Abstract
In preliminary studies, the generation of thrombin in vivo was found to induce a 92% loss of functional activity of factor IX (F.IX) despite the detection by Western blotting of a product resembling activated F.IX (F.IXa) and a 25% increase in F.IX antigen levels (Hoogendoorn et al, Thromb Haemost 69:1127, 1993 [abstr]). These changes were associated with evidence of increased elastase availability. To study the possibility that these two observations were related, a detailed physical and functional characterization of the hydrolysis of purified human F.IX by human neutrophil elastase (HNE) was performed in vitro. An activated partial thromboplastin time (aPTT) clotting assay demonstrated that, although HNE eliminated the potential of F.IX to be activated, it only marginally reduced the F.IXa activity. Reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) indicated that HNE treatment of F.IX generated cleavage products of 30 and 20 kD that could not be distinguished from the respective heavy and light chain peptides that were identified in parallel studies when F.IX was activated by activated bovine F.XI (F.XIa), one of its physiological activators. In addition, nonreducing SDS-PAGE demonstrated that HNE-treated F.IX formed no complexes with antithrombin III (ATIII) in the presence of heparin. Furthermore, HNE-treated F.IX was unable to (1) bind the active site probe p-aminobenzamidine; (2) hydrolyze the synthetic peptide substrate CH3SO2-Leu-Gly-Arg-p-nitroanilide; and (3) activate human factor X (F.X). In contrast to dansyl-Glu-Gly-Arg-chloromethyl ketone (dEGR)-inactivated F.IXa, HNE-treated F.IX (0.01 to 10,000 pmol/L) failed to inhibit the clotting activity of F.IXa (10 pmol/L) in the aPTT. NH2-terminal sequencing indicated that HNE cleaved human F.IX at Thr140, Thr144, Ile164, Thr172, and Val181. The cleavages at Thr140/Thr144 and at Thr172/Val181 are both very close to the normal F.XIa -(Arg145) and β-(Arg180) cleavage sites, respectively. In summary, the results suggest that the activatability of F.IX is eliminated after cleavage by HNE and that the inability of HNE-treated F.IX to support F.IXa-like coagulant function is a consequence of improper active site formation. These in vitro observations support the possibility that increased HNE cleavage of F.IX in vivo may contribute to the disregulation of hemostasis that occurs in conditions such as disseminated intravascular coagulation (DIC).
© 1998 by The American Society of Hematology.
THE COAGULATION CASCADE consists of a series of proteolytic reactions in which blood coagulation enzymes are formed from their inactive zymogens.1 The generation of the terminal enzyme, thrombin, mediates the conversion of fibrinogen to fibrin, which in turn provides the essential reinforcement for the unstable primary platelet hemostatic plug. Thrombin has ubiquitous roles in hemostasis as well as other biological systems.2The infusion of a combination of two components of the prothrombinase complex, activated factor X (F.Xa) and a negatively charged phospholipid surface provided by phosphatidylcholine/phosphatidylserine (PCPS) vesicles, induces a substantial conversion of prothrombin to thrombin in vivo in a number of different animal species, including nonhuman primates. This model has been used to study the coagulant and fibrinolytic responses that occur consequent to acute prothrombin activation in vivo.3-5 At relatively high levels of F.Xa and PCPS, significant disregulation of hemostasis may occur and the clinical and laboratory responses observed demonstrate a remarkable similarity to those seen in the human condition, disseminated intravascular coagulation (DIC).6 During a detailed evaluation of the changes in individual coagulation factor concentrations and functions in this model, a major reduction in the functional activity of factor IX (F.IX) was observed. In contrast, a quantitative immunological assay suggested that this was due to a qualitative change rather than consumption. Immediately after thrombin generation, a lower molecular mass F.IX species was identified by F.IX Western blotting, suggesting the generation of its normal activated product (F.IXa).7 However, these findings were surprising given that F.IXa would have been expected to form a higher apparent molecular mass complex with antithrombin-III (ATIII) in a plasma milieu.8 This finding suggested that perhaps F.IXa had been generated and subsequently degraded to an inactive protease. Alternatively, a protease other than its physiological activators F.XIa or F.VIIa and tissue factor may have been responsible for generating a product that, although physically similar to F.IXa by electrophoretic and Western blotting characteristics, was devoid of coagulant function. Takaki et al9 have previously demonstrated that human neutrophil elastase (HNE) can hydrolyze purified human F.IX in vitro to produce a product with this profile. In our model, increased levels of elastase/α1-proteinase inhibitor complexes and elastase-specific fibrinogen degradation products were observed immediately after the generation of thrombin in vivo, suggesting increased elastase availability. Moreover, although a coincidental association could not be excluded, a profound decrease in the circulating leukocyte count occurred at this time, suggesting that neutrophil activation and/or lysis could provide the source of this protease.7
Although Takaki et al9 demonstrated the generation of a functionally impaired but structurally similar HNE-derived cleavage product of F.IX, the specific cleavage sites were not documented. F.IX is a single-chain plasma glycoprotein (molecular weight [Mr] 57,000), the amino acid sequence of which is known.10 F.IX is activated in a two-step process by F.XIa. Initial cleavage at the α-site (Arg145-Ala146) produces a two-chain inactive intermediate of 46 kD (F.IX-α). Cleavage at the β-site (Arg180-Val181) provides the fully active F.IXa-β. The former cleavage occurs rapidly, with the latter being slower and rate limiting.11 The active enzyme consists of an N-terminal 18-kD light chain (residues 1-145) disulphide bonded to a C-terminal 28 kD (residues 181-415) heavy chain.12 Cleavage at Arg145-Ala146 appears to be of importance with regard to the binding interaction between F.IX and its essential cofactor, factor VIIIa (F.VIIIa),13,14 whereas cleavage at Arg180-Val181 appears to be important for the formation of the catalytic pocket surrounding the active site serine (Ser365).15 In the following studies, we have characterized the physical and functional changes that are observed after the hydrolysis of human F.IX by HNE in vitro. The findings demonstrate that, although the electrophoretic characteristics of the HNE cleavage products of F.IX observed are remarkably similar to those after treatment with F.XIa, major functional differences result.
MATERIALS AND METHODS
Materials
All reagents used were of analytical grade. Human F.IX and F.X were purified from fresh frozen plasma according to Bajaj et al.16 Bovine F.XIa (F.XIa) was obtained from Enzyme Research Laboratories Inc (South Bend, IN). Human F.IX (4 μmol/L) was activated to F.IXa by incubation with F.XIa at 500 nmol/L for 2 hours at 37°C according to Lenting et al.13Phosphatidylcholine/phosphatidylserine (PCPS) vesicles (75%:25% wt/wt, respectively) were prepared according to Barenholz et al.17 Bovine serum albumin (BSA; RIA grade, fraction V), porcine intestinal mucosa heparin (grade I), N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide (Succ-AAPF-pNA), N-methoxy-succinyl-Ala-Ala-Pro-Val-p-nitroanilide (MeSucc-AAPV-pNA), rabbit brain cephalin, p-aminobenzamidine (pAB), and Coomassie Blue R 250 were obtained from Sigma Chemical Co (St Louis, MO). CH3SO2-D-Leu-Gly-Arg-p-nitroanilide (CH3SO2-LGR-pNA) was from Diagnostica Stago (Asnières, France). The F.Xa peptide substrate, S-2222 was from DiaPharma Group, Inc (Franklin, OH). Polyethylene glycol 8000 was from VWR Scientific (Mississauga, Ontario, Canada). β-mercaptoethanol (βME) was obtained from Fisher Scientific (Fair Lawn, NJ). Recombinant human ATIII was a generous gift from Genentech (San Bruno, CA). This was purified further before use by heparin-sepharose chromatography.18 Purified HNE was obtained from Biodesign International (Kennebunk, ME). Purity of greater than 97% was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and densitometry. It exhibited negligible activity against the cathepsin G substrate, Succ-AAPF-pNA, whereas its activity against the elastase substrate MeSucc-AAPV-pNA was completely inhibited by either an elastase-specific monoclonal antibody (Pel Freeze, Rogers, AR) or Methoxy-succinyl-Ala-Ala-Pro-Val chloromethyl ketone (AAPV-CMK; Enzyme Systems Products, Livermore, CA). 1,5 dansyl-glutamyl-glycyl-arginine-chloromethylketone dihydrochloride (dEGR) was obtained from Calbiochem (San Diego, CA).
Methods
Proteolysis of F.IX or F.IXa by HNE.
Purified F.IX or F.IXa (4.0 μmol/L of each) was incubated at 37°C with HNE (400 nmol/L) in 50 mmol/L HEPES/0.15 mol/L NaCl, pH 7.4 (HBS), containing 5.0 mmol/L CaCl2 (HBS/Ca). At varying time intervals of incubation, two aliquots were withdrawn from the same reaction mixture. One was added to an equal volume of SDS solubilizing solution and heated for 2 minutes at 90°C for SDS-PAGE (see below). The other was added to AAPV-CMK (10 μmol/L final concentration) in HBS and incubated on ice for 10 minutes for subsequent assay of F.IX clotting activity (see below). Control incubations indicated that 10 μmol/L AAPV-CMK completely inactivated the HNE under these conditions, but it had no effect on the F.IX/IXa clotting activity at the concentrations of F.IX/IXa assayed (see below).
Factor IX clotting assay.
An activated partial thromboplastin time (aPTT) clotting assay was performed with a Coag-a-mate XC-Plus (Organon Teknika Inc, Scarborough, Ontario, Canada) and F.IX-deficient human plasma (Biopool, Burlington, Ontario, Canada), as previously described.19 Normal human pooled plasma was used as the standard, assigning 1 unit of F.IX activity per milliliter of plasma.
SDS-PAGE.
SDS-PAGE was performed according to the method of Neville.20 Samples for SDS-PAGE were added to an equal volume of 50 mmol/L Tris-HCl (pH 6.14)/1.0% SDS/10.0% (wt/vol) glycerol/0.1 mg/mL bromophenol blue either with (reducing) or without (nonreducing) 2.0% βME added (SDS solubilizing solution). These samples were then heated at 90°C for 2 minutes and approximately 4.0 μg of F.IX protein was loaded per lane using 1.5 mm 5% to 15% or 5% to 20% linear polyacrylamide gradient SDS gels. The cleavage products were visualized after staining overnight with 0.0016% Coomassie Blue in 5% acetic acid and 7.5% ethanol. The gels were destained for 1 hour in 18% methanol and 9% acetic acid and then soaked in 15% methanol and 5% glycerol for 1 hour. The gels were then photographed and dried between two sheets of BioGel Wrap (Biodesign, Carmel, NY) overnight at 37°C.
NH2-terminal sequencing of the cleavage products of F.IX after HNE hydrolysis.
F.IX (4 μmol/L) was incubated with HNE (400 nmol/L) for 2 and 30 minutes in HBS/Ca at 37°C in a final volume of 887 μL as outlined above. The two digests were quenched by adding AAPV-CMK (10 μmol/L final concentration) and incubation on ice for 10 minutes. The samples were then separately dialyzed versus 2 L of 0.2 N acetic acid at 4°C for 5 to 6 hours with one change. The samples were then frozen at −70°C for 1 hour and lyophilized to dryness. The samples were then electrophoresed using a 1.5-mm 5% to 15% linear polyacrylamide gradient SDS gel employing the Neville system20 under reducing conditions. Electrophoretic transfer of the cleavage products onto an Immobilon-P membrane (Millipore, Bedford, MA) was performed using a Trans Blot apparatus (Hoeffer Scientific, San Francisco, CA) for 2 hours at 500 mA in 200 mmol/L glycine/25 mmol/L Tris/0.1% SDS/20% methanol, pH 8.3. After transfer, the membrane was stained for 5 minutes with 0.25% Coomassie Blue in 50% methanol and 10% acetic acid, destained in 50% methanol and 10% acetic acid (2 times for 5 minutes), rinsed with distilled water (2 times for 5 minutes), air dried overnight, and stored at −20°C. The NH2-terminal sequencing was performed by Dr Teng Song Chen at the Biotechnology Service Centre at the Hospital for Sick Children (Toronto, Ontario, Canada). Amino acid sequences of the specific F.IX fragments generated after F.IX cleavage by HNE were determined directly from the Coomassie Blue-stained blot by automated Edman degradation using a Porton Gas-Phase Microsequencer (Model 2090; Tarzana, CA) with on-line phenylthiohydantoin (PTH) analysis. The PTH-amino acids released at each cycle were determined by high-performance liquid chromatography (HPLC) analysis after comparison of retention times to those of PTH-amino acid standards excluding cysteine.
Binding of pAB to F.IX cleaved with F.XIa or HNE.
The pAB binding studies were performed according to a modification of the method described by Lin et al.21 Briefly, F.IX (4 μmol/L) was incubated with F.XIa (500 nmol/L) or HNE (400 nmol/L) in the presence of 4 μmol/L pAB in HBS, pH 7.4 containing 0.5% (wt/vol) polyethylene glycol 8000 and 5 mmol/L CaCl2. The reactions (250 μL) were performed in a 0.3 mL quartz cuvette incubated at 37°C with a Perkin Elmer LS50B Luminescence Spectrometer (Perkin Elmer, Beaconsfield, UK), using excitation and emission wavelengths of 336 and 376 nm, respectively, and a 350 nm cutoff filter in the emission beam. Aliquots of the reaction mixtures each containing 4 μg of F.IX protein were removed after 0, 0.5, 2, 5, 10, 20, 40, and 60 minutes of incubation with F.XIa or HNE and subsequently analyzed by reducing SDS-PAGE. The results are presented after subtracting the fluorescence values for control reactions containing either F.XIa or HNE but without added F.IX.
Cleavage of CH3SO2-LGR-pNA peptide.
F.IX (4.0 μmol/L) was incubated in HBS/Ca at 37°C either alone for 30 minutes (control) or with 400 nmol/L HNE for 30 minutes or with 500 nmol/L F.XIa for 2 hours. The HNE was inactivated by addition of AAPV-CMK (10 μmol/L final concentration) and incubation on ice for 10 minutes. Hydrolysis of CH3SO2-LGR-pNA by the different F.IX samples was assayed in 0.2% (wt/vol) BSA, 0.1 mol/L NaCl, 0.01 mol/L CaCl2, 0.05 mol/L HEPES, pH 8.4. Peptide cleavage was initiated by the addition of 50 μL of 2 mmol/L CH3SO2-LGR-pNA peptide to 50 μL of F.IX sample in a 96-well flat-bottom plate (Dynatech Immulon, VWR Scientific, Mississauga, Ontario, Canada). The initial rates of p-nitroaniline (pNA) produced were determined using different F.IX concentrations (0.125 to 1.0 μmol/L) at 405 nm and 37°C using a SpectroMax 250 Plate Reader (Molecular Devices, Sunnyvale, CA). The molar concentration of pNA generated over time was determined using a path length of 0.30 cm for 100 μL volume and a molar extinction coefficient of 9.65 × 103 mol/L−1cm−1 for pNA.22 The initial rates of pNA production (in micromoles per liter per minute) are expressed after the subtraction of absorbance at 405 nm for reactions performed in the absence of added F.IX (F.IX or HNE-treated F.IX reactions) or in the presence of F.XIa but without added F.IX (F.IXa reaction).
Activation of human F.X.
The activation of human F.X by F.IX, HNE-treated F.IX, or F.IXa (see above under Cleavage of CH3SO2-LGR-pNA Peptide for production of the different F.IX samples) was determined by monitoring the cleavage of the F.Xa chromogenic substrate S-2222 at 405 nm in a 96-well microplate using a SpectroMax 250 Plate Reader at 37°C (see above). The reactions (200 μL) contained 25 or 250 nmol/L F.IX sample, 600 nmol/L F.X, 30 μmol/L PCPS vesicles, 0.4 mmol/L S-2222, and 10 mmol/L CaCl2 in HBS, pH 7.4. The reaction rates were determined according to the equation: A405 = at2 + V 0 t, where a is the acceleration rate, A405 is the absorbance at 405 nm minus the value at time = 0, and V0 is the rate of S2222 hydrolysis in the absence of F.X.23 The acceleration rates were determined by nonlinear regression of the data using SYSTAT (Evanston, IL).
Effect of HNE on the interaction of F.IX and F.IXa with ATIII.
F.IX (4.0 μmol/L) was incubated in HBS/Ca at 37°C either alone for 30 minutes (F.IX control) or with 400 nmol/L HNE for 30 minutes (F.IXe) or with 500 nmol/L F.XIa for 2 hours (F.IXa). The HNE was inactivated by addition of AAPV-CMK (10 μmol/L final concentration) and incubation on ice for 10 minutes. In some instances, F.IXa and F.IXe (4 μmol/L of each) were further incubated at 37°C with HNE (400 nmol/L for 30 minutes, F.IXa/e) and F.XIa (500 nmol/L for 2 hours, F.IXe/a), respectively. The HNE was inactivated as described above. The F.IX samples were then either diluted HBS/BSA for the aPTT clotting assay or prepared directly for nonreducing SDS-PAGE or further incubated with ATIII (5 μmol/L) and heparin (2.0 mg/mL) for 30 minutes at 37°C, after which they were prepared for nonreducing SDS-PAGE. The gels were stained with 0.0016% Coomassie Blue overnight before being photographed and then dried between two sheets of BioGel Wrap (Biodesign) at 37°C.
Effect of HNE-treated F.IX or dEGR-F.IXa on the aPTT clotting activity of F.IXa.
F.IX (4 μmol/L) was incubated at 37°C in HBS/Ca either with 400 nmol/L HNE for 30 minutes (F.IXe) or with 500 nmol/L F.XIa for 2 hours (F.IXa). The HNE was inactivated after the addition of AAPV-CMK to 10 μmol/L (final concentration) and incubation on ice for 10 minutes. A portion of the F.IXa reaction was treated with a fivefold molar excess of dEGR-CMK (20 μmol/L) for 20 minutes at 20°C to generate dEGR-F.IXa followed by further incubation for 30 minutes at 37°C to inactivate any unreacted inhibitor. Control reactions indicated that incubation of 20 μmol/L dEGR-CMK alone in HBS/Ca under the above conditions had no effect compared with HBS/BSA alone in the aPTT clotting assay at the concentrations of F.IXe/dEGR-F.IXa assayed. The different samples were then assayed over 0.01 to 10,000 pmol/L in the aPTT clotting assay with a fixed amount of F.IXa (10 pmol/L). The results are presented as log-log plots of clot time (in seconds) versus competitor concentration (in picomoles per liter).
All studies except for NH2-terminal sequencing were performed on at least three separate occasions and, unless otherwise stated, the results presented are representative of those obtained in each case.
RESULTS
The Effect of HNE on the aPTT Procoagulant Activity of F.IX or F.IXa
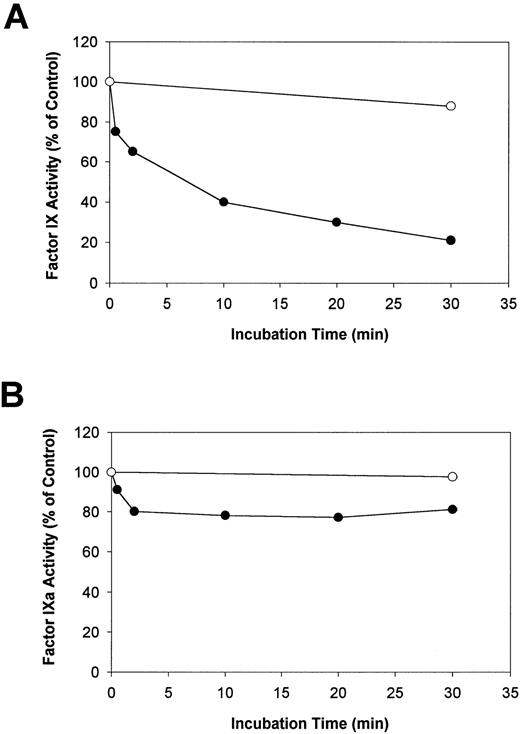
Human F.IX or F.IXa was incubated with HNE in the presence of Ca2+, and aliquots were removed after various time intervals to measure the resultant procoagulant activity using an aPTT clotting assay. In addition, other aliquots were removed simultaneously from the same reaction mixtures for analysis by SDS-PAGE under reducing conditions (see below). Preliminary experiments indicated that in the presence of HBS, pH 7.4, and 5.0 mmol/L Ca2+ (HBS/Ca), the addition of 5 or 50 nmol/L HNE to F.IX (4 μmol/L) decreased the resultant F.IX-aPTT clotting activity from 100% at zero time to 82% or 76%, respectively, after 30 minutes at 37°C. Therefore, because HNE predominately inactivated the F.IX clotting activity, the [HNE] was raised to 400 nmol/L to increase the reaction rate. The effect of HNE on the aPTT clotting activity of F.IX and F.IXa under these conditions is shown in Fig 1A and B, respectively. In reactions containing F.IX (4 μmol/L) in HBS/Ca, the addition of 400 nmol/L HNE decreased the aPTT clotting activity of F.IX from 100% to 40% after 10 minutes and to 20% after 30 minutes. The F.IX-aPTT clotting activity in the absence of HNE was 88% of the initial level after 30 minutes at 37°C (Fig 1A).
The effect of HNE on F.IX and F.IXa as determined by aPTT clotting assay. Purified F.IX or F.IXa (4 μmol/L of each) was incubated either alone (control) or with HNE (400 nmol/L) in HBS/Ca at 37°C. After various times, two simultaneous aliquots of the same reaction mixtures were withdrawn and assayed for either aPTT clotting activity after the addition of 10 μmol/L AAPV-CMK (A and B) or analyzed by reducing SDS-PAGE (see Fig 2 below). The relative F.IX and F.IXa aPTT clotting activity (normalized to the time = 0 sample in each case) versus incubation time with (•) or without (○) added HNE is plotted in (A) and (B), respectively.
The effect of HNE on F.IX and F.IXa as determined by aPTT clotting assay. Purified F.IX or F.IXa (4 μmol/L of each) was incubated either alone (control) or with HNE (400 nmol/L) in HBS/Ca at 37°C. After various times, two simultaneous aliquots of the same reaction mixtures were withdrawn and assayed for either aPTT clotting activity after the addition of 10 μmol/L AAPV-CMK (A and B) or analyzed by reducing SDS-PAGE (see Fig 2 below). The relative F.IX and F.IXa aPTT clotting activity (normalized to the time = 0 sample in each case) versus incubation time with (•) or without (○) added HNE is plotted in (A) and (B), respectively.
In similar experiments, 400 nmol/L HNE decreased the aPTT clotting activity of F.IXa only marginally (Fig 1B). Incubation of F.IXa (4 μmol/L) in HBS/Ca with 400 nmol/L HNE resulted in a marginal (10% to 20%) decrease in the F.IXa-aPTT clotting activity from the initial level over the first 0.5 to 2 minutes (Fig 1B). Thereafter, the clotting activity of F.IXa remained unchanged such that approximately 80% of the initial activity remained after 30 minutes. The aPTT clotting activity of F.IXa in the absence of HNE after 30 minutes was 98% of the initial activity.
Reducing SDS-PAGE Analysis of HNE Cleavage of F.IX or F.IXa
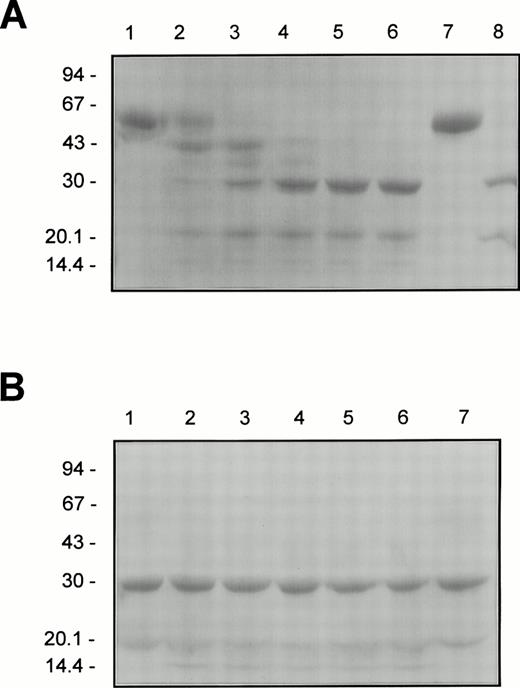
To study directly the HNE cleavage of F.IX and F.IXa in relation to the changes in their aPTT clotting activities, electrophoretic analysis was conducted under denaturing and reducing conditions using the same reaction mixtures and times of sampling that were used for the aPTT clotting assays (see Fig 1, above). The results for F.IX or F.IXa (4 μmol/L of each) incubated with HNE (400 nmol/L) in HBS/Ca are illustrated in Fig 2A and B, respectively. The initial HNE-dependent decrease in the F.IX clotting activity at 0.5 and 2.0 minutes correlated with the cleavage of intact F.IX and the generation of at least 5 protein fragments with apparent molecular masses of 44, 34, 30, 20, and 17 kD (Fig 2A, lanes 1 through 3). Subsequently, over the next 28 minutes, during which time HNE had the greatest inactivation effect on the F.IX-aPTT clotting activity, the 44- and 34-kD protein fragments became progressively converted into three more stable protein products with apparent molecular masses of 30, 20, and 17 kD (Fig 2A, lanes 4 through 6). The generation of these three stable protein products of F.IX upon incubation with HNE correlated very well with the decrease in the F.IX clotting activity as determined by densitometry of the Coomassie Blue-stained gel (results not shown). Interestingly, the 30- and 20-kD protein fragments of F.IX generated by HNE cleavage comigrated with the heavy and light chains, respectively, generated upon activating F.IX with F.XIa (compare Fig2A, lanes 4 through 6 with lane 8).
The cleavage pattern of F.IX and F.IXa treated with HNE as determined by reducing SDS-PAGE. Purified F.IX or F.IXa (4 μmol/L of each) was incubated with HNE (400 nmol/L) as described in the legend to Fig 1. Aliquots (4 μg protein) of F.IX (A) or F.IXa (B) were removed into reducing SDS sample after 0, 0.5, 2, 10, 20, and 30 minutes of incubation at 37°C with HNE (lanes 1 through 6, respectively), heated for 2 minutes at 90°C, electrophoresed in a 5% to 15% linear polyacrylamide gradient SDS gel according to Neville,20 and stained with Coomassie Blue. F.IX and F.IXa (A, lanes 7 and 8, respectively) or F.IXa (B, lane 7) incubated under the conditions given above for 30 minutes at 37°C but in the absence of added HNE is also shown. The positions of the molecular weight markers (in kilodaltons) are indicated to the left of each panel.
The cleavage pattern of F.IX and F.IXa treated with HNE as determined by reducing SDS-PAGE. Purified F.IX or F.IXa (4 μmol/L of each) was incubated with HNE (400 nmol/L) as described in the legend to Fig 1. Aliquots (4 μg protein) of F.IX (A) or F.IXa (B) were removed into reducing SDS sample after 0, 0.5, 2, 10, 20, and 30 minutes of incubation at 37°C with HNE (lanes 1 through 6, respectively), heated for 2 minutes at 90°C, electrophoresed in a 5% to 15% linear polyacrylamide gradient SDS gel according to Neville,20 and stained with Coomassie Blue. F.IX and F.IXa (A, lanes 7 and 8, respectively) or F.IXa (B, lane 7) incubated under the conditions given above for 30 minutes at 37°C but in the absence of added HNE is also shown. The positions of the molecular weight markers (in kilodaltons) are indicated to the left of each panel.
The 10% to 20% decrease in the aPTT clotting activity of F.IXa after incubation with HNE for 0.5 to 2 minutes coincided with the production of a protein fragment with apparent molecular mass of approximately 17 kD (Fig 2B, lanes 1 through 3). Over the next 28 minutes of incubation, the initial 20% decrease in the clotting activity of F.IXa before achieving more stable values (Fig 1B) correlated well with unchanged levels of the three protein species with apparent molecular masses of 30, 20, and 17 kD (Fig 2B, lanes 4 through 6). As judged by densitometry, the staining intensities of these three protein species remained unchanged from samples taken during the 28-minute time interval between 2 and 30 minutes of incubation with HNE (results not shown).
NH2-Terminal Sequencing of F.IX After Cleavage With HNE
The results of the NH2-terminal sequencing of the products of human F.IX after cleavage with HNE are shown in Table 1 and a schematic diagram of the position of the HNE cleavage sites within the F.IX molecule is shown in Fig 3. After comparison of the NH2-terminal amino acid sequences determined in this study to the previously reported amino acid sequence for human F.IX,10 the data indicate that HNE cleaves human F.IX at least at five positions: Thr140, Thr144, Ile164, Thr172, and Val181 (Table1). There are at least two more HNE cleavage sites within the extreme C-terminal 100 amino acids of the heavy chain of F.IX; however, unambiguous NH2-terminal amino acid sequences were only observed for the five proteolytic products of apparent molecular mass of 44, 34, 30, 20, and 17 kD in determination of the HNE cleavage sites listed above.
NH2-Terminal Sequence of Human F. IX-Derived Fragments After HNE Digestion
| Cycle No. . | 44 kD-150 . | 34 kD . | 30 kD . | 20 kD . | 17 kD . |
|---|---|---|---|---|---|
| 1 | S (15.0)/R (7.0) | L (30.0)/Y (8.0) | V (42.0)/Q (12.0) | Y (59.0)/V (11.0) | V (4.0) |
| 2 | K (29.0)/A (9.0) | D (28.0)/N (10.0) | G (43.0)/S (16.0) | N (62.0)/G (9.0) | G (4.0) |
| 3 | L (17.0)/E (8.0) | N (4.0)/S (6.0) | G (35.0)/F (14.0) | S (37.0)/G (10.0) | G (5.0) |
| 4 | T (23.0)/A (30.0) | I (25.0)/G (8.0) | E (34.0)/N (19.0) | G (15.0)/E (8.0) | E (4.0) |
| 5 | R (23.0)/V (10.0) | T (14.0)/K (13.0) | D (49.0) | K (36.0)/D (10.0) | D (8.0) |
| 6 | A (12.0)/F (8.0) | Q (11.0)/L (8.0) | A (30.0)/F (14.0) | L (28.0)/A (8.0) | |
| 7 | E (11.0)/P (6.0) | S (12.0)/E (4.0) |
| Cycle No. . | 44 kD-150 . | 34 kD . | 30 kD . | 20 kD . | 17 kD . |
|---|---|---|---|---|---|
| 1 | S (15.0)/R (7.0) | L (30.0)/Y (8.0) | V (42.0)/Q (12.0) | Y (59.0)/V (11.0) | V (4.0) |
| 2 | K (29.0)/A (9.0) | D (28.0)/N (10.0) | G (43.0)/S (16.0) | N (62.0)/G (9.0) | G (4.0) |
| 3 | L (17.0)/E (8.0) | N (4.0)/S (6.0) | G (35.0)/F (14.0) | S (37.0)/G (10.0) | G (5.0) |
| 4 | T (23.0)/A (30.0) | I (25.0)/G (8.0) | E (34.0)/N (19.0) | G (15.0)/E (8.0) | E (4.0) |
| 5 | R (23.0)/V (10.0) | T (14.0)/K (13.0) | D (49.0) | K (36.0)/D (10.0) | D (8.0) |
| 6 | A (12.0)/F (8.0) | Q (11.0)/L (8.0) | A (30.0)/F (14.0) | L (28.0)/A (8.0) | |
| 7 | E (11.0)/P (6.0) | S (12.0)/E (4.0) |
NH2-terminal sequencing of human F.IX-derived fragments after HNE cleavage. Human F.IX (4 μmol/L) was incubated with HNE (400 nmol/L) for 2 and 30 minutes at 37°C in HBS, pH 7.4, with 5.0 mmol/L CaCl2 as described in the Materials and Methods. Approximately 100 μg (1,750 pmol) was used for each sample reaction. The apparent molecular mass of the specific fragments is shown along with the picomoles of the amino acid identified (in parentheses) after each number of cycles performed.
Fragments 44 kD, 34 kD, 30 kD, 20 kD, and 17 kD represent the HNE-derived cleavage products of F.IX.
A schematic comparison of F.XIa and HNE cleavage of human F.IX. Human F.IX is composed of a light chain (residues 1-145), an activation peptide (residues 146-180), and a heavy chain (residues 181-415). The light chain is composed of a γ-carboxylglutamyl-(GLA) containing domain, two domains with homology to human epidermal growth factor (EGF), and a linker (L) domain.10 The activation peptide (AP) is cleaved upon F.IX activation by F.XIa. The catalytic domain (CD) contains the active site formed by the triad of Asp269, His221, and Ser365residues. The results of the NH2-terminal sequencing indicate that HNE inactivates human F.IX coagulant activity after cleaving at Thr140, Thr144, Ile164, Thr172, and Val181. Human F.IX is shown with the position of the cleavage sites for either F.XIa (top) or HNE (bottom).
A schematic comparison of F.XIa and HNE cleavage of human F.IX. Human F.IX is composed of a light chain (residues 1-145), an activation peptide (residues 146-180), and a heavy chain (residues 181-415). The light chain is composed of a γ-carboxylglutamyl-(GLA) containing domain, two domains with homology to human epidermal growth factor (EGF), and a linker (L) domain.10 The activation peptide (AP) is cleaved upon F.IX activation by F.XIa. The catalytic domain (CD) contains the active site formed by the triad of Asp269, His221, and Ser365residues. The results of the NH2-terminal sequencing indicate that HNE inactivates human F.IX coagulant activity after cleaving at Thr140, Thr144, Ile164, Thr172, and Val181. Human F.IX is shown with the position of the cleavage sites for either F.XIa (top) or HNE (bottom).
The 44-kD intermediate protein fragment contained two amino acid sequences matching the seven amino acids in human F.IX from Ser141 to Glu147 (main product) and from Arg145 to Pro151 (minor product), indicating HNE cleavage at Thr140 and Thr144, respectively (Table 1). The 34-kD intermediate protein fragment also contained two amino acid sequences matching the seven amino acids in F.IX from Leu165 to Ser171 (main product) and from Tyr1 to Glu7 (minor product), indicating HNE cleavage at Ile164 and the N-terminal seven amino acids of intact F.IX, respectively. The 30-kD product fragment contained two amino acid sequences which matched the sequence of six amino acids in F.IX from Val182 to Ala187 (main product) and from Gln173 to Phe178 (minor product) indicating HNE cleavage at Val181 and Thr172, respectively. The 20-kD product fragment contained two amino acid sequences that matched the sequence of six amino acids in F.IX from Tyr1 to Leu6 (main product) and Val182 to Ala187 (minor product), indicating the first six amino acids of the N-terminus of intact F.IX and HNE cleavage at Val181, respectively. The 17-kD product fragment contained an amino acid sequence that matched a sequence of five amino acids from Val182 to Asp186indicating HNE cleavage at Val181. The fact that both the 20- and 17-kD peptide fragments of F.IX generated by HNE cleavage possessed the same NH2-terminal amino acid sequence as the 30-kD F.IX fragment suggests that at least two more HNE cleavage sites in human F.IX would be predicted to occur downstream of Val181 in the extreme C-terminal 100 to 150 amino acid residues of the heavy chain. However, peptide products of apparent molecular mass of 10 to 13 kD were not present in sufficient quantities and/or resolved completely under these experimental conditions for unambiguous sequence determination.
Fluorescence Analysis of F.IXa or HNE-Treated F.IX Interaction With pAB
The binding of the fluorescent active site probe pAB to the active site region of either F.IXa and HNE-treated F.IX was monitored by measuring the fluorescence change upon cleavage of F.IX with F.XIa and HNE, respectively. The relative increase in fluorescence is proportional to the extent of exposure of the active site. Cleavage of F.IX by F.XIa resulted in a time-dependent increase in the pAB fluorescence to maximal levels approximately 40 to 60 minutes after protease addition (Fig 4A). The maximal pAB fluorescence values achieved after 40 to 60 minutes after the addition of F.XIa to F.IX correlated well with the generation of the 30- and 23-kD heavy and light chain peptides, respectively, as determined by reducing SDS-PAGE (Fig 4B, lanes 7 and 8) and densitometry (results not shown). In contrast, there was no increase in pAB fluorescence over a similar time course with F.IX treated with HNE (Fig 4A), despite it being cleaved into five protein fragments of apparent molecular mass of 45, 35, 30, 23, and 12 kD as determined by reducing SDS-PAGE (Fig 4C, lanes 2 through 8). The failure of HNE-treated F.IX to bind pAB and exhibit increased fluorescence suggests the presence of an abnormal conformation of the active site compared with F.IXa.
Cleavage of F.IX by HNE or F.XIa in the presence of pAB. F.IX (4.0 μmol/L) was incubated at 37°C in the presence of pAB (4.0 μmol/L) and after approximately 100 seconds, F.XIa (500 nmol/L) or HNE (400 nmol/L) was added and the incubations continued. (A) shows the fluorescence intensity values recorded for F.IX treated with F.XIa (•) or HNE (○) with excitation and emission wavelengths of 336 and 376 nm, respectively, and a cutoff filter of 350 nm in the emission beam using a Perkin Elmer LS50B Luminescence Spectrometer. (B) (F.XIa-treated F.IX) and (C) (HNE-treated F.IX) show reducing SDS-PAGE analysis of aliquots (4 μg protein each) removed from the reactions in (A) after 0, 0.5, 2, 5, 10, 20, 40, and 60 minutes of incubation with protease (lanes 1 through 8, respectively, in each case) using 5% to 20% linear polyacrylamide gradient gels after staining with Coomassie Blue. The positions of molecular weight markers (in kilodaltons) are indicated at the left of each panel.
Cleavage of F.IX by HNE or F.XIa in the presence of pAB. F.IX (4.0 μmol/L) was incubated at 37°C in the presence of pAB (4.0 μmol/L) and after approximately 100 seconds, F.XIa (500 nmol/L) or HNE (400 nmol/L) was added and the incubations continued. (A) shows the fluorescence intensity values recorded for F.IX treated with F.XIa (•) or HNE (○) with excitation and emission wavelengths of 336 and 376 nm, respectively, and a cutoff filter of 350 nm in the emission beam using a Perkin Elmer LS50B Luminescence Spectrometer. (B) (F.XIa-treated F.IX) and (C) (HNE-treated F.IX) show reducing SDS-PAGE analysis of aliquots (4 μg protein each) removed from the reactions in (A) after 0, 0.5, 2, 5, 10, 20, 40, and 60 minutes of incubation with protease (lanes 1 through 8, respectively, in each case) using 5% to 20% linear polyacrylamide gradient gels after staining with Coomassie Blue. The positions of molecular weight markers (in kilodaltons) are indicated at the left of each panel.
Cleavage of CH3-SO2-LGR-pNA Peptide by F.IX, HNE-Treated F.IX, and F.IXa
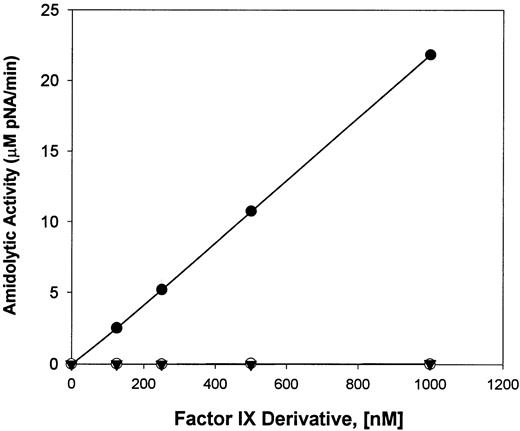
Reactions of F.IX, HNE-treated F.IX, and F.IXa were assayed for their ability to hydrolyze the synthetic peptide, CH3-SO2-LGR-pNA; the results are shown in Fig 5. The results indicate that, although no peptide hydrolysis was observed with 0.125 to 1.0 μmol/L of either F.IX zymogen or HNE-treated F.IX, F.IXa (0.125 to 1.0 μmol/L) cleaved this peptide substrate under the conditions used at initial rates similar to those reported by other investigators.13
Cleavage of CH3SO2-LGR-pNA peptide by different F.IX derivatives. The amidolytic activity (micromoles per liter of pNA released per minute) of various concentrations (0.125 to 1.0 μmol/L) of F.IX (▾) HNE-treated F.IX (○) and F.IX incubated with F.XIa (•) was determined from the initial rates of hydrolysis of 1 mmol/L CH3SO2-LGR-pNA peptide using a SpectroMax 250 Plate Reader to record the absorbance values at 405 nm.
Cleavage of CH3SO2-LGR-pNA peptide by different F.IX derivatives. The amidolytic activity (micromoles per liter of pNA released per minute) of various concentrations (0.125 to 1.0 μmol/L) of F.IX (▾) HNE-treated F.IX (○) and F.IX incubated with F.XIa (•) was determined from the initial rates of hydrolysis of 1 mmol/L CH3SO2-LGR-pNA peptide using a SpectroMax 250 Plate Reader to record the absorbance values at 405 nm.
Activation of Human Factor X by F.IX, HNE-Treated F.IX, and FIXa
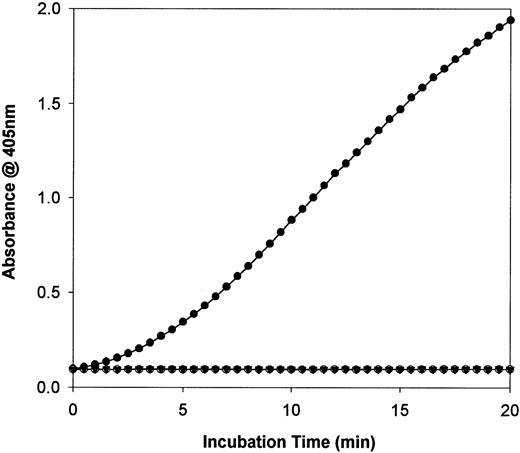
F.IX, HNE-treated F.IX, and F.IXa were also assayed for their ability to activate the physiological protein substrate for the tenasecomplex (F.IXa/F.VIIIa/phospholipid/Ca2+), F.X. The results are shown in Fig 6. Two different concentrations (25 or 250 nmol/L) of F.IX, HNE-treated F.IX, or F.IXa were each incubated with F.X at 37°C in the presence of phospholipid, calcium, and the chromogenic substrate for activated F.X (F.Xa), S-2222. As shown in Fig6, under the conditions used, both the F.IX zymogen and HNE-treated F.IX (each at 250 nmol/L) were each unable to activate F.X and lead to S-2222 hydrolysis. In contrast, F.IXa was able to activate F.X and the calculated reaction rate for 250 nmol/L F.IXa was 6.2 × 10−3 min−2, which agrees well with previously published studies.21
Activation of human factor X by F.IX derivatives. The hydrolysis of chromogenic substrate S-2222 (0.4 mmol/L) by factor Xa (F.Xa) generated with 250 nmol/L of F.IX (▾), HNE-treated F.IX (○), or F.XIa-treated F.IX (•) is shown after using a SpectroMax 250 Plate Reader to record absorbance changes at 405 nm.
Activation of human factor X by F.IX derivatives. The hydrolysis of chromogenic substrate S-2222 (0.4 mmol/L) by factor Xa (F.Xa) generated with 250 nmol/L of F.IX (▾), HNE-treated F.IX (○), or F.XIa-treated F.IX (•) is shown after using a SpectroMax 250 Plate Reader to record absorbance changes at 405 nm.
The Effect of HNE on F.IX and F.IXa Complexation With ATIII in the Presence of Heparin and aPTT Clotting Activity
Reaction mixtures of F.IX, F.IXa, HNE-treated F.IX (F.IXe), F.IXa treated with HNE (F.IXa/e), or HNE-treated F.IX subsequently incubated with F.XIa (F.IXe/a) were subjected to nonreducing SDS-PAGE before and after treatment with ATIII in the presence of heparin; the results are shown in Fig 7A and B. Additionally, aliquots from the same reaction mixtures used for the ATIII/heparin interaction studies were also assayed over a range of concentrations using the aPTT clotting assay; the results are shown in Fig 7C.
Effect of HNE or F.XIa on F.IX complexation with ATIII in the presence of heparin and aPTT activity. The different F.IX samples were generated as described in the Materials and Methods. Aliquots of the samples were solubilized before and after incubation with ATIII (5 μmol/L) and heparin (2 mg/mL) for 30 minutes at 37°C for nonreducing SDS-PAGE (A and B, respectively). In addition, these same F.IX samples were also diluted in HBS/BSA and assayed for aPTT clotting activity (C). (A and B) Nonreducing SDS-PAGE analysis of F.IX derivatives before and after incubation with ATIII in the presence of heparin, respectively: F.IX alone (lane 1), F.IXa (lane 2), F.IX treated with HNE (lane 3), F.IXa treated with HNE (lane 4), and F.IX treated with HNE then F.XIa (lane 5). Also shown in (B) is ATIII and heparin alone (lane 6). A 5% to 15% linear polyacrylamide gradient gel was used and the positions of the molecular weight markers (in kilodaltons) are indicated at the left of (A) and (B). (C) Log aPTT clot time (in seconds) versus log F.IX derivative concentration (in picomoles per liter): HBS/BSA buffer alone (•); F.IX (○); HNE-treated F.IX (▿); F.IX treated with HNE then F.XIa (□); F.IXa (▾); F.IXa treated with HNE (▪).
Effect of HNE or F.XIa on F.IX complexation with ATIII in the presence of heparin and aPTT activity. The different F.IX samples were generated as described in the Materials and Methods. Aliquots of the samples were solubilized before and after incubation with ATIII (5 μmol/L) and heparin (2 mg/mL) for 30 minutes at 37°C for nonreducing SDS-PAGE (A and B, respectively). In addition, these same F.IX samples were also diluted in HBS/BSA and assayed for aPTT clotting activity (C). (A and B) Nonreducing SDS-PAGE analysis of F.IX derivatives before and after incubation with ATIII in the presence of heparin, respectively: F.IX alone (lane 1), F.IXa (lane 2), F.IX treated with HNE (lane 3), F.IXa treated with HNE (lane 4), and F.IX treated with HNE then F.XIa (lane 5). Also shown in (B) is ATIII and heparin alone (lane 6). A 5% to 15% linear polyacrylamide gradient gel was used and the positions of the molecular weight markers (in kilodaltons) are indicated at the left of (A) and (B). (C) Log aPTT clot time (in seconds) versus log F.IX derivative concentration (in picomoles per liter): HBS/BSA buffer alone (•); F.IX (○); HNE-treated F.IX (▿); F.IX treated with HNE then F.XIa (□); F.IXa (▾); F.IXa treated with HNE (▪).
Treatment of F.IX with HNE (F.IXe) generated a proteolytic product of apparent molecular mass of 47 kD that comigrated with F.IX activated with F.XIa (F.IXa) under denaturing and nonreducing conditions (Fig 7A, compare lanes 2 and 3). Incubation of F.IXa with HNE (F.IXa/e) or HNE-treated F.IX with FXIa (F.IXe/a) did not change the resultant apparent molecular weight of the protein product from that observed with F.IXa or F.IXe, respectively (Fig 7A, compare lanes 2 and 4 and lanes 3 and 5).
F.IXa formed at least two larger SDS-resistant complexes with ATIII in the presence of heparin under nonreducing conditons of apparent molecular mass of 94 and 85 kD (Fig 7B, lane 2). In contrast, F.IXe did not form these two higher apparent molecular mass species upon incubation with ATIII in the presence of heparin (Fig 7B, lane 3). F.IX did not form higher apparent molecular mass species when incubated with ATIII and heparin (Fig 7B, lane 1). Incubation of F.IXa/e with ATIII and heparin resulted in the production of similar levels of the two SDS-resistant complexes with ATIII of apparent molecular mass of 94 and 85 kD that were observed upon incubation of F.IXa alone with ATIII and heparin (Fig 7B, compare lanes 2 and 4). The two different species observed after incubation of F.IXa with ATIII/heparin agree with previous studies.21 However, F.IXe/a was not able to form the SDS-resistant complexes with ATIII and heparin that was observed with F.IXa or F.IXa/e and the staining pattern closely simulated F.IXe alone (Fig 7B, compare lanes 2 and 4 with lanes 3 and 5). The decreased formation of the larger apparent molecular mass species observed with F.IXe compared with F.IXa is in agreement with other studies that showed decreased binding of elastase-treated F.IX to ATIII/heparin compared with F.IXa.9 The lack of appearance of larger apparent molecular mass complexes with ATIII and heparin for the F.IXe and F.IXe/a samples was not attributable to incomplete HNE inactivation by 10 μmol/L AAPV-CMK and inactivation of the ATIII, because each of these samples possessed negligible activity against Me-Succ-AAPV-pNA peptide even upon overnight incubation (results not shown). Furthermore, the pattern of F.IXa/e complexation with ATIII and heparin was very similar to that of F.IXa in which no HNE was added (Fig 7B, compare lanes 2 and 4).
The Effect of HNE on the APTT Procoagulant Activity of F.IX and F.IXa
Aliquots of the same reaction mixtures of F.IX, F.IXa, F.IXe, F.IXa/e, and F.IXe/a used for the ATIII/heparin binding studies (see above) were also assayed for their aPTT coagulant activities over a range of concentrations; the results are shown in Fig 7C. The times of F.IX treatment with HNE or F.XIa were chosen to preclude any residual F.IX and its intermediates from being present in the different preparations. F.IXe had an aPTT clotting activity that closely approximated both F.IXe/a and the HBS/BSA buffer control, yet each was significantly less active than untreated F.IX (Fig 7C). F.IXa and F.IXa/e were the two most active species and had approximately equal aPTT clotting activity over the concentrations assayed (Fig 7C). In summary, these results suggest that HNE cleavage of F.IX eliminated its potential to be activated by F.XIa and, conversely, HNE treatment of F.IXa does not largely affect its resultant aPTT clotting activity (see also Fig 1A and B, above).
The Effect of HNE-Treated F.IX and dEGR-F.IXa on the aPTT Clotting Activity of F.IXa
The effect of various amounts of either HNE-treated F.IX or dEGR-F.IXa (each at 0.01 to 10,000 pmol/L) on the aPTT clotting activity of a fixed level of F.IXa (10 pmol/L) was examined; the results are displayed in Fig 8. The results demonstrate that, in striking contrast to dEGR-F.IXa, which progressively prolonged the aPTT clot times of 10 pmol/L F.IXa at concentrations greater than 10 pmol/L, assay of HNE-treated F.IX over this same concentration range failed to alter the resultant aPTT clot times (Fig 8). The lack of a competitive prolongation of the F.IXa aPTT clot times after varying [HNE-treated F.IX] with fixed [F.IXa] suggests that the HNE-derived cleavage products of F.IX failed to block the assembly of F.IXa into the tenase (F.IXa/F.VIIIa/Ca2+) complex.
The effect of HNE-treated F.IX and dEGR-F.IXa on the aPTT clotting activity of F.IXa. F.IX was treated with HNE or F.XIa (to generate F.IXa) as described in the Materials and Methods. A portion of the F.IXa reaction was further treated with dEGR-CMK to generate dEGR-F.IXa. The aPTT clotting activity of a fixed amount of F.IXa (10 pmol/L) was determined using varying amounts (0.01 to 10,000 pmol/L) of either HNE-treated F.IX (•) or dEGR-F.IXa (○) as competitor. A Log-Log plot of APTT clot time (in seconds) versus competitor concentration (in picomoles per liter) is shown.
The effect of HNE-treated F.IX and dEGR-F.IXa on the aPTT clotting activity of F.IXa. F.IX was treated with HNE or F.XIa (to generate F.IXa) as described in the Materials and Methods. A portion of the F.IXa reaction was further treated with dEGR-CMK to generate dEGR-F.IXa. The aPTT clotting activity of a fixed amount of F.IXa (10 pmol/L) was determined using varying amounts (0.01 to 10,000 pmol/L) of either HNE-treated F.IX (•) or dEGR-F.IXa (○) as competitor. A Log-Log plot of APTT clot time (in seconds) versus competitor concentration (in picomoles per liter) is shown.
DISCUSSION
This study has characterized in detail the cleavage events involved in the HNE-dependent inactivation of F.IX coagulant function in vitro. The use of purified components in a controlled system has allowed for the correlation between the specific HNE cleavage sites within the F.IX molecule with the resultant product’s physical and catalytic properties. The results suggest that, although HNE hydrolysis of F.IX generates a F.IXa-like species in terms of its electrophoretic characteristics, the product does not possess enzymatic activity and is not activatable.
The critically important role that F.IX plays in the coagulation cascade is emphasized by the severe bleeding disorder that results from its congenital deficiency in Christmas disease (hemophilia B).24 With the exception of the acquired deficiency associated with liver disease,25 little attention appears to have been paid to other pathological mechanisms, including DIC, that may lead to its decreased bioavailability. The data reported here confirm and expand on the information provided by Takaki et al9 with regard to the cleavage and inactivation of F.IX by human neutrophil elastase. Whereas HNE induces relatively subtle physical differences when compared with the hydrolysis of F.IX by one of its physiological activators, F.XIa, the functional consequences of these changes are profound.
A preferred F.IX cleavage site for HNE appeared to be Val181-Val182, as determined by NH2-terminal sequencing (Table 1). This specificity is in line with the known preference of HNE to hydrolyze substrates after valine residues.26 It has also been suggested that this region of the molecule, close to the physiological β-cleavage site, is exposed and susceptible to protease cleavage, whereas the remainder of the molecule is sterically protected.27 However, in addition, there was also unambiguous evidence of cleavage at Thr140-Ser141 and Thr144-Arg145 and Thr172-Gln173. As shown in Figs 2 and 7, it is not surprising that these relatively small differences between the products of HNE versus F.XIa-induced F.IX cleavage cannot be delineated by standard electrophoretic techniques under both reducing and nonreducing conditions, respectively.
HNE hydrolysis of F.IX does not generate a functional active site, as inferred by the absence of aPTT coagulant activity, pAB fluorescence, catalytic activity against both peptide and the physiological protein substrate, F.X, as well as its inability to complex with ATIII in the presence of heparin. The HNE-dependent inactivation of human F.IX coagulant activity and reduction in ATIII/heparin binding reported here agrees well with previous observations of others.9 With respect to the x-ray structure of porcine F.IXa, it is likely that the ammonium group of Val181 at the amino-terminal of the heavy chain, after cleavage at the β-site (Arg180-Val181) by F.XIa, forms a buried salt bridge with Asp364 adjacent to the active site serine (Ser365) that is necessary for development of an active serine protease.28 After HNE cleavage between residues Val181 and Val182, Val181 would not be available for proper ion pair formation and this may explain the absence of pAB binding and catalytic activity towards both the CH3-SO2-LGR-pNA peptide and F.X protein compared with F.IXa. In addition, HNE cleavage of F.IX at Thr140 and Thr144 may be significant in mediating at least part of the HNE-induced inactivation of its aPTT clotting activity given recent evidence that cleavage at the α-site (Arg145-Ala146), is required to expose the F.IX binding site for F.VIII via its light chain.13,14 It is possible that cleavage upstream of the α-site may disrupt the ordered assembly of the tenase complex and thus have a substantial detrimental effect on catalytic efficiency.29 However, in comparison with dEGR-F.IXa, the inability of 0.01 to 10,000 pmol/L HNE-treated F.IX to prolong the aPTT clotting activity of 10 pmol/L F.IXa suggests that the product(s) of HNE cleavage of F.IX do not affect F.IXa assembly into the tenase complex. Finally, given the similarity between the inactivation peptides produced by the HNE and the activation peptide released by F.XIa, F.IX activation assays based on the measurement of the circulating levels of the activation peptide should be interpreted with caution when increased bioavailability of HNE cannot be excluded. However, it is possible that HNE cleavage of human F.IX at Ile164 may be sufficiently distant from F.XIa cleavage of F.IX at Arg145 and Arg180 to be diagnostic of HNE cleavage in vivo using antibodies directed against a synthetic peptide beginning at Leu165. This possibility requires further study in the future.
These data support the view that in vitro HNE hydrolyzes F.IX to produce a product that, although physically similar to F.IXa, has no coagulant function. Should this occur in vivo, hemostasis would be significantly compromised. In contrast, F.IXa was only partially inactivated by HNE, as indicated by a marginally decreased aPTT clotting activity. Other data reported here indicate that F.XIa cannot rescue the clotting activity of F.IX treated with HNE. These results are entirely in agreement with that expected from the NH2-terminal sequencing data. The results reported here suggest that F.IXa is more resistant to the HNE-dependent inactivation of its clotting activity than the F.IX zymogen. Therefore, the net procoagulant activity of F.IX in the context of the data presented here is a complex interplay between the functional concentrations of HNE and F.XIa that become exposed to the F.IX zymogen.
Point mutations where Phe is substituted for Val181 or Leu for Val182 result in severe or mild hemophilia B, respectively.30,31 Similarly, mutations at or around the α-cleavage site are also associated with this disorder, although such cases are usually mild with regard to clinical severity.32,33 Although increased elastase availability has been documented in DIC,34 there are no reports characterizing F.IX structure and function in this context. This will be required to confirm or refute the possibility that the changes induced by HNE in vitro described here may occur in this condition in vivo. However, in an experimental animal model of DIC, such evidence is available.7 F.IX Western blotting and functional assay demonstrated the appearance of an F.IX degradation product with identical characteristics to that described here. Increased elastase availability was confirmed by the finding of elevated levels of the protease in complex with its primary physiological inhibitor, α1-proteinase inhibitor (α1-PI).7 Although this would also suggest that the normal relatively high plasma level (20 μmol/L) of its natural inhibitor α1-PI would preclude inappropriate degradation of proteins such as F.IX, evidence does exist that elastase may escape physiological inhibitory control when bound to either a protein substrate, elastin,35 or the neutrophil membrane upon cellular activation.36 The finding of increased levels of elastase-specific fibrinogen degradation products in the experimental DIC model described provides further evidence in support of elastase being primarily responsible for the changes in F.IX observed.7 These data, if confirmed in the human condition, may lead to the development of therapeutic strategies to inhibit more selectively elastase degradation of F.IX and other coagulation factors that are either consumed or degraded during DIC.37The use of such therapies, initially in the experimental model described, could in turn validate the role and importance of elastase in the pathogenesis of DIC.
ACKNOWLEDGMENT
The authors acknowledge helpful discussions with Dr Koen Mertens (Central Laboratory of the Netherlands Blood Transfusion Service) and Dr Mark Hatton (McMaster University). The technical assistance of Marilyn Garrett is gratefully acknowledged as is the support of Barbara Saunders in the preparation of the manuscript.
Supported by a grant from the Medical Research Council of Canada (MA-7667) and The Bayer/Canadian Red Cross Society Research and Development Fund (Grant No. 9610). A.R.G. is a Distinguished Research Professor of the Heart & Stroke Foundation of Ontario.
A preliminary report of these investigations was presented at the XVth Congress of the International Society on Thrombosis and Haemostasis, Jerusalem, Israel, June 11-16, 1995.
Address reprint requests to John A. Samis, PhD, The Department of Pathology, Botterell Hall, Rm A222, Queen’s University, Kingston, Ontario, Canada K7L 3N6; e-mail: samisj@post.queensu.ca.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal