Abstract
A novel cell line was established from a patient with a leukemic-state nasal angiocentric natural killer (NK) cell lymphoma with systemic skin infiltration. The morphology of the leukemic cells was large-granular-lymphocyte (LGL), and their immunophenotype was CD2+, CD3−, CD5+, CD7+, CD16−, CD56+, and CD57−. The presence of Epstein-Barr viral (EBV) genome was shown in specimens from the patient’s nose, skin, and peripheral blood by in situ hybridization using an EBV-encoded small RNA-1 probe or by Southern blotting using a terminal-repeat probe of the EBV genome. Leukemic cells were cocultured with a mouse stromal cell line (SPY3-2) in the presence of 100 U/mL recombinant human interleukin-2 and a novel stromal cell-independent cell line, NK-YS, was established. The NK-YS cells showed LGL morphology and expressed surface CD2, CD5, CD7, CD25, CD56, and CD95. The NK-YS cells retained cytotoxicity against K562 and Jurkat cells. A Southern blotting using a terminal-repeat probe of EBV showed that NK-YS and fresh leukemic cells had a clonal EBV genome, whereas the T-cell receptor β and γ chain genes of NK-YS were not rearranged. In an immunocytochemical analysis, the NK-YS cells showed a type-II latent infection of EBV. The NK-YS cells preserved the original characteristics of NK cell lymphoma/leukemia and will be a useful tool for the study of biological characteristics of EBV-associated nasal angiocentric NK cell lymphoma/leukemia.
© 1998 by The American Society of Hematology.
LETHAL MIDLINE granulomatosis and lymphomatoid granulomatosis have each been thought of as a type of granulomatous angiitis similar to Wegener’s granulomatosis.1-3 Histological and immunohistochemical studies performed for lethal midline granulomatosis showed that these lymphoid cells infiltrating in an angiodestructive manner showed atypical morphologies and a phenotype of mature T cells.4,5The disease was thus regarded as a kind of peripheral T-cell lymphoma termed as angiocentric immunoproliferative lesions.6
However, many cases of nasal angiocentric immunoproliferative lesions have been described in Asian countries, and they are reported to be immunophenotyped as CD3−CD56+, not rearranged for T-cell receptor genes, and to be carrying the Epstein-Barr viral (EBV) genome. Accordingly, this lymphoma is now considered a clonal proliferative disorder of natural killer (NK) cells associated with EBV infection.7-15 In a recent workshop held in Hong Kong on nasal and related extranodal angiocentric T/NK cell lymphomas, both Western and Asian types of angiocentric lymphomas were confirmed to be a distinct clinicopathologic entity, ie, nasal and extranodal angiocentric T/NK cell lymphoma.16 The diagnostic criteria of nasal angiocentric T/NK cell lymphoma include (1) angiocentric infiltration with or without necrosis, (2) extranodal infiltration into skin, soft tissue, testis, upper respiratory tract, or gastrointestinal tract, (3) EBV association, (4) an immunophenotype of CD56+CD3− (NK cell type) or CD56−CD3+ (T cell type), and (5) no rearrangement of T-cell receptor genes (NK cell type).16 It is well known that nasal angiocentric T/NK cell lymphoma associated with EBV infection is refractory to conventional chemotherapy, and its clinical course is highly aggressive.17
We recently treated a patient with typical nasal angiocentric NK cell lymphoma, who developed skin infiltration and leukemia with proliferation of large granular lymphocytes (LGLs). We cocultured leukemic cells from the patient with a mouse stromal cell line (SPY3-2)18 in the presence of recombinant human interleukin 2 (rhIL-2). After a 3-month cultivation, the leukemic cells grew independent of the stromal cells, and a novel cell line, NK-YS, was established. This is the first cell line of NK leukemic cells originating from a typical case of EBV-associated nasal angiocentric NK cell lymphoma/leukemia. In the present study, we characterized the biological features of this unique cell line at the cellular and molecular levels.
MATERIALS AND METHODS
Case report.
A 19-year-old Japanese female had been complaining of persistent nasal obstruction with serous rhinorrhea and low-grade fever for 2 months. A left nasal obstructive tumor was found and diagnosed by biopsy as a nasal angiocentric lymphoma (Fig 1A). Immunohistochemical staining and in situ hybridization of the paraffin-embedded specimens disclosed that the lymphoma cells were positive for polyclonal CD3, CD45RO, and EBV-encoded small RNA-1 (EBER-1). The patient was referred to us for treatment of the disease. Her own and her family’s histories were not contributory. The physical examination showed no significant findings except swelling in the left root of the nose, and laboratory tests showed no remarkable abnormalities. A magnetic resonance imaging analysis of the head and neck showed the tumor located in the nasal cavity without the involvement of cervical lymph nodes, tonsils, or the central nervous system. Chest and abdominal computed tomography scans disclosed no involvement in these areas. Bone marrow involvement was not detected in the aspirated specimen. The patient was initially treated with conventional chemotherapy, the CHOP (cyclophosphamide, adriamycin, vincristine, and prednisolone) regimen, but no response was achieved. Local radiotherapy at a total dose of 50 Gy was then used, and a considerable regression of the nasal tumor was observed. However, at the end of the radiation therapy the patient developed multiple erythematous or purpuric skin lesions with ulceration. A biopsied specimen of the skin lesion (Fig 1B) showed that lymphoma cells invaded both the dermis and epidermis. These infiltrating lymphoma cells on frozen sections were shown to be immunohistochemically positive for CD56, but negative for CD3, CD4, CD8, CD16, negative CD19, CD20, and CD57. Southern blotting analysis of DNA extracted from the skin lesion showed no rearrangement of the immunoglobulin heavy chain gene or T-cell receptor β and γ chain genes. In situ hybridization disclosed the presence of EBER-1 in the nuclei of the lymphoma cells. These results indicated that this lymphoma was compatible with the diagnostic criteria of angiocentric NK cell lymphoma. The skin lesions showed spontaneous regression and relapse in an alternating fashion until the final stage, ie, the development of liver infiltration and leukemic change. Seven days after the seventh course of chemotherapy, the patient’s white blood cell count was increased to 1 × 104/mm3; 99% of the cells were abnormal LGLs having coarse azurophilic granules (Fig 1C). These abnormal LGLs expressed CD2, CD5, CD7, and CD56 antigens (Table 1) and EBER-1 (data not shown), but were negative for CD3. A monoclonal band of EBV terminal repeat was documented. These characteristics were compatible with those of the skin lesions stated previously.
Pathology of lymphoma in the (A) nose, (B) skin, and (C) peripheral blood of the patient. (A) Original nasal angiocentric lymphoma shows vascular infiltration with prominent necrosis (hematoxylin-eosin [HE] staining, original magnification × 40). (B) Lymphoma cells infiltrate into the epidermis and dermis (HE staining, original magnification × 40). (C) Leukemic cells in the terminal stage show the morphology of LGLs with coarse azurophilic granules and abundant cytoplasm (May-Grünwald Giemsa staining, original magnification × 500).
Pathology of lymphoma in the (A) nose, (B) skin, and (C) peripheral blood of the patient. (A) Original nasal angiocentric lymphoma shows vascular infiltration with prominent necrosis (hematoxylin-eosin [HE] staining, original magnification × 40). (B) Lymphoma cells infiltrate into the epidermis and dermis (HE staining, original magnification × 40). (C) Leukemic cells in the terminal stage show the morphology of LGLs with coarse azurophilic granules and abundant cytoplasm (May-Grünwald Giemsa staining, original magnification × 500).
Phenotypic Analysis of Freshly Isolated Natural Killer (NK) Leukemic Cells and the Cell Line NK-YS
| Antigen . | Fresh Leukemic Cells . | NK-YS-150 . |
|---|---|---|
| CD1a | − | − |
| CD2 | +++ | ++ |
| CD3 | − | − |
| CD4 | − | − |
| CD5 | +++ | ++ |
| CD6 | − | − |
| CD7 | +++ | ++ |
| CD8 | − | − |
| CD10 | − | − |
| CD11a | ND | +++ |
| CD11b | ND | − |
| CD11c | ND | ++ |
| CD14 | − | − |
| CD15 | − | − |
| CD16 | − | − |
| CD19 | − | − |
| CD20 | − | − |
| CD21 | − | − |
| CD25 | − | +++ |
| CD33 | − | − |
| CD34 | − | − |
| CD38 | +++ | ++ |
| CD45 | ND | +++ |
| CD56 | +++ | +++ |
| CD57 | − | − |
| CD58 | + | ++ |
| CD95 (Fas) | ND | +++ |
| TcR αβ | ND | − |
| TcR γδ | ND | − |
| Sm-Ig (κ + λ) | ND | − |
| Antigen . | Fresh Leukemic Cells . | NK-YS-150 . |
|---|---|---|
| CD1a | − | − |
| CD2 | +++ | ++ |
| CD3 | − | − |
| CD4 | − | − |
| CD5 | +++ | ++ |
| CD6 | − | − |
| CD7 | +++ | ++ |
| CD8 | − | − |
| CD10 | − | − |
| CD11a | ND | +++ |
| CD11b | ND | − |
| CD11c | ND | ++ |
| CD14 | − | − |
| CD15 | − | − |
| CD16 | − | − |
| CD19 | − | − |
| CD20 | − | − |
| CD21 | − | − |
| CD25 | − | +++ |
| CD33 | − | − |
| CD34 | − | − |
| CD38 | +++ | ++ |
| CD45 | ND | +++ |
| CD56 | +++ | +++ |
| CD57 | − | − |
| CD58 | + | ++ |
| CD95 (Fas) | ND | +++ |
| TcR αβ | ND | − |
| TcR γδ | ND | − |
| Sm-Ig (κ + λ) | ND | − |
Abbreviations: ND, not determined.
+++, >75% positive; ++, 10% to 75% positive; +, <10% positive; −, negative.
Establishment of NK cell lymphoma/leukemia cell line.
Mononuclear cells were isolated by the Ficoll-Hypaque method from the patient’s peripheral blood after the appearance of circulating malignant cells, and were suspended in Iscove’s modified Dulbecco’s medium (IMDM; GIBCO, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; Sanko Junyaku Co, Tokyo, Japan). This cell preparation was composed of more than 99% CD56-positive cells. The mononuclear cells (1 × 106) were inoculated into a 25-cm2 culture flask (Falcon 3013; Becton Dickinson, Oxnard, CA) with or without a confluent layer of the mouse spleen stromal cell line SPY3-2 as a feeder layer in 5 mL of IMDM supplemented with 10% FBS containing 100 units of rhIL-2 (Shionogi and Co, Osaka, Japan).18 This flask was maintained in a humidified atmosphere at 37°C with 5% CO2 in air. Half of the medium was collected twice a week and centrifuged at 1,000 rpm for 3 minutes in a plastic tube and the pellet was resuspended in 2.5 mL of the fresh medium. This suspension was returned to the initial culture flask. After 2 weeks of culture, the feeder layer was found to be destroyed or killed by the cultured leukemic cells when observed under a phase-contrast microscope; the leukemic cell suspension was then transferred to a newly prepared culture flask with a confluent monolayer of SPY3-2. After we confirmed the proliferation of NK leukemia cells in the culture fed by SPY3-2 at 12 weeks, we cloned them by limiting dilution and established a cell line, NK-YS, which could proliferate in the liquid culture system independently of the stromal cell line used for a feeder layer.
Morphological evaluation.
Cytocentrifuge smears of peripheral blood mononuclear cells from the patient and cells from NK-YS cells were prepared and stained with May-Grünwald Giemsa for observation under a light microscope. For electron microscopic observation, cell pellets from NK-YS were fixed in 1.5% glutaraldehyde for 2 hours, followed by postfixation with 1% osmium tetraoxide for 2 hours. The preparations were then dehydrated in graded ethanol, and embedded in SPUR. Ultra-thin sections were made for double-staining with uranyl acetate and lead citrate. The stained sections were observed under a JEM 1010 electron microscope (NIHON DENSHI, Tokyo, Japan) at 70 kV.
Flow cytometric analysis.
Leukemic cells from the patient and NK-YS cells were analyzed by single-color immunofluorescence with a flow cytometer (FACS Calibour; Becton Dickinson, Mountain View, CA) for the expression of surface markers. The fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated antibodies used were as follows: Leu5b (CD2), Leu4 (CD3), Leu3a (CD4), Leu1 (CD5), Leu9 (CD7), Leu2a (CD8), CALLA (CD10), LFA1α (CD11a), Leu15 (CD11b), LeuM5 (CD11c), LeuM7 (CD13), LeuM3 (CD14), LeuM1 (CD15), Leu11 (CD16), Leu12 (CD19), Leu16 (CD20), CR2 (CD21), IL2R (CD25), LeuM9 (CD33), HPCA1 (CD34), HLe1 (CD45), Leu19 (CD56), Leu7 (CD57), TCR α/β, and SmIg (κ + λ) from Becton-Dickinson; OKT6 (CD1) from Ortho Diagnostic Systems (Raritan, NJ); B1 (CD21) from Coulter Immunology (Hialeah, FL); LFA3 (CD58), Fas (CD95) from Immunotech (Marseilles, France); TCR γ/δ from T Cell Sciences (Cambridge, MA). Throughout the flow cytometric analysis, FITC- or PE-conjugated mouse IgG was used as the negative control.
Cytotoxic assay.
The conventional 51Cr release assay was used to evaluate the NK cell activity of NK-YS cells.19 Briefly, NK-YS cells were cultured with IMDM supplemented with 10% FBS and 100 U/mL of rhIL-2 for 3 days, and then they were mixed and incubated for 4 hours with 51Cr-labeled target cells such as K562 cells or Jurkat cells at various effector/target ratios. The specific release of51Cr from the target cells into the supernatant was measured with a gamma counter. All experiments were performed in triplicate, and the percentage lysis was determined with the following equation: (experimental mean cpm − spontaneous release mean cpm)/(total release mean cpm − spontaneous release mean cpm) × 100 = % specific lysis.
Inteferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) production.
One million NK-YS cells or control Jurkat cells were cultured with IMDM supplemented with 10% FBS and 100 U/mL of rhIL-2 for 48 hours, and at the end of the culture period, the culture media were harvested by centrifugation at 500g for 5 minutes at 4°C after the culture. The concentrations of IFN-γ and TNF-α in these culture media were determined by an enzyme-linked immunosorbent assay (ELISA; EASIA kit; MEDGENIX, Fleurus, Belgium).
Chromosomal analysis.
NK-YS cells in the logarithmic phase of cell growth were arrested with 0.01 mg of colcemid for 30 minutes before hypotonic treatment with 0.075 mol/L KCl. The cells were fixed with a mixture of methanol and acetic acid at a 3:1 ratio. Cell suspensions in the fixatives were dropped onto a glass slide and flame dried. A conventional G-banding method was used for karyotyping.20
Southern blotting analysis of T-cell receptor genes.
The rearrangement of the T-cell receptor β and γ chain genes was evaluated according to the standard method.12,14 21Briefly, 5 μg DNA from NK-YS cells was extracted according to the standard methods, digested with EcoRI, BamHI, orHindIII, electrophoresed through a 0.6% agarose gel and transferred onto a nitrocellulose filter. DNA extracted by human placenta was used as the negative control. The filter was hybridized with a 32P-labeled Cβ1 or Jγ probe and washed under appropriate stringency conditions, and bands were visualized by autoradiography.
Detection of EBV genome.
DNA samples from NK-YS cells, an EBV-transformed B lymphoblastoid cell line (LAD),22,23 as a positive control and Jurkat cells as a negative control were extracted according to the standard method, digested with EcoRI and PstI restriction enzymes, electrophoresed, and blotted to nitrocellulose filters. These filters were hybridized with a 32P-labeled cDNA probe of the EBV terminal repeat and washed under appropriate stringency conditions, and the bands were then visualized by autoradiography.24
Immunocytochemical study for EBV products.
To investigate and determine the latent infection pattern of EBV, we stained NK-YS cells with anti-EBV nuclear antigen-2 (EBNA-2; Novocostra LAB, Newcastle-upon-Tyne, UK), anti-latent membrane protein-1 (LMP-1; Novocostra), and anti-ZEBRA (immediate-early BZLF1 gene product) antibody (Dakopatts, Glostrup, Denmark) using an avidin-biotin-peroxidase complex (ABC) method.18Cytocentrifuge smears of NK-YS and LAD cells (positive control) were stained. Briefly, NK-YS cells were cytocentrifuged at 1,500 rpm, and the slide preparations were air dried and fixed with acetone for 5 minutes at 4°C. These slides were pretreated with normal serum and incubated with the primary antibody for 2 hours. After sufficient washing with phosphate-buffered saline (PBS), the slides were incubated with a biotinylated secondary antibody for 30 minutes. After a further washing with PBS, they were incubated with ABC for 60 minutes. After further washing with PBS, they were stained for peroxidase activity with 0.2 mg/mL of 3,3 diaminobenzidine tetrahydrochloride and 0.0075% of hydrogen peroxide in PBS.18 We then observed the stained slides under a light microscope.
RT-PCR analysis for mRNA expression of EBNA-2 and LMP-1.
Total RNA was extracted from LAD cells, NK-YS cells, and Jurkat cells with an RNA Zol kit (Biotex, Houston, TX). First strand cDNA was synthesized from 4 μg of the total RNA with oligo-dT primers (Promega, Madison, WI) and reverse transcriptase (Superscript II; GIBCO-BRL, Gaithersburg, MD) in a total of 20 μL of the reaction buffer. Two microliters of the reaction buffer containing the cDNA sample was amplified by using polymerase chain reaction (PCR) Core Kit (Boehringer Mannheim, Indianapolis, IN) and specific oligonucleotide primers for EBNA-2; 5′-AGG CTG CCC ACC CTG AGG AT-3′, 5′-GCC ACC TGG CAG CCC TAA AG-3′, and LMP-1; 5′-CGG AAG AGG TGG AAA ACA AA-3′, 5′-GTG GGG GTC GTC ATC ATC TC-3′, according to the previous reports.25 This primer pair of LMP-1 can detect the deletion in LMP-1 gene product.25 The PCR parameters for EBNA-2 and LMP-1 were identical. The PCR cycle was repeated 30 times, and a fraction of each sample was electrophoresed on a 2% agarose gel, stained with ethidium bromide, and visualized under ultraviolet illumination.
RESULTS
Establishment of the NK-YS cell line.
In the primary culture of leukemic cells with a stromal cell line, SPY3-2, the leukemic cells proliferated slowly during the first 6 weeks of culture. Thereafter, the proliferation rate accelerated, and the total number of leukemic cells reached a level more than 1 × 108 cells at 12 weeks of the primary culture. Some of these cells were cryopreserved for subsequent studies. In the coculture, some of the leukemic cells adhered to stromal cells and migrated under the stromal cell layer. Within 2 weeks after the start of coculture, the adherent stromal cell layer was destroyed; first, the stromal cells in contact with each other shrank and detached, then the cell membranes of the stromal cells broke and the debris of the stromal cells resided in the adherent layer, and finally no stromal cells were seen. This leukemic cell-mediated cytotoxicity against a stromal cell layer was never observed in cocultures with other myeloid or B lymphoid leukemic cell lines (data not shown). Thus, this cytotoxicity was considered to be derived from the natural killing activity of the NK leukemic cells. At 12 weeks of the primary culture, the leukemic cells showed stable proliferation, growing independently of the stromal cells. We then performed a cloning procedure using a limiting dilution method and established the stromal cell–independent cell line NK-YS. The doubling time of this cell line was approximately 48 hours (data not shown). The cytotoxicity of the NK-YS cells against the stromal cells was retained after the cloning procedure.
Morphology.
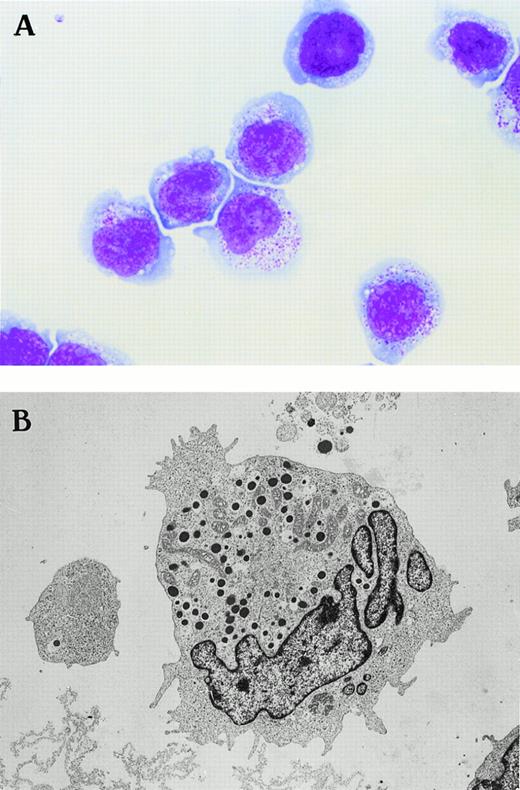
NK-YS cells showed LGL morphology when evaluated by light microscope. They have large nuclei with coarse chromatin and conspicuous nucleoli, and they have abundant basophilic cytoplasm with many azurophilic granules (Fig 2A). These azurophilic granules were negative for peroxidase staining (data not shown).
Morphology of the novel human NK cell line of NK-YS (A) under light microscopy (May-Grünwald Giemsa staining, original magnification × 500) and (B) under electron microscopy (original magnification × 3,800). The NK-YS cells show LGL morphology identical to that of leukemic cells in the peripheral blood of the patient from whom the cell line was derived.
Morphology of the novel human NK cell line of NK-YS (A) under light microscopy (May-Grünwald Giemsa staining, original magnification × 500) and (B) under electron microscopy (original magnification × 3,800). The NK-YS cells show LGL morphology identical to that of leukemic cells in the peripheral blood of the patient from whom the cell line was derived.
When NK-YS cells were evaluated by electron microscope, they showed dense granules 500 μm in diameter corresponding to the azurophilic granules, and the granules were also negative for peroxidase staining under electron microscopic observation (data not shown). The cells showed mitochondria and polysomes in the cytoplasm. They were characterized by convoluted nuclei with prominent polarity against dense granules and pseudopodia (Fig 2B).
Immunophenotyping.
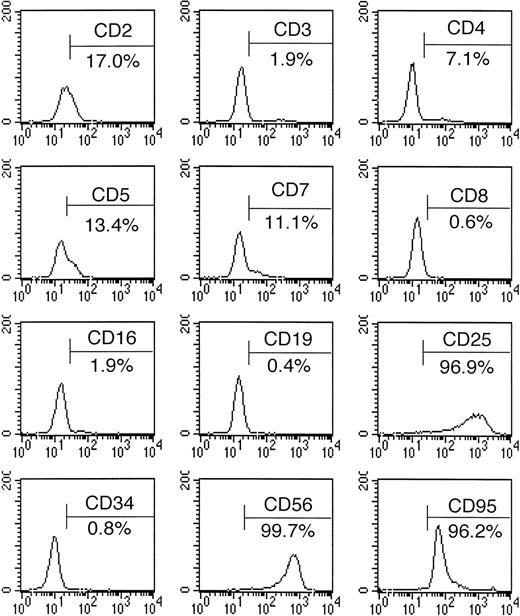
The immunophenotyping results are summarized in Table 1 and Fig3. The NK-YS cells expressed CD2, CD5, CD7, CD25, CD56, CD58, and CD95 (Fas) antigens but did not show detectable levels of surface CD3, CD4, CD8, CD16, or CD21 antigens. Leukemic cells from the peripheral blood expressed CD2, CD5, CD7, and CD56. Thus, the NK-YS cells reflected the phenotype of the original leukemic cells. CD25 (IL-2 receptor α) seemed to be induced during the culture process. CD95 (Fas) expression was observed in 96% of the NK-YS cells.
Surface antigen expression of NK-YS cells, analyzed by flow cytometry after the cells were stained with FITC- or PE-labeled antibodies.
Surface antigen expression of NK-YS cells, analyzed by flow cytometry after the cells were stained with FITC- or PE-labeled antibodies.
Cytotoxic activity.
The cytotoxic activity of NK-YS cells against K562 and Jurkat cells is shown in Fig 4. The cytotoxicity increased linearly along with the logarithm of effector/target ratios. The degree of the cytotoxicity against K562 cells was not different from that against Jurkat cells.
Assay of the natural killing activity of NK-YS against K562 and Jurkat cells. The specific lysis (%) was plotted against the effector/target ratio. The effector/target ratio was altered from 1:1 to 40:1. The NK-YS cells show natural killing activity against the K562 and the Jurkat cells.
Assay of the natural killing activity of NK-YS against K562 and Jurkat cells. The specific lysis (%) was plotted against the effector/target ratio. The effector/target ratio was altered from 1:1 to 40:1. The NK-YS cells show natural killing activity against the K562 and the Jurkat cells.
Production of IFN-γ and TNF-α.
High levels of IFN-γ were produced by NK-YS cells when cultured in the standard culture medium containing rhIL-2. The TNF-α concentration in the culture medium was undetectable with the ELISA used in this study (Table 2).
Chromosomal analysis.
The fresh leukemic cells showed a complex karyotype of 46, XX, der(4)t(1;4)(q12;p16) in 7 of the 10 metaphases analyzed (data not shown). Additional chromosomal abnormalities such as −9 and +mar1, add(3)(q21) and add(15)(p17), or add(5)(p11), −7, and +mar2 were also observed. The G-banding analysis showed that the NK-YS cells had a karyotype of 46, XX, add (3) (q26. 2), der(4)t(1;4)(q12;p16) in 10 of the 10 metaphases analyzed (Fig5). The NK-YS cells preserved the common chromosomal abnormalities of der(4)t(1;4)(q12;p16) observed in the original leukemic cells.
Karyotype of the NK-YS cells. The karyotype is 46, XX, add (3) (q26.2), der(4)t(1;4)(q12;p16) according to the ISCN system.
Karyotype of the NK-YS cells. The karyotype is 46, XX, add (3) (q26.2), der(4)t(1;4)(q12;p16) according to the ISCN system.
Southern blotting analysis of T-cell receptor genes.
The T-cell receptor β and γ chain genes of the NK-YS cells showed a germline configuration. (Fig 6). This observation was identical to those of biopsied samples from the skin lesion as noted in the case report (data not shown). Because the sample of fresh leukemic cells was limited, the rearrangement of the T-cell receptor β and γ chain genes in those cells was not analyzed.
Southern blotting analysis of the NK-YS genome for the rearrangement of the T-cell receptor (A) β and (B) γ chain genes. DNA extracted from human placenta was used as a negative control (NC). (A) BamHI- or EcoRI-digested DNA was electrophorased in the left or right lane, respectively. (B) EcoRI-,BamHI-, or HindIII-digested DNA was electrophorased in the left, center, or right lane, respectively. The NK-YS cells show the germ-line configuration of T-cell receptor β and γ chain genes.
Southern blotting analysis of the NK-YS genome for the rearrangement of the T-cell receptor (A) β and (B) γ chain genes. DNA extracted from human placenta was used as a negative control (NC). (A) BamHI- or EcoRI-digested DNA was electrophorased in the left or right lane, respectively. (B) EcoRI-,BamHI-, or HindIII-digested DNA was electrophorased in the left, center, or right lane, respectively. The NK-YS cells show the germ-line configuration of T-cell receptor β and γ chain genes.
Detection of EBV terminal repeat.
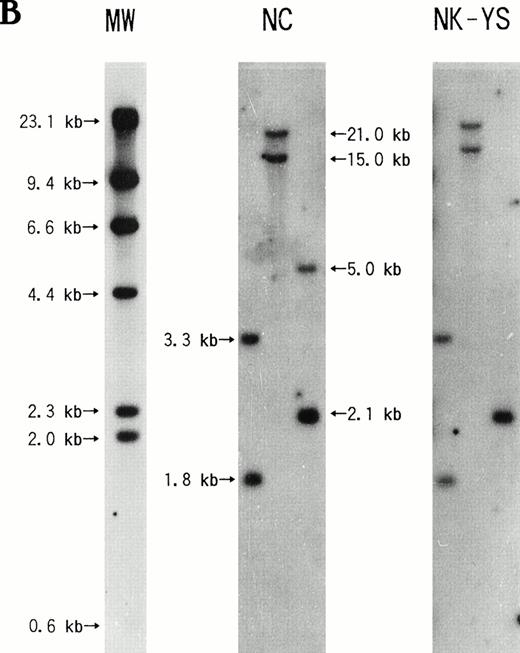
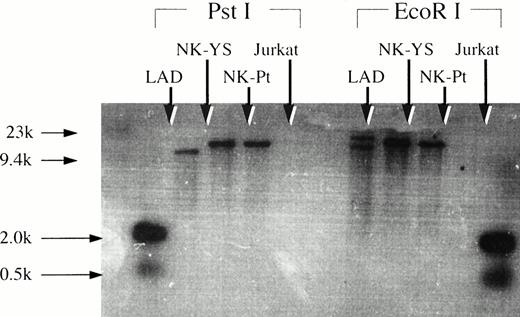
The Southern blotting analysis showed that freshly isolated leukemic cells and the NK-YS cells had an identical monoclonal band of the EBV terminal repeat (Fig 7), indicating that the fresh leukemic cells and NK-YS cells are derived from the same clone and that the EBV genome in the NK-YS cells was infected in vivo rather than during the culture process in vitro.
Southern blotting analysis of genomic DNA extracted from NK-YS cells and fresh leukemic cells after restriction enzyme digestion using EcoRI and PstI with the EBV terminal repeat. EBV-transformed B lymphoblastoid cell line, LAD cells, and Jurkat cells were used for positive and negative controls, respectively. The NK-YS cells showed a monoclonal band of EBV terminal repeat identical to that of the fresh leukemic cells, but not identical to that of the LAD cells.
Southern blotting analysis of genomic DNA extracted from NK-YS cells and fresh leukemic cells after restriction enzyme digestion using EcoRI and PstI with the EBV terminal repeat. EBV-transformed B lymphoblastoid cell line, LAD cells, and Jurkat cells were used for positive and negative controls, respectively. The NK-YS cells showed a monoclonal band of EBV terminal repeat identical to that of the fresh leukemic cells, but not identical to that of the LAD cells.
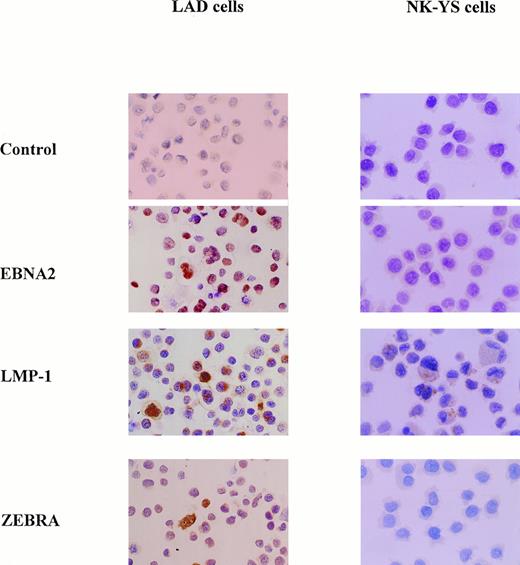
Expression of EBV latent proteins and replication-associated proteins.
The immunocytochemical analysis showed that the NK-YS cells expressed a low level of LMP-1 but not EBNA-2 or ZEBRA protein in contrast to the LAD cells, which expressed EBNA-2, LMP-1, and ZEBRA proteins (Fig8). These results indicated that the NK-YS cells had a type-II latent infection of EBV, whereas the LAD cells had a type-III latent infection.26 The lack of ZEBRA protein production indicated that EBV did not replicate in the NK-YS cells.27-29
Expression of EB viral latent proteins by NK-YS cells and by LAD cells used as a positive control. The LAD cells expressed EBNA-2, LMP-1, and ZEBRA proteins. NK-YS cells expressed a low level of LMP-1, but no EBNA-2 or ZEBRA protein (original magnification × 200).
Expression of EB viral latent proteins by NK-YS cells and by LAD cells used as a positive control. The LAD cells expressed EBNA-2, LMP-1, and ZEBRA proteins. NK-YS cells expressed a low level of LMP-1, but no EBNA-2 or ZEBRA protein (original magnification × 200).
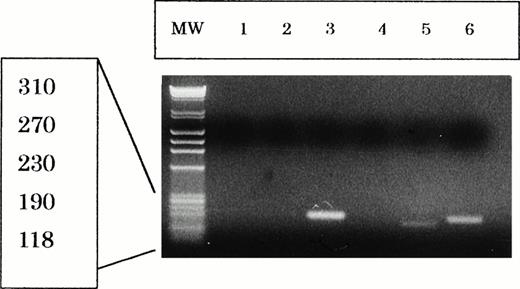
Expression of EBNA-2 and LMP-1 mRNA.
By RT-PCR, it was shown that the NK-YS cells expressed low levels of LMP-1 mRNA but no EBNA-2 mRNA, whereas the LAD cells expressed high levels of EBNA-2 and LMP-1 mRNA (Fig 9). This result is compatible with the immunocytochemistry. The LMP-1 reverse transcription PCR products of the NK-YS cells were about 30 bp shorter than those of the LAD cells. This result suggests that EBV in the NK-YS may have a deletion in the LMP-1 gene.25
Reverse transcription-PCR analysis of EBNA-2 and LMP-1 mRNA expression in Jurkat cells (lane 1 and 4), NK-YS cells (lane 2 and 5), and LAD cells (lane 3 and 6). Ten microliters of reaction solution was applied to each lane of gel. The LAD cells expressed both EBNA-2 (195 bp) and LMP-1 (160 bp) mRNA. NK-YS cells expressed a low level of LMP-1 (130 bp) mRNA with about 30 bp deletion, but no EBNA-2 mRNA. ø X174 HaeIII digest was electrophorased in the MW lane.
Reverse transcription-PCR analysis of EBNA-2 and LMP-1 mRNA expression in Jurkat cells (lane 1 and 4), NK-YS cells (lane 2 and 5), and LAD cells (lane 3 and 6). Ten microliters of reaction solution was applied to each lane of gel. The LAD cells expressed both EBNA-2 (195 bp) and LMP-1 (160 bp) mRNA. NK-YS cells expressed a low level of LMP-1 (130 bp) mRNA with about 30 bp deletion, but no EBNA-2 mRNA. ø X174 HaeIII digest was electrophorased in the MW lane.
DISCUSSION
We described the establishment of a novel cell line, NK-YS, derived from a patient with NK cell lymphoma/leukemia. The clinical manifestations and course of this patient were typical for EBV-associated nasal angiocentric NK cell lymphoma, as reported previously.16 Although the patient responded transiently to radiotherapy, the disease was resistant to conventional chemotherapy and progressed to disseminated skin infiltration and peripheral blood in the terminal stage. By Southern blotting analysis using an EBV terminal repeat probe, the NK-YS cells possessed the clonality identical to that of the leukemic cells in the patient’s peripheral blood, but it was not confirmed that leukemic cells proliferating in the peripheral blood originated from the nasal lymphoma, because the biopsied specimen from the nasal lesion was not available for investigation. However, the presence of EBER-1 in the nasal and skin lesions of the lymphoma and in the leukemic cells strongly suggests that all shared the common origin of lymphoma/leukemia cells.
Several cell lines of NK leukemia have been reported.30-33Among them, the presence of EBV genome was shown in a cell line (YT) established in Japan. However, in that case, the patient’s diagnosis was lymphoblastic lymphoma with thymoma. The morphological, phenotypic, and cytogenetic characteristics of the original lymphoma cells of the YT cell line and their association with EBV infection were not fully described.31,34 Other cell lines were established from aggressive NK leukemia in Western countries. Among them, the NK92 cell line was reported not to carry the EBV genome,32 but the association with EBV infection was not determined in other cell lines.30,33 NK-YS is the first cell line of an NK lineage originating from a clinically documented EBV-associated nasal angiocentric NK cell lymphoma and retaining all of the cytological characteristics of NK cell lymphoma reported by Jaffe.16The vigorous cytotoxicity of NK-YS cells against the stromal cell line used for the feeder layer and against leukemic cell lines suggests that lymphoma cells may be actively involved in the prominent necrosis of the nasal lesion, which is the histological hallmark of nasal angiocentric NK cell lymphoma. Thus, NK-YS cells fulfill the requirements for a representative cell line of EBV-associated nasal angiocentric T/NK cell lymphoma.
It was recently reported that the apoptosis of normal NK cells and cells of NK92 cell line can be induced in vitro when they are stimulated with both IL-2 and IL-12; this apoptosis is mediated by high levels of IFN-γ and TNF-α produced by these NK cells.35However, stimulation with IL-2 alone did not induce the apoptosis of the NK cells. When NK-YS cells were cultured with 100 U/mL of IL-2, they produced high levels of IFN-γ but not TNF-α, as mentioned previously. This IFN-γ production seems to be much higher than that of normal NK cells and the NK92 cell line stimulated with IL-2 and IL-12.35 This result indicates that NK-YS cells are highly resistant to apoptosis inducible by IFN-γ. This resistance to apoptotic stimulation by IFN-γ seems to be one of the important features of NK-YS cells.
NK cell malignancy associated with a cytogenetic abnormality in the long arm of chromosome 1 has been reported. Fernandez et al30 reported a case of NK-LGL leukemia with the chromosomal duplication of (1) (q21; q31), and Robertson33et al described another case showing add (1) (q42). Similarly, the chromosomal abnormality in the NK-YS cells and original leukemic cells involved der(4)t(1;4)(q12;p16) in the chromosomal analysis, although the pattern and location of these abnormalities in the long arm of chromosome 1 were not consistent. It is not yet known whether a specific oncogene or antioncogene for NK cell malignancy is involved in the long arm of chromosome 1.
African Burkitt’s lymphoma is the first lymphoid malignancy in which the EBV genome was shown in relation to the development of the disease.36 However, the role of EBV products in this classic EBV-associated lymphoid malignancy has not been fully documented. Recent studies clarified the roles of EBNA-2 and LMP-1 in the development of the immortality of EBV-infected B cells.37,38 The role of EBV products in relation to the immortality of NK cells has not been investigated to our knowledge. The infection status of EBV in NK-YS cells indicated type-II latency26 as shown in Fig 8, which is consistent with previous observations of nasal angiocentric T/NK cell lymphomas,7,16 and the deleted LMP-1 of the NK-YS cells may play an important role in the transformation of NK-cells.25This cell line should be of value in pursuing genes involved in NK cell lymphomagenesis and in understanding the role of the EBV gene products in nasal NK lymphoma biology.
ACKNOWLEDGMENT
We thank Dr T. Takenouchi, Chiba Prefectural Cancer Center, for providing the DNA probe for EBV terminal repeat. We thank Mrs T. Takabatake for her excellent technical support and Mrs Y. Watanabe for her assistance in the laboratory. We also thank the laboratory staff of Matsuo Hospital for providing technical support. We thank Mrs R. Oonishi and Mr Oohashi, SRL laboratory, for their assistance in the chromosomal analysis of cell line.
Address reprint requests to Junjiro Tsuchiyama, MD, Department of Internal Medicine, Okayama Municipal Hospital, 6-10 Amase, Okayama 700, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. Pathology of lymphoma in the (A) nose, (B) skin, and (C) peripheral blood of the patient. (A) Original nasal angiocentric lymphoma shows vascular infiltration with prominent necrosis (hematoxylin-eosin [HE] staining, original magnification × 40). (B) Lymphoma cells infiltrate into the epidermis and dermis (HE staining, original magnification × 40). (C) Leukemic cells in the terminal stage show the morphology of LGLs with coarse azurophilic granules and abundant cytoplasm (May-Grünwald Giemsa staining, original magnification × 500).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/4/10.1182_blood.v92.4.1374/5/m_blod416tsu1acz.jpeg?Expires=1763490301&Signature=z7t75UCjJLXDPLPWnFBmxNYK4g2duGCrWzckiTP9kPsxWzM6O7b~dzA4ZsnKxp6BvyicZHCwqdMqosdRpDWU2KM6CM6xgGV3ldt4aJEMoPjokEoXNW4sv3gFTDu3W5kq648G5nt7tnYkZCCUA9Z15DVr2Kf2sgwBo6LAJrTyBqS1M4gnU5pyuXEUh~YqlwEEfrT9LawfBIkimJbNIU8~i~7GmbHramuRJIVyF4ZQS7eO8LZNrz6ZRMf1nFnoDoSbjZ7QZQer4-gYVPs~0xt3WYvFJ~qq~c4Z-aXa5qiyEkfc1jqXWmEiJkJkihX8RQF4HTZbRAIqep1bgRytsZVIOw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal