Abstract
The purpose of this study was to compare the relative risk of second malignancies in a cohort of patients with hairy cell leukemia (HCL) against the normal population. Potential effects of type of treatment and duration of follow-up and the site distribution of cancer were also examined. Between 1976 and 1996, 117 patients were diagnosed with HCL in British Columbia who were referred to the British Columbia Cancer Agency (BCCA) for treatment. All additional malignancies were traced using a provincial population-based cancer registry and follow-up records from the BCCA. There were 90 men and 27 women. Median age at diagnosis was 53 years. The median follow-up time was 68 months. Twenty-three patients underwent primary splenectomy, 65 received interferon alpha, 24 deoxycoformycin, and 67 cladribine (2-chlorodeoxyadenosine). Thirty-six patients had an additional malignancy (30.7%) with a total of 44 tumors. Six patients (5.1%) had two or more malignancies. Twenty-five patients had malignancies diagnosed after HCL (21.3%), three concurrent with HCL (2.6%), and 12 preceding HCL (10.2%). Second tumors (n = 28 tumors) occurred at a median of 40 months after HCL (range, 3 to 167). The relative rate (RR) of second malignancy among men and women was 2.91 (P < .001) and 1.65 (P = .23), respectively, compared with age and secular trend-matched controls. There were eight prostate cancers, nine nonmelanoma skin cancers, two lung cancers, and four gastrointestinal adenocarcinomas. The RR (90% confidence interval [CI]) in the various treatment groups were: splenectomy (RR = 0.21 to 3.81), purine analogues (RR = 0.60 to 5.69), interferon then purine analogues (RR = 1.60 to 4.31), interferon alone (RR = 1.57 to 8.40). Cancer risk peaked at 2 years after HCL (RR = 4.13) and fell steadily afterwards, reaching a RR of 1.82 at 6 years. Twenty patients died, six due to HCL, 10 due to second malignancies, and four of unrelated causes. HCL patients appear to be inherently prone to malignancies. This appears to be more related to HCL tumor burden than to genetic predisposition or treatment effect. RR tends to fall with time after effective treatment. However, close monitoring for and vigorous prevention of cancer in HCL patients is advisable.
© 1998 by The American Society of Hematology.
HAIRY CELL LEUKEMIA (HCL) is a relatively uncommon chronic lymphoid leukemia characterized by circulating abnormal lymphocytes of distinctive morphology, immunophenotype, and cytochemical properties.1 The treatment of HCL has been revolutionized by the sequential introduction of splenectomy,2 interferon (IFN),3and purine analogues4 as the standards of treatment. With the use of cladribine (2-chlorodeoxyadenosine, 2CDA) for treatment, a sustained complete remission can be attained for over 90% of patients and many may well be cured.5 2CDA has also been successful as secondary treatment in patients relapsing from other forms of treatment or earlier courses of 2CDA.6
With prolonged survival, it has been recognized that many HCL patients have a tendency to develop further malignancies. Scattered case reports7-10 and individual single center series11-13 described the range of malignancies found. However, inconsistent reporting policies and incomplete follow-up have rendered it difficult to quantify the risk. The Lymphoma Tumor Group of the British Columbia Cancer Agency (BCCA) is referred all cases of HCL within the province for pathological review, tertiary oncology care, and follow-up. All malignancies are required to be reported to a provincial population-based cancer registry maintained by the BCCA. Here we report on 117 patients with HCL diagnosed over a 20-year period and followed prospectively. The analysis takes advantage of the cancer registry data from within our stable geographically defined at risk population. This allowed us to quantify relative risks for male and female cancer incidence compared with the general provincial population over a defined period of observation.
MATERIALS AND METHODS
The clinical records of 117 patients with HCL were reviewed for clinical, family, and smoking history. All pathologic material was reviewed by an experienced hematopathologist at BCCA. Cases were included only if they demonstrated characteristic morphology in peripheral blood or bone marrow smears, typical bone marrow biopsy histology, positivity for CD11c, CD25, and CD103 by flow cytometric immunophenotyping (when available) and cytochemical positivity for tartrate resistant acid phosphatase (TRAP). Corresponding computerized files of all malignancies among the 117 HCL patients were requested from the Cancer Registry for cross-reference to the date and nature of the other malignancies. Mortality data on all deceased cases were also obtained.
Standardized cancer incidence ratios (SIRs) were used to compare the cancer incidence of the HCL patients with those of the BC general population. Cancer incidence rates in BC were calculated by 5-year age groups and 5-year calendar periods dating back to 1969, using rates derived from the population-based BC Cancer Registry.14Tests of significance for the SIRs were calculated assuming the observed number of second malignancies followed a Poisson distribution with mean given by the expected number of second malignancies based on BC population rates.15 One sided t-test and 90% confidence intervals (CI) corresponding to a 5% significance level were used. Actuarial proportion of patients without a second malignancy was also calculated.16
RESULTS
Between 1976 and 1996, 117 patients presented to BCCA with HCL. There were 90 men and 27 women (77% v 23%). Taking the mean male and female population of BC between 1975 and 1991 as 1.42 and 1.45 million, respectively, the crude incidence rate was 3.16 and 0.93 per million per year for men and women. Median age was 53 years (range, 23 to 85). The median follow-up time was 68 months (range, 1 to 212). Three patients were lost to follow-up and not available for complete analysis.
The study group included patients enrolled in four clinical trials: a phase I study of lymphoblastoid IFN,17 a phase III trial of IFN alpha at two different doses,18 a trial of splenectomy versus IFN alpha,19 and a trial of IFN alpha versus 2-deoxycoformycin (DCF).20 From 1976 to 1981, 23 patients (19.7%) had splenectomy as part of the primary treatment, 14 as part of combination treatment. From 1979 to 1992, 65 patients (55.5%) received IFN (0.2 MU/m2 to 2 MU/m2 three times weekly) as part of first line treatment. Twenty-one of these were subsequently treated with 2CDA for disease persistence or relapse. From 1984 to 1991, 24 cases (20.5%) were treated with DCF (4 mg/m2 every 2 weeks). From 1991, 46 cases were initially treated with 2CDA (0.1 mg/kg/day for 7 days). In total, 67 cases (57.2%) received 2CDA at some time. Three cases were not treated. Fifty-six patients (47.8%) received more than one treatment modality.
Thirty-six patients had an additional malignancy (30.7%) with a total of 44 separate neoplasms (Table 1). Six patients (5.1%) had two or more malignancies in addition to HCL. Twenty-five cases (21.3%) presented after HCL, three cases (2.6%) concurrent with HCL, and 12 cases (10.2%) preceded HCL. The preceding tumors (n = 13) occurred at a median of 85 months before HCL (range, 1 to 336). Antineoplastic treatments given before the diagnosis of HCL included chemotherapy in five cases, radiotherapy in two cases, and excision in six cases. Second tumors (n = 28) occurred at a median of 40 months after HCL (range, 3 to 167). There were seven prostate cancers, nine nonmelanoma skin cancers, two lung cancers, and four gastrointestinal adenocarcinomas after HCL. Eleven patients had a history of cancer in first degree relatives. Three patients were smokers (including the two who developed lung cancers) and 13 were ex-smokers.
Demographic and Clinical Details of 36 HCL Patients With Further Malignancies
| Case . | Sex/ Age . | Treatments . | HCLdx . | Second Ca . | Site . | Treatment . | Pre . | Post . | Smoker . | FamHx . | Death . | Cause . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F/49 | 2CDA | Jan-93 | HD NS | Stage IIIA | chemo RT | −336 | quit 10 y | ||||
| Squamous ca IS | cervix | cone excise | −60 | |||||||||
| 2 | F/64 | 2CDA | Jul-87 | Squamous ca | cervix | cone excise | −249 | |||||
| 3 | M/54 | IF 2CDA | Aug-79 | Melanoma | back | wide excision | −176 | |||||
| 4 | M/42 | 2CDA | Sep-94 | Sarcoma | arm | wide excision | −116 | |||||
| 5 | F/82 | IFN | Jun-80 | Adenocarcinoma | breast | OT | −114 | mo ca liver | Sep-85 | HCL | ||
| 6 | M/55 | IFN DCF | Dec-88 | Carcinoid | rectum dissem | OT RT | −89 | 1 pack | sis ca colon | |||
| Squamous ca | lung dissem | RT | 22 | Mar-91 | ca lung | |||||||
| 7 | M/65 | IFN DCF | Mar-83 | Basal cell ca | ear | excision | −85 | fa ca stomach | ||||
| Adenocarcinoma | stomach dissem | palliation | 7 | May-94 | ca stomach | |||||||
| 8 | F/46 | IFN DCF | Feb-83 | PNET | multiple lower leg | wide excision | −78 | |||||
| 9 | M/64 | IFN | May-91 | Basal cell ca | eyelid | excision | −48 | |||||
| Adenocarcinoma | prostate | radical OT | 35 | |||||||||
| 10 | M/63 | IFN | May-84 | Basal cell ca | face multiple | excision | −24 | quit 30 y | mo ca breast | Jun-89 | HCL | |
| 11 | M/37 | IFN 2CDA | Sep-90 | Squamous ca | trunk | excision | −18 | quit 10 y | ||||
| Basal cell ca | cheek | excision | 40 | |||||||||
| 12 | M/65 | 2CDA | May-92 | Adenocarcinoma | lung | RT | −1 | ½ pack | Jul-93 | ca lung | ||
| 13 | M/56 | IFN 2CDA | Nov-89 | Adenoma | thyroid | excision | 0 | mo ca breast | ||||
| 14 | M/57 | 2CDA | Jul-95 | MGUS | blood | none | 0 | |||||
| 15 | M/78 | IFN 2CDA | Apr-96 | Adenocarcinoma | prostate dissem | hormone | 0 | quit 30 y | Nov-96 | ca prost | ||
| 16 | F/85 | Vinblastine | Oct-84 | HD NS | Stage IIIA | vinblastine | 3 | sis ca liver | Aug-85 | HD | ||
| 17 | M/55 | IFN DCF | Apr-87 | Squamous ca | ear | excision | 15 | quit 26 y | mo ca colon | |||
| 18 | M/47 | Splen | Nov-79 | Melanoma | scalp brain | excision RT | 19 | Oct-82 | melanoma | |||
| 19 | M/75 | IFN 2CDA | Dec-90 | Adenocarcinoma | prostate | OT | 23 | Sep-93 | stroke | |||
| 20 | M/70 | IFN | Oct-85 | Squamous ca | lung | RT | 24 | quit 1 y | May-96 | ca lung | ||
| 21 | F/46 | IFN DCF 2CDA | Feb-84 | Melanoma | forearm | wide excision | 27 | quit 16 y | fa ca pros/colon | Jul-91 | HCL | |
| 22 | M/66 | 2CDA | Mar-92 | Adenocarcinoma | prostate | hormone | 27 | |||||
| 23 | M/68 | IFN | Jul-80 | Squamous ca | multiple | multiple excision | 27 | Oct-92 | ca parotid | |||
| Adenosquamous | parotid | Radical OT RT | 113 | |||||||||
| Basal cell ca | multiple | multiple excision | 118 | |||||||||
| Adenocarcinoma | prostate | hormone | 143 | |||||||||
| 24 | M/46 | IFN DCF | Mar-84 | Sarcoma | nasopharynx | excision | 36 | quit 6 y | ||||
| 25 | F/70 | IFN 2CDA | May-91 | Adenocarcinoma | colon dissem | OT palliation | 41 | Jul-94 | ca colon | |||
| 26 | M/65 | 2CDA | Nov-91 | Adenocarcinoma | prostate | Radical RT | 49 | quit 30 y | ||||
| 27 | M/46 | IFN | Nov-79 | Acute leukemia | blood | palliation | 65 | Apr-85 | leukemia | |||
| 28 | M/46 | IFN DCF 2CDA | Sep-86 | Squamous ca | shoulder | excision | 68 | fa leukemia | ||||
| 29 | M/43 | S IFN 2CDA | Mar-86 | Meningioma | pituitary | OT RT | 70 | |||||
| 30 | M/66 | S IFN DCF | Sep-86 | Adenocarcinoma | cecum dissem | palliation | 99 | fa ca prostate | Dec-94 | HCL | ||
| 31 | F/58 | DCF | Dec-84 | Renal cell ca | kidney dissem | OT RT | 114 | quit 20 y | ||||
| 32 | M/48 | IFN 2CDA | Aug-80 | Basal cell ca | neck | excision | 125 | |||||
| 33 | M/52 | IFN | Jun-79 | Adenocarcinoma | prostate | TURP RT | 127 | quit 20 y | fa ca esophagus | |||
| 34 | M/44 | IFN DCF 2CDA | Sep-85 | Adenocarcinoma | prostate | RT | 139 | quit 15 y | ||||
| 35 | M/62 | IFN 2CDA | Jun-81 | Squamous ca | nose | excision | 151 | 1 pack | ||||
| 36 | M/60 | S | Jan-80 | Adenocarcinoma | cecum | resection | 167 | quit 32 y |
| Case . | Sex/ Age . | Treatments . | HCLdx . | Second Ca . | Site . | Treatment . | Pre . | Post . | Smoker . | FamHx . | Death . | Cause . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F/49 | 2CDA | Jan-93 | HD NS | Stage IIIA | chemo RT | −336 | quit 10 y | ||||
| Squamous ca IS | cervix | cone excise | −60 | |||||||||
| 2 | F/64 | 2CDA | Jul-87 | Squamous ca | cervix | cone excise | −249 | |||||
| 3 | M/54 | IF 2CDA | Aug-79 | Melanoma | back | wide excision | −176 | |||||
| 4 | M/42 | 2CDA | Sep-94 | Sarcoma | arm | wide excision | −116 | |||||
| 5 | F/82 | IFN | Jun-80 | Adenocarcinoma | breast | OT | −114 | mo ca liver | Sep-85 | HCL | ||
| 6 | M/55 | IFN DCF | Dec-88 | Carcinoid | rectum dissem | OT RT | −89 | 1 pack | sis ca colon | |||
| Squamous ca | lung dissem | RT | 22 | Mar-91 | ca lung | |||||||
| 7 | M/65 | IFN DCF | Mar-83 | Basal cell ca | ear | excision | −85 | fa ca stomach | ||||
| Adenocarcinoma | stomach dissem | palliation | 7 | May-94 | ca stomach | |||||||
| 8 | F/46 | IFN DCF | Feb-83 | PNET | multiple lower leg | wide excision | −78 | |||||
| 9 | M/64 | IFN | May-91 | Basal cell ca | eyelid | excision | −48 | |||||
| Adenocarcinoma | prostate | radical OT | 35 | |||||||||
| 10 | M/63 | IFN | May-84 | Basal cell ca | face multiple | excision | −24 | quit 30 y | mo ca breast | Jun-89 | HCL | |
| 11 | M/37 | IFN 2CDA | Sep-90 | Squamous ca | trunk | excision | −18 | quit 10 y | ||||
| Basal cell ca | cheek | excision | 40 | |||||||||
| 12 | M/65 | 2CDA | May-92 | Adenocarcinoma | lung | RT | −1 | ½ pack | Jul-93 | ca lung | ||
| 13 | M/56 | IFN 2CDA | Nov-89 | Adenoma | thyroid | excision | 0 | mo ca breast | ||||
| 14 | M/57 | 2CDA | Jul-95 | MGUS | blood | none | 0 | |||||
| 15 | M/78 | IFN 2CDA | Apr-96 | Adenocarcinoma | prostate dissem | hormone | 0 | quit 30 y | Nov-96 | ca prost | ||
| 16 | F/85 | Vinblastine | Oct-84 | HD NS | Stage IIIA | vinblastine | 3 | sis ca liver | Aug-85 | HD | ||
| 17 | M/55 | IFN DCF | Apr-87 | Squamous ca | ear | excision | 15 | quit 26 y | mo ca colon | |||
| 18 | M/47 | Splen | Nov-79 | Melanoma | scalp brain | excision RT | 19 | Oct-82 | melanoma | |||
| 19 | M/75 | IFN 2CDA | Dec-90 | Adenocarcinoma | prostate | OT | 23 | Sep-93 | stroke | |||
| 20 | M/70 | IFN | Oct-85 | Squamous ca | lung | RT | 24 | quit 1 y | May-96 | ca lung | ||
| 21 | F/46 | IFN DCF 2CDA | Feb-84 | Melanoma | forearm | wide excision | 27 | quit 16 y | fa ca pros/colon | Jul-91 | HCL | |
| 22 | M/66 | 2CDA | Mar-92 | Adenocarcinoma | prostate | hormone | 27 | |||||
| 23 | M/68 | IFN | Jul-80 | Squamous ca | multiple | multiple excision | 27 | Oct-92 | ca parotid | |||
| Adenosquamous | parotid | Radical OT RT | 113 | |||||||||
| Basal cell ca | multiple | multiple excision | 118 | |||||||||
| Adenocarcinoma | prostate | hormone | 143 | |||||||||
| 24 | M/46 | IFN DCF | Mar-84 | Sarcoma | nasopharynx | excision | 36 | quit 6 y | ||||
| 25 | F/70 | IFN 2CDA | May-91 | Adenocarcinoma | colon dissem | OT palliation | 41 | Jul-94 | ca colon | |||
| 26 | M/65 | 2CDA | Nov-91 | Adenocarcinoma | prostate | Radical RT | 49 | quit 30 y | ||||
| 27 | M/46 | IFN | Nov-79 | Acute leukemia | blood | palliation | 65 | Apr-85 | leukemia | |||
| 28 | M/46 | IFN DCF 2CDA | Sep-86 | Squamous ca | shoulder | excision | 68 | fa leukemia | ||||
| 29 | M/43 | S IFN 2CDA | Mar-86 | Meningioma | pituitary | OT RT | 70 | |||||
| 30 | M/66 | S IFN DCF | Sep-86 | Adenocarcinoma | cecum dissem | palliation | 99 | fa ca prostate | Dec-94 | HCL | ||
| 31 | F/58 | DCF | Dec-84 | Renal cell ca | kidney dissem | OT RT | 114 | quit 20 y | ||||
| 32 | M/48 | IFN 2CDA | Aug-80 | Basal cell ca | neck | excision | 125 | |||||
| 33 | M/52 | IFN | Jun-79 | Adenocarcinoma | prostate | TURP RT | 127 | quit 20 y | fa ca esophagus | |||
| 34 | M/44 | IFN DCF 2CDA | Sep-85 | Adenocarcinoma | prostate | RT | 139 | quit 15 y | ||||
| 35 | M/62 | IFN 2CDA | Jun-81 | Squamous ca | nose | excision | 151 | 1 pack | ||||
| 36 | M/60 | S | Jan-80 | Adenocarcinoma | cecum | resection | 167 | quit 32 y |
Thyroid adenoma and MGUS are reported as malignancy in both the study and control population by Cancer Registry codings.
Abbreviations: HCL dx, date of diagnosis of HCL; ca, carcinoma; Pre, months before diagnosis of HCL; Post, months after diagnosis of HCL; Fam Hx, family history of malignancy; F, female; 2CDA, cladrabine; HD, Hodgkin’s disease; NS, nodular sclerosis; chemo, chemotherapy; RT, radiotherapy; IS, in situ; M, male; IFN, interferon; OT, surgical operation; mo, mother; dissem, disseminated; sis, sister; fa, father; DCF, deoxycoformycin; PNET, peripheral neuroectoderm tumor; MGUS, monoclonal gammopathy of unknown significance; S, splenectomy.
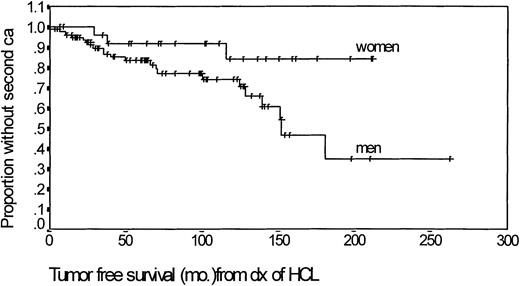
Among 87 men, the actuarial time to a 50% risk of developing a second cancer was 150 months (Fig 1). The corresponding risk at 150 months was 15% in the 27 women. Among cases with a subsequent malignancy, 10 had been treated with splenectomy, seven with IFN, seven with 2CDA, and four with DCF. Twenty patients died, 10 (50%) due to second cancer, six due to HCL, and four of unrelated causes.
Kaplan Meier actuarial analysis of the time to development of a second malignancy in male and female patients with HCL.
Kaplan Meier actuarial analysis of the time to development of a second malignancy in male and female patients with HCL.
The increased risks for second malignancies were calculated including and excluding skin malignancies and results were similar (Table 2). Among 87 men, there was an increased relative rate (RR) of 2.91 for all cancers (P < .001, 90% CI 1.97 to 4.15). The RR in 27 women was 1.65, but did not reach statistical significance. Taking all patients into consideration, the RR is 2.60 times that of an age, geographical, and secular period matched background population (P < .001). When nonmelanoma skin cancers and carcinoma in situ were excluded, the RRs were similar (men RR = 3.03; women RR = 2.30, overall RR = 2.86). Among the subtypes of cancers, the RR for prostate cancer and colon cancer were 3.95 (P < .001) and 5.83 (P = .016), respectively. The number of events in the other cancer groups was too low for accurate estimation of relative risks. The RR varied with time (Table 2). The increased risk of second malignancy peaked between 1 and 2 years after diagnosis (RR = 4.13; P = .001; CI 2.05 to 7.45). It then fell steadily to a level of RR = 1.82 at 6 years and beyond. An increase in relative risk is found with all four types and combinations of treatment (RR 1.25 to 4.80) (Table 2). The increase in risk was highest and statistically significant in the groups treated with IFN (90% CI RR = 1.57 to 8.40 ) or IFN and purine analogues (90% CI RR = 1.60 to 4.31). However, the different groups have been followed up for variable times and the 90% CIs for RR of all four groups overlapped extensively.
Increased Risk in Second Malignancy in HCL Patients by Sex, Cancer Subtype, Time After Diagnosis and Treatment Type
| . | Observed . | Expected . | Ratio . | 90% CI . | P Value . |
|---|---|---|---|---|---|
| All cancers | |||||
| Male | 22 | 7.57 | 2.91 | 1.97-4.15 | <.001 |
| Female | 4 | 2.42 | 1.65 | 0.56-3.78 | .226 |
| Both | 26 | 9.99 | 2.60 | 1.82-3.61 | <.001 |
| Exclude all squamous/basal cell ca of skin and ca in situ | |||||
| Male | 17 | 5.61 | 3.03 | 1.93-4.54 | <.001 |
| Female | 4 | 1.74 | 2.30 | 0.78-5.25 | .099 |
| Both | 21 | 7.35 | 2.86 | 1.91-4.11 | <.001 |
| Cancer subtypes* | |||||
| Colon | 3 | 0.51 | 5.83 | 1.57-15.04 | .016 |
| Lung | 2 | 1.28 | 1.28 | 0.27-4.92 | .365 |
| Prostate | 6 | 1.52 | 3.95 | 1.72-7.78 | <.001 |
| Kidney | 1 | 0.19 | 5.33 | 0.21-25.20 | .171 |
| Year after HCL diagnosis | |||||
| 0 to 1 | 3 | 1.07 | 2.80 | 0.76-7.22 | .094 |
| 1 to 2 | 8 | 1.94 | 4.13 | 2.05-7.45 | .001 |
| 3 to 5 | 5 | 2.20 | 2.27 | 0.89-4.77 | .073 |
| >6 | 7 | 3.85 | 1.82 | 0.85-3.42 | .095 |
| Type of treatment† | |||||
| IFN | 5 | 1.25 | 4.00 | 1.57-8.40 | .010 |
| IFN and PA | 13 | 4.80 | 2.71 | 1.60-4.31 | .001 |
| PA | 3 | 1.36 | 2.21 | 0.60-5.69 | .160 |
| Splenectomy | 2 | 1.65 | 1.21 | 0.21-3.81 | .340 |
| . | Observed . | Expected . | Ratio . | 90% CI . | P Value . |
|---|---|---|---|---|---|
| All cancers | |||||
| Male | 22 | 7.57 | 2.91 | 1.97-4.15 | <.001 |
| Female | 4 | 2.42 | 1.65 | 0.56-3.78 | .226 |
| Both | 26 | 9.99 | 2.60 | 1.82-3.61 | <.001 |
| Exclude all squamous/basal cell ca of skin and ca in situ | |||||
| Male | 17 | 5.61 | 3.03 | 1.93-4.54 | <.001 |
| Female | 4 | 1.74 | 2.30 | 0.78-5.25 | .099 |
| Both | 21 | 7.35 | 2.86 | 1.91-4.11 | <.001 |
| Cancer subtypes* | |||||
| Colon | 3 | 0.51 | 5.83 | 1.57-15.04 | .016 |
| Lung | 2 | 1.28 | 1.28 | 0.27-4.92 | .365 |
| Prostate | 6 | 1.52 | 3.95 | 1.72-7.78 | <.001 |
| Kidney | 1 | 0.19 | 5.33 | 0.21-25.20 | .171 |
| Year after HCL diagnosis | |||||
| 0 to 1 | 3 | 1.07 | 2.80 | 0.76-7.22 | .094 |
| 1 to 2 | 8 | 1.94 | 4.13 | 2.05-7.45 | .001 |
| 3 to 5 | 5 | 2.20 | 2.27 | 0.89-4.77 | .073 |
| >6 | 7 | 3.85 | 1.82 | 0.85-3.42 | .095 |
| Type of treatment† | |||||
| IFN | 5 | 1.25 | 4.00 | 1.57-8.40 | .010 |
| IFN and PA | 13 | 4.80 | 2.71 | 1.60-4.31 | .001 |
| PA | 3 | 1.36 | 2.21 | 0.60-5.69 | .160 |
| Splenectomy | 2 | 1.65 | 1.21 | 0.21-3.81 | .340 |
Abbreviations: CI, confidence interval; ca, carcinoma; IFN, interferon; PA, purine analogue.
Event censored at time of development of first secondary cancer for both the study and control populations. Record of events for both populations drawn from the same database.
Patients with other types or combinations of treatment excluded from analysis.
DISCUSSION
We report a series of HCL patients from a stable geographically defined population treated sequentially according to different protocols over a period of 20 years. The age, sex ratio, and overall outcome were not different from previous large reported series. However, we find one of the highest rates (22%) of further malignancies among these patients. Previous series have reported an incidence varying from 2% to 19% (Table3).11-13,21-29 Our ability to detect a high incidence may be attributed to a long and complete follow-up for most cases. The published literature is, however, heterogeneous in many ways. Follow-up periods varied and some series excluded skin malignancies.13,24 In several series, second cancers were reported only as part of mortality data.25-27Four studies compared the treatment population with SIRs from national cancer registries and either did12 or did not13,28,29 demonstrate significantly increased risk. Our study is based on a stable geographically defined population with a matched control population both from a single Canadian province. The only other population-based epidemiological study conducted in Los Angeles county showed similar results, reporting an annual incidence of HCL of 2.9 and 0.6 per million among men and women.24 The incidence of multiple primary cancers within the study period was 14%, twice that of other cancer subtypes in the study. However, the absolute values of the RR were not reported. Incidence was again highest immediately around the time of HCL diagnosis.
Published Series on Secondary Malignancies in HCL Patients
| Author . | Year . | No. . | Age . | M:F . | FU . | Treatment . | 2nd Ca . | % . | Skin . | Pros . | Lung . | GI . | Hem . | NHL . | HD . | RCC . | Br . | Sar . | Others . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jacobs7 | 1975-1984 | 172 | NA | NA | 60 | S/Chl/IFN | 14 | 7.5 | 5 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | |||
| Bernstein24 | 1972-1987 | 208 | NA | 4.0:1 | NA | NA | 30 | 14.4 | 3 | 6 | 4 | 1 | 16 | ||||||
| Berman23 | 1984-1990 | 35 | 52 | 6.0:1 | 44 | IFN | 1 | 2 | 1 | ||||||||||
| Smith26 | 1984-1990 | 56 | 52 | 4.6:1 | 72 | S/IFN | 3 | 5 | 2 | 1 | |||||||||
| Kraut4 | 1987-1994 | 24 | NA | NA | 82 | DCF | 4 | 16 | 3 | 1 | |||||||||
| Kamperier12 | 1983-1986 | 69 | 53 | 7.6:1 | 91 | Chl/DCF/IFN | 13 | 19 | 1 | 2 | 3 | 3 | 1 | 1 | 2 | ||||
| Troussard28 | 1966-1993 | 107 | 51 | 8.7:1 | 102 | IFN | 11 | 10.2 | 3 | 1 | 1 | 2 | 2 | 2 | |||||
| Frassoldati25 | 1965-1990 | 725 | 54 | 3.9:1 | NA | S/IFN/DCF/2CDA | 22 | 3.7 | 3 | 3 | 3 | 5 | 1 | 3 | 2 | 2 | |||
| Pawson29 | 1988-1995 | 200 | 48 | 4.9:1 | 65 | IFN/DCF/2CDA | 8 | 4 | 2 | 2 | 1 | 1 | 2 | ||||||
| Lauria27 | 1991-1994 | 40 | 54 | 3.0:1 | 48 | IFN/2CDA | 2 | 5 | 1 | 1 | |||||||||
| Kurzrock13 | 1968-1995 | 350 | 50 | 4.3:1 | 72 | IFN/DCF/2CDA | 26 | 7.4 | 6 | 4 | 2 | 4 | 2 | 3 | 1 | 1 | 2 | 3 | |
| Au | 1976-1996 | 117 | 53 | 3.3:1 | 68 | S/IFN/DCF/2CDA | 25 | 22 | 9 | 8 | 2 | 4 | 1 | 1 | 1 | 1 | 1 | 5 | |
| Total | 2,103 | 159 | 31 | 23 | 16 | 23 | 10 | 12 | 3 | 4 | 8 | 4 | 35 |
| Author . | Year . | No. . | Age . | M:F . | FU . | Treatment . | 2nd Ca . | % . | Skin . | Pros . | Lung . | GI . | Hem . | NHL . | HD . | RCC . | Br . | Sar . | Others . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jacobs7 | 1975-1984 | 172 | NA | NA | 60 | S/Chl/IFN | 14 | 7.5 | 5 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | |||
| Bernstein24 | 1972-1987 | 208 | NA | 4.0:1 | NA | NA | 30 | 14.4 | 3 | 6 | 4 | 1 | 16 | ||||||
| Berman23 | 1984-1990 | 35 | 52 | 6.0:1 | 44 | IFN | 1 | 2 | 1 | ||||||||||
| Smith26 | 1984-1990 | 56 | 52 | 4.6:1 | 72 | S/IFN | 3 | 5 | 2 | 1 | |||||||||
| Kraut4 | 1987-1994 | 24 | NA | NA | 82 | DCF | 4 | 16 | 3 | 1 | |||||||||
| Kamperier12 | 1983-1986 | 69 | 53 | 7.6:1 | 91 | Chl/DCF/IFN | 13 | 19 | 1 | 2 | 3 | 3 | 1 | 1 | 2 | ||||
| Troussard28 | 1966-1993 | 107 | 51 | 8.7:1 | 102 | IFN | 11 | 10.2 | 3 | 1 | 1 | 2 | 2 | 2 | |||||
| Frassoldati25 | 1965-1990 | 725 | 54 | 3.9:1 | NA | S/IFN/DCF/2CDA | 22 | 3.7 | 3 | 3 | 3 | 5 | 1 | 3 | 2 | 2 | |||
| Pawson29 | 1988-1995 | 200 | 48 | 4.9:1 | 65 | IFN/DCF/2CDA | 8 | 4 | 2 | 2 | 1 | 1 | 2 | ||||||
| Lauria27 | 1991-1994 | 40 | 54 | 3.0:1 | 48 | IFN/2CDA | 2 | 5 | 1 | 1 | |||||||||
| Kurzrock13 | 1968-1995 | 350 | 50 | 4.3:1 | 72 | IFN/DCF/2CDA | 26 | 7.4 | 6 | 4 | 2 | 4 | 2 | 3 | 1 | 1 | 2 | 3 | |
| Au | 1976-1996 | 117 | 53 | 3.3:1 | 68 | S/IFN/DCF/2CDA | 25 | 22 | 9 | 8 | 2 | 4 | 1 | 1 | 1 | 1 | 1 | 5 | |
| Total | 2,103 | 159 | 31 | 23 | 16 | 23 | 10 | 12 | 3 | 4 | 8 | 4 | 35 |
Abbreviations: No., number of patients in study; M:F, male to female ratio; FU, median follow up time in months; 2nd Ca, number of second malignancies. Pros, prostate cancer; GI, gastrointestinal cancer; Hem, hematological malignancy; NHL, non-Hodgkin’s lymphoma; HD, Hodgkin’s disease; RCC, renal cell carcinoma; Br, breast cancer; Sar, sarcoma; NA, not available; S, splenectomy; Chl, chlormabucil; IFN, interferon.
Our results show that the incidence of malignancy is high both before and after the diagnosis of HCL. A comparison with the provincial population shows that this is neither due to local screening or referral bias, nor due to the increasing age of the cohort or extended follow-up. Increase in incidence of second cancers has also been noted in other lymphoproliferative disorders such as chronic lymphocytic leukemia (CLL), but with a much lower relative risk (RR 1.32 to 1.40).30 The increased cancer risk in HCL patients may be related to immunosuppression due to HCL31 or its treatment.32,33 It has been proposed previously that better treatment may have allowed time for more secondary malignancies to develop in an aging population.34 However, the development of second malignancies is highest in the period up to 2 years after diagnosis. Taking into account the lag time for a neoplasm from initiation to reach a clinically detectable level, these data support the theory that the cancer risk could at least be partly related to immunosuppression or some other effect associated with the HCL clone itself. A residual risk persisted for up to 6 years posttreatment. The steady fall in RR, however, suggests that the risk is reduced by effective treatment. The high incidence of malignancy occurring before (10.2%) and concurrent (2.6%) with HCL also suggests some pretreatment predisposition to cancer. High incidence of malignancies preceding and concurrent with HCL has also been noted in another population-based study.24 Five of our patients had a total of three to five separate malignancies in their clinical histories. Such cases have also been noted in previous series (2 of 13 in Kampmeier et al12 and seven of 26 in Kurzrock et al13). The combined data argue strongly for an inherent predisposition to malignancies due to the HCL clone itself.
In previous studies, IFN and purine analogues have not been shown to cause more cancers compared with splenectomy.13 28 Our results showed an increased risk of second malignancies associated with all types and combinations of treatments (Table 2), including splenectomy alone. This risk reached statistical significance only in the groups treated with IFN alone or IFN and purine analogues. This may have been influenced by differences in the follow-up time and numbers of patients in the groups. The 90% CI of all four groups overlapped, so our data do not provide evidence that IFN or purine analogue caused more malignancies compared with splenectomy. However, it is interesting to note that the least effective treatment of HCL, that is, IFN, was associated in our data with the highest residual risk of second neoplasm. More effective treatment with purine analogues, and longer time from that effective treatment, both correlated with lowered risk of second cancer, suggesting that it is the HCL itself that increases predisposition to second cancers.
The distribution of various types of cancer followed that of the general population. Previous reports of preferential increase in leukemia12 and lymphoma13 were not supported by our data. The large number of case reports of secondary lymphomas after HCL may be largely due to reporting bias due to availability of clonality analyses.35-39 The high incidence of adenocarcinomas of the gastrointestinal tract and lung-based carcinomas has been previously recognized (Table 3). The incidence of prostate cancers (seven secondary cases, one concurrent case; median age, 68) is also significantly higher than the control population. A high incidence of skin malignancies has been reported from series that included data on skin cancers (Table 3). Our data further show that the RR for second cancer is increased by the same extent whether or not skin malignancies are included.
HCL is one of the few highly treatable disseminated cancers and only six (5.1%) of our patients died of complications related to the disease. However, more than twice that number died of second malignancies. The observation that the incidence of second neoplasms is highest near and immediately after the diagnosis of HCL is intriguing. Given the indolent course of HCL, it is conceivable that the disease causes a progressive impact that increases with the leukemia burden. This peaks near diagnosis when the burden is highest and diminishes after the elimination of HCL. If this is true, longer follow-up of cured patients should show a continuous decline in RR of secondary cancer to control levels. Such an observation would have important implications for the relationship between the lymphoid system and the development of other neoplasms and carcinogenesis in general. It is also important in a clinical sense in that meticulous follow-up, vigorous prevention, and early detection of malignancy in HCL patients may improve their survival outcome.
ACKNOWLEDGMENT
The authors thank Colleen Wong, Lisa Dykman, and Sherry Malsbury for data management and their colleagues in the Lymphoma Tumor Group, BCCA, for the clinical management of the patients.
W.Y.A. is supported by a Croucher Foundation Fellowship.
Address reprint requests to Joseph M. Connors, MD, British Columbia Cancer Agency, 600 W 10th Ave, Vancouver, BC V5Z 4E6, Canada.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal