.1% bovine serum albumin (BSA), 1% aprotinin, 5 mmol/L EDTA, 50 mmol/L NaF, 5 umol/L phenylarsine oxide (PAO) and 100 umol/L sodium ortho-vanadate. For immune precipitates done in the presence of PAP, 1.8 units of PAP were added to the lysates that were then warmed to 30°C for 10 minutes before proceeding to the next step. Cell lysates were then centrifuged at 10,000g for 30 minutes at 4°C to bring down the cellular debris. To precipitate the Cbl, Sos, or SLP-76 proteins along with their associated proteins, we added polyclonal rabbit anti-Cbl, rabbit anti-Sos, or goat anti–SLP-76 antisera to these lysates and then incubated them at 0°C for 1 hour with occassional gentle mixing. Formalin-fixed Staphylococcus aureus, (30 μL to 50 μL of a 10% solution first washed with EB buffer) was then added and further incubated for an additional hour at 0°C with occasional gentle mixing. The resultant immune complexes were washed three times with EB buffer, resolved on 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred to nitrocellulose for immunoblot analysis. Membranes were incubated with the specified primary antibody at a dilution of 1:1000 overnight, followed by horseradish peroxidase (1:10,000 dilution), or alkaline phosphatase (1:5000 dilution) conjugated secondary antibody. Proteins were visualized using an enhanced chemiluminescence detection system (ECL, Amersham Corp, Arlington Heights, IL) or alkaline phosphatase colorimetric development. To reprobe membranes, they were blocked with 5% nonfat milk for 1 hour and then reblotted with a different primary antibody.
GST-fusion Protein Experiments.
GST-fusion protein constructs representing amino acids 225-265 and amino acids 268-416 of SLP-76 were subcloned into Escherichia coli expression plasmids pGEX. The E. coli was grown to an optical density of 0.5 to 0.6 at 37°C. Synthesis of GST-fusion proteins was then induced with 0.2 mmol/L isopropyl β-Δ-thiogalactopyranoside. After a 2-hour induction, bacteria were harvested and lysed. GST-fusion proteins were affinity purified with glutathione sepharose beads and then eluted with 20 mmol/L Glutathione and dialyzed against 50 mmol/L Tris, pH 8.0. Purified proteins were quantitated by Bradford assay and confirmed by Coomassie blue staining on SDS/polyacrylamide gels. U937IF lysates with and without FcγRI stimulation by MoAb 197 whole-mouse IgG and RαM F(ab′)2 fragment were prepared as described above. The cell lysates were then mixed with the different GST-fusion proteins (10 μg/mL) and incubated for 1 hour at 0°C with occasional gentle mixing. Glutathione sepharose beads were then added to the lysates and incubated at 0°C for an additional hour with occasional gentle mixing. The mixture was gently washed three times with pulldown-wash buffer (50 mmol/L Tris; HCL, pH 7.5, 150 mmol/L NaCl; 1 mmol/L EDTA, 0.1% Tween-20, 10 mmol/L NaF, 1% NP-40), resolved on 12.5% SDS-PAGE and electrotransferred to nitrocellulose paper. Immunoblots were then performed as described above. The gel was stained with Coomassie blue to confirm that equivalent amounts of GST and GST-protein were used in the binding experiments.
RESULTS
SLP-76 is tyrosine phosphorylated on FcγRI stimulation.
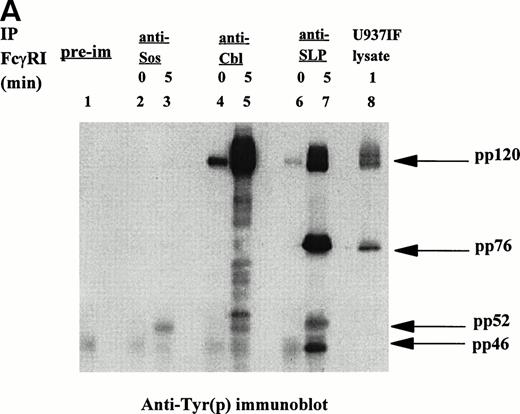
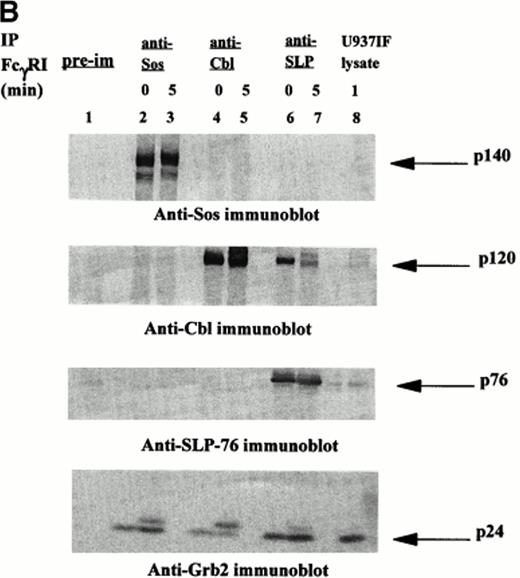
Signal transduction events through the ITAM are mediated via associations between adapter proteins, many of which are phosphoproteins. Ravichandran et al43 reported that these interactions result in formation of molecular complexes containing Shc and Grb2, which serve to localize the guanine nucleotide exchange factor Sos to the cell membrane in which the activation of Ras likely occurs. Recent identification of the SLP-76 phosphoprotein associated with Grb2 in TCR signaling prompted our interest in this molecule and its potential role in FcγRI signaling. We examined whether SLP-76 was tyrosine phosphorylated after FcγRI stimulation in U937 cells. U937 (2 × 107) cells differentiated in IFN for 4 to 5 days were first incubated with MoAb 32.2 (F(ab′)2 fragment of IgG) followed by stimulation with RαM F(ab′)2 fragment for 5 minutes or no stimulation. The cells were then lysed and immunoprecipitated with polyclonal goat anti–SLP-76 antibody and analyzed by Western Blot. Antiphosphotyrosine immunoblot was performed (Fig 1A). Marked tyrosine phosphorylation of a 76 kD protein was detected in the U937IF cells stimulated with anti-FcγRI (Fig 1A, lane 5). The 76 kD protein was not phosphorylated in resting cells (Fig 1A, lane 4). To confirm the identity of the 76 kD phosphoprotein, the membrane was reprobed with anti–SLP-76 antibody. Both stimulated cells and cells at rest brought down an equivalent amount of SLP-76 (Fig 1B, middle panel). Two bands of SLP-76 are consistently observed in our SLP-76 immunoprecipitates. The slower migrating isoform is the predicted tyrosine phosphorylated species. The 76 kD immunoreactive bands of the SLP-76 immunoblot superimposed on the 76 kD tyrosine phosphorylated bands on the SLP-76 immunoprecipitate. The lower portion of the antiphosphotyrosine blot in Fig 1A was probed with anti-Grb2, confirming that SLP-76 is associated with Grb2 in myeloid cells (Fig 1B, lower panel). The band that appears just above the Grb2 bands in the Fig 1B lower panel as previously described, is identified as light chain of IgG.32 The figure also suggests that there is an inducible component to the Grb2-SLP-76 interaction as Grb2 binding to SLP-76 is slightly increased on FcγRI activation (lanes 4 and 5). This increased binding of SLP-76 and Grb2 after receptor activation becomes more evident in Fig2B and 3B. This is in contrast to the Grb2-Cbl interaction in which Grb2 binding to Cbl remains the same regardless of FcγRI receptor stimulation (lanes 2 and 3). The heavy band at 120 kD in Fig 1A, lanes 2 and 3, represent tyrosine phosphorylated Cbl from Cbl immune precipitates, which superimposes on the anti-Cbl immunoblot represented in Fig 1B, upper panel. The preimmune cells shown in Fig 1A and 1B represent unstimulated U937IF cells immune precipitated with rabbit IgG. Preimmune samples were made with stimulated U937IF cells immune precipitated with goat antiserum exhibit identical results (data not shown). From these data we conclude that SLP-76 and Cbl are involved in FcγRI signaling and we hypothesize that SLP-76-Grb2 interaction may functionally link FcγRI to activation of Ras.
[SLP-76 and Cbl are tyrosine phosphorylated upon FcγRI stimulation.] Immunoprecipitation was performed with anti-Cbl and anti–SLP-76 antibody from lysates of resting U937IF cells or cells stimulated by anti-FcγRI cross-linking with MoAb 32.2 F(ab′)2 fragment. Proteins were resolved by SDS/PAGE, transferred to nitrocellulose membrane, and immunoblotted. (A) Antiphosphotyrosine immunoblot. Lane 1 represents precipitation with preimmune antisera. Lanes 2 and 3 represent anti-Cbl IP of U937IF cells at rest and after 5-minute stimulation, respectively. Lanes 4 and 5 represent anti–SLP-76 IP of U937IF cells at rest and after 5-minute stimulation, respectively. Lane 6 represents whole-cell lysate (1 × 106 cell equivalents) of stimulated U937IF cells. (B) Same membrane as in Fig 1A was blocked and reprobed. Upper panel represents anti-Cbl immunoblot. Middle panel represents anti–SLP-76 immunoblot. Lower panel represents anti-Grb2 immunoblot.
[SLP-76 and Cbl are tyrosine phosphorylated upon FcγRI stimulation.] Immunoprecipitation was performed with anti-Cbl and anti–SLP-76 antibody from lysates of resting U937IF cells or cells stimulated by anti-FcγRI cross-linking with MoAb 32.2 F(ab′)2 fragment. Proteins were resolved by SDS/PAGE, transferred to nitrocellulose membrane, and immunoblotted. (A) Antiphosphotyrosine immunoblot. Lane 1 represents precipitation with preimmune antisera. Lanes 2 and 3 represent anti-Cbl IP of U937IF cells at rest and after 5-minute stimulation, respectively. Lanes 4 and 5 represent anti–SLP-76 IP of U937IF cells at rest and after 5-minute stimulation, respectively. Lane 6 represents whole-cell lysate (1 × 106 cell equivalents) of stimulated U937IF cells. (B) Same membrane as in Fig 1A was blocked and reprobed. Upper panel represents anti-Cbl immunoblot. Middle panel represents anti–SLP-76 immunoblot. Lower panel represents anti-Grb2 immunoblot.
[Kinetics of SLP-76-Cbl interaction following FcγRI stimulation.] Immunoprecipitation was performed with anti–SLP-76 antibody from lysates of resting U937IF cells or cells stimulated by anti-FcγRI cross-linking with MoAb 32.2 F(ab′)2 fragment for varying periods of time ranging from 30 seconds to 30 minutes. Proteins were resolved by SDS/PAGE, transferred to nitrocellulose membrane, and immunoblotted. (A) Antiphosphotyrosine immunoblot. Lane 1 represents precipitation with preimmune antisera. Lane 2 represents U937IF cells at rest. Lanes 3-7 represent U937IF cells stimulated for 30 seconds, 1 minutes, 5 minutes, 10 minutes, and 30 minutes, respectively. Lane 8 represents whole-cell lysate (1 × 106 cell equivalents) of stimulated U937IF cells. (B) Same membrane as in Fig 2A was blocked and reprobed. Upper panel represents anti-Cbl immunoblot. Middle panel represents anti–SLP-76 immunoblot. Lower panel represents anti-Grb2 immunoblot.
[Kinetics of SLP-76-Cbl interaction following FcγRI stimulation.] Immunoprecipitation was performed with anti–SLP-76 antibody from lysates of resting U937IF cells or cells stimulated by anti-FcγRI cross-linking with MoAb 32.2 F(ab′)2 fragment for varying periods of time ranging from 30 seconds to 30 minutes. Proteins were resolved by SDS/PAGE, transferred to nitrocellulose membrane, and immunoblotted. (A) Antiphosphotyrosine immunoblot. Lane 1 represents precipitation with preimmune antisera. Lane 2 represents U937IF cells at rest. Lanes 3-7 represent U937IF cells stimulated for 30 seconds, 1 minutes, 5 minutes, 10 minutes, and 30 minutes, respectively. Lane 8 represents whole-cell lysate (1 × 106 cell equivalents) of stimulated U937IF cells. (B) Same membrane as in Fig 2A was blocked and reprobed. Upper panel represents anti-Cbl immunoblot. Middle panel represents anti–SLP-76 immunoblot. Lower panel represents anti-Grb2 immunoblot.
[Biochemical characterization of SLP-76-Cbl and SLP-76-Shc interactions.] Immunoprecipitation was performed with anti–SLP-76 antibody from lysates of resting U937IF cells or cells stimulated by anti–FcγRI cross-linking with MoAb 32.2 F(ab′)2 fragment for varying periods of time ranging from 30 seconds to 30 minutes in the presence or absence of PAP. Proteins were resolved by SDS/PAGE, transferred to nitrocellulose membrane, and immunoblotted. (A) Antiphosphotyrosine immunoblot. Lane 1 represents precipitation with preimmune antisera. Lanes 2-6 represent anti–SLP-76 IP of U937IF cells at rest and after 30 seconds, 2 min, 10 min and 30 min stimulation respectively. Lanes 7-11 represent anti-SLP-76 IP of U937IF cells at rest and after 30 seconds, 2 minutes, 10 minutes, and 30 minutes stimulation, respectively, to which 1.8 units PAP was added to the cell lysates. Lane 12 represents whole-cell lysate (1 × 106 cell equivalents) of stimulated U937IF cells. (B) Same membrane as in Fig 3A was blocked and reprobed. Upper panel represents anti-Cbl immunoblot. Second panel represents anti–SLP-76 immunoblot. Third panel represents anti-Shc immunoblot. Lower panel represents anti-Grb2 immunoblot.
[Biochemical characterization of SLP-76-Cbl and SLP-76-Shc interactions.] Immunoprecipitation was performed with anti–SLP-76 antibody from lysates of resting U937IF cells or cells stimulated by anti–FcγRI cross-linking with MoAb 32.2 F(ab′)2 fragment for varying periods of time ranging from 30 seconds to 30 minutes in the presence or absence of PAP. Proteins were resolved by SDS/PAGE, transferred to nitrocellulose membrane, and immunoblotted. (A) Antiphosphotyrosine immunoblot. Lane 1 represents precipitation with preimmune antisera. Lanes 2-6 represent anti–SLP-76 IP of U937IF cells at rest and after 30 seconds, 2 min, 10 min and 30 min stimulation respectively. Lanes 7-11 represent anti-SLP-76 IP of U937IF cells at rest and after 30 seconds, 2 minutes, 10 minutes, and 30 minutes stimulation, respectively, to which 1.8 units PAP was added to the cell lysates. Lane 12 represents whole-cell lysate (1 × 106 cell equivalents) of stimulated U937IF cells. (B) Same membrane as in Fig 3A was blocked and reprobed. Upper panel represents anti-Cbl immunoblot. Second panel represents anti–SLP-76 immunoblot. Third panel represents anti-Shc immunoblot. Lower panel represents anti-Grb2 immunoblot.
SLP-76 associates with Cbl in a constitutive manner in myeloid cells.
A 120 kD phosphoprotein was noted to coprecipitate with SLP-76 in FcγRI-stimulated cells (Fig 1A, lane 5). This portion of the membrane in Fig 1A was reprobed with anti-Cbl antibody. The 120 kD immunoreactive bands on the Cbl immunoblot superimpose on the 120 kD bands noted on the antiphosphotyrosine blot, confirming the identification of these bands as Cbl (Fig 1B, upper panel). Cbl is present not only in FcγRI-stimulated cells but also in U937IF cells at rest. However, Cbl is a single band at rest and a double band on receptor stimulation (Fig 1B, upper panel, lanes 4 and 5). Extensive studies in our lab have established that this pattern of mobility shift of the Cbl band is due to tyrosine phosphorylation of Cbl. The sum of the two bands in Fig 1B (upper panel, lane 5) appears to be equivalent to the single band in Fig 1B (upper panel, lane 4).
SLP-76 immunoprecipitates performed in another myeloid cell line, THP-1 cells (data not shown), showed similar results. Taken together, these data show that SLP-76 immunoprecipitated from FcγRI-activated myeloid cells is tyrosine phosphorylated and coprecipitates Cbl. SLP-76 is not detected in Cbl immunoprecipitates (Fig 1A and 1B, lanes 2 and 3). The anti-Cbl antibody used in our Cbl immunoprecipitates is directed against the extreme carboxy-terminal region of the molecule and does not immunoprecipitate SLP-76. The reason for our inability to coimmunoprecipitate SLP-76 with Cbl is unclear but it is plausible that this C-terminal region of Cbl is the region of SLP-76-Cbl binding in vivo. These data provide the first evidence that Cbl-SLP-76 and Grb2 can form a trimolecular signaling complex in vivo.
Kinetics of SLP-76 and Cbl phosphorylation and binding in myeloid cells.
Kinetic experiments were performed to confirm SLP-76-Cbl interaction in myeloid cells. In Fig 2, SLP-76 was immunoprecipitated from U937IF cell lysates as described above except that the cells were stimulated with anti-FcγRI (32.2 MoAb F(ab′)2 fragment of IgG) followed by stimulation with RαM F(ab′)2 fragment for varying periods of time ranging from 30 seconds to 30 minutes. Western Blot analysis with antiphosphotyrosine was then performed. Within 30 seconds of FcγRI stimulation, SLP-76 becomes tyrosine phosphorylated. This phosphorylation reaches a peak at 5 minutes to 10 minutes and then gradually diminishes, but is still present at 30 minutes (Fig 2A, lanes 3-7, center bands). Similarly, Cbl also becomes rapidly tyrosine phosphorylated on FcγRI stimulation and peaks at 5 minutes to 10 minutes, but then becomes dephosphorylated on tyrosine by 30 minutes (Fig 2A, lanes 3-7, upper bands). Importantly, the pattern of pp120 tyrosine phosphorylation observed in SLP-76 immunoprecipitates was identical to the kinetic pattern of Cbl tyrosine phosphorylation observed in Cbl immunoprecipitates.35 A phosphoprotein of 35 kD is also noted to coimmunoprecipitate with SLP-76 on FcγRI stimulation and follows a similar kinetic pattern of tyrosine phosphorylation. The identity of this phosphoprotein remains unknown.
The membrane in Fig 2A probed with anti–SLP-76 antibody, confirmed equivalent amounts of SLP-76 in all lanes except the preimmune (Fig 2B, middle panel). The preimmune (lane 1) represents unstimulated U937IF lysate immunoprecipitated with goat antiserum. The upper portion of the membrane was probed with anti-Cbl antibody. Unstimulated U937IF cells show a single-banded Cbl (Fig 2B, upper panel, lane 2). The more rapidly migrating band of Cbl appears stronger in the early stimulation lanes (Fig 2B, upper panel, lanes 3-4). The slower migrating band becomes more evident as stimulation progresses (Fig 2B, upper panel, lanes 5-7). Of note is that at 30 minutes, when the antiphosphotyrosine band of Cbl is no longer evident, a double band persists on the anti-Cbl blot (Fig 2A, lane 7 and Fig 2B, upper panel, lane 7). This may represent a serine/threonine phosphorylated form of Cbl as reported by Liu et al.44 The relative amount of Cbl in each lane appears very similar, suggesting a constitutive interaction between SLP-76 and Cbl but with inducible tyrosine phosphorylation. The lower portion of the membrane in Fig 2A was probed with anti-Grb2 antibody. The no stimulation lane does not contain as much Grb2 as the FcγRI stimulated lanes. The quantity of Grb2 associated with SLP-76 reaches a peak at around 5 to 10 minutes and persists at 30 minutes after FcγRI-receptor crosslinking (Fig 2B, lower panel). This pattern suggests an inducible component to the interaction between SLP-76 and Grb2. The kinetics of SLP-76-Grb2 interaction parallels the kinetics of SLP-76 phosphorylation, which also parallels the kinetics of Cbl phosphorylation. These kinetic data, along with the results shown in Fig 1B and 3B, support the formation of a constitutive SLP-76-Grb2 complex in vivo that becomes augmented on FcγRI aggregation.
Biochemical analysis of SLP-76-Cbl-Grb2-Shc interactions.
To further characterize the Cbl-SLP-76 and SLP-76-Grb2 interactions, we determined whether these associations after FcγRI-receptor stimulation were phosphorylation dependent. Lysates of U937IF cells at rest and after varying periods of FcγRI-receptor stimulation with anti-FcγRI (32.2 MoAb F(ab′)2 fragment of IgG) followed by stimulation with RαM F(ab′)2 fragment (from 30 seconds to 30 minutes) were immunoprecipitated with anti–SLP-76 in the presence and absence of PAP. PAP is known to dephosphorylate phosphotyrosine and phosphoserine/phosphothreonine residues. Proteins were isolated by Western Blot and antiphosphotyrosine immunoblot was performed as described above. As expected, no tyrosine phosphorylation was evident in the immunoprecipitates that had been previously PAP treated (Figure 3A, lanes 7-11). Of note is that the phosphoprotein bands migrating at 46 kD and 52 kD were also not evident in the PAP-treated immunoprecipitates, suggesting that these bands associate with SLP-76 via phosphorylation-dependent interactions. The membrane in Fig 3A was then reprobed with anti-Cbl, anti–SLP-76, anti-Shc, and anti-Grb2. The PAP-treated immunoprecipitates continue to show an association between Cbl and SLP-76, however the slower migrating Cbl band appears attenuated during the earlier timepoints in the presence of PAP compared with the non-PAP–containing immunoprecipitates (Fig3B, upper panel, lanes 2 to 6 v lanes 7-11). Equivalent amounts of SLP-76 are confirmed by anti–SLP-76 Western Blot (Fig 3B, second panel, lanes 2-11). The portion of the membrane in Fig 3A corresponding to pp46 and pp52 was probed with anti-Shc antibody. The immunoreactive bands at 46 kD and 52 kD superimpose on the 46 kD and 52 kD tyrosine phosphorylated bands noted in Fig 3A, confirming the presence of Shc in the SLP-76 immunoprecipitates on FcγRI stimulation (Fig 3B, third panel, lanes 3-6). In parallel experiments in which the membrane in Fig3B (third panel) was probed with secondary antimouse antibody alone, no Shc immunoreactive bands were observed, confirming that pp46 and pp52 in Fig 3A represent Shc (data not shown). The interaction between SLP-76 and Shc is not detected in the PAP-treated immunoprecipitates, suggesting that the interaction is phosphorylation dependent (Fig 3B, third panel, lanes 8-11). PAP treatment diminishes the interaction between SLP-76 and Grb2 in both stimulated and unstimulated cells (Fig3B, lower panel, lanes 2 to 6 v lanes 7-11). These data suggest that there is a component of the SLP-76-Grb2 interaction that is phosphorylation dependent and potentially mediated through the SH2 domain of Grb2. Alternatively, this phosphorylation-dependent component of the SLP-76-Grb2 interaction could occur via another phosphoprotein. Motto et al37 have mapped the Grb2-binding site on SLP-76 to amino acids 224-244 in the proline-rich region, which shows that the association between these two molecules is mediated through the SH3 domain of Grb2. Our PAP data suggest that SLP-76 and Grb2 associate in a constitutive manner because the interaction is observed in U937IF cells at rest. However, this interaction becomes augmented on FcγRI receptor stimulation, suggesting an inducible association. The anti-Grb2 blots of Fig 1B, 2B, and 3B all consistently show this increased association between SLP-76 and Grb2 on FcγRI-receptor stimulation. This interaction possibly occurs through the Grb2 SH2 domain, likely via the interaction of another phosphoprotein that recruits more Grb2. Based on the results in Fig 3A and 3B, a possible candidate would be tyrosine-phosphorylated Shc that is bound to both Grb2 and Cbl. We conclude from these data that Shc protein is tyrosine phosphorylated after FcγRI activation, which likely induces Shc to bind to a SLP-76-Cbl-Grb2 complex.
The Grb2-binding domain of SLP-76 binds Shc but not Cbl.
To further elucidate the regions of SLP-76 involved in Cbl and Grb2 binding, pull-down binding experiments were performed. U937IF cell lysates prepared at rest and after 1-minute stimulation of the FcγRI receptor with MoAb 197 followed by RαM F(ab′)2 fragment were incubated with SLP-76 GST-fusion proteins representing the Grb2 binding-domain (amino acid residues 225-265) and the proline-rich region that does not associate with Grb2 (amino acid residues 268-416) as mapped by Motto et al.37 In FcγRI-stimulated cells, a double-banded phosphoprotein of molecular weights 46 kD and 52 kD is pulled down by the SLP-76 GST-fusion protein representing the Grb2-binding domain (data not shown). To identify these 46 kD and 52 kD proteins, this region of the membrane was reprobed with anti-Shc primary antibody and developed via an AP-colorimetric system as described above. Both bands immunoreacted with anti-Shc antibody (Fig4, center panel, lane 5). The data confirm that Grb2 is pulled down by the SLP-76 GST-fusion protein corresponding to the Grb2-binding domain. This domain is also responsible for binding Shc in FcγRI- as well as FcγRII-stimulated cells (Fig 4, lower panel, lanes 4-7). Cbl does not appear to be part of this potential trimolecular complex, nor is it associated with the proline-rich region of SLP-76 that does not bind Grb2 (Fig 4, upper panel, lanes 4-7). The exact Cbl-binding site on SLP-76 remains to be determined. Preliminary GST-fusion protein data from our lab suggests that the carboxy terminal SH2 domain of SLP-76 is not involved in Cbl binding (data not shown). These data provide evidence that the Grb2-binding region of SLP-76 is responsible for FcγRI- and perhaps FcγRII-augmented association of Shc and SLP-76 observed in Fig 3B.
[The Grb2-binding domain of SLP-76 does not bind Cbl.] U937IF cells at rest and stimulated by anti-FcγRI cross-linking with MoAb 197 were incubated with SLP-76 GST-fusion proteins representing the Grb2-binding domain (amino acid residues 225-265) and the proline-rich region that does not associate with Grb2 (amino acid residues 268-416). Lane 1 represents precipitation with preimmune antisera. Lanes 2 and 3 represent incubation of GST with U937IF cells at rest and after 5 minute stimulation, respectively. Lanes 4 and 5 represent incubation of SLP-76 GST-fusion protein aa 225-268 with U937IF cells at rest and after 5 minute stimulation, respectively. Lanes 6 and 7 represent incubation of SLP-76 GST-fusion protein aa 268-416 with U937IF cells at rest and after 5 minutes stimulation, respectively. Lane 8 represents whole-cell lysate (1 × 106 cell equivalents) of stimulated U937IF cells. Upper panel represents anti-Cbl immunoblot. Middle panel represents anti-Shc immunoblot. Lower panel represents anti-Grb2 immunoblot.
[The Grb2-binding domain of SLP-76 does not bind Cbl.] U937IF cells at rest and stimulated by anti-FcγRI cross-linking with MoAb 197 were incubated with SLP-76 GST-fusion proteins representing the Grb2-binding domain (amino acid residues 225-265) and the proline-rich region that does not associate with Grb2 (amino acid residues 268-416). Lane 1 represents precipitation with preimmune antisera. Lanes 2 and 3 represent incubation of GST with U937IF cells at rest and after 5 minute stimulation, respectively. Lanes 4 and 5 represent incubation of SLP-76 GST-fusion protein aa 225-268 with U937IF cells at rest and after 5 minute stimulation, respectively. Lanes 6 and 7 represent incubation of SLP-76 GST-fusion protein aa 268-416 with U937IF cells at rest and after 5 minutes stimulation, respectively. Lane 8 represents whole-cell lysate (1 × 106 cell equivalents) of stimulated U937IF cells. Upper panel represents anti-Cbl immunoblot. Middle panel represents anti-Shc immunoblot. Lower panel represents anti-Grb2 immunoblot.
Characterization of SLP-76-Cbl-Grb2 and Grb2-Sos complexes in vivo.
It has been shown that Grb2 binds to the guanine nucleotide exchange factor Sos, leading to activation of Ras.10,45,46 The tyrosine phosphorylation of Shc, followed by its interaction with the Grb2-SH2 domain results in recruitment of Sos to the receptor-signaling complex.9,47 Previous data from our lab implicated Shc, Grb2, Raf-1, and MAP Kinase in FcγRI signaling in U937IF cells.32 Herein, we observe a novel association between Cbl, SLP-76, Grb2, and Shc. Because Grb2 and Shc are known to interact with the Ras guanine nucleotide exchange factor Sos, we set out to determine whether Sos was also bound to this likely multimolecular complex. U937IF cell lysates prepared at rest and after a 5 minute stimulation of the FcγRI receptor were subjected to immunoprecipitations with anti-Sos, anti-Cbl and anti–SLP-76 antibody. Proteins were isolated by Western Blot and then immunoblotted with antiphosphotyrosine, anti-Sos, anti-Cbl, anti–SLP-76, anti-Shc and anti-Grb2. The antiphosphotyrosine immunoblot is virtually identical to Fig 1A with the exception of the very faint phosphoprotein band migrating at 52 kD in the FcγRI-stimulated lane of the anti-Sos immunoprecipitate (Fig 5A, lane 3). Anti-Shc immunoblot of this region identifies the 52 kD band as Shc (data not shown). SLP-76 immunoprecipitates do not contain Sos despite the preservation of a protein complex containing SLP-76-Cbl-Grb2 and Grb2-Shc. The reciprocal immunoprecipitates with Sos do not contain SLP-76 (Fig 5B, first and third panel, lanes 2 and 3 and lanes 6 and 7). Grb2 is present in all immunoprecipitates, both in resting and stimulated cells (Figure 5B, lower panel, lanes 2-7). However, in the Sos and SLP-76 immunoprecipitates, more Grb2 is recruited in the FcγRI-receptor stimulated cells (Fig 5B, lower panel, lanes 2 and 3, 6 and 7). This pattern of increased SLP-76-Grb2 binding on FcγRI-receptor stimulation is consistent with that noted in Fig 1, 2, and 3 as described above. These results suggest that a multimolecular complex consisting of Cbl-SLP-76-Grb2-Shc is distinct from a trimolecular complex containing Sos-Grb2-Shc. The data provide the first evidence that the aforementioned quatramolecular complex exists in nature and stimulates inquiry into the potential roles of these distinct molecular complexes in FcγRI signaling.
Characterization of SLP-76-Grb2 and Grb2-Sos signaling complexes. Immunoprecipitation was performed with anti-Sos, anti-Cbl, and anti–SLP-76 antibody from lysates of resting U937IF cells or cells stimulated by anti-FcγRI cross-linking with MoAb 32.2 F(ab′)2 fragment. Proteins were resolved by SDS/PAGE, transferred to nitrocellulose membrane, and immunoblotted. (A) Antiphosphotyrosine immunoblot. Lane 1 represents precipitation with preimmune antisera. Lanes 2 and 3 represent anti-Sos IP of U937IF cells at rest and after 5 minutes stimulation, respectively. Lanes 4 and 5 represent anti-Cbl IP of U937IF cells at rest and after 5 minutes stimulation, respectively. Lanes 6 and 7 represent anti–SLP-76 IP of U937IF cells at rest and after 5 minutes stimulation, respectively. Lane 8 represents whole-cell lysate (1 × 106 cell equivalents) of stimulated U937IF cells. (B) Same membrane as in Fig 5A was blocked and reprobed. Upper panel represents anti-Sos immunoblot. Second panel represents anti-Cbl immunoblot. Third panel represents anti–SLP-76 immunoblot. Lower panel represents anti-Grb2 immunoblot.
Characterization of SLP-76-Grb2 and Grb2-Sos signaling complexes. Immunoprecipitation was performed with anti-Sos, anti-Cbl, and anti–SLP-76 antibody from lysates of resting U937IF cells or cells stimulated by anti-FcγRI cross-linking with MoAb 32.2 F(ab′)2 fragment. Proteins were resolved by SDS/PAGE, transferred to nitrocellulose membrane, and immunoblotted. (A) Antiphosphotyrosine immunoblot. Lane 1 represents precipitation with preimmune antisera. Lanes 2 and 3 represent anti-Sos IP of U937IF cells at rest and after 5 minutes stimulation, respectively. Lanes 4 and 5 represent anti-Cbl IP of U937IF cells at rest and after 5 minutes stimulation, respectively. Lanes 6 and 7 represent anti–SLP-76 IP of U937IF cells at rest and after 5 minutes stimulation, respectively. Lane 8 represents whole-cell lysate (1 × 106 cell equivalents) of stimulated U937IF cells. (B) Same membrane as in Fig 5A was blocked and reprobed. Upper panel represents anti-Sos immunoblot. Second panel represents anti-Cbl immunoblot. Third panel represents anti–SLP-76 immunoblot. Lower panel represents anti-Grb2 immunoblot.
DISCUSSION
Complex adapter proteins such as Cbl, Shc, and SLP-76 are substrates for receptor-coupled tyrosine kinases implicated in ITAM–based signaling events. These proteins contain multiple domains that are capable of forming complexes with other signaling molecules, especially phosphoproteins and SH2 containing adapter proteins. This propensity for phosphorylation and multimolecular complex formation serves to regulate the proteins to which they bind and ultimately to control signaling output from aggregated receptors. We set out to examine specific protein-protein interactions identified as substrates for protein tyrosine kinases induced by FcγRI-receptor stimulation in myeloid cells.
In this report, we show tyrosine phosphorylation of SLP-76, Cbl, and Shc on FcγRI-receptor stimulation and a novel constitutive interaction between Cbl and SLP-76. Phosphorylation of SLP-76 has been reported in lymphocytes on TCR and BCR stimulation as well as myeloid leukemia cells and platelets on FcεRI- and FcγRIIA-receptor stimulation, respectively.37,40-42 The kinase responsible for SLP-76 phosphorylation after TCR stimulation is believed to be ZAP-70, a member of the Syk family kinases.48Interestingly, Cbl also is a substrate for ZAP-70 tyrosine phosphorylation after TCR stimulation and evidence suggests that the association of the Cbl PTB domain with ZAP-70/Syk facilitates direct or indirect regulation of tyrosine kinase function.25,49Previous data from our lab and others show that the FcγRI receptor also signals through the protein tyrosine kinase Syk.6 50The kinetics of SLP-76 phosphorylation parallels the kinetics of Cbl phosphorylation, suggesting a link between these two adapter proteins in the FcγRI-signaling cascade.
Tyrosine phosphorylation serves to generate docking sites for SH2-containing proteins, thereby linking upstream receptor activation to downstream effector molecules and ultimately to transcriptional events. One such SH2-containing molecule is Shc, which we have shown to associate with SLP-76 in a tyrosine-phosphorylation–dependent manner. Shc is known to associate with a molecular complex containing Grb2 and Sos, the guanine nucleotide exchange factor responsible for converting GDP Ras to its active GTP form.9 Data from our laboratory established that the FcγRI receptor signals through a Shc-Grb2 complex leading to activation of Raf-1 and MAP Kinase.32 35 In the present study, we report a novel phosphorylation-dependent association between SLP-76 and Shc that is induced on FcγRI activation. Previous experiments in other signaling systems (EGF, B- and T-cell receptor) have documented SLP-76-Grb2 binding but have not observed the association between SLP-76 and Shc. It is likely that the SLP-76-Shc interaction is mediated via the binding of the Grb2-SH2 domain to tyrosine-phosphorylated Shc, because it is eliminated in the presence of PAP when Shc is dephosphorylated. Further support for this interaction is evidenced by our GST-fusion protein-binding data (Fig 4) indicating that the Grb2-binding domain of SLP-76 (amino acid residues 225-265) also associates with Shc under conditions of stimulation.
The interaction between SLP-76, Shc, and Grb2 would suggest that SLP-76 may also be found in a multimolecular complex containing Sos, which also associates with Shc and Grb2. However, our data indicate that SLP-76 is not found in such a multimolecular Ras-activating complex. The association of Grb2 and Shc with SLP-76 and Cbl may serve as a repository for Grb2 and Shc, regulating their association with Sos and subsequent activation of Ras. There are several lines of evidence in support of the involvement of Cbl in the regulation of Ras. First, inCaenorhabditis elegans, sli-1, a Cbl homolog acts as a negative regulator of the Ras homolog, Let60, possibly by regulating the activity of Sem5, a Grb2 homolog.51 Second, Liu et al22 reported that transient overexpression of a transforming Cbl mutant was able to increase NFAT activity, showing that Cbl is involved in Ras-dependent T-cell–signaling pathways leading to transcriptional activation of IL-2. Third, Cbl overexpression in conjunction with a Ras-sensitive AP1 reporter resulted in inhibition of TCR-induced ERK2 activation and a T-cell activation-induced exchange of Cbl for Sos on Grb2.52 The above mentioned data from Rellahan et al,52 along with data from our lab, can be interpreted as evidence that Cbl functions as an adapter shield or exchanger for Grb2 in regulation of Grb2-Sos.35 Evidence also exists in TCR signaling supporting involvement of SLP-76 in the regulation of Ras. A functional link between SLP-76 and activation of Ras and calcium pathways has been suggested by Wardenburg et al48 who have shown that overexpression of wild-type SLP-76 leads to a hyperactive TCR, whereas expression of a SLP-76 molecule that is unable to be tyrosine phosphorylated results in attenuated TCR function. Musci et al53 have shown that SLP-76 functions upstream of ERK, which is downstream of Ras and does not involve calcium-dependent pathways. All three of the major SLP-76 domains (amino terminal phosphotyrosines, central proline-rich Grb2-binding domain, and carboxy terminal SH2 domain) are required for optimal activation of T cells. Others have linked SLP-76 to transcriptional activation of the IL-2 gene through association with the SH2 domain of Vav.37,38,54 Cbl has also been shown to associate with the SH2 domain of Vav, which also contains a guanine nucleotide exchange factor (GNEF) domain.55
Our data provides the first evidence for an inducible interaction between SLP-76 and Shc, occurring only on FcγRI-receptor activation and a constitutive interaction between SLP-76 and Cbl. We postulate that these complex adapter proteins through their protein-protein interactions, function as a repository for key molecules involved in the activation of Ras, thereby serving to regulate Ras. Work is underway to elucidate the functional significance of these protein-protein interactions and determine the structural and functional motifs required for SLP-76-Cbl and SLP-76-Grb2-Shc interactions that contribute to the regulation of Ras in myeloid cells.
ACKNOWLEDGMENT
The authors thank Drs Wade Kyono, Rae Kil Park, and Anat Epstein for their suggestions and careful review of the manuscript.
Supported in part by grants from the National Institutes of Health (R01CA75637-01 and R01GM53256) and performed in the Neil Bogart Memorial Laboratories, which are supported by the T.J. Martell Foundation for Leukemia, Cancer, and AIDS Research. D.L.D. is supported by the Childrens Hospital Career Development Award, a grant from the Robert E. and May R. Wright Stop Cancer Foundation, and a grant from the American Cancer Society (RPG-98-244-01-LBC). G.A.K. is an Established Investigator for the American Heart Association.
Address correspondence to Donald L. Durden, MD, PhD, Department of Pediatrics, Division of Hematology-Oncology, Childrens Hospital Los Angeles, 4650 Sunset Blvd MS#57, Los Angeles, CA 90027; e-mail:ddurden%smtpgate@chlais.usc.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. [SLP-76 and Cbl are tyrosine phosphorylated upon FcγRI stimulation.] Immunoprecipitation was performed with anti-Cbl and anti–SLP-76 antibody from lysates of resting U937IF cells or cells stimulated by anti-FcγRI cross-linking with MoAb 32.2 F(ab′)2 fragment. Proteins were resolved by SDS/PAGE, transferred to nitrocellulose membrane, and immunoblotted. (A) Antiphosphotyrosine immunoblot. Lane 1 represents precipitation with preimmune antisera. Lanes 2 and 3 represent anti-Cbl IP of U937IF cells at rest and after 5-minute stimulation, respectively. Lanes 4 and 5 represent anti–SLP-76 IP of U937IF cells at rest and after 5-minute stimulation, respectively. Lane 6 represents whole-cell lysate (1 × 106 cell equivalents) of stimulated U937IF cells. (B) Same membrane as in Fig 1A was blocked and reprobed. Upper panel represents anti-Cbl immunoblot. Middle panel represents anti–SLP-76 immunoblot. Lower panel represents anti-Grb2 immunoblot.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/5/10.1182_blood.v92.5.1697/4/m_blod41706001aw.jpeg?Expires=1763583353&Signature=4w1-2pYJ8261qDexR4WKJHMmW8sO82jYZarCb4CKwf44teu3P5mYO4w0Y-49eTVHpoxiKGwjhENlHKRAZoIOgsukyqWGtLfRbX0jX2MzrCZAO-SG6-lKHDjmblc2F~LcQQDGiOfwdVAhCQZXeWGvFSUxRQ~o~tLbBFJxkcjqA7mjvaPyqTbMi8rKEOxLJYRJrasLKW80lYZvMyKmUA-lKQWROfO3Q~nSGPvhtSaMine57hm44AtfnTWW2vRf1xtzDGlDlaPv1-YcypFWQWs5ghl9dSa2cjR2eFl222fEGQe~qUZHT2oY~x2yXTSW2ZRRrKzUm2rwaRd~2W7-qhMZeA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. [SLP-76 and Cbl are tyrosine phosphorylated upon FcγRI stimulation.] Immunoprecipitation was performed with anti-Cbl and anti–SLP-76 antibody from lysates of resting U937IF cells or cells stimulated by anti-FcγRI cross-linking with MoAb 32.2 F(ab′)2 fragment. Proteins were resolved by SDS/PAGE, transferred to nitrocellulose membrane, and immunoblotted. (A) Antiphosphotyrosine immunoblot. Lane 1 represents precipitation with preimmune antisera. Lanes 2 and 3 represent anti-Cbl IP of U937IF cells at rest and after 5-minute stimulation, respectively. Lanes 4 and 5 represent anti–SLP-76 IP of U937IF cells at rest and after 5-minute stimulation, respectively. Lane 6 represents whole-cell lysate (1 × 106 cell equivalents) of stimulated U937IF cells. (B) Same membrane as in Fig 1A was blocked and reprobed. Upper panel represents anti-Cbl immunoblot. Middle panel represents anti–SLP-76 immunoblot. Lower panel represents anti-Grb2 immunoblot.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/5/10.1182_blood.v92.5.1697/4/m_blod41706001bw.jpeg?Expires=1763583353&Signature=1meWLx4g0YX3mq1xcVMQ59d-h-IF5pX0PEft06c0qZlw9bK3GWKbMvRXM0B-gb1BX13FXGZcO~W3PEEFhOvhQgTjiPkKmUe61nU9arQBcwjjO6NjOwj5vsfSVQ0SeXDeZ2TpfpUXqSmzR3OgPDP5dLeyB0oylVzDasD2deIXqq0oAfs8tNnK57UZYN6y-4mDQPNp3QUKSu1mHvYxMgASVu5bFU4dr3BhalW3-AbYbwT2034uYYUdlUqlsWmszDsMOX9fCBGLM3m5wfXYU-w-TUsb4X-hqLJGXmUeX4t~ILp1COiKBDr-OAyopP3BjZmYlWhLzYDWT3BPYyDyhETlyw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. [Kinetics of SLP-76-Cbl interaction following FcγRI stimulation.] Immunoprecipitation was performed with anti–SLP-76 antibody from lysates of resting U937IF cells or cells stimulated by anti-FcγRI cross-linking with MoAb 32.2 F(ab′)2 fragment for varying periods of time ranging from 30 seconds to 30 minutes. Proteins were resolved by SDS/PAGE, transferred to nitrocellulose membrane, and immunoblotted. (A) Antiphosphotyrosine immunoblot. Lane 1 represents precipitation with preimmune antisera. Lane 2 represents U937IF cells at rest. Lanes 3-7 represent U937IF cells stimulated for 30 seconds, 1 minutes, 5 minutes, 10 minutes, and 30 minutes, respectively. Lane 8 represents whole-cell lysate (1 × 106 cell equivalents) of stimulated U937IF cells. (B) Same membrane as in Fig 2A was blocked and reprobed. Upper panel represents anti-Cbl immunoblot. Middle panel represents anti–SLP-76 immunoblot. Lower panel represents anti-Grb2 immunoblot.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/5/10.1182_blood.v92.5.1697/4/m_blod41706002aw.jpeg?Expires=1763583353&Signature=l6b~0-vsAZ9TqV81DLGXSAiu3lnRhGiC4Qrxbuo-eXCMK0ZNk67FoJTpRLiwB6zDW521qqDU9ZktuGDAS5k-tD5dkz7swh6AhEvbXrBs-1gtpnXDKTj80dvvAEh0T3HjVYEQNemA3tVVYVXqI-3lgnNq19F-KO36LkC7R6iw52xhFuYuuRY1jCpTb8gDHP4VQAAHNRaWn3xCU8J5Z9Dy6PamGYO4B2T63kDe3cBXJzhHWQuY0-KujsJtK7vPDZzl~YAifgOlRJjam4bPJuXnYf2TdAc4u-6tkXzw~mTXH9KrFA8oH1fYoH~pQxPKyqHkgDrT43f-pDk6sLqRJt~kiw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. [Kinetics of SLP-76-Cbl interaction following FcγRI stimulation.] Immunoprecipitation was performed with anti–SLP-76 antibody from lysates of resting U937IF cells or cells stimulated by anti-FcγRI cross-linking with MoAb 32.2 F(ab′)2 fragment for varying periods of time ranging from 30 seconds to 30 minutes. Proteins were resolved by SDS/PAGE, transferred to nitrocellulose membrane, and immunoblotted. (A) Antiphosphotyrosine immunoblot. Lane 1 represents precipitation with preimmune antisera. Lane 2 represents U937IF cells at rest. Lanes 3-7 represent U937IF cells stimulated for 30 seconds, 1 minutes, 5 minutes, 10 minutes, and 30 minutes, respectively. Lane 8 represents whole-cell lysate (1 × 106 cell equivalents) of stimulated U937IF cells. (B) Same membrane as in Fig 2A was blocked and reprobed. Upper panel represents anti-Cbl immunoblot. Middle panel represents anti–SLP-76 immunoblot. Lower panel represents anti-Grb2 immunoblot.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/5/10.1182_blood.v92.5.1697/4/m_blod41706002bw.jpeg?Expires=1763583353&Signature=wfQYaZKxKQlDE-lpvKh9YksHtXfTcv1O7iJ-xKQKGbW1lOq7YfonJmKeBJrcJCCigChmkpoHEn4K0fDAyHuwzBYZIo~wAWOO0MoTwW2arlchPASt2Q30sVK8y90rFYh3jNuWH-lTlsycAz01P8EpSZYxmE2oqmmG0WAnngpO2yV9NlTrp41IScOUVU47bM4XnW8v-h4t6S3U2wuWg7d5I8WbhD9rvKMJ4pQR4BA18LzXWEhVSNSj0liomPN3eZoYNEdH8Vo8HwYyJtLwT7RfcT7Fz99-yZmgl5X~mulK5B-9pwfuXiAAd-0wVMum5aQ2CM8FEfuuZ6XroGcDRM7k3Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. [Biochemical characterization of SLP-76-Cbl and SLP-76-Shc interactions.] Immunoprecipitation was performed with anti–SLP-76 antibody from lysates of resting U937IF cells or cells stimulated by anti–FcγRI cross-linking with MoAb 32.2 F(ab′)2 fragment for varying periods of time ranging from 30 seconds to 30 minutes in the presence or absence of PAP. Proteins were resolved by SDS/PAGE, transferred to nitrocellulose membrane, and immunoblotted. (A) Antiphosphotyrosine immunoblot. Lane 1 represents precipitation with preimmune antisera. Lanes 2-6 represent anti–SLP-76 IP of U937IF cells at rest and after 30 seconds, 2 min, 10 min and 30 min stimulation respectively. Lanes 7-11 represent anti-SLP-76 IP of U937IF cells at rest and after 30 seconds, 2 minutes, 10 minutes, and 30 minutes stimulation, respectively, to which 1.8 units PAP was added to the cell lysates. Lane 12 represents whole-cell lysate (1 × 106 cell equivalents) of stimulated U937IF cells. (B) Same membrane as in Fig 3A was blocked and reprobed. Upper panel represents anti-Cbl immunoblot. Second panel represents anti–SLP-76 immunoblot. Third panel represents anti-Shc immunoblot. Lower panel represents anti-Grb2 immunoblot.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/5/10.1182_blood.v92.5.1697/4/m_blod41706003aw.jpeg?Expires=1763583353&Signature=rzERc-d5GmcXRMy1h-cLfcqORYjkxNhllHdsbXiopk~g-ozvB5DxWbPasF7m2Nh889kWwNGFTQngUspyylUsXY0U~02DFZyluX58pi37UtUqBUMdjv4x8JvDhVJCYEkkkmBlNaPBk9fSYKV1gOBsZDtoK3hx7w9YPG2AlVTM1wj~SLM7HeTQsbzbTP16bMaxgG2m-jOfVnhGK281J1jlIFdaqYrXG8exSKkNTyilCrmQ3cRmDHUMpoRMe0rrUQi2aK8Jx5bOAVYAlv5sqCHwUDLMn2FyMT112821WcXwVWYj9JIDU9K-cRxQy05mclCDkaLKHQEa0rwzVoisWHny5A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. [Biochemical characterization of SLP-76-Cbl and SLP-76-Shc interactions.] Immunoprecipitation was performed with anti–SLP-76 antibody from lysates of resting U937IF cells or cells stimulated by anti–FcγRI cross-linking with MoAb 32.2 F(ab′)2 fragment for varying periods of time ranging from 30 seconds to 30 minutes in the presence or absence of PAP. Proteins were resolved by SDS/PAGE, transferred to nitrocellulose membrane, and immunoblotted. (A) Antiphosphotyrosine immunoblot. Lane 1 represents precipitation with preimmune antisera. Lanes 2-6 represent anti–SLP-76 IP of U937IF cells at rest and after 30 seconds, 2 min, 10 min and 30 min stimulation respectively. Lanes 7-11 represent anti-SLP-76 IP of U937IF cells at rest and after 30 seconds, 2 minutes, 10 minutes, and 30 minutes stimulation, respectively, to which 1.8 units PAP was added to the cell lysates. Lane 12 represents whole-cell lysate (1 × 106 cell equivalents) of stimulated U937IF cells. (B) Same membrane as in Fig 3A was blocked and reprobed. Upper panel represents anti-Cbl immunoblot. Second panel represents anti–SLP-76 immunoblot. Third panel represents anti-Shc immunoblot. Lower panel represents anti-Grb2 immunoblot.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/5/10.1182_blood.v92.5.1697/4/m_blod41706003bw.jpeg?Expires=1763583353&Signature=CTLYZyFuOLz6EqNJdxGHsyFjnaqGIBxA7ZmZTMeRZCanYn26Bgq3o4iPmqiFdrKroE6fEXC9v6JqteYBp8lW2EfrNhN2WfFn6YqIr5U0O-3jrHO6jspSWGgCHYAnn8njJk73~Bl0LV0Z6nuwRBoYTX3rTV6VmBgyVFJZKg3rpAdZm0OYpKFhYPeVjV-aShEp-JyVZCbcPCIFNuw3SM24zHsbR6juOGLuQ4Gfu3JXH7xYK6ZKHq362lWevkZWDWU7U4MLkNRzqQiKI92tkMpLUsClt~7XjWC5aFpgmr5qaX~SENRXoGkQroFnXLgqClw8O52tZpem0sGNMgcEQ6vJVQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. [The Grb2-binding domain of SLP-76 does not bind Cbl.] U937IF cells at rest and stimulated by anti-FcγRI cross-linking with MoAb 197 were incubated with SLP-76 GST-fusion proteins representing the Grb2-binding domain (amino acid residues 225-265) and the proline-rich region that does not associate with Grb2 (amino acid residues 268-416). Lane 1 represents precipitation with preimmune antisera. Lanes 2 and 3 represent incubation of GST with U937IF cells at rest and after 5 minute stimulation, respectively. Lanes 4 and 5 represent incubation of SLP-76 GST-fusion protein aa 225-268 with U937IF cells at rest and after 5 minute stimulation, respectively. Lanes 6 and 7 represent incubation of SLP-76 GST-fusion protein aa 268-416 with U937IF cells at rest and after 5 minutes stimulation, respectively. Lane 8 represents whole-cell lysate (1 × 106 cell equivalents) of stimulated U937IF cells. Upper panel represents anti-Cbl immunoblot. Middle panel represents anti-Shc immunoblot. Lower panel represents anti-Grb2 immunoblot.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/5/10.1182_blood.v92.5.1697/4/m_blod41706004w.jpeg?Expires=1763583353&Signature=slRdf4RUvonUJ~s5GS65wmgi4zD1NT-Qt42InB-gx~YbJF2ww4QFkVlnCMKhT73CBXhTJ7BOdWMtWPKH7MZ3-G8wP3v1Cwb7tsT3bOmqbG1kNSQOX~r29so94h2lQkWiUV8pRsfERV5b1skPcqkZgNifTPQA9u02S5SjtCFBxQnO7-O6C-h~AvWH7DSrK4~lddkRGupLXgFXfwid68B-8oGagLr7K~pkuBu5Wd33vqPvjzrZacxWMVfvf3dcAkyrAIJN3d4qTvc16hxDas6Te8pj1sYqoRyCR4d-53pyEc~IBWEO2sWCOfQXZCYdsh0~7j7S6UnWYonD-BvWuQkU0A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal