Abstract
The antiphospholipid syndrome is a thrombophilic condition marked by antibodies that recognize anionic phospholipid-protein cofactor complexes. We recently reported that exposure to IgG fractions from antiphospholipid patients reduces the level of annexin-V, a phospholipid-binding anticoagulant protein, on cultured trophoblasts and endothelial cells and accelerates coagulation of plasma exposed to these cells. Therefore, we asked whether antiphospholipid antibodies might directly reduce annexin-V binding to noncellular phospholipid substrates. Using ellipsometry, we found that antiphospholipid IgGs reduce the quantity of annexin-V bound to phospholipid bilayers; this reduction is dependent on the presence of β2-glycoprotein I. Also, exposure to plasmas containing antiphospholipid antibodies reduces annexin-V binding to phosphatidyl serine-coated microtiter plates, frozen thawed washed platelets, activated partial thromboplastin time (aPTT) reagent and prothrombin time reagent and reduces the anticoagulant effect of the protein. These studies show that antiphospholipid antibodies interfere with the binding of annexin-V to anionic phospholipid and with its anticoagulant activity. This acceleration of coagulation, due to reduced binding of annexin V, stands in marked contrast to the “lupus anticoagulant effect” previously described in these patients. These results are the first direct demonstration of the displacement of annexin-V and the consequent acceleration of coagulation on noncellular phospholipid surfaces by antiphospholipid antibodies.
© 1998 by The American Society of Hematology.
THE PRESENCE of antibodies in blood, which recognize anionic phospholipids or anionic phospholipid-protein complexes, has been associated with a thrombophilic syndrome, the antiphospholipid antibody (aPL) syndrome. This syndrome is characterized by arterial and venous thrombosis or by recurrent pregnancy loss, attributed to placental thrombosis.1-4Paradoxically, these antibodies may manifest as “lupus anticoagulants” in vitro by inhibiting phospholipid-dependent blood coagulation.3 5 Yet, in vivo, these “anticoagulants” are associated with thrombotic manifestations, and not with bleeding disorders.
Annexin-V (placental anticoagulant protein-I, vascular anticoagulant-α) has potent anticoagulant properties in vitro that are based on its high affinity for anionic phospholipids and its capacity to displace coagulation factors from phospholipid surfaces.6 We previously reported that annexin-V, which is normally present on the apical surface of placental syncytiotrophoblasts,7 is markedly reduced on apical membranes of placental villi from aPL patients.8,9 Also, IgG fractions from aPL patients reduce the quantity of annexin-V on cultured trophoblasts and endothelial cells and accelerate the coagulation of plasma exposed to these cells.10 The latter findings raised the possibility that cultured cells might not be necessary to observe this effect and that aPL antibodies might similarly reduce annexin-V binding to noncellular phospholipid-coated surfaces and to phospholipid preparations used for conventional clinical coagulation tests.
We therefore investigated the effects of aPL IgG preparations and aPL plasmas on annexin-V adsorption to phospholipid-coated surfaces. We also studied the effects of aPL IgG fractions and plasmas on the binding of annexin-V and coagulation on frozen thawed washed platelets (as prepared for the activated partial thromboplastin time [aPTT] test known as the “platelet neutralization procedure”) using methods similar to those we had previously used with cultured trophoblasts and endothelial cells.10 We then moved to studies using readily available phospholipid reagents used for routine clinical coagulation reactions.
MATERIALS AND METHODS
Sources of IgGs and plasmas.
IgG antibodies were isolated from the citrated plasma of three patients with severe antiphospholipid syndrome (previously described in Rand et al10) and three normal control subjects using protein G as described.11 For studies with plasmas, citrated specimens were collected from 10 patients with aPL syndrome and 10 non-aPL controls at Mount Sinai Medical Center. The IgGs and plasmas were tested for the presence of antibodies against β2-glycoprotein I (β2-GPI), prothrombin and annexin-V with standardized nitrocellulose (Schleicher & Schuell, Keene, NH) dot-blots containing varying quantities of the proteins, up to 1 μg. All three of the purified aPL IgGs recognized β2-GPI directly, one of the three recognized purified human prothrombin, and none recognized annexin-V directly. Of the 10 aPL plasmas, nine contained immunoglobulin that recognized β2-GPI, one recognized prothrombin alone, and none recognized annexin-V directly.
To provide the same standard plasma for the second stage of coagulation tests, plasmas from three normal blood bank donors were pooled, aliquotted and stored at −40°C for all coagulation studies.
Annexin-V.
Annexin-V was purified from human placentas as described.12The identity of the protein was confirmed by immunoblot analysis using a previously characterized affinity-purified monospecific polyclonal rabbit anti-annexin–V IgG.7
For studies of annexin-V binding to phosphatidyl serine-coated microtiter plates (below), the protein was labeled with biotin as previously described.13 Annexin-V was dialyzed at 4°C against buffer containing 0.05 mol/L boric acid, 0.1 mol/L NaCl, pH 8.5. Biotin-NHS (Calbiochem-Novabiochem Corp, La Jolla, CA) was then added to the annexin-V at a 1:2 molar ratio of annexin-V to biotin-NHS. The reaction was performed for 30 minutes at 4°C and quenched with 10 mmol/L glycine. The biotinylated annexin-V was then dialyzed against Tris-buffered saline (TBS) buffer (0.05 mol/L Tris, 0.1 mol/L NaCl, pH 7.4). The concentration of biotinylated annexin-V was determined by absorbance at 280 nm and aliquots were stored at −70°C.
Ellipsometry studies.
We studied the effects of aPL antibodies on phospholipid-bound annexin-V with computer-assisted ellipsometry using methods similar to those previously published,6 as follows: planar phospholipid bilayers were applied to silicon slides as described.14,15 A 5-mmol/L vesicle mixture of 30% 1,2-dioleoyl-sn-glycero-3-phosphatidylserine and 70% 1,2-dioleoyl-sn-glycero-3-phosphocholine (PS/PC) (Avanti Polar-Lipids, Inc, Alabaster, AL) was dried under nitrogen and sonicated (Sonic Dismembrator model F60; Fisher Scientific, Pittsburgh, PA) in HEPES buffer (0.01 mol/L HEPES, 0.14 mol/L NaCl, pH 7.5) at 0°C until the suspension was completely clear. Silicon slides of 1 by 4 cm and 0.4 mm thickness were cut from silicon wafers (Wacker Chemie, Munich, Germany, n-type, phosphor-doped). The slides were thoroughly cleaned with detergent (Sparkleen; Fisher Scientific) and water. They were then kept overnight in 30% chromic sulfuric acid, flushed with water, and stored in 50% alcohol-water until use, when they were rinsed with distilled water and then dipped into a container containing stirring HEPES buffer composed of 0.01 mol/L HEPES, 0.14 mol/L NaCl, 0.1% bovine serum albumin (BSA), 5 mmol/L CaCl2, pH 7.5. The sonicated PS/PC vesicles (final concentration 50 μmol/L) were then added and stirred for 10 minutes. Each slide was flushed with the latter HEPES buffer and transferred to an ellipsometer cuvette containing the stirring buffer. Adsorption of annexin-V (2 μg/mL), (β2-GPI) (2 μg/mL, kindly provided by Dr K. McCrae, Temple University, Philadelphia, PA) and IgG preparations (100 μg/mL) to the phospholipid bilayers on the silicon slide were observed after the serial addition of each of the proteins. The mass of the PS/PC bilayer (≈0.4 μg/cm2) was measured and subtracted for these curves. After adsorption had reached equilibrium, residual annexin-V was desorbed from the phospholipid bilayers on the silicon slide by the addition of 0.5 mol/L EDTA (to a final concentration of 6 mmol/L) and measured. Addition of EDTA had no effect on the adsorptions of IgGs or β2-GPI, alone or in combination. The completeness of desorption of annexin-V from the surface by EDTA was further confirmed by subsequently solubilizing the phospholipid bilayers with 0.1% sodium dodecyl sulfate (SDS). The SDS-solubilized samples were checked for annexin-V by standard immunoblot with a monospecific polyclonal rabbit anti–annexin-V IgG following 0.1% SDS-12% polyacrylamide gel electrophoresis, as described.7
Annexin-V binding to phosphatidyl serine-coated microtiter plates.
Microtiter plates (Nunc-Immuno Plate, MaxiSorp Surface) (Fisher Scientific) were coated with phosphatidyl serine (PS) (Avanti Polar-Lipids, Inc) as previously described.16 17 Citrated plasma samples (50 μL) were added to each well in duplicate and incubated for 30 minutes at room temperature. The wells were emptied and washed four times with phosphate-buffered saline (PBS) buffer, pH 7.4. 50 μL of biotinylated-annexin V (1 μg/mL in TBS buffer containing 0.1 % BSA and 5 mmol/L CaCl2, pH 7.4) was then added to each well and incubated for 30 minutes at room temperature. The wells were washed four times with PBS buffer, followed by 50 μL of phosphatase-labeled streptavidin (0.5 μg/mL in TBS buffer containing 0.1 % BSA) (Kirkegaard & Perry Laboratories, Gaithersburg, MD), which was incubated for 30 minutes at room temperature. The wells were then emptied and washed four times with PBS buffer, pH 7.4. A total of 50 μL of p-nitrophenyl phosphate substrate (1 mg/mL in DEA buffer) (Sigma Chemical Co, St Louis, MO) was added and incubated for approximately 30 minutes at room temperature, after which the optical absorbance was read at 405 nm with a kinetic microplate reader (Molecular Devices, Menlo Park, CA).
Platelets.
Platelets were prepared for coagulation studies after the method previously described for the platelet aPTT test (“platelet neutralization procedure”).18 Pooled platelets from blood bank donors were washed three times in TBS buffer (0.15 mol/L NaCl, 0.02 mol/L Tris) containing 1 mg/mL glucose, pH 7.4, resuspended to a density of 2 × 105/μL in the buffer, aliquotted, and stored at −70°C for all studies.
Annexin-V assays and coagulation studies with washed platelets.
The effects of aPL IgGs on the quantity of platelet-associated annexin-V were performed with modified methods similar to those described for cultured trophoblasts and endothelial cells.10 aPL and control IgGs were added to normal citrated plasma to a final concentration of 5 mg/mL. Aliquots of frozen thawed washed platelets (1 × 108) were incubated with 100 μL of the plasmas at 4°C for 2 hours, as previously described.10 The platelets were washed three times and resuspended in HEPES buffer containing 5 mmol/L CaCl2, pH 7.4. Annexin-V was then added to the platelets in the HEPES buffer containing 5 mmol/L CaCl2 to a final concentration of 20 μg/mL (determined after pilot studies with varying concentrations of annexin-V) and incubated at 4°C for an additional 15 minutes. The platelets were then centrifuged and washed three times in the same buffer. After the final centrifugation, to dissociate annexin-V, the platelets were resuspended in the HEPES buffer containing 1 mmol/L EGTA. The quantity of platelet-associated annexin-V was determined by an enzyme-linked immunosorbent assay (ELISA),10 as described. The results of the assays were expressed as ng of annexin-V/106 platelets.
We investigated the effects of aPL IgG on coagulation using platelets exposed to aPL or control IgG. For these experiments the IgGs were added to pooled normal plasma to a concentration of 5 mg/mL. Thawed washed platelets (120 μL), at a density of 2 × 105/μL in the TBS buffer described above, were added to 120 μL of IgG-containing plasma and preincubated for 10 minutes at 37°C. The platelets were centrifuged, washed two times, and resuspended in 120 μL of the TBS buffer. A 50-μL volume of the platelet suspension was then added to 50 μL of pooled normal plasma, incubated for 30 seconds at 37°C in a ST4 Coagulation Instrument (American Bioproducts Co, Parsipanny, NJ). A total of 50 μL of Celite (5 g/L in TBS buffer consists of 0.05 mol/L Tris, 0.1 mol/L NaCl, pH 7.4) was added and the mixture was incubated for 60 seconds. A volume of 50 μL of 0.02 mol/L CaCl2 or 0.02 mol/L CaCl2 containing annexin-V at 20 μg/mL was then added, the times until clot formation were measured, and the mean times of duplicate tests were reported. Coagulation times of specimens without and with annexin-V were recorded, along with the net prolongations (ie, coagulation time in the presence of annexin-V minus coagulation time in the absence of annexin-V), which reflected the anticoagulant activity of the annexin-V.
Annexin-V assays and coagulation studies with aPTT reagent.
We have studied the effects of aPL IgGs on the quantity of annexin-V associated with aPTT reagent-phospholipid (Actin FS) (Baxter Diagnostics Inc, Deerfield, IL). A total of 100 μL of the aPTT reagent-phospholipid was incubated with 100 μL of aPL patient plasma or control plasma for 15 minutes at 37°C. The mixture of aPTT reagent-phospholipid and plasma was sedimented with a microcentrifuge (Model 5451; Brinkmann Instruments, Inc, Westbury, NY) at 15,000g for 20 minutes at room temperature. The plasma-treated–phospholipid pellets were washed three times with HEPES buffer (0.01 mol/L HEPES, 0.14 mol/L NaCl, 0.1% BSA, pH 7.4) and resuspended in HEPES buffer containing 5 mmol/L CaCl2. Annexin-V was then added into the phospholipid suspension to a final concentration of 2 μg/mL and incubated at 37°C for 10 minutes. After centrifugation, the phospholipid pellets were washed three times in HEPES buffer containing 5 mmol/L CaCl2. The phospholipid-associated annexin-V was then dissociated from the aPTT reagent-phospholipid with HEPES buffer containing 5 mmol/L EDTA and assayed by ELISA as described above. The results of the assays were expressed as nanograms of annexin-V/50 μL aliquot of aPTT reagent-phospholipid.
The effect of aPL IgG on coagulation with aPTT reagent-phospholipid was determined using a two-stage assay, in which the first stage exposed phospholipid to potential aPL antibodies in the test plasma, and the second stage measured coagulation times with the phospholipid using a pooled normal plasma. We designed this assay using the pooled normal plasma for the second stage to deal with the variable baseline coagulation times of individual patients. A total of 50 μL of the aPTT reagent-phospholipid was added to 50 μL of aPL patient or control citrated plasma. The mixture was sedimented with the microcentrifuge, as described above. The pellets were then washed once in the TBS buffer and resuspended in 220 μL of this buffer. A total of 50 μL of this plasma treated-phospholipid suspension was added to 50 μL pooled normal plasma and incubated for 120 seconds at 37°C in the ST4 Coagulation Instrument, after which 50 μL of 0.02 mol/L CaCl2 or 0.02 mol/L CaCl2 containing annexin-V (30 μg/mL) was added. The times until clot formation of duplicate specimens were monitored and reported, as described for the platelets above.
Annexin-V assays and coagulation studies with prothrombin time reagent.
The effect of aPL IgG on the quantity of annexin-V associated with prothrombin time (PT) reagent (tissue factor-phospholipid complex) was also studied. PT reagent (Thromboplastin-C Plus; Baxter Diagnostics Inc) was washed three times with TBS buffer by sedimentaion and resuspension with the microcentrifuge for 20 minutes at 15,000g, as described for aPTT reagent-phospholipid. A total of 100 μL of the washed phospholipid of PT reagent was incubated with 100 μL of aPL or control IgG-containing plasma (at a concentration of 5 mg/mL, prepared as for the platelets described above) for 15 minutes at 37°C. The mixture was then centrifuged and the pellets of IgG-treated PT reagent-phospholipid were then treated in the same way as described above for aPTT reagent-phospholipid. The phospholipid-associated annexin-V was dissociated from the PT reagent-phospholipid by the HEPES buffer containing 5 mmol/L EDTA and was assayed by ELISA, as described above. The results were reported as described for the aPTT reagent above.
To investigate the effects of aPL IgG on coagulation with PT reagent, a two-stage assay, similar to the aPTT assay described above, was used. The PT reagent (50 μL) was washed three times in TBS buffer to remove calcium and was added to 50 μL of aPL patient or control plasma. The mixtures were centrifuged as described above and the pellets were washed once in the TBS buffer and resuspended in 220 μL of this buffer. A total of 50 μL of the suspension was then added to 50 μL pooled normal plasma and incubated for 120 seconds at 37°C in the ST4 Coagulation Instrument. A 50-μL volume of 0.02 mol/L CaCl2 or 0.02 mol/L CaCl2 containing annexin-V (30 μg/mL) was added. The times until clot formation of duplicate specimens were monitored and recorded as described above.
Fluorimetric determination of annexin-V binding to phospholipid.
The effect of aPL plasma on the binding of fluorescein isothiocyanate (FITC)-conjugated annexin-V to phospholipid was also determined. A total of 50 μL of aPTT reagent-phospholipid was added to 50 μL of aPL or control plasma and sedimented in the microcentrifuge as described above. The pellets of plasma-treated–phospholipid were washed once in the TBS buffer, pH 7.4, and then resuspended in 55 μL of the above buffer containing 5 mmol/L CaCl2 and 1 μg/mL of FITC-conjugated annexin-V (Clontech Laboratories Inc, Palo Alto, CA). The mixture was incubated for 5 minutes at room temperature and centrifuged as described above, after which the supernatants were collected and the pellets were resuspended in 55 μL of the TBS buffer containing 10 mmol/L EDTA. The levels of labeled annexin V in the supernatant and on the aPTT reagent-phospholipid were measured in terms of relative fluorescence intensity with an SLM/Amico SPF-500C spectrofluorimeter (Milton Co, Rochester, NY). The samples were contained in 50 μL quartz microcuvettes, thermostated at 20°C. Excitation was at 490 nm with a 4-nm band-pass and emission was detected at 523 nm with a 20-nm band-pass. The results were reported as relative fluorescence units (RFU). A standard curve of fluorescence intensity versus FITC-annexin-V concentration was linear between 0 and 2 μg/mL of protein.
Statistical analysis.
Statistical analyses were performed using the Student’s two-tailedt-test.
RESULTS
Ellipsometry studies.
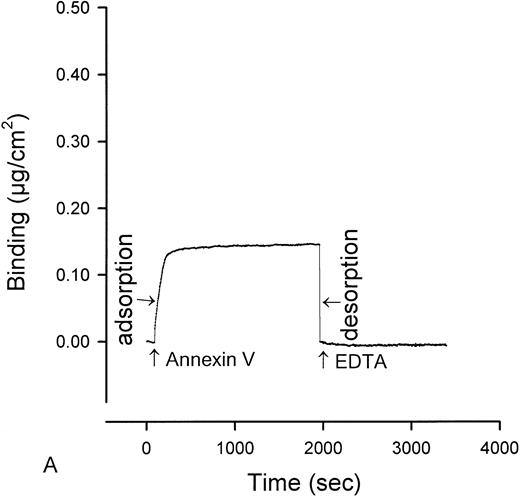
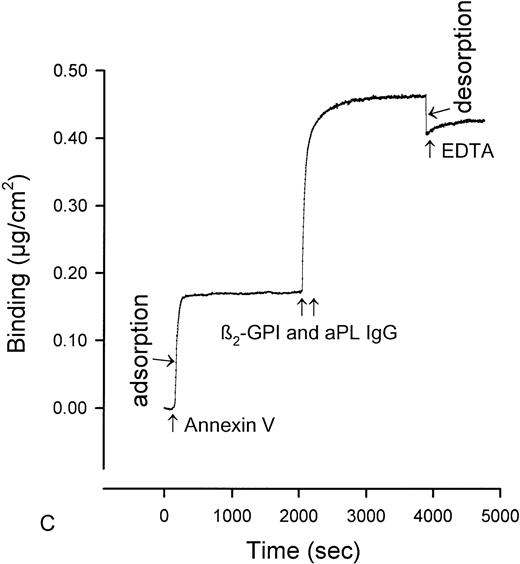
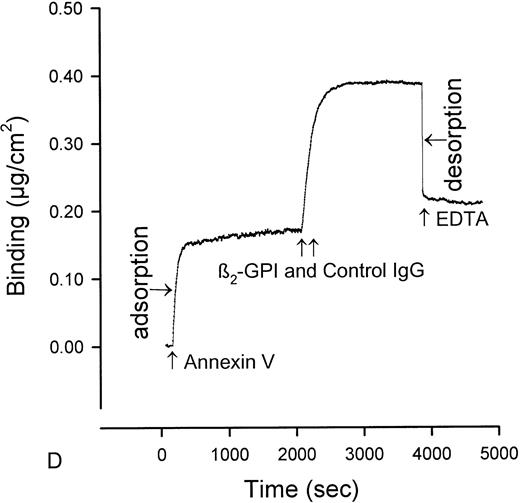
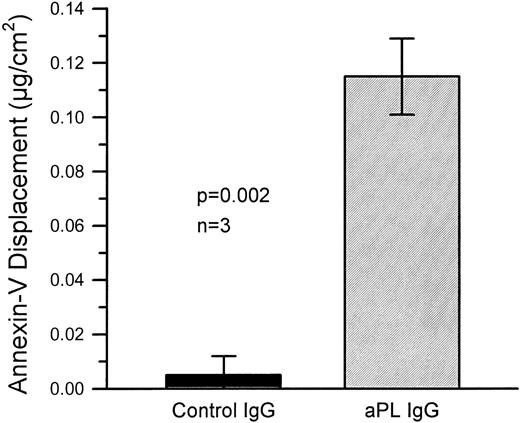
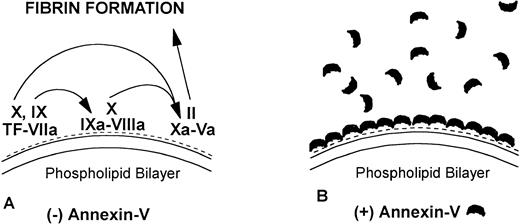
The quantity of annexin-V adsorbed to PS/PC phospholipid bilayers on silicon slides was determined by ellipsometry, and the amount of annexin-V remaining on the phospholipid bilayers after addition of IgG preparations was determined by adding EDTA to desorb the residual annexin-V. All of the annexin-V, which bound to the phospholipid surface, was subsequently desorbed by addition of the EDTA (Fig 1A). Because the adsorptions of IgGs and β2-GPI were not affected by EDTA, we were able to use EDTA-induced desorption to measure the amount of annexin-V remaining on the phospholipid surface after addition of aPL IgG antibodies. Measurement of the binding of three pairs of aPL IgG from aPL patients to the planar phospholipid bilayers of PS/PC-bound annexin-V showed that aPL IgG binding to the phospholipid bilayers, in the presence of β2-GPI cofactor, displaced 0.115 ± 0.014 μg/cm2 (mean ± standard error of mean [SEM]) annexin-V from the phospholipid surface (Figs 1C and2). In contrast, control IgG with the cofactor did not displace annexin-V (0.005 ± 0.007 μg/cm2, P = .002) (Fig 1D and 2). Also, aPL IgG without the cofactor did not reduce adsorbed annexin-V (Fig 1B). Immunoblotting of SDS-solubilized slides after the EDTA desorption showed no residual annexin-V (data not shown).
Ellipsometry studies of effects of aPL IgG and cofactor on displacement of annexin-V from PS/PC phospholipid bilayers. (A) Shows the rapid adsorption of annexin-V to the PS/PC (30%/70%) phospholipid bilayer. Treatment with EDTA and measurement of the desorption of this protein can be used to measure the amount of annexin-V on the phospholipid surface. As shown, this calcium-dependent binding protein is completely desorbed from the phospholipid surface by addition of 6 mmol/L EDTA. (B) Shows that incubation of the annexin-V–coated phospholipid bilayer with a polyclonal human aPL IgG in the absence of β2-GPI does not displace the annexin-V, ie, the quantity of annexin-V desorbed after treatment with EDTA matches the quantity of annexin-V, which had originally adsorbed. (C) Incubation of the annexin-V–coated phospholipid bilayer with β2-GPI followed by polyclonal aPL IgG results in a significant reduction of the quantity of annexin-V on the bilayer. This is reflected by the marked reduction of the amount of annexin-V, which desorbs after treatment with EDTA. (D) In contrast, treatment of the phospholipid bilayer with the β2-GPI cofactor followed by a control (non-aPL) IgG fraction does not change the quantity of annexin-V on the phospholipid surface at all, ie, the quantity of annexin-V that is desorbed by treatment with EDTA is the same as the quantity that had been adsorbed in the first place.
Ellipsometry studies of effects of aPL IgG and cofactor on displacement of annexin-V from PS/PC phospholipid bilayers. (A) Shows the rapid adsorption of annexin-V to the PS/PC (30%/70%) phospholipid bilayer. Treatment with EDTA and measurement of the desorption of this protein can be used to measure the amount of annexin-V on the phospholipid surface. As shown, this calcium-dependent binding protein is completely desorbed from the phospholipid surface by addition of 6 mmol/L EDTA. (B) Shows that incubation of the annexin-V–coated phospholipid bilayer with a polyclonal human aPL IgG in the absence of β2-GPI does not displace the annexin-V, ie, the quantity of annexin-V desorbed after treatment with EDTA matches the quantity of annexin-V, which had originally adsorbed. (C) Incubation of the annexin-V–coated phospholipid bilayer with β2-GPI followed by polyclonal aPL IgG results in a significant reduction of the quantity of annexin-V on the bilayer. This is reflected by the marked reduction of the amount of annexin-V, which desorbs after treatment with EDTA. (D) In contrast, treatment of the phospholipid bilayer with the β2-GPI cofactor followed by a control (non-aPL) IgG fraction does not change the quantity of annexin-V on the phospholipid surface at all, ie, the quantity of annexin-V that is desorbed by treatment with EDTA is the same as the quantity that had been adsorbed in the first place.
Quantitative displacement of annexin-V from the PS/PC phospholipid bilayers by aPL IgG preparations in the presence of β2-GPI cofactor. The combination of aPL IgG with β2-GPI significantly displace annexin-V from the bilayers as compared with control IgG with β2-GPI. The mean (±SEM) quantity of annexin-V displaced by three different aPL syndrome patients’ IgG fractions was 0.115 ± 0.014 μg/cm2 as compared with no significant displacement by three different control IgG preparations (0.005 ± 0.007 μg/cm2, P = .002). These data demonstrate the displacement of annexin-V from the phospholipid bilayer surface by aPL IgG in the presence of β2-GPI.
Quantitative displacement of annexin-V from the PS/PC phospholipid bilayers by aPL IgG preparations in the presence of β2-GPI cofactor. The combination of aPL IgG with β2-GPI significantly displace annexin-V from the bilayers as compared with control IgG with β2-GPI. The mean (±SEM) quantity of annexin-V displaced by three different aPL syndrome patients’ IgG fractions was 0.115 ± 0.014 μg/cm2 as compared with no significant displacement by three different control IgG preparations (0.005 ± 0.007 μg/cm2, P = .002). These data demonstrate the displacement of annexin-V from the phospholipid bilayer surface by aPL IgG in the presence of β2-GPI.
Annexin-V binding to phosphatidyl serine-coated microtiter plates.
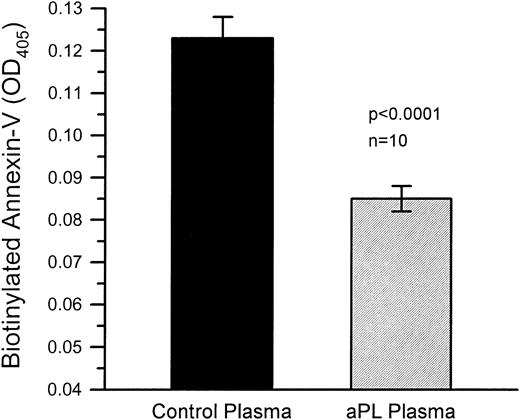
PS-coated microtiter plates treated with aPL plasmas bound significantly less biotin-labeled annexin-V than PS-coated microtiter plates, which had been treated with control plasmas (Fig 3). The mean optical density (OD; ±SEM) of the aPL plasma-treated wells was 0.085 ± 0.003 and 0.123 ± 0.005 for the wells teated with control plasmas (n = 10 for each group, P < .0001).
Annexin-V binding to microtiter plates coated with phosphatidyl serine (PS). PS-coated microtiter plate wells treated with aPL plasmas bound significantly less biotin-labeled annexin-V than PS-coated microtiter plate wells, which had been treated with control plasmas. Annexin-V was detected by addition of phosphatase-labeled streptavidin followed by p-nitrophenyl phosphate substrate. The mean OD (±SEM) of the aPL plasma-treated wells was 0.085 ± 0.003 and 0.123 ± 0.005 for the wells treated with control plasmas (n = 10 for each group, P < .0001).
Annexin-V binding to microtiter plates coated with phosphatidyl serine (PS). PS-coated microtiter plate wells treated with aPL plasmas bound significantly less biotin-labeled annexin-V than PS-coated microtiter plate wells, which had been treated with control plasmas. Annexin-V was detected by addition of phosphatase-labeled streptavidin followed by p-nitrophenyl phosphate substrate. The mean OD (±SEM) of the aPL plasma-treated wells was 0.085 ± 0.003 and 0.123 ± 0.005 for the wells treated with control plasmas (n = 10 for each group, P < .0001).
Platelets.
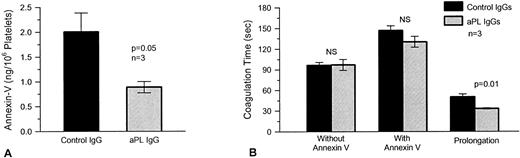
Frozen thawed washed platelets, which had been incubated with annexin-V after preincubation with aPL IgG preparations from three different patients, had significantly less annexin-V bound to their surfaces (mean ± SEM, 0.89 ± 0.12 ng/106 platelets) than platelets, which had been preexposed to control IgGs (2.01 ± 0.38 ng/106 platelets, P = .05; n = 3) (Fig 4A). Although annexin-V increased the coagulation times with both aPL and control IgG treatments, the protein had less of an anticoagulant effect with aPL IgG-treated platelets, ie, there was significantly less prolongation with thawed washed platelets, which had been preincubated with aPL IgG (mean ± SEM, 33.2 ± 0.9 seconds longer than coagulation time in absence of annexin-V) compared with control IgG (50.4 ± 4.1 seconds longer than coagulation time in absence of annexin-V, P = .01; n = 3) (Fig 4B).
The effects of aPL IgG on the quantity of platelet surface annexin-V and plasma coagulation. (A) Washed human platelets were exposed to three different aPL and control IgG preparations in plasma, following which the platelets were incubated with annexin-V (20 μg/mL), in the presence of calcium, as described in Materials and Methods. Surface annexin-V was then dissociated with EGTA and measured by ELISA. Platelets preexposed to aPL IgG had significantly less annexin-V on their surfaces (mean ± SEM, 0.89 ± 0.12 ng/106 platelets) as compared with controls (2.01 ± 0.38 ng/106 platelets, P = .05). (B) Plasma coagulation times were determined using platelets, which had been preexposed to the aPL and control IgGs in plasma. The platelets were added to pooled normal plasma, which was recalcified in the presence and absence of added annexin-V (20 μg/mL). Annexin-V lengthened the coagulation times of pooled normal plasma with both control and aPL IgG-treated platelets. The net prolongation, compared with the coagulation time without annexin-V, was significantly less with the aPL-treated platelets (mean prolongation ± SEM, 33.2 ± 0.9 seconds) as compared with controls (50.4 ± 4.1 seconds, n = 3, P = .01).
The effects of aPL IgG on the quantity of platelet surface annexin-V and plasma coagulation. (A) Washed human platelets were exposed to three different aPL and control IgG preparations in plasma, following which the platelets were incubated with annexin-V (20 μg/mL), in the presence of calcium, as described in Materials and Methods. Surface annexin-V was then dissociated with EGTA and measured by ELISA. Platelets preexposed to aPL IgG had significantly less annexin-V on their surfaces (mean ± SEM, 0.89 ± 0.12 ng/106 platelets) as compared with controls (2.01 ± 0.38 ng/106 platelets, P = .05). (B) Plasma coagulation times were determined using platelets, which had been preexposed to the aPL and control IgGs in plasma. The platelets were added to pooled normal plasma, which was recalcified in the presence and absence of added annexin-V (20 μg/mL). Annexin-V lengthened the coagulation times of pooled normal plasma with both control and aPL IgG-treated platelets. The net prolongation, compared with the coagulation time without annexin-V, was significantly less with the aPL-treated platelets (mean prolongation ± SEM, 33.2 ± 0.9 seconds) as compared with controls (50.4 ± 4.1 seconds, n = 3, P = .01).
aPTT reagent.
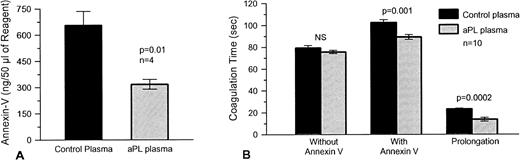
We found that aPTT reagent-phospholipid preexposed to aPL plasma bound significantly less annexin V (mean ± SEM, 318 ± 28 ng/50 μL aliquot of reagent) than controls (656 ± 80 ng/50 μL aliquot of reagent, P = .01; n= 4) (Fig 5A). We then designed a two-stage test, in which aPTT reagent-phospholipid was first incubated with individual test plasma, then washed and, for the second stage, exposed to a pooled normal plasma, which was then recalcified in the presence and absence of added annexin-V. We found that preexposure of aPTT reagent-phospholipid to plasmas from aPL syndrome patients significantly accelerated the coagulation of pooled normal plasma in the presence of annexin-V (mean ± SEM, 89.2 ± 2.2 seconds) as compared with control plasmas (mean ± SEM, 102.5 ± 2.6 seconds; P = .001, n = 10) (Fig 5B). Also, there was a commensurate decrease in the annexin-V–induced anticoagulant effect, as assessed by prolongation of the coagulation time, of the aPL treated reagent as compared with the reagent, which had been preincubated with control plasma (mean ± SEM, 13.6 ± 1.8 seconds for aPL patients and 23.1 ± 0.8 seconds for controls, P = .0002) (Fig 5B).
The effects of aPL plasmas on annexin-V bound to aPTT reagent-phospholipid and plasma coagulation with this reagent. (A) aPTT reagent-phospholipid was exposed to four different aPL and control plasmas and then to annexin-V (2 μg/mL), after which surface annexin-V was dissociated with EDTA and measured by ELISA. aPTT reagent-phospholipid, which had been preexposed to aPL-plasmas, had significantly less annexin-V (mean ± SEM, 318 ± 28 ng/50 μL aliquot of reagent) as compared with controls (656 ± 80 ng/50 μL aliquot of reagent, P = .01). (B) Plasma coagulation times were determined using aPTT reagent-phospholipid exposed to the aPL and control plasmas (n = 10 for each group) in the first stage and then in the second stage, to pooled normal plasma in the presence and absence of added annexin-V (30 μg/mL). Annexin-V delayed the coagulation times of aPTT reagent exposed to both types of plasmas. In the presence of annexin-V, the coagulation times of the aPTT reagent, which had been preexposed to aPL-plasma, was significantly faster (mean ± SEM, 89.2 ± 2.2 seconds) than reagent exposed to the control plasma (102.5 ± 2.6 seconds, P = .001). Also, there was a significant decrease in the net prolongation of the coagulation times induced by annexin-V (mean ± SEM, 13.6 ± 1.8 seconds for aPL plasmas versus 23.1 ± 0.8 seconds for controls, P = .0002).
The effects of aPL plasmas on annexin-V bound to aPTT reagent-phospholipid and plasma coagulation with this reagent. (A) aPTT reagent-phospholipid was exposed to four different aPL and control plasmas and then to annexin-V (2 μg/mL), after which surface annexin-V was dissociated with EDTA and measured by ELISA. aPTT reagent-phospholipid, which had been preexposed to aPL-plasmas, had significantly less annexin-V (mean ± SEM, 318 ± 28 ng/50 μL aliquot of reagent) as compared with controls (656 ± 80 ng/50 μL aliquot of reagent, P = .01). (B) Plasma coagulation times were determined using aPTT reagent-phospholipid exposed to the aPL and control plasmas (n = 10 for each group) in the first stage and then in the second stage, to pooled normal plasma in the presence and absence of added annexin-V (30 μg/mL). Annexin-V delayed the coagulation times of aPTT reagent exposed to both types of plasmas. In the presence of annexin-V, the coagulation times of the aPTT reagent, which had been preexposed to aPL-plasma, was significantly faster (mean ± SEM, 89.2 ± 2.2 seconds) than reagent exposed to the control plasma (102.5 ± 2.6 seconds, P = .001). Also, there was a significant decrease in the net prolongation of the coagulation times induced by annexin-V (mean ± SEM, 13.6 ± 1.8 seconds for aPL plasmas versus 23.1 ± 0.8 seconds for controls, P = .0002).
Prothrombin time reagent (tissue factor-phospholipid).
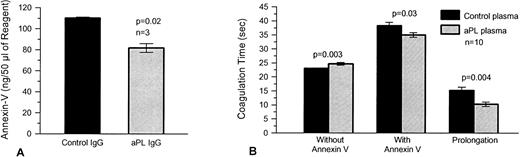
Similar experiments with tissue factor-phospholipid suspensions (PT reagent) also showed significantly less binding of annexin V to PT reagent-phospholipid, which had been preexposed to aPL IgG fractions (mean ± SEM, 82 ± 4 ng/50 μL aliquot of reagent) than controls (110 ± 1 ng/50 μL aliquot of reagent, P = .02, n = 3) (Fig 6A). In the presence of annexin-V, PT reagent, which had been preexposed to aPL plasmas, accelerated the subsequent coagulation of pooled normal plasma (mean ± SEM, 35.0 ± 0.8 seconds) as compared with controls (38.3 ± 1.2 seconds, P = .03, n = 10) (Fig 6B). There was a corresponding decrease in the annexin-V–induced anticoagulant effect with PT reagent, which had been preincubated with aPL plasma (mean ± SEM, 10.3 ± 0.8 seconds) as compared with controls (15.2 ± 1.2 seconds, P = .004). In contrast, in the absence of annexin-V, aPL-treated prothrombin time reagent caused a small but significant slowing of coagulation compared with prothrombin time reagent, which had been preincubated with control plasma (mean ± SEM, 24.7 ± 0.5 seconds for aPL-treated reagent compared with 23.1 ± 0.1 seconds for controls, P = .003) (Fig 6B).
The effects of aPL plasmas on annexin-V bound to prothrombin time reagent (tissue factor-phospholipid complex) and on plasma coagulation. (A) Prothrombin time reagent was preexposed to three different aPL and control IgG preparations in plasma and then to annexin-V (2 μg/mL), after which surface annexin-V was dissociated with EDTA and measured by ELISA. Prothrombin time reagent preexposed to aPL IgG-containing plasmas had significantly less annexin-V (mean ± SEM, 82 ± 4 ng/50 μL aliquot of reagent) compared with controls (110 ± 1 ng/50 μL aliquot of reagent, P = .02). (B) Plasma coagulation times were determined using prothrombin time reagent, which was exposed to the aPL and control plasmas (n = 10 for each group), in the first stage and in the second stage to pooled normal plasma in the presence and absence of annexin-V (30 μg/mL). There was a small but significant prolongation of coagulation time when the prothrombin time reagent was exposed to the aPL plasmas in the absence of annexin-V (P = .003). Addition of annexin-V resulted in prolongation of coagulation times with both types of reagent (ie, exposure to control and aPL plasmas). However, in contrast to the results without annexin-V, the coagulation times of the prothrombin time reagent, which had been preexposed to aPL-plasma, were significantly shortened (mean ± SEM, 35.0 ± 0.8 seconds compared with 38.3 ± 1.2 seconds for control plasmas, P = .03). There was a concomitant significant decrease in the net prolongation of the coagulation times using PT reagent, which had been pretreated with aPL plasma (10.3 ± 0.8 seconds compared with 15.2±1.2 seconds for control plasmas, P = .004).
The effects of aPL plasmas on annexin-V bound to prothrombin time reagent (tissue factor-phospholipid complex) and on plasma coagulation. (A) Prothrombin time reagent was preexposed to three different aPL and control IgG preparations in plasma and then to annexin-V (2 μg/mL), after which surface annexin-V was dissociated with EDTA and measured by ELISA. Prothrombin time reagent preexposed to aPL IgG-containing plasmas had significantly less annexin-V (mean ± SEM, 82 ± 4 ng/50 μL aliquot of reagent) compared with controls (110 ± 1 ng/50 μL aliquot of reagent, P = .02). (B) Plasma coagulation times were determined using prothrombin time reagent, which was exposed to the aPL and control plasmas (n = 10 for each group), in the first stage and in the second stage to pooled normal plasma in the presence and absence of annexin-V (30 μg/mL). There was a small but significant prolongation of coagulation time when the prothrombin time reagent was exposed to the aPL plasmas in the absence of annexin-V (P = .003). Addition of annexin-V resulted in prolongation of coagulation times with both types of reagent (ie, exposure to control and aPL plasmas). However, in contrast to the results without annexin-V, the coagulation times of the prothrombin time reagent, which had been preexposed to aPL-plasma, were significantly shortened (mean ± SEM, 35.0 ± 0.8 seconds compared with 38.3 ± 1.2 seconds for control plasmas, P = .03). There was a concomitant significant decrease in the net prolongation of the coagulation times using PT reagent, which had been pretreated with aPL plasma (10.3 ± 0.8 seconds compared with 15.2±1.2 seconds for control plasmas, P = .004).
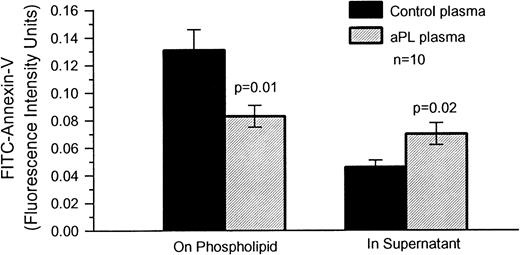
FITC-Annexin-V binding to phospholipid.
We found that preexposure of aPTT reagent-phospholipid to aPL plasma significantly reduced the amount of FITC-conjugated annexin-V, which subsequently bound to the phospholipid (mean ± SEM, 0.083 ± 0.008 RFU) as compared with control plasmas (0.131 ± 0.015 RFU, P = .01, n = 10) (Fig 7), and increased the amount of labeled annexin-V in the supernatant (mean ± SEM, 0.070 ± 0.008 RFU for aPL exposed phospholipid and 0.046 ± 0.005 RFU for control plasmas, P = .02) (Fig7).
The effects of aPL plasmas on the binding of FITC–annexin-V to aPTT reagent-phospholipid. aPTT reagent was incubated with aPL and control plasmas (n = 10 for each group), after which 1 μg/mL of FITC–annexin-V was added. Annexin-V bound to the aPTT reagent and in the fluid phase were quantified by spectrofluorimetry, as decribed in Materials and Methods. There was a significant decrease of the amount of annexin-V associated with the aPTT reagent, which had been preincubated with aPL plasmas (mean ± SEM, 0.083 ± 0.008 RFU/50 μL aliquot of reagent) compared with controls plasmas (0.131 ± 0.015 RFU/50 μL aliquot of reagent,P = .01). In contrast, there was a significant increase in the amount of labeled annexin-V remaining in the supernatant of aPTT reagent, which had been preincubated with aPL plasmas (mean ± SEM, 0.070 ± 0.008 RFU/50 μL aliquot of reagent) as compared with control plasmas (0.046 ± 0.005 RFU/50 μL aliquot of reagent,P = .02).
The effects of aPL plasmas on the binding of FITC–annexin-V to aPTT reagent-phospholipid. aPTT reagent was incubated with aPL and control plasmas (n = 10 for each group), after which 1 μg/mL of FITC–annexin-V was added. Annexin-V bound to the aPTT reagent and in the fluid phase were quantified by spectrofluorimetry, as decribed in Materials and Methods. There was a significant decrease of the amount of annexin-V associated with the aPTT reagent, which had been preincubated with aPL plasmas (mean ± SEM, 0.083 ± 0.008 RFU/50 μL aliquot of reagent) compared with controls plasmas (0.131 ± 0.015 RFU/50 μL aliquot of reagent,P = .01). In contrast, there was a significant increase in the amount of labeled annexin-V remaining in the supernatant of aPTT reagent, which had been preincubated with aPL plasmas (mean ± SEM, 0.070 ± 0.008 RFU/50 μL aliquot of reagent) as compared with control plasmas (0.046 ± 0.005 RFU/50 μL aliquot of reagent,P = .02).
DISCUSSION
We recently described a new thrombogenic mechanism in the aPL syndrome—that IgG fractions from aPL patients reduce the quantity of annexin-V on cultured trophoblasts and endothelial cells and accelerate the coagulation of plasma added to these cells.10 In this report, we extend our previous observations with cultured cells and demonstrate that cultured cells are not necessary to observe this effect, which also occurs with phospholipid-coated surfaces, frozen thawed washed platelets, and ordinary phospholipid suspensions used for conventional clinical coagulation tests, which were treated similarly to the cells in our previous study.10 It should be noted that in the present study, the experiments with platelets were not designed to study unactivated and activated platelets, but rather platelet phospholipid for coagulation testing, as previously described.18
Using ellipsometry, we provide direct evidence that aPL IgG preparations, in the presence of β2-GPI, displace annexin-V from PS/PC phospholipid bilayers. Interestingly, both the aPL IgG and β2-GPI are necessary for the displacement to occur. In accordance with this finding, incubating PS-coated microtiter plate wells with aPL patient plasmas also results in the significant reduction of the quantity of labeled annexin-V, which binds to that surface.
Similarly, exposing different sources of phospholipids used for coagulation reactions, such as washed frozen thawed platelet suspensions, aPTT reagent, and prothrombin time reagent, to aPL antibodies reduces the quantity of annexin-V, which binds to these phospholipid surfaces. As with the previously reported cell culture experiments,10 the aPL antibodies also accelerate coagulation in the presence of annexin-V, reducing the potent anticoagulant activity of this phospholipid-binding protein.
These results, which demonstrate accelerated coagulation by aPL antibodies when annexin-V is present in coagulation reactions, stand in contrast to the “lupus anticoagulant” phenomenon, which was first described in 1952.19 To our knowledge, our results are the first direct demonstration of the displacement of annexin-V and the acceleration of coagulation on noncellular phospholipid surfaces by antiphospholipid antibodies and provide a methodology, which may prove suitable for clinical testing. Because annexin-V has the highest affinity for phospholipid among the various members of the annexin family,20 we speculate that antiphospholipid antibodies may also have a similar effect on displacing the lower affinity annexins, such as annexin-II, which might also have antithrombotic properties,21 from phospholipids. Previously, using a different system that investigated the inhibition of activated protein C by aPL antibodies, Smirnov et al22 were able to show accelerated clotting in some aPL plasmas. They found that clotting initiated by factor X activating enzyme from Russell’s viper venom was faster in some aPL patient plasma when high concentrations of activated protein C and phosphatidyl ethanolamine were present.
Because aPL antibodies are capable of reducing the binding of annexin-V to anionic phospholipids in vitro and in cell culture, we propose that this effect may occur in the in vivo situations and processes where anionic phospholipids are exposed to flowing blood. These include the surface of placental villi,23 activated platelets,24 apoptotic cells and cellular particles,25 and disrupted erythrocyte membranes.26 In all of these settings, the presence of aPL antibodies could inhibit the binding of circulating annexin-V, which would in turn result in a membrane surface, which is more thrombogenic.
In light of these data, how are we to understand the prothrombotic effect of the aPL antibodies? Even if aPL antibodies displace annexin-V from the phospholipid surface, what difference ought it make whether the phospholipid is bound by annexin-V or by aPL antibody-cofactor complexes, ie, shouldn’t the phospholipid still remain unavailable for coagulation reactions? The mechanism by which aPL antibodies accelerate coagulation in the presence of annexin-V is probably due to topographical differences between the binding of the two ligands to phospholipid. Annexin-V binds in clusters on the phospholipid surface,14 while aPL antibodies disrupt this carpet of annexin-V, which shields the surface and allow enough room on the phospholipid surface for coagulation factors such as prothrombin to bind.27 Thus, the prothrombotic effect of the aPL antibodies is due to their increasing the net quantity of available anionic phospholipid for coagulation reactions by displacing annexin-V from the surface. We propose that the paradoxical “lupus anticoagulant” phenomenon is a reflection of the effects of the aPL antibodies in the presence of coagulation protein, but in the absence of annexin-V; in this situation, the antibodies will prolong coagulation by decreasing the net quantity of phospholipids available for coagulation reactions (because annexin-V is not present). In reflecting the relative inhibitory effects of high affinity antiphospholipid-protein complexes, in the absence of annexin-V, to the binding of coagulation factors, we propose that the “lupus anticoagulant” phenomenon may indeed serve as a surrogate marker for antibodies whose actual pathophysiogic function lies in their capacity to displace annexin-V.
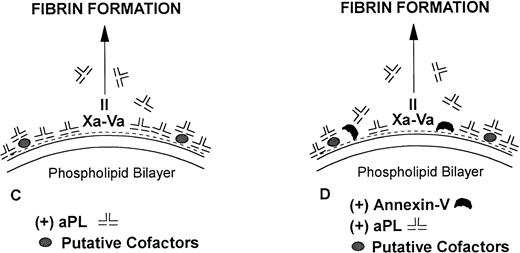
We propose the following model (Fig 8), which advances the one we had previously described10: Annexin-V plays a physiologic role in inhibiting blood coagulation reactions on vascular surfaces by shielding highly thrombogenic anionic phospholipids from coagulation enzyme complexes. We hypothesize that aPL syndrome patients have antibodies with a sufficient affinity for anionic phospholipids (and/or presumptive cofactors such as β2-GPI) to disrupt or prevent the assembly of this annexin-V protective shield. This results in a net increase of exposed phospholipid and permits a more procoagulant topography to be available for coagulation reactions. Thus, coagulation systems, which include annexin-V, will demonstrate a relative acceleration of coagulation, a “lupus procoagulant effect”, due to displacement of annexin-V by aPL syndrome antibodies. In contrast, in the absence of added annexin-V, only the relatively inhibitory effects of the antibodies on phospholipid-dependent coagulation reactions are observed (as compared with when antibodies are absent), thereby resulting in the classical “lupus anticoagulant” effect.
Model for mechanisms of the “lupus anticoagulant effect” and the inhibition of annexin-V and acceleration of coagulation by antiphospholipid antibodies. (A) Anionic phospholipids (negative charges), when exposed on the apical surface of the cell membrane bilayer, serve as potent cofactors for the assembly of three different coagulation complexes: the tissue factor (TF)-VIIa complex, the IXa-VIIIacomplex and the Xa-Va complex, and thereby accelerating blood coagulation. The TF complexes yield either factor IXa or factor Xa, the IXa complex yields factor Xa, and the Xa formed from both of these reactions is the active enzyme in the prothrombinase complex, which yields factor IIa (thrombin), which in turn cleaves fibrinogen to form fibrin. (B) Annexin-V, in the absence of aPL antibodies, forms clusters, which bind with high affinity to the anionic phospholipid surface and shield the surface from the assembly of the phospholipid-dependent coagulation complexes, thereby inhibiting coagulation reactions. (C) In the absence of annexin-V, aPL antibodies can prolong the coagulation times, compared with control antibodies, by reducing the access of coagulation factors to anionic phospholipids. This may result in a “lupus anticoagulation effect.” (D) In the presence of annexin-V, antiphospholipid antibodies, either directly or via interaction with protein-phospholipid cofactors, disrupt the ability of annexin-V to cluster on the phospholipid surface, resulting in a net increase of the amount of anionic phospholipid available for promoting coagulation reactions. This manifests in the net acceleration of coagulation in vitro and in thrombophilia in vivo.
Model for mechanisms of the “lupus anticoagulant effect” and the inhibition of annexin-V and acceleration of coagulation by antiphospholipid antibodies. (A) Anionic phospholipids (negative charges), when exposed on the apical surface of the cell membrane bilayer, serve as potent cofactors for the assembly of three different coagulation complexes: the tissue factor (TF)-VIIa complex, the IXa-VIIIacomplex and the Xa-Va complex, and thereby accelerating blood coagulation. The TF complexes yield either factor IXa or factor Xa, the IXa complex yields factor Xa, and the Xa formed from both of these reactions is the active enzyme in the prothrombinase complex, which yields factor IIa (thrombin), which in turn cleaves fibrinogen to form fibrin. (B) Annexin-V, in the absence of aPL antibodies, forms clusters, which bind with high affinity to the anionic phospholipid surface and shield the surface from the assembly of the phospholipid-dependent coagulation complexes, thereby inhibiting coagulation reactions. (C) In the absence of annexin-V, aPL antibodies can prolong the coagulation times, compared with control antibodies, by reducing the access of coagulation factors to anionic phospholipids. This may result in a “lupus anticoagulation effect.” (D) In the presence of annexin-V, antiphospholipid antibodies, either directly or via interaction with protein-phospholipid cofactors, disrupt the ability of annexin-V to cluster on the phospholipid surface, resulting in a net increase of the amount of anionic phospholipid available for promoting coagulation reactions. This manifests in the net acceleration of coagulation in vitro and in thrombophilia in vivo.
In conclusion, we have demonstrated that, in addition to their effects on cultured trophoblasts and endothelial cells, IgGs and plasmas from patients with the aPL syndrome also reduce annexin-V on noncellular phospholipid-coated surfaces and accelerate coagulation with phospholipids used for standard clinical coagulation assays. Further studies of large numbers of well-characterized aPL and control patients will be necessary to learn whether the methods developed in this study may be suitable for clinical tests.
ACKNOWLEDGMENT
This article is dedicated to the memory of Dr Richard Gorlin, the consummate physician, scientist, and teacher.
Supported in part by Grants No. HL-29019 and AI-24671 from the National Institutes of Health.
Address reprint requests to Jacob H. Rand, MD, Hematology Division, Mount Sinai School of Medicine, Box 1079, 5 E 98th St, New York, NY 10029; e-mail:j_rand@smtplink.mssm.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal