Abstract
We have synthesized a novel six-coordinate metal chelator from the triamine cis-1,3,5-triaminocyclohexane by the addition of a 2-pyridylmethyl pendant arm on each nitrogen, which we term tachpyr. The experiments described here were designed to explore whether this compound exhibits potential antitumor activity. When added to MBT2 or T24 cultured bladder cancer cells, tachpyr was profoundly cytotoxic, with an IC50 of approximately 4.6 μmol/L compared with 70 μmol/L for desferioxamine. To explore the mode of action of tachpyr, several metal complexes were prepared, including Fe(II), Ca(II), Mn(II), Mg(II), Cu(II), and Zn(II) tachpyr complexes. Of these, the Zn(II), Cu(II), and Fe(II) complexes were without toxic effect, whereas the Ca(II), Mn(II), and Mg(II) complexes remained cytotoxic. To further probe the role of Zn(II) and Cu(II) chelation in the cytotoxicity of tachpyr, sterically hindered tachpyr derivatives were prepared through N-alkylation of tachpyr. These derivatives were unable to strongly bind Fe(III) or Fe(II) but were able to bind Zn(II) and Cu(II). When added to cells, these sterically hindered tachpyr derivatives were nontoxic, consistent with a role of iron depletion in the cytotoxic mechanism of tachpyr. Further, the addition of tachpyr to proliferating cultures resulted in an early and selective inhibition of ferritin synthesis, an iron storage protein whose translation is critically dependent on intracellular iron pools. Taken together, these experiments suggest that tachpyr is a cytotoxic metal chelator that targets intracellular iron, and that the use of tachpyr in cancer therapy deserves further exploration.
© 1998 by The American Society of Hematology.
IRON IS ESSENTIAL FOR the catalytic activity of numerous critical enzymes, including respiratory chain enzymes and ribonucleotide reductase, the rate limiting step in DNA synthesis. Thus, iron is absolutely required for mammalian cell growth. The close linkage between cell proliferation and iron has suggested that iron deprivation strategies may be useful in inhibiting tumor cell growth. For example, antitransferrin receptor antibodies, which inhibit a major pathway of cellular iron uptake, are being explored for their potential to inhibit growth of hematopoietic and nonhematopoietic tumors.1,2 Desferioxamine, a potent iron chelator, is currently in clinical trials in combined chemotherapy of neuroblastoma3,4 and prostate cancer. Similarly, gallium nitrate, which blocks iron incorporation by binding to the transferrin receptor, is being used in combined chemotherapy of bladder cancer5,6 and lymphoma.7
The design of iron chelators for use in tumor treatment is a relatively unexplored field. Desferioxamine, a bacterial siderophore and potent iron chelator used in conditions of iron overload, has been used in some studies of antiproliferative effects of iron chelation.8-10 However, desferioxamine suffers from several limitations.11 Systematic examination of the ability of alternative iron chelators to function as antiproliferative agents has largely been limited to chelators of the pyridoxyl isonicotonyl hydrazide (PIH) family.12 This group of compounds has been shown to be effective in mobilizing iron in vitro and in vivo. A recent study of the antiproliferative effect of PIH and its analogs on cultured human neuroblastoma cells identified several analogs with an IC50 lower than that of desferioxamine and a potential correlation between lipophilicity and cytotoxic effects.13Cytotoxicity was not uniformly correlated with iron chelation efficacy, suggesting that the ability to permeate cells and target selected essential pools of iron may be critical components of the antiproliferative activity of iron chelators.13
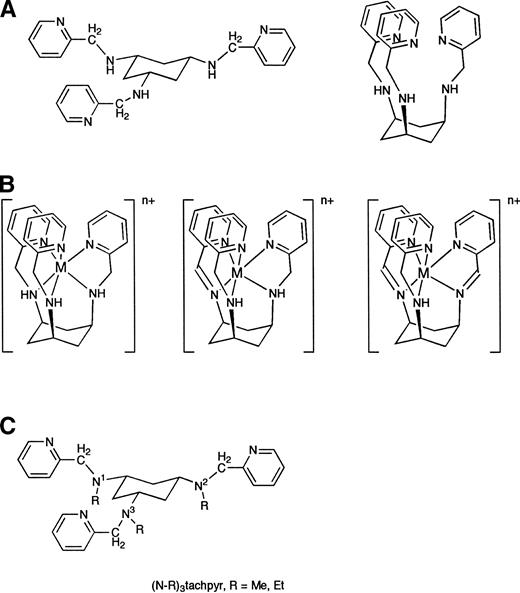
We have synthesized a novel metal chelator based on the framework triamine cis-1,3,5-triaminocyclohexane, which we term tachpyr.14 Tachpyr has three aminopyridine arms attached to a cyclohexyl ring (Fig 1). It is a lipophilic molecule that undergoes a cyclohexyl ring flip (Fig 1a) to give a chelator with hexadentate octahedral geometry. Tachpyr accepts metals of radius from 0.76 Å to 0.94 Å with minimal distortion.15
Structure of tachpyr in the open and closed conformations (A), its metal complexes (B), and N-alkylated derivatives (C).
Structure of tachpyr in the open and closed conformations (A), its metal complexes (B), and N-alkylated derivatives (C).
Tachpyr differs from desferioxamine and PIH in chemical structure and properties. For example, unlike either of these chelators, tachpyr reacts with Fe(III) to yield an Fe(II) complex in a redox process that oxidizes tachpyr (G. Park et al, manuscript in preparation). The experiments described here were designed to explore whether this compound exhibits potential antitumor activity. We report that tachpyr shows cytotoxic activity consistent with a potential application in antitumor therapy.
MATERIALS AND METHODS
Preparation of tachpyr and its derivatives.
Tachpyr, (N-Me)3tachpyr, and (N-Et)3tachpyr were synthesized fromcis-1,3,5-triaminocyclohexane14 (G. Park et al, manuscript in preparation). To purify and facilitate handling of chelators, their nitrate salts were prepared through treatment of the chelator with excess nitric acid in ethanol. Typically, 8 molar equivalents of concentrated nitric acid was added to a solution of approximately 5 × 10−3 mol of tachpyr in 1 mL of anhydrous ethanol resulting in the formation of a pale green precipitate. This precipitate was washed twice with approximately 8 mL of anhydrous diethyl ether and dried under reduced pressure (5 × 10−2 Torr). The compositions of the nitrate salts were determined by elemental analysis and were found to be tachpyr.5HNO3.H2O, (N-Me)3tachpyr.6HNO3, and (N-Et)3tachpyr.6HNO3.
Metal complexes of tachpyr were prepared from reaction of the appropriate metal salt with the chelator in methanol solution, followed by slow diffusion of diethyl ether into the reaction solution. Subsequently, recrystallization was conducted to achieve sufficient purity as determined by combustion analyses16 (G. Park et al, manuscript in preparation). They are represented as M[tachpyr][X]2 (M = Ca(II), Mg(II), Cu(II), X = Cl−; M = Mn(II), Zn(II), X = ClO4−). Further description of the structures and reactivity of these complexes will appear elsewhere; however, their formulations are represented by the leftmost structure of Fig 1b in all cases except for the Fe(II) complex, for which the rightmost two structures of Fig 1b apply. Compounds were dissolved in phosphate buffered saline, pH 7.4, before use in cytotoxicity assays.
Cytotoxicity assays.
MBT2 and T24 are mouse and human bladder cancer cell lines, respectively. MBT2 cells were a generous gift from Dr N. Lattime (Thomas Jefferson University, Philadelphia, PA), and T24 cells were obtained from the American Type Culture Collection. MRC-5 cells were a gift from H. Blau (Stanford University, Stanford, CA). Cells were grown in RPMI medium (MBT2) or DME medium (T24 and MRC-5) containing 10% fetal bovine serum in a humidified chamber containing 5% CO2. A total of 5 × 103 cells were plated in 96-well tissue culture dishes and allowed to attach overnight before test compounds were added. Six replicate cultures were used for each point. After 72 hours (or less, in time course studies), viable cells remaining were assessed using a methytetrazolium (MTT) assay, in which (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide is added to the medium and the formation of a reduced product assayed by measuring the optical density at 560/650 nm after 3 hours. Color formation is proportional to viable cell number.17IC50 was defined as the concentration required to inhibit viability by 50% and was calculated by using the Multiple Drug-Effect Analysis Program (Biosoft, Ferguson, MO). The IC50 of tachpyr was not significantly affected by the addition of 100 μg/mL iron-saturated transferrin to the medium (not shown).
In some experiments, cell survival was assessed by using a clonegenic assay, which measures the ability of cells to divide and form visible colonies of approximately 50 or more cells. For these experiments, replicate plates of cells were prepared and various concentrations of tachpyr added to the growth medium (control cells were plated at the same time and received no drug). At the end of the incubation period, surviving cells were removed from the dish by trypsin, diluted, and replated in growth medium without drug. Colonies were allowed to grow for 7 days. The growth medium was removed, the plates fixed with 0.5% gluteraldehyde, stained with 1% crystal violet, and colonies were counted.
Ferritin synthesis.
Ferritin synthesis was assessed after 7 or 16 hours of exposure of cells to media containing either no additions (control), tachpyr, desferioxamine, or ferric nitrolotriacetate. Cells were metabolically labeled for the last 2 hours of incubation with [35]S-translabel (ICN), and ferritin visualized by immunoprecipitation and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), as previously described.18
RESULTS
Tachpyr is toxic to proliferating bladder cancer cells.
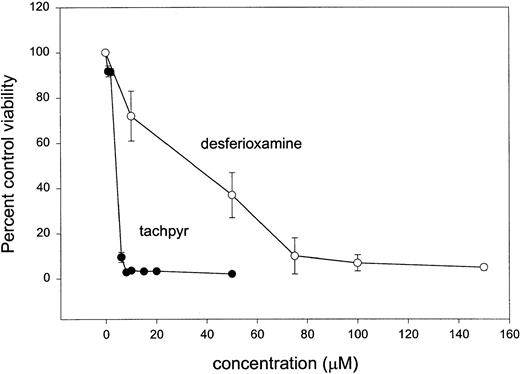
To explore the fundamental biological properties of tachpyr, and to assess whether these were consistent with an iron-depleting mode of action, we measured the effects of tachpyr on growing cells in culture and compared them with desferioxamine, a known iron chelator. MBT2, a mouse bladder tumor cell line, was incubated with varying doses of tachpyr or desferioxamine for 72 hours, and effects on viability were assessed using an MTT dye reduction assay.17 As seen in Fig 2, cell growth was inhibited by both tachpyr and desferioxamine. Tachpyr was more potent than desferioxamine: In four independent experiments, the average IC50 of tachpyr was 4.6 ± 2.0 μmol/L (standard error), compared with 70 μmol/L for desferioxamine.
Comparative cytotoxic effect of tachpyr and desferioxamine on cultured MBT2 bladder cancer cells. Cells were incubated with varying doses of desferioxamine mesylate or tachpyr for 72 hours and viability was assessed by using an MTT assay as described in the Materials and Methods. Calculated IC50s from this data are 3.6 μmol/L for tachpyr and 70 μmol/L for desferioxamine. Mean IC50 of tachpyr in four independent experiments was 4.6 ± 2.0 μmol/L (standard error).
Comparative cytotoxic effect of tachpyr and desferioxamine on cultured MBT2 bladder cancer cells. Cells were incubated with varying doses of desferioxamine mesylate or tachpyr for 72 hours and viability was assessed by using an MTT assay as described in the Materials and Methods. Calculated IC50s from this data are 3.6 μmol/L for tachpyr and 70 μmol/L for desferioxamine. Mean IC50 of tachpyr in four independent experiments was 4.6 ± 2.0 μmol/L (standard error).
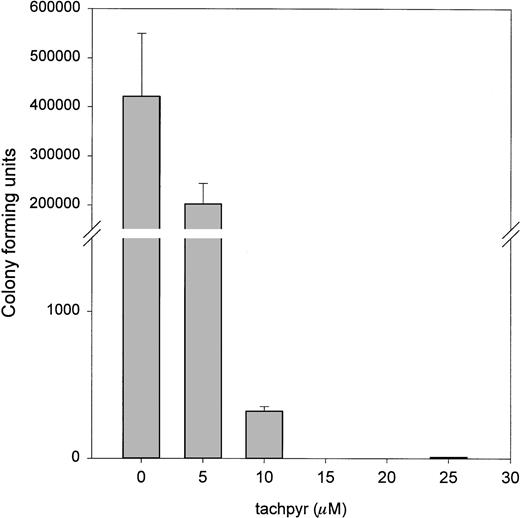
The MTT assay is widely used to measure drug effects on cell viability.13,19 20 It is generally believed to measure mitochondrial function. To confirm that the results of the MTT assay did not reflect an interference with mitochondrial activity, but a true effect of tachpyr on cell viability, we also measured effects of tachpyr using a clonegenic assay. As seen in Fig 3, this assay confirmed that viability, as measured by colony-forming ability, was also sharply reduced by tachpyr.
Tachpyr inhibits colony-forming ability of treated cells. MBT2 cells were incubated for 72 hours with tachpyr and viability was assessed by using a clonegenic assay.
Tachpyr inhibits colony-forming ability of treated cells. MBT2 cells were incubated for 72 hours with tachpyr and viability was assessed by using a clonegenic assay.
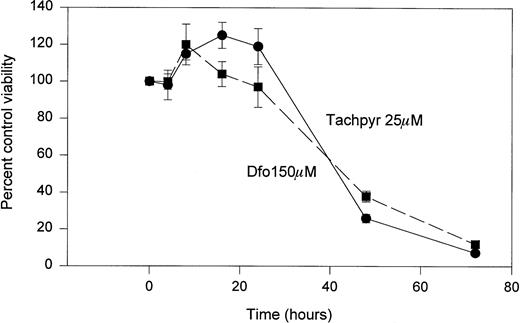
As shown in Fig 4, a time course study showed that tachpyr was not immediately cytotoxic to cells. Rather, a delay of approximately 24 hours was observed before the cytotoxic action of tachpyr became manifest. This time course exactly paralleled that of desferioxamine, a known iron chelator (Fig 4).
Time course of cytotoxicity of tachpyr and desferioxamine. MBT2 cells were incubated with 150 μmol/L (dfo) or 25 μmol/L tachpyr for various lengths of time and survival was measured by using an MTT assay. Viability at time 0 was defined as 100%.
Time course of cytotoxicity of tachpyr and desferioxamine. MBT2 cells were incubated with 150 μmol/L (dfo) or 25 μmol/L tachpyr for various lengths of time and survival was measured by using an MTT assay. Viability at time 0 was defined as 100%.
Effects of tachpyr/metal complexes on cell viability.
To explore whether iron deprivation contributed to tachpyr cytotoxicity, a number of metal complexes of tachpyr were prepared and their effects on MBT2 cells were compared with those of tachpyr. As shown in Figs 5 and6, the Ca(II), Mn(II), and Mg(II) complexes of tachpyr were toxic, whereas the Zn(II), Fe(II), and Cu(II) complexes were not. Initially, these findings appear consistent with the hypothesis that tachpyr functions via the complexation of Zn(II), Fe(II), or Cu(II). However, it is also possible that the association between Zn(II), Cu(II), or Fe(II) and tachpyr may be sufficiently strong to prevent the formation of free ligand. This in turn would restrict the amount of tachpyr available to deplete intracellular iron pools. Further support of this hypothesis comes from studies with sterically hindered chelators that bind Zn(II) and Cu(II) but not Fe(II) (see below). In either case, results with tachpyr/metal complexes suggest that depletion of Ca(II), Mn(II), or Mg(II) does not play a major role in the cytotoxicity of tachpyr.
Comparative cytotoxicity of tachpyr and Fe[tachpyr]Cl2. MBT2 cells were treated for 72 hours with either tachpyr (▩) or Fe[tachpyr]Cl2 (▨) before viability was assessed using an MTT assay.
Comparative cytotoxicity of tachpyr and Fe[tachpyr]Cl2. MBT2 cells were treated for 72 hours with either tachpyr (▩) or Fe[tachpyr]Cl2 (▨) before viability was assessed using an MTT assay.
Comparative cytotoxicity of tachpyr and its metal derivatives. Viability was assessed after 72 hours exposure to the indicated compounds as described in the Materials and Methods. (◍) tachpyr; (○) [Mg]tachpyr; (▾) [Mn]tachpyr; (▿) [Cu]tachpyr; (▩) [Zn]tachpyr; (□) [Ca]tachpyr.
Comparative cytotoxicity of tachpyr and its metal derivatives. Viability was assessed after 72 hours exposure to the indicated compounds as described in the Materials and Methods. (◍) tachpyr; (○) [Mg]tachpyr; (▾) [Mn]tachpyr; (▿) [Cu]tachpyr; (▩) [Zn]tachpyr; (□) [Ca]tachpyr.
Tachpyr derivatives unable to bind iron are not toxic.
To assess further whether iron chelation was involved in the cytotoxic mechanism of tachpyr, we prepared alkylated derivatives of tachpyr in which methyl or ethyl groups were substituted for hydrogen at the amino nitrogens N1, N2, and N3 (Fig 1c). To probe the chemical and structural effects of this change on complexation properties, the single-crystal X-ray structures of the Ni(II) complexes Ni[tachpyr]Cl2 and Ni[(N-Me)3tachpyr][ClO4]2 were compared. The bond distance between the metal and the amine nitrogens of the respective tachpyr chelator were lengthened from 2.102(4) to 2.168(4) Å on methylation of tachpyr (G. Park et al, manuscript in preparation). This bond lengthening shows the steric effect of the methyl group on nitrogen, an effect that weakens the association of (N-Me)3tachpyr with metal ions, and in particular affects the reaction of tachpyr with iron. Thus, we have been able to prepare the complexes Cu[(N-Me)3tachpyr][ClO4]2, Zn[(N-Me)3tachpyr][ClO4]2, and Zn[(N-Et)3tachpyr][ClO4]2, although it has not been possible to observe a strong association between Fe(II) or Fe(III) and (N-Me)3tachpyr (G. Park et al, unpublished observations). Figure 7compares the growth-inhibitory ability of these alkylated tachpyr derivatives with the parent compound. In contrast to tachpyr, which was cytotoxic, these derivatives were completely without effect on the viability of MBT2 cells. This observation is consistent with a role of iron chelation, as opposed to zinc or copper chelation, in the cytotoxic effects of tachpyr.
N-alkylated derivatives of tachpyr are not cytotoxic. Viability was assessed after 72 hours treatment with the indicated compounds.
N-alkylated derivatives of tachpyr are not cytotoxic. Viability was assessed after 72 hours treatment with the indicated compounds.
Tachpyr inhibits ferritin synthesis. Cells were treated with 20 μmol/L tachpyr (tach), 150 μmol/L desferioxamine (def) or 200 μmol/L ferric nitrilotriacetate (iron) in growth medium for 16 hours. Cells were labeled with [35]S amino acids for the last 2 hours of treatment. Ferritin was isolated by immunoprecipitation and analyzed by SDS-PAGE. The H and L subunits of ferritin are shown (lower and upper bands, respectively).
Tachpyr inhibits ferritin synthesis. Cells were treated with 20 μmol/L tachpyr (tach), 150 μmol/L desferioxamine (def) or 200 μmol/L ferric nitrilotriacetate (iron) in growth medium for 16 hours. Cells were labeled with [35]S amino acids for the last 2 hours of treatment. Ferritin was isolated by immunoprecipitation and analyzed by SDS-PAGE. The H and L subunits of ferritin are shown (lower and upper bands, respectively).
Tachpyr inhibits ferritin synthesis.
To directly examine whether iron represented an important intracellular target of tachpyr, we assessed whether tachpyr affected synthesis of ferritin, a major iron storage protein whose synthesis is critically dependent on intracellular concentrations of iron.21 Desferioxamine, an iron chelator known to inhibit ferritin synthesis, was used as a positive control. Cells were treated for 16 hours, labeled with [35]S amino acids, and ferritin isolated by immunoprecipitation and SDS-PAGE. As shown in Fig 8, under these conditions tachpyr repressed ferritin synthesis as effectively as desferioxamine. This was a specific effect and did not reflect a generalized inhibition of protein synthesis, because equal numbers of trichloroacetic acid-precipitable counts were immunoprecipitated in all cases. A similar inhibition of ferritin synthesis was seen after 7 hours of treatment (not shown), a timepoint that precedes all cytotoxic effects of tachpyr (Fig 2). These results suggest that intracellular iron depletion is an early, proximal event initiated by tachpyr.
Effects of tachpyr are not restricted by cell type.
To ensure that effects of tachpyr were not restricted to a particular cell type, the effect of tachpyr on the viability of T24 bladder cancer cells and MRC-5 normal human diploid fibroblasts was also assessed. As shown in Table 1, both cell types were sensitive to tachpyr. However, the IC50 of T24 cells was 4.3 μmol/L, whereas that of MRC-5 cells was 30.5 μmol/L, approximately sevenfold higher than observed for the two tumor cell lines.
IC50 of Tachpyr in Different Cell Types
| Cell Type . | IC50 (μM) . |
|---|---|
| MBT2 | 2.2 |
| T24 | 4.3 |
| MRC-5 | 30.5 |
| Cell Type . | IC50 (μM) . |
|---|---|
| MBT2 | 2.2 |
| T24 | 4.3 |
| MRC-5 | 30.5 |
Cells were seeded in 96-well plates, exposed to various doses of tachpyr for 72 hours, and cytotoxicity assessed by using an MTT assay as described in the Materials and Methods. Each point was the average of six replicate plates. IC50 was calculated by using the Multiple Drug-Effect Analysis Program (Biosoft).
DISCUSSION
Tachpyr is a metal ligand with a unique chemical structure relative to other ligands that have been explored as antitumor therapeutics. It differs from the PIH chelator family13 in containing exclusively nitrogen donor ligands (Fig 1). In this regard, tachpyr also differs from natural siderophores, such as desferioxamine, as well as most chemical chelators (eg, L1, CP94, DTPA, DBED, and EDTA22), which generally contain oxygen donor atoms. Additionally, tachpyr differs from PIH in denticity, being hexadentate rather than bidentate. Our preliminary results suggest that the chemical features of tachpyr may allow it to both bind and reduce iron, properties that may profoundly influence its biological activity (G. Park et al, manuscript in preparation). Because it is chemically unrelated to previously studied metal chelators, the biological properties of tachpyr were unknown. The experiments described here were designed to assess biological targets of tachpyr and its potential as an antitumor therapeutic.
Our results show a striking cytotoxic effect of tachpyr. In the cells studied, the IC50 of 4.6 μmol/L was lower than that of desferioxamine (70 μmol/L), suggesting an effective dose in the range reported for the most effective PIH analogs (1-7 μmol/L).13 We have measured the lipophilicity (n-octanol-water partition coefficient; log Poct/H2O) of tachpyr as −0.10 (F. Lu et al, manuscript in preparation), a value consistent with an ability to penetrate biological membranes. Thus, the relative decrease in IC50of tachpyr relative to desferioxamine may in part be attributable to its enhanced permeability relative to desferioxamine. Alternatively or additionally, tachpyr may have greater access to key intracellular iron pools, enabling it to achieve equivalent cytotoxicity at lower doses.
A number of lines of evidence point to iron as a major target of tachpyr. Tachpyr has the potential to chelate several biologically important metals including Ca(II), Mg(II), Mn(II), Fe(II), Cu(II), and Zn(II). Although we cannot definitively exclude a role of chelation of other metals in the effects of tachpyr, our toxicity studies of tachpyr complexes of the above metals are consistent with the suggestion that iron is a major target of tachpyr. Thus Ca[tachpyr]Cl2, Mg(tachpyr)Cl2, and Mn(tachpyr)[ClO4]2 retained their toxicity, whereas Fe[tachpyr]Cl2, Cu(tachpyr)Cl2, and Zn(tachpyr)[ClO4]2 were nontoxic (Figs 5 and6). Assuming that metal-tachpyr complexes do not cross the cell membrane because they are cationic,23 we postulate that the toxicity of the Ca(II), Mn(II), and Mg(II) complexes arises from the liberation of free tachpyr by transmetallation of the metal ion to a ligand with greater affinity for these metals (such as apotransferrin). We further hypothesize that this reaction does not occur appreciably with Zn(II), Cu(II), and Fe(II) complexes of tachpyr because of their greater inherent stability. These hypotheses are in accord with the well-established theoretical affinities of nitrogen-donor ligands (such as tachpyr) for metal ions, which predict that Ca(II), Mn(II), and Mg(II) are less tightly bound than Fe(II), Zn(II), or Cu(II).24 Toxicity studies with N-alkylated derivatives of tachpyr extend these conclusions by showing that N-alkylation blocks the cytotoxicity of tachpyr. Because N-alkylation inhibits the reaction of tachpyr with iron but not with zinc or copper, these results suggest that Zn(II) or Cu(II) are not major cellular targets of tachpyr (Fig7). Taken together, these experiments are consistent with a mechanism in which iron chelation leads to cytotoxicity.
The lipophilicity constant of uncomplexed tachpyr is consistent with a mode of action involving penetration of the cell and chelation of an intracellular iron pool. A reduction of the intracellular labile iron pool is also consistent with the observed ability of tachpyr to inhibit ferritin synthesis. The translation of ferritin mRNA is regulated by an intracellular pool of chelatable iron.21 Thus, when iron levels are low, ferritin synthesis is decreased; conversely, when iron levels are high, ferritin synthesis increases. This regulatory response to iron is posttranscriptional and is caused by the recruitment of stored message from monosomes to polysomes in the presence of iron,21 a process mediated by iron regulatory element-binding proteins (IRPs), proteins that bind to a region (the iron regulatory element [IRE]) of the 5′UTR of the ferritin mRNA and regulate its translation.25-27 The observation that treatment of MBT2 cells results in an early and selective repression of ferritin synthesis is consistent with iron depletion as a major consequence of tachpyr activity.
Based on previous results suggesting that bladder cancer may be particularly susceptible to iron depletion therapy,9 28-30the experiments described here assessed the cytotoxic potential of tachpyr on cultured murine bladder cancer cells. The observed cytotoxic effects were not restricted to these MBT2 cells, but were also seen in other cell types examined, including the human bladder cancer cell line T24 (Table 1) as well as breast cancer cell lines (not shown). Normal diploid fibroblasts were sixfold to sevenfold more resistant to tachpyr than the tumor cell lines we tested (Table 1). Although the significance of this difference will remain unclear until more cell lines are examined, it is tempting to speculate that despite the cytotoxic effects of tachpyr on normal cell types, cancer cells may exhibit a heightened sensitivity to tachpyr that can be exploited therapeutically.
ACKNOWLEDGMENT
The authors thank F.H. Lu for preparation of the N-alkyl tachpyr ligands, N. Ye for the preparation of tachpyr derivatives, and R. Ma for superb technical assistance. We thank the NIH for the support of F.H. Lu through the STRP (student training research program) fellowship program during 1997.
Received November 17, 1997; accepted April 10, 1998.
Supported in part by Grant No. DK 42412 from the National Institutes of Health (F.M.T. and S.V.T.) and by Grant No. DK42412-09S1 (S.P.W.).
Address reprint requests to S.V. Torti, PhD, The Wake Forest University School of Medicine, Department of Biochemistry, Wake Forest University, Medical Center Boulevard, Winston-Salem, NC 27157-1082.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 5. Comparative cytotoxicity of tachpyr and Fe[tachpyr]Cl2. MBT2 cells were treated for 72 hours with either tachpyr (▩) or Fe[tachpyr]Cl2 (▨) before viability was assessed using an MTT assay.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/4/10.1182_blood.v92.4.1384/5/m_blod41630005x.jpeg?Expires=1769300887&Signature=kxMaIbg7VR~9idqzmxf4ygNSgDlIRxY~DFTOUfT~qsX0mHxXFI7chroluGT4XNWRG8z4ed1jjzlbtCvblMzDBYOvq9DbeX2AOr1S9DaIQ~NIDHBhZJC2rbknX5u3A~xCBfmbUNKkvDAz~41jrq-~YM4r-pHRNay8YoziCAic4D4i64hL5E1EsQE~pCLY0u~uq76rAtHXc3VCvzKoK4ggxeTmtWlV4jTvsSo9irlMHx6eGxQbu6PnkaWYgB8hjsUTv-Rucl5IFGnBdb~b8CHWymO3JJqAhlh5nB3ReP6v3hhJib3j43YLBmzyyza6hvvlg6g~i3549SJ9L7ftBWiEFA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Comparative cytotoxicity of tachpyr and its metal derivatives. Viability was assessed after 72 hours exposure to the indicated compounds as described in the Materials and Methods. (◍) tachpyr; (○) [Mg]tachpyr; (▾) [Mn]tachpyr; (▿) [Cu]tachpyr; (▩) [Zn]tachpyr; (□) [Ca]tachpyr.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/4/10.1182_blood.v92.4.1384/5/m_blod41630006x.jpeg?Expires=1769300887&Signature=dWuORsqhK~WjZC-npjTrv6R64uPkUtBehZKmzYLU7lvtwSE-zJU3khKs9Hv3ghPD7aWNvz1A-Upe~S-sdsrjPDM-b0XhosP9Bvz01zidBUb5mrIt7~ADNP8TGaEaivp5o5p8Lu8qUG-SYMpx6ug6rmwo95fCOk-pJvhQbAwpgwCVVQCVcicr482YK203rF3XGFEztlREK9ttCKiH8-J7XBbEemOooHQ-1xOUMJzFweg8-omPd6oV4~NW8OZqXudMnQBs5RTqvBNExTmL0rfp7nC6ztY8abkA~kOniRsC9w0nuXr9Kg10lCt7Pi7HeGDZxaw7JdE0vyTagtLU7paBFw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 8. Tachpyr inhibits ferritin synthesis. Cells were treated with 20 μmol/L tachpyr (tach), 150 μmol/L desferioxamine (def) or 200 μmol/L ferric nitrilotriacetate (iron) in growth medium for 16 hours. Cells were labeled with [35]S amino acids for the last 2 hours of treatment. Ferritin was isolated by immunoprecipitation and analyzed by SDS-PAGE. The H and L subunits of ferritin are shown (lower and upper bands, respectively).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/4/10.1182_blood.v92.4.1384/5/m_blod41630008w.jpeg?Expires=1769300887&Signature=UfhU-Gw3K0EPXWfM2RGjYpbBa7gtHXVvpSFoxGQmsfPQLcAXS7oR0uT96d76Zvp60FoCXgxe1fTgznR1xHYwh4~QD~iIlU9Kgj-ogemRMHtWJDgRcQVwAFrBu626xJ2oxlyYrWE~iC91XJMI4h1BO4k9c~lLs~~y6Z4yRSmx-j3CvMfBqE0TJawZnmO2S6ijRXOktxUkxv~0mKmk3dkIiZwpDy7ESp27~-vq3wGDEhlG5ViKz4fMPOZjnt9zSgDgxaNFrStXL09HqJjI7iv8zQtMAck5O5xT-3dpNnJOUF14Irg8dpGWpXvMXucdrCHIceRB7BVU-WGMDmoq0~S~aw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal