Abstract
The function and the outside-in signaling pathways of IIbβ3 were examined in relation to cell adhesion using a megakaryoblastic leukemia cell line, CMK. After 12-O-tetradecanoylphorbol-13-acetate (TPA) treatment, the cells adhered to the culture plate and underwent megakaryocytic differentiation with expression of IIbβ3. Binding of soluble fibrinogen to the cells via IIbβ3 was dependent on cell adhesion. Cell detaching reduced the affinity of this integrin for soluble fibrinogen, although its surface expression was almost unchanged. In contrast, detached cells became tightly adherent to the fibrinogen-coated plate (solid-phase fibrinogen). The same ligand, fibrinogen, present either in soluble or solid-phase form, triggered differential signaling pathways mediated by IIbβ3. By the stimulation with soluble fibrinogen, Syk was tyrosine-phosphorylated but FAK was dephosphorylated, whereas solid-phase fibrinogen promptly caused tyrosine phosphorylation of FAK followed by delayed phosphorylation of Syk. In addition, the binding of soluble fibrinogen to the cells adherent to fibrinogen-coated plate resulted in tyrosine phosphorylation of integrin β3 and a complex formation of integrin β3 with Syk. This implies the cooperation of both soluble and solid-phase fibrinogen-mediated signaling pathways.
© 1998 by The American Society of Hematology.
INTEGRIN αIIBβ3 EXPRESSED on platelet megakaryocyte-lineage cells is one of the integrin family, α- and β-heterodimeric cell surface receptors and is involved in the interactions between extracellular matrix and the cytoskeleton.1-3 In the process of platelet activation, αIIbβ3 undergoes conformational changes from the resting to the activated form (inside-out signaling). In the activated platelets, soluble fibrinogen binds to αIIbβ3 and triggers outside-in signaling during platelet aggregation, but in the resting form, αIIbβ3 only adheres to fibrinogen-coated plate (binding of the solid-phase fibrinogen).4,5 The function of αIIbβ3 in megakaryocytes has been limitedly characterized as the αIIbβ3-mediated endocytosis of fibrinogen into α granules6 and the modulation of αIIbβ3 activity by thrombopoietin.7 Several studies using cell lines have shown that the cytoplasmic domains of αIIbβ3 are essential for signaling response8-10 and that αIIbβ3-mediated cell adhesion is enhanced by the treatment with 12-O-tetradecanoylphorbol-13-acetate (TPA).11,12 In platelet aggregation, tyrosine phosphorylation of β3 might be important in initiating outside-in signaling through αIIbβ3.13
FAK and Syk are nonreceptor protein tyrosine kinases involved in αIIbβ3-mediated signaling. FAK is found in the focal adhesion and appears to be activated in response to integrin-mediated adhesion. In platelets, FAK is reported to be activated dependently on αIIbβ3-mediated aggregation, whereas the ligation of αIIbβ3 is not sufficient to stimulate FAK phosphorylation.14,15 In fact, a few reports showed that clustering of αIIbβ3 with some anti-αIIbβ3 antibodies does not promote FAK phosphorylation.16 17
Syk is expressed exclusively in hematopoietic cells.18,19In a number of studies on the activation of Syk, the functional roles of Syk in B-cell receptor (BCR) and some Fc receptors have been extensively examined in association with the immune receptor tyrosine activation motifs (ITAMs).20-22 In platelets, we and others have reported that Syk is activated by a variety of stimulants23-25 and translocates into the cytoskeleton in two steps, one of which is dependent on αIIbβ3-mediated aggregation.26 In addition, Clark et al27 have reported that Syk is activated via engagement of αIIbβ3 by fibrinogen only when the anti–ligand-induced binding site (anti-LIBS) monoclonal antibody (MoAb) that alters αIIbβ3 to the activated form is used. Recently Gao et al28 showed that Syk activation mediated by αIIbβ3 is triggered by the binding of soluble fibrinogen and does not require phosphorylated ITAMs. As for other integrin-mediated signaling, Syk was reported to be activated after the ligation of β1 or β2 integrins.29 30
In this study, we address the possibility of differential signaling pathways mediated by αIIbβ3 when it is engaged via fibrinogen in different forms, either in soluble or solid-phase form, using a megakaryoblastic leukemia cell line, CMK.31
MATERIALS AND METHODS
Antibodies.
Anti-Syk MoAb 101 was kindly provided by Wako Pure Chemicals (Tokyo, Japan). Polyclonal antibodies against Syk (sc-573, sc-929) and against FAK (sc-557) were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Antiphosphotyrosine MoAb 4G10 was purchased from Upstate Biotechnology Inc (Lake Placid, NY), antiphosphotyrosine MoAb PY-20 from Transduction Laboratories (Lexington, KY), anti-αIIbβ3 MoAb P2 from Immunotech (Marseille, France),32 anti-αIIbβ3 MoAb for immunophenotyping analysis from DAKO Japan (Kyoto, Japan), fluorescein-labeled anti-αIIbβ3 MoAb specific for the activated form, fluorescein isothiocyanate (FITC)-PAC1, from Becton Dickinson (San Jose, CA),33 anti-integrin β3 MoAb for immunoprecipitation from Southern Biotechnology Associates Inc (Birmingham, AL), and anti-integrin β3 and anti-integrin αIIb MoAb for immunoblotting from Affiniti Research Products Ltd (Nottingham, UK).
Reagents.
Human fibrinogen was kindly provided by Yoshitomi Pharmaceutical Industries Ltd (Osaka, Japan). TPA, Arg-Gly-Asp-Ser (RGDS), and Arg-Gly-Glu-Ser (RGES) peptides were purchased from Sigma (St Louis, MO). The disulfide-linked peptide, cyclo(S,S)-Mpr.(ε-phenylimidyl-Lys)GDWPPen-NH2 (cyclic-KGD) was kindly provided by Dr Robert M. Scarborough (COR Therapeutics Inc, South San Francisco, CA).34 Cytochalasin D was purchased from Wako Pure Chemicals (Tokyo, Japan),35 poly-L-lysine from Nacalai Tesque (Kyoto, Japan), and fluorescein-C6-succunimidyl ester (FXS) from PanVera Corp (Madison, WI).
Cell culture.
The CMK cell line, provided by Dr T. Sato (Chiba University, Chiba, Japan), was maintained in RPMI 1640 medium with 10% heat-inactivated fetal calf serum (FCS).31 This cell line was derived from a child with megakaryoblastic leukemia and has the property of expressing some characteristics of mature megakaryocytes after TPA treatment.36 The cells were induced to differentiation by the addition of 20 nmol/L TPA and harvested at the indicated times.
The stimulation with soluble and solid-phase fibrinogen.
We added the indicated concentrations of fibrinogen dissolved in RPMI 1640 without FCS either to the adherent cells formed after TPA treatment for 3 days (D3-adherent cells) or to the cells detached mechanically by use of a cell scraper after the same TPA treatment (D3-detached cells). To remove the influence of TPA, the medium was exchanged 1 day before the stimulation with fibrinogen. In some cases, the cells were preincubated with cytochalasin D at 37°C or with cyclic-KGD, RGDS or RGES peptides at 4°C for 15 minutes. To examine the cell adhesion to solid-phase fibrinogen or poly-L-lysine, the culture plates were precoated with phosphate-buffered saline (PBS) containing 100 μg/mL fibrinogen or 10 μg/mL poly-L-lysine overnight at 4°C, incubated with 1% bovine serum albumin (BSA) in PBS for 1 hour at 37°C to block nonspecific binding, and washed with PBS before use. The BSA precoated plates were used as a negative control. The detached cells were reincubated on the plates precoated with fibrinogen, poly-L-lysine, or BSA for the indicated times.
Immunoprecipitation.
Usually, cells were lysed in a nonionic detergent buffer (1% Triton X-100, 50 mmol/L Tris/HCl, pH 7.5, 150 mmol/L NaCl, 5 mmol/L EDTA, 1 mmol/L sodium vanadate, 1 mmol/L phenylmethylsulfonyl fluoride, and 10 μg/mL leupeptin). In some cases, sodium dodecyl sulfate (SDS)-containing lysis buffer (0.05% SDS, 0.5% sodium deoxycholate, 50 mmol/L Tris/HCl, pH 7.5, 150 mmol/L NaCl, 5 mmol/L EDTA, 1 mmol/L sodium vanadate, 1 mmol/L phenylmethylsulfonyl fluoride, and 10 μg/mL leupeptin) was used. The cell lysates were centrifuged and the supernatants were incubated with the indicated antibodies for 2 hours at 4°C, after which protein A-Sepharose (Pharmacia Biotech, Uppsala, Sweden) was added for 1 hour at 4°C. The precipitates were washed 3 times with the indicated lysis buffer. For immunoblotting, the precipitates were boiled with electrophoresis SDS sample buffer (2% SDS, 10% glycerol, 5% 2-mercaptoethanol, 0.001% bromophenol blue, and 62.5 mmol/L Tris/HCl, pH 6.8) for 3 minutes. In vitro kinase assay of Syk was performed as previously described.23
Immunoblotting.
For the day course study, whole cell lysates were prepared by boiling with electrophoresis SDS sample buffer for 3 minutes. Whole cell lysates or immunoprecipitates were separated by SDS-PAGE and transferred onto polyvinylidine difluoride membranes (Immobilon; Millipore Corp, Bedford, MA). The membrane blots were blocked with 5% skim milk in T-PBS (PBS containing 0.05% Tween 20) or low-salt Tris buffered-saline containing Tween 20 (low-salt T-TBS; 10 mmol/L Tris/HCl, pH 7.5, 100 mmol/L NaCl containing 0.05% Tween 20; for the detection of tyrosine phosphorylation of integrin β3) for 1 hour at 37°C and then incubated with primary antibodies in T-PBS or low-salt T-TBS for 1 hour at room temperature. After washing, membranes were incubated with horseradish peroxidase-conjugated goat antimouse IgG or antirabbit IgG polyclonal antibodies in T-PBS or low-salt T-TBS for 30 minutes at room temperature. After washing, the enhanced chemiluminescence (ECL) assay was performed and the positive bands were detected using x-ray films.
Flow cytometry.
To measure the αIIbβ3-mediated binding of soluble fibrinogen to CMK cells, fibrinogen was labeled with FXS according to the manufacturer’s method. Total αIIbβ3 expression was determined using anti-αIIbβ3 MoAb and fluorescein-conjugated antimouse IgG polyclonal antibody. FITC-PAC1, fluorescein-labeled anti-αIIbβ3 MoAb was used to detect the activated form. Each binding assay was performed for 30 minutes at room temperature. The binding assay for adherent cells was performed under the adherent condition and then the cells were detached mechanically for flow cytometric analyses. Flow cytometry was performed on a Becton Dickinson FACScan with Lysys II software. In the case of inhibition assay, anti-αIIbβ3 MoAb was pretreated before the addition of FXS-labeled fibrinogen.
RESULTS
TPA-induced differentiation of CMK cells.
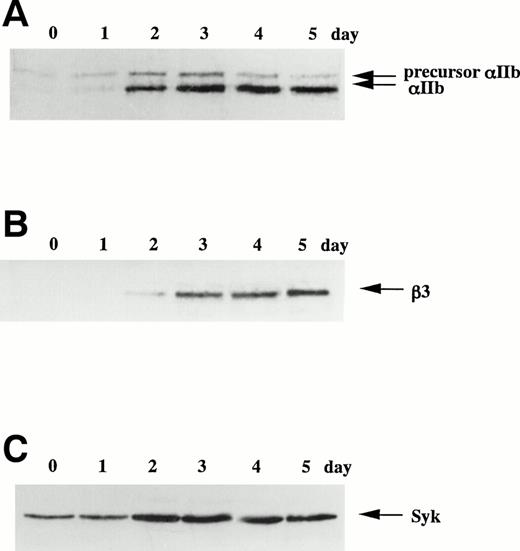
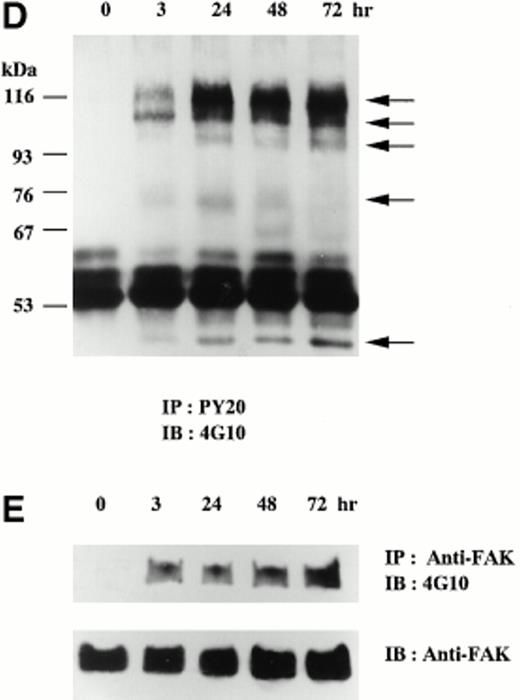
When CMK cells were cultured in suspension, the percentage of αIIbβ3-positive cells was less than 10% by immunophenotyping analysis (data not shown). In contrast, on the addition of 20 nmol/L TPA, most of CMK cells adhered to the plate by 3 hours. From day 3, cell spreading became prominent and the αIIbβ3-positivity increased to greater than 90%, but the cell growth was completely arrested (data not shown). The expression of integrin αIIb, β3 and protein tyrosine kinase Syk and FAK was examined by immunoblotting analyses using corresponding antibodies before and after TPA treatment (Fig 1). In Fig 1A, the upper band was considered as the precursor of αIIb. The bands of αIIb and β3 that were hardly detected on day 0 became detectable with time and were constant after day 3 (Fig 1A and B). In contrast, the band of Syk was already detectable on day 0 and increased about fourfold after day 2 (Fig 1C). The expression of FAK was unchanged in this day course study (data not shown). Next, protein tyrosine phosphorylation of whole cell lysates was analyzed. Tyrosine-phosphorylated proteins of 115, 105, 95, 75, and 50 kD were newly detected after the addition of TPA (Fig 1D). The 75-kD band reached a maximal level at 24 hours and then decreased, whereas the other tyrosine-phosphorylated bands were constant throughout the time course study. In the same lysates, tyrosine phosphorylation of FAK was detected at 3 hours after the addition of TPA, and the phosphorylation was maintained up to 72 hours (Fig 1E).
TPA-induced differentiation of CMK cells on day course study. After addition of 20 nmol/L TPA to CMK cells, whole cell lysates were subjected to immunoblotting analyses with (A) anti-integrin IIb MoAb, (B) anti-integrin β3 MoAb, or (C) anti-Syk polyclonal antibody. In each lane, 30 μg protein of cell lysates was loaded. The cell lysates were next immunoprecipitated with (D) antiphosphotyrosine MoAb (PY20) or (E) anti-FAK polyclonal antibody. Immunoblotting analyses were performed with antiphosphotyrosine MoAb (4G10) as described in the Materials and Methods. In (D), arrows indicate the positions of the tyrosine-phosphorylated protein bands. In (E), the blots were stripped and reprobed with the anti-FAK polyclonal antibody. Results are representative of 3 experiments.
TPA-induced differentiation of CMK cells on day course study. After addition of 20 nmol/L TPA to CMK cells, whole cell lysates were subjected to immunoblotting analyses with (A) anti-integrin IIb MoAb, (B) anti-integrin β3 MoAb, or (C) anti-Syk polyclonal antibody. In each lane, 30 μg protein of cell lysates was loaded. The cell lysates were next immunoprecipitated with (D) antiphosphotyrosine MoAb (PY20) or (E) anti-FAK polyclonal antibody. Immunoblotting analyses were performed with antiphosphotyrosine MoAb (4G10) as described in the Materials and Methods. In (D), arrows indicate the positions of the tyrosine-phosphorylated protein bands. In (E), the blots were stripped and reprobed with the anti-FAK polyclonal antibody. Results are representative of 3 experiments.
Binding of anti-αIIbβ3 MoAb and soluble fibrinogen to TPA-induced CMK cells.
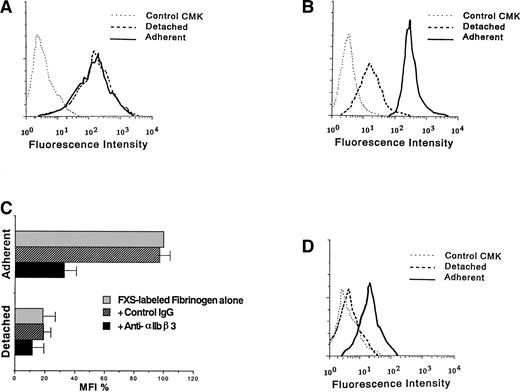
The effect of cell adhesion on ligand binding to αIIbβ3 was examined by flow cytometry. Surface expression of αIIbβ3 was almost the same between D3-adherent and D3-detached cells (Fig 2A). In contrast, the binding of FXS-labeled fibrinogen to D3-adherent cells was higher than that to D3-detached cells (Fig 2B). To confirm that the binding is mediated by αIIbβ3, cells were pretreated with anti-αIIbβ3 MoAb or control mouse IgG for 5 minutes at room temperature before the addition of FXS-labeled fibrinogen. As shown in Fig 2C, anti-αIIbβ3 MoAb but not control IgG showed a suppressive effect. Additionally, a larger amount of FITC-PAC1 MoAb specific for the activated form of αIIbβ3 could bind to αIIbβ3 on the surface of D3-adherent than D3-detached cells (Fig 2D). These results suggested that αIIbβ3 on the surface of TPA-induced CMK cells exists in the activated form dependently on cell adhesion.
Binding of anti-IIbβ3 MoAb and soluble fibrinogen to TPA-induced CMK cells. D3-adherent, D3-detached, and control CMK cells were incubated with (A) anti-IIbβ3 complex MoAb or (B) 200 μg/mL FXS-labeled fibrinogen for 30 minutes at room temperature, and flow cytometry was performed. D3-adherent cells were harvested by detaching them mechanically after the binding reaction. (C) D3-adherent and D3-detached cells were pretreated with 40 μg/mL of anti-IIbβ3 MoAb or control mouse IgG for 5 minutes at room temperature and then treated with 50 μg/mL FXS-labeled fibrinogen, after which flow cytometry was performed. The MFI of FXS-labeled fibrinogen bound to D3-adherent cells was taken as 100%, and results represent the mean ± SD of 3 independent experiments. (D) D3-adherent, D3-detached, and control CMK cells were incubated with the activation-dependent anti-IIbβ3 MoAb, PAC1 as described above, and flow cytometry was performed. Results of (A), (B), and (D) are representative of 3 experiments.
Binding of anti-IIbβ3 MoAb and soluble fibrinogen to TPA-induced CMK cells. D3-adherent, D3-detached, and control CMK cells were incubated with (A) anti-IIbβ3 complex MoAb or (B) 200 μg/mL FXS-labeled fibrinogen for 30 minutes at room temperature, and flow cytometry was performed. D3-adherent cells were harvested by detaching them mechanically after the binding reaction. (C) D3-adherent and D3-detached cells were pretreated with 40 μg/mL of anti-IIbβ3 MoAb or control mouse IgG for 5 minutes at room temperature and then treated with 50 μg/mL FXS-labeled fibrinogen, after which flow cytometry was performed. The MFI of FXS-labeled fibrinogen bound to D3-adherent cells was taken as 100%, and results represent the mean ± SD of 3 independent experiments. (D) D3-adherent, D3-detached, and control CMK cells were incubated with the activation-dependent anti-IIbβ3 MoAb, PAC1 as described above, and flow cytometry was performed. Results of (A), (B), and (D) are representative of 3 experiments.
Tyrosine phosphorylation of D3-adherent cells and D3-detached cells after the stimulation with soluble or solid-phase fibrinogen.
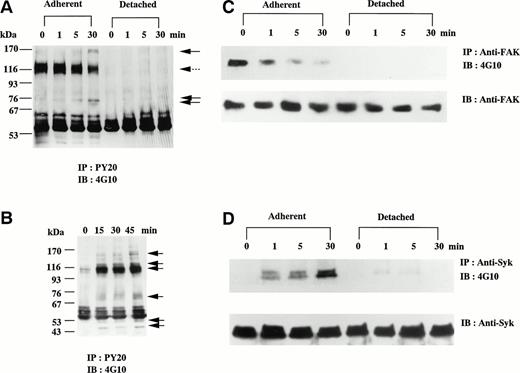
Protein tyrosine phosphorylation in D3-adherent and D3-detached cells was analyzed before and after the stimulation with soluble fibrinogen. To remove the direct influence of TPA, the medium was exchanged 1 day before the experiments. In D3-adherent cells, the addition of soluble fibrinogen resulted in gradual shrinking of the cells contrary to cell spreading and increased tyrosine phosphorylation of several proteins, including 160∼150-kD, 75-kD, and approximately 72-kD bands, in contrast to the reduction of 115-kD band (Fig 3A). Reprobe assay confirmed that 72-kD band contained Syk (data not shown). As expected from the result of Fig 2, soluble fibrinogen could not trigger any signal in D3-detached cells, whereas 115-kD proteins were tyrosine-dephosphorylated just by detaching (Fig 3A). We then examined whether solid-phase fibrinogen binds to these cells and triggers the outside-in signaling. D3-detached cells were further incubated on the plate precoated with fibrinogen. After 15 minutes, most of the cells adhered to the fibrinogen plate (the cells on fibrinogen plate), but few cells adhered to the BSA-coated plate (negative control). Figure 3B shows that tyrosine phosphorylation of 150-kD, 120-kD, 115-kD, 75∼70-kD, 50-kD, and 45-kD proteins was significantly increased by adhesion to the fibrinogen plate.
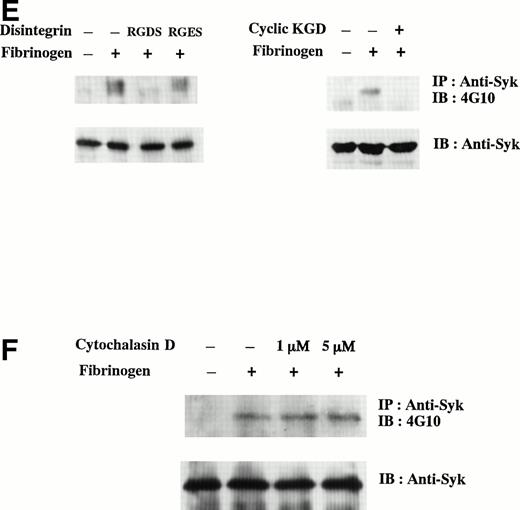
Tyrosine phosphorylation of D3-adherent cells and D3-detached cells after the stimulation with soluble and solid-phase fibrinogen. (A) D3-adherent and D3-detached cells were stimulated with 1 mg/mL soluble fibrinogen for the indicated times. (B) D3-detached cells were incubated on the fibrinogen plate for the indicated times (0, 15, 30, and 45 minutes). In (A) and (B), the cell lysates were immunoprecipitated with antiphosphotyrosine MoAb (PY20) and immunoblotting analysis was performed with antiphosphotyrosine MoAb (4G10) as described in the Materials and Methods. The positions of the molecular markers are shown to the left in kilodaltons. Solid arrows and a broken arrow indicate the positions of the tyrosine-phosphorylated protein bands and the tyrosine-dephosphorylated protein band, respectively. In (C) and (D), D3-adherent and D3-detached cells were stimulated with 1 mg/mL soluble fibrinogen for the indicated times. The cell lysates were immunoprecipitated with (C) anti-FAK polyclonal antibody or (D) anti-Syk MoAb, and immunoblotting analysis was performed with antiphosphotyrosine MoAb (4G10). The blots were stripped and reprobed with the indicated antibodies. (E) The effects of disintegrin peptides on tyrosine phosphorylation of Syk were examined. D3-adherent cells were treated with 5 mmol/L RGDS, 5 mmol/L RGES, or 40 μmol/L cyclic-KGD peptide for 15 minutes at 4°C and stimulated with 1 mg/mL soluble fibrinogen for 5 minutes, and then tyrosine phosphorylation of Syk was examined as described above. (F) The effect of cytochalasin D on tyrosine phosphorylation of Syk was examined. D3-adherent cells were pretreated with 1 or 5 μmol/L cytochalasin D for 15 minutes at 37°C and then stimulated with 1 mg/mL soluble fibrinogen for 5 minutes, and tyrosine phosphorylation of Syk was examined as described above. Results are representative of 4 experiments.
Tyrosine phosphorylation of D3-adherent cells and D3-detached cells after the stimulation with soluble and solid-phase fibrinogen. (A) D3-adherent and D3-detached cells were stimulated with 1 mg/mL soluble fibrinogen for the indicated times. (B) D3-detached cells were incubated on the fibrinogen plate for the indicated times (0, 15, 30, and 45 minutes). In (A) and (B), the cell lysates were immunoprecipitated with antiphosphotyrosine MoAb (PY20) and immunoblotting analysis was performed with antiphosphotyrosine MoAb (4G10) as described in the Materials and Methods. The positions of the molecular markers are shown to the left in kilodaltons. Solid arrows and a broken arrow indicate the positions of the tyrosine-phosphorylated protein bands and the tyrosine-dephosphorylated protein band, respectively. In (C) and (D), D3-adherent and D3-detached cells were stimulated with 1 mg/mL soluble fibrinogen for the indicated times. The cell lysates were immunoprecipitated with (C) anti-FAK polyclonal antibody or (D) anti-Syk MoAb, and immunoblotting analysis was performed with antiphosphotyrosine MoAb (4G10). The blots were stripped and reprobed with the indicated antibodies. (E) The effects of disintegrin peptides on tyrosine phosphorylation of Syk were examined. D3-adherent cells were treated with 5 mmol/L RGDS, 5 mmol/L RGES, or 40 μmol/L cyclic-KGD peptide for 15 minutes at 4°C and stimulated with 1 mg/mL soluble fibrinogen for 5 minutes, and then tyrosine phosphorylation of Syk was examined as described above. (F) The effect of cytochalasin D on tyrosine phosphorylation of Syk was examined. D3-adherent cells were pretreated with 1 or 5 μmol/L cytochalasin D for 15 minutes at 37°C and then stimulated with 1 mg/mL soluble fibrinogen for 5 minutes, and tyrosine phosphorylation of Syk was examined as described above. Results are representative of 4 experiments.
The effects of soluble fibrinogen on tyrosine phosphorylation of FAK and Syk were examined. By the stimulation with soluble fibrinogen, tyrosine phosphorylation of FAK was gradually reduced in D3-adherent cells (Fig 3C). In D3-detached cells, FAK was tyrosine-dephosphorylated just by detaching and soluble fibrinogen showed no effect (Fig 3C). In contrast to FAK, soluble fibrinogen increased tyrosine phosphorylation of Syk, which was readily detected at 1 minute and reached a maximal level at 30 minutes in D3-adherent cells (Fig 3D). As for D3-detached cells, Syk was only faintly tyrosine-phosphorylated, in marked contrast to the results of D3-adherent cells (Fig 3D). In TPA-untreated control CMK cells, soluble fibrinogen did not induce tyrosine phosphorylation of Syk (data not shown). Syk activity assayed in vitro showed a good correlation with tyrosine phosphorylation induced by fibrinogen (data not shown).
To further confirm that tyrosine phosphorylation of Syk is specifically mediated by αIIbβ3, the effects of disintegrin peptides were examined. In addition to RGDS and control RGES peptides, cyclic-KGD, a disulfide-linked peptide that has a potent affinity and high specificity for αIIbβ3,34 was used. As shown in Fig 3E, soluble fibrinogen-induced tyrosine phosphorylation of Syk was suppressed by 5 mmol/L RGDS peptide but not by RGES peptide. Cyclic-KGD peptide (40 μmol/L) suppressed the phosphorylation of Syk to almost the level of 5 mmol/L RGDS. To further investigate whether the activation of Syk occurs via direct αIIbβ3-mediated signaling or indirect changes of cytoskeleton triggered by αIIbβ3-mediated signaling, we pretreated the cells with 1 or 5 μmol/L cytochalasin D, an actin polymerization inhibitor. Cytochalasin D failed to inhibit the tyrosine phosphorylation of Syk (Fig 3F). These results indicated that Syk is directly activated by the ligation of soluble fibrinogen and does not require subsequent cytoskeletal reorganization.
Binding of soluble fibrinogen to the cells on fibrinogen plate.
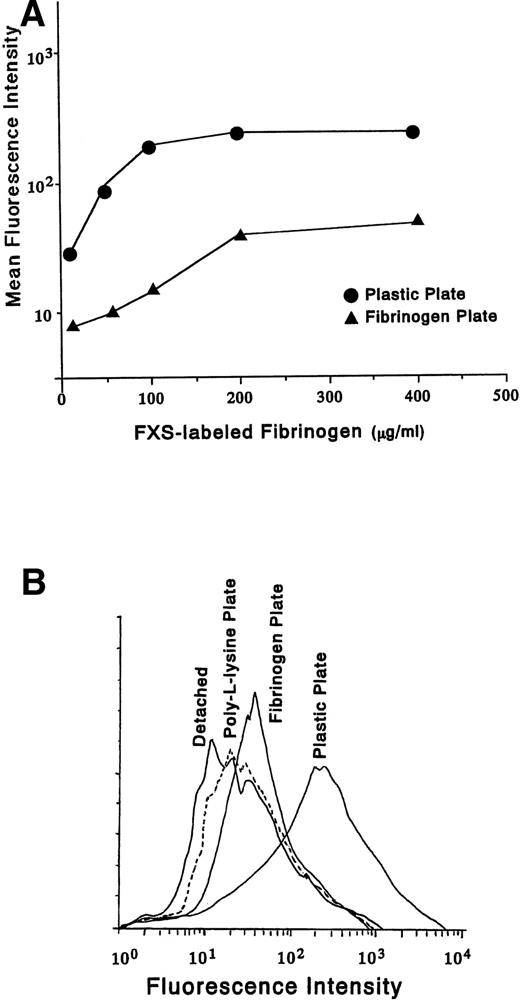
We next examined whether soluble fibrinogen has an ability to bind to the cells on fibrinogen plate. Varying concentrations of FXS-labeled fibrinogen were added to D3-adherent cells (plastic plate) and the cells on fibrinogen plate (Fig 4A). The binding was quantitated as the mean fluorescence intensity (MFI). FXS-labeled fibrinogen showed saturation binding at a concentration of about 100 μg/mL in D3-adherent cells and 200 μg/mL in the cells on fibrinogen plate, respectively. At the saturating concentration, MFI of the cells on fibrinogen plate was reduced to about one sixth as compared with that of D3-adherent cells. The decreased binding of fibrinogen to the cells on fibrinogen plate may be due simply to fewer available receptors for binding. Figure 4B shows the binding of FXS-labeled fibrinogen to D3-detached cells, the cells on poly-L-lysine plate, the cells on fibrinogen plate, and D3-adherent cells (plastic plate). The histogram of the cells on fibrinogen plate was shifted to the right as compared with that of the D3-detached cells or the cells on poly-L-lysine plate, which indicated that soluble fibrinogen can bind to the cells on fibrinogen plate.
Binding of soluble fibrinogen to the cells on fibrinogen plate. (A) FXS-labeled fibrinogen was added to D3-adherent cells and the cells on the fibrinogen plate at the indicated concentrations and the binding was characterized by flow cytometry. The MFIs from the histograms for each concentration of FXS-labeled fibrinogen were plotted graphically. (B) FXS-labeled fibrinogen (200 μg/mL) was added either to the D3-detached cells (Detached), the cells on poly-L-lysine plate (broken line), the cells on fibrinogen plate, or D3-adherent cells (Plastic Plate) for 30 minutes at room temperature. Adherent cells were detached mechanically after the binding reaction and subjected to flow cytometry. In (C) and (D), D3-detached cells were incubated on the fibrinogen plate for the indicated times (0, 15, 30, and 45 minutes). The cell lysates were immunoprecipitated with (C) anti-FAK polyclonal antibody or (D) anti-Syk MoAb, and immunoblotting analysis was performed with antiphosphotyrosine MoAb (4G10). The blots were stripped and reprobed with indicated antibodies. +Soluble Fg. indicates the stimulation with 1 mg/mL soluble fibrinogen for 5 minutes. PL shows the result of plating on poly-L-lysine plate for 30 minutes. Ad+Soluble Fg. and De+Soluble Fg. are the results of 1 mg/mL soluble fibrinogen stimulation on D3-adherent cells and D3-detached cells, respectively. Results are representative of 3 experiments.
Binding of soluble fibrinogen to the cells on fibrinogen plate. (A) FXS-labeled fibrinogen was added to D3-adherent cells and the cells on the fibrinogen plate at the indicated concentrations and the binding was characterized by flow cytometry. The MFIs from the histograms for each concentration of FXS-labeled fibrinogen were plotted graphically. (B) FXS-labeled fibrinogen (200 μg/mL) was added either to the D3-detached cells (Detached), the cells on poly-L-lysine plate (broken line), the cells on fibrinogen plate, or D3-adherent cells (Plastic Plate) for 30 minutes at room temperature. Adherent cells were detached mechanically after the binding reaction and subjected to flow cytometry. In (C) and (D), D3-detached cells were incubated on the fibrinogen plate for the indicated times (0, 15, 30, and 45 minutes). The cell lysates were immunoprecipitated with (C) anti-FAK polyclonal antibody or (D) anti-Syk MoAb, and immunoblotting analysis was performed with antiphosphotyrosine MoAb (4G10). The blots were stripped and reprobed with indicated antibodies. +Soluble Fg. indicates the stimulation with 1 mg/mL soluble fibrinogen for 5 minutes. PL shows the result of plating on poly-L-lysine plate for 30 minutes. Ad+Soluble Fg. and De+Soluble Fg. are the results of 1 mg/mL soluble fibrinogen stimulation on D3-adherent cells and D3-detached cells, respectively. Results are representative of 3 experiments.
Because we found that binding of solid-phase fibrinogen triggered protein tyrosine phosphorylation of D3-detached cells (Fig 3B), we further examined the effect of solid-phase fibrinogen on tyrosine phosphorylation of FAK and Syk. Tyrosine phosphorylation of FAK was promptly induced by adhesion to fibrinogen plate but not by adhesion to poly-L-lysine plate (Fig 4C) and was suppressed by pretreatment with 5 μmol/L cytochalasin D (data not shown). In contrast, Syk was gradually tyrosine-phosphorylated by adhesion to fibrinogen plate and the degree of phosphorylation was less than that for soluble fibrinogen to D3-adherent cells (Fig 4D, 30 v Ad+Soluble Fg.). Therefore, the patterns of tyrosine phosphorylation triggered by soluble and solid-phase fibrinogen were different. The effect of additional binding of soluble fibrinogen to the cells on fibrinogen plate on tyrosine phosphorylation of FAK and Syk was examined next. Tyrosine phosphorylation of FAK was reduced (Fig 4C, 30 v 30+Soluble Fg.), whereas Syk phosphorylation was even more enhanced (Fig 4D, 30v 30+Soluble Fg.) and the phosphorylation level was higher than that of D3-adherent cells (Fig 4D, 30+Soluble Fg. v Ad+Soluble Fg.).
Association of Syk with αIIbβ3 was detected only after the costimulation with soluble and solid-phase fibrinogen.
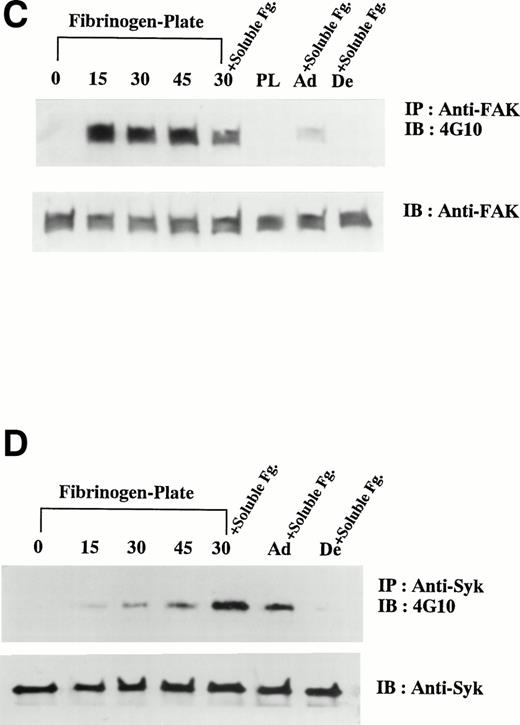
To examine the effects of additional stimulation with soluble fibrinogen on the cells on fibrinogen plate, coimmunoprecipitation assays between αIIbβ3 and Syk were performed. Anti-αIIbβ3 complex MoAb P2 coimmunoprecipitated Syk and anti-Syk MoAb 101 also coimmunoprecipitated integrin αIIb with Syk after the additional binding of soluble fibrinogen (Fig 5A and B). P2 immunoprecipitated both integrin αIIb (Fig 5B) and β3 (data not shown) in 1% Triton X-100 lysis buffer containing EDTA, as previously reported by McGregor et al.32 Because the coimmunoprecipitation between integrin β3 and Syk was not detected using Triton X-100 lysis buffer, we used SDS-containing lysis buffer to obtain nonionic detergent-insoluble fractions. Antiphosphotyrosine MoAb PY20 immunoprecipitated a trace amount of Syk in the cells on fibrinogen plate and then a greater amount on additional stimulation with soluble fibrinogen (Fig 5C). From the same lysates, anti-β3 MoAb coimmunoprecipitated Syk (Fig 5C) and, conversely, anti-Syk antibody coimmunoprecipitated integrin β3 after the additional stimulation with soluble fibrinogen (Fig 5D).
Association of Syk with integrin IIbβ3 after the costimulation with soluble and solid-phase fibrinogen. D3-detached cells (0), the cells on fibrinogen plate incubated for 30 minutes (30), and the cells on fibrinogen plate after the additional stimulation with 1 mg/mL soluble fibrinogen for 5 minutes (30+Soluble Fg.) were lysed and immunoprecipitated with indicated antibodies. In (A) and (B), the cells were lysed in 1% Triton X-100 lysis buffer. The immunoblotting analysis for each immunoprecipitate was performed with (A) anti-Syk polyclonal antibody or (B) anti-integrin IIb MoAb. In (C) and (D), the cells were lysed in 0.05% SDS-containing buffer. The immunoblotting analysis for each immunoprecipitate was performed with (C) anti-Syk polyclonal antibody or (D) anti-integrin β3 MoAb. Results are representative of 3 experiments.
Association of Syk with integrin IIbβ3 after the costimulation with soluble and solid-phase fibrinogen. D3-detached cells (0), the cells on fibrinogen plate incubated for 30 minutes (30), and the cells on fibrinogen plate after the additional stimulation with 1 mg/mL soluble fibrinogen for 5 minutes (30+Soluble Fg.) were lysed and immunoprecipitated with indicated antibodies. In (A) and (B), the cells were lysed in 1% Triton X-100 lysis buffer. The immunoblotting analysis for each immunoprecipitate was performed with (A) anti-Syk polyclonal antibody or (B) anti-integrin IIb MoAb. In (C) and (D), the cells were lysed in 0.05% SDS-containing buffer. The immunoblotting analysis for each immunoprecipitate was performed with (C) anti-Syk polyclonal antibody or (D) anti-integrin β3 MoAb. Results are representative of 3 experiments.
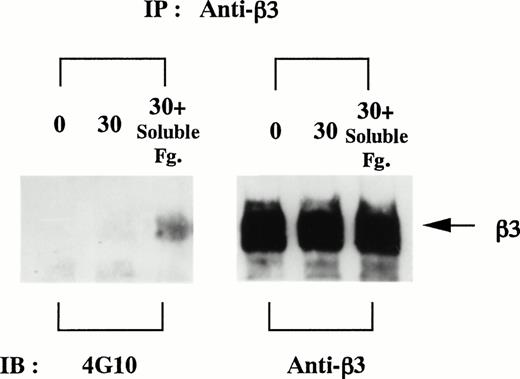
Tyrosine phosphorylation of integrin β3 after the costimulation with soluble and solid-phase fibrinogen.
Between the cytoplasmic tails of integrin αIIb and β3, only β3 contains 2 tyrosine residues. Tyrosine phosphorylaton of integrin β3 was detected only on additional stimulation with soluble fibrinogen on the cells on fibrinogen plate by the use of SDS-containing lysis buffer (Fig 6).
Integrin β3 is tyrosine-phosphorylated after the costimulation with soluble and solid-phase fibrinogen. D3-detached cells (0), the cells on fibrinogen plate incubated for 30 minutes (30), and the cells on fibrinogen plate after the additional stimulation with 1 mg/mL soluble fibrinogen for 5 minutes (30+Soluble Fg.) were lysed in 0.05% SDS-containing buffer and immunoprecipitated with anti-integrin β3 MoAb. Aliquots of each condition were equally divided and electrophoresed on the same gel in duplicate. Immunoblotting analyses were performed as described in the Materials and Methods. Results are representative of 3 experiments.
Integrin β3 is tyrosine-phosphorylated after the costimulation with soluble and solid-phase fibrinogen. D3-detached cells (0), the cells on fibrinogen plate incubated for 30 minutes (30), and the cells on fibrinogen plate after the additional stimulation with 1 mg/mL soluble fibrinogen for 5 minutes (30+Soluble Fg.) were lysed in 0.05% SDS-containing buffer and immunoprecipitated with anti-integrin β3 MoAb. Aliquots of each condition were equally divided and electrophoresed on the same gel in duplicate. Immunoblotting analyses were performed as described in the Materials and Methods. Results are representative of 3 experiments.
DISCUSSION
CMK cells are known to undergo megakaryocytic differentiation after TPA treatment. During this process, cell growth is arrested, the cells become adherent to the plastic plate, and integrin αIIbβ3 is strongly expressed. In the present study, we examined the function and the outside-in signaling pathways of the newly expressed αIIbβ3 in relation to cell adhesion. CMK cells are reported to express several other integrins such as VLA-4,37 and these molecules might be related to cell adhesion under FCS-containing conditions. In this study, we focused on the relation of αIIbβ3-mediated signaling with cell adhesion and came to the following conclusions: (1) binding of soluble fibrinogen to αIIbβ3 expressed on the surface of TPA-treated CMK cells depends on cell adhesion; (2) the same ligand, fibrinogen, present either in soluble or solid-phase form, triggers differential signaling pathways mediated by αIIbβ3; and (3) as a result of the cooperation of both soluble and solid-phase fibrinogen-mediated signaling pathways, integrin β3 is tyrosine-phosphorylated and forms a complex with Syk.
In platelets, agonist-initiated signals convert αIIbβ3 from a resting form to an activated form by increasing its affinity for ligands such as fibrinogen. In CMK cells, soluble fibrinogen binds to αIIbβ3 on the surface of D3-adherent cells without any other stimulations but not on the surface of D3-detached cells. Because TPA is removed, its direct effect might be excluded. One possibility is that newly expressed αIIbβ3 works as an activated form, a functional receptor for soluble fibrinogen, in the adhesion process of megakaryocytic differentiation after TPA treatment. In fact, activation-dependent anti-αIIbβ3 antibody, PAC1, bound preferably to adherent cells. The elevated tyrosine phosphorylation observed in TPA-treated cells may lead to increased αIIbβ3 affinity. Cell detaching resulted in decreased tyrosine phosphorylation of FAK and some other proteins concomitant with the decrease in the affinity of αIIbβ3 for soluble fibrinogen. In contrast, D3-detached cells promptly adhered to fibrinogen plate, indicating binding of solid-phase fibrinogen. These results are consistent with the report that solid-phase but not soluble fibrinogen binds to αIIbβ3 in the resting form in platelets.4
Outside-in signaling triggered by soluble fibrinogen is different in several respects from that of solid-phase fibrinogen, even if the same integrin is mediated. The ligation of soluble fibrinogen to D3-adherent cells resulted in reduced cell spreading and increase in tyrosine phosphorylation of Syk and several proteins, whereas that of some other proteins, including FAK, was decreased. To characterize the mechanism of Syk activation, 2 types of inhibitors, cytochalasin D, an actin polymerization inhibitor and cyclic-KGD peptide, a specific inhibitor of αIIbβ3 were used (Fig 3E and F). Syk appeared to be directly activated and tyrosine-phosphorylated by the ligation of soluble fibrinogen, and the activation occurred at the upstream of the cytoskeletal reorganization. On the other hand, the ligation of solid-phase fibrinogen, namely adhesion to fibrinogen plate, brought about cell spreading and prompt tyrosine phosphorylation of FAK. As for Syk, the ligation of solid-phase fibrinogen caused delayed and faint tyrosine phosphorylation as compared with the result of soluble fibrinogen (Fig 4D). It was also reported that platelet adhesion to fibrinogen triggers only faint Syk tyrosine phosphorylation.38 Postbinding events such as the reorganization of cytoskeletal proteins or phosphorylation of other kinases might be needed for Syk activaton in the case of solid-phase fibrinogen. Thus, there may exist different signaling pathways mediated by αIIbβ3, although it is engaged with the same ligand, fibrinogen, present either in soluble or solid-phase form. This hypothesis is consistent with the results of previous reports by Pelletier et al16 and Defilippi et al.17 They showed that different ligands trigger different actin organization and tyrosine phosphorylation of FAK using a panel of anti-αIIbβ3 antibodies. In the present study, we also propose the presence of different mechanisms involving tyrosine phosphorylation of Syk triggered by soluble or solid-phase fibrinogen.
The binding of soluble fibrinogen to the cells adherent to solid-phase fibrinogen gave rise to more enhanced tyrosine phosphorylation of Syk than the binding to D3-adherent cells (Fig 4D). In addition, costimulation of both forms of fibrinogen enabled us to detect coprecipitation of integrin β3 with Syk and tyrosine phosphorylation of β3, which were not detected by the stimulation with a single form of fibrinogen. These results were obtained only with SDS-containing lysis buffer and not with nonionic detergent lysis buffer. The lysis in SDS may have protected the phosphorylated β3 protein from dephosphorylation, as Law et al13 indicated. Furthermore, these molecules may be associated with cytoskeleton, but exactly how integrin β3 and Syk exist in a complex form is unknown.
For β1 integrin, Miyamoto et al2 reported that both integrin clustering and occupancy accumulate a number of cytoskeletal proteins and signaling molecules, including tyrosine kinases, in adhesion complexes. We address the possibility that similar accumulation induced by adhesion to solid-phase fibrinogen mediates more effective signaling on additional stimulation with soluble fibrinogen. In platelets, Law et al13 showed that integrin β3 is tyrosine-phosphorylated after aggregation and some Src family kinases and Syk have the ability to phosphorylate β3 in vitro. Although they failed to detect an association of Syk with β3 in vivo or in vitro, Syk might be a candidate for tyrosine kinase phosphorylating β3. In this study, we suggest that β3 is tyrosine-phosphorylated as a result of outside-in signaling triggered by both soluble and solid-phase fibrinogen and that this phosphorylation leads to a stable complex containing Syk and cytoskeletal molecules.
Finally, we propose that costimulation of soluble and solid-phase fibrinogen on αIIbβ3 is related to megakaryocytic maturation and function, because this integrin is expressed exclusively in platelet-megakaryocyte lineage cells. Zauli et al7 showed the effect of thrombopoietin on αIIbβ3-dependent adhesion of magakaryocytic cells. Handagama et al6 reported that αIIbβ3 in mature megakaryocytes is the receptor of fibrinogen for endocytosis. In the process of megakaryocytic differentiation, cells come to express αIIbβ3 and adhere to extracellular matrix proteins containing the RGD motif through αIIbβ3 and other integrins. Further studies are needed to evaluate the signaling mechanisms of integrins in the process of cell adhesion and megakaryocytic differentiation.
ACKNOWLEDGMENT
The authors thank Dr Robert M. Scarborough (COR Therapeutics Inc) for providing a cyclic peptide, Dr Ryukichi Ryo (Kobe University, Kobe, Japan) for discussions, and Yukie Tanaka for technical support.
Supported in part by Grants-in-Aid from the Ministry of Education, Science and Culture of Japan for General Scientific Research and Scientific Research on Priority Areas, by the Yamanouchi Foundation for Research on Metabolic Disorders, and in part by Fukui Prefectural Universities Research Foundation for the Promotion of Sciences through Contract.
Address reprint requests to Hirohei Yamamura, MD, Department of Biochemistry, Kobe University School of Medicine, Chuo-ku, Kobe 650, Japan; e-mail: yamamura@kobe-u.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal