Abstract
One hundred seventy-four patients with progressive or advanced chronic lymphocytic leukemia (CLL) have received initial therapy with fludarabine as a single agent or fludarabine combined with prednisone. The overall response rate was 78% and the median survival was 63 months. No difference in response rate or survival was noted in the 71 patients receiving fludarabine as a single agent compared with the 103 patients who received prednisone in addition. The median time to progression of responders was 31 months and the overall median survival was 74 months. Patients over the age of 70 years had shorter survivals. Patients with advanced stage disease (Rai III and IV) had a somewhat shorter survival than earlier stage patients. More than half the patients who relapsed after fludarabine therapy responded to salvage treatment, usually with fludarabine-based regimens. Second remissions were more common in patients who had achieved a complete remission on their initial treatment. The CD4 and CD8 T-lymphocyte subpopulations decreased to levels in the range of 150 to 200/μL after the first 3 courses of treatment. Although recovery towards normal levels was slow, the incidence of infections was low in patients in remission (1 episode of infection for every 3.33 patient years at risk) and decreased with time off treatment. There was no association of infections or febrile episodes with the use of corticosteroids or the CD4 count at the end of treatment and a poor correlation with the increase in CD4 counts during remission. Infectious episodes were less common in patients who had a complete response compared with partial responders. Richter’s transformation occurred in 9 patients and Hodgkin’s disease occurred in 4 patients. Five other patients died from other second malignancies. Fludarabine appears to be an effective initial induction therapy with a reasonable safety profile for patients with CLL.
© 1998 by The American Society of Hematology.
THE TRADITIONAL MANAGEMENT of patients with chronic lymphocytic leukemia (CLL) needing therapy is the use of chlorambucil with or without corticosteroids.1-4 Other alkylating agent-based regimens that have been used are cyclophosphamide, vincristine, and prednisone (CVP)5; cyclophosphamide, vincristine, prednisone, and adriamycin with or without ara-C (CHOP, POACH)6,7; cyclophosphamide, adriamycin, and prednisone (CAP)8; or multiple alkylating agent regimens such as the M2 regimen.9 Comparative studies have been performed that have not demonstrated superiority of any one regimen in previously untreated patients. The response rates to therapy have varied from 50% to 80%, with the majority of the responses being partial remissions.1-9
More recently, the purine analogs, fludarabine,10-162-chlorodeoxyadenosine,17 and pentostatin,18 have been explored in previously treated patients with CLL. Fludarabine, in particular, had a very high response rate, with a substantial number of complete remissions in previously treated patients with CLL.10-16 Increasingly purine analogs are being used as initial therapy for patients with CLL.11,18,19 Two reports from the M.D. Anderson Cancer Center (MDACC; Houston, TX) using fludarabine with or without prednisone have already been published, but long-term follow-up data of these studies are not available.11,20 In particular, little information has been provided regarding time to progression in responding patients and the results of re-treatment with fludarabine or other regimens. Randomized comparisons between fludarabine and chlorambucil or between fludarabine, CAP, and CHOP regimens21-23 have recently been conducted in the United States and Europe.
The purpose of this report is to present an overall evaluation of the response to fludarabine regimens as initial therapy of patients with CLL, the response rate, toxicity, time to progression, results of re-treatment, and long-term survival.
PATIENTS AND METHODS
Three different fludarabine studies form the basis of this report. The first used fludarabine at 25 to 30 mg/m2 daily for 5 days every 4 weeks administered as part of a phase I/II clinical trial (FLU-PhI-II).10 The next regimen was the combination of fludarabine at 30 mg/m2/d for 5 days combined with prednisone at 30 mg/m2/d for 5 days every 4 weeks (FLU+Pred).11 After completion of the fludarabine + prednisone protocol, subsequent patients were treated with fludarabine at 30 mg/m2/d for 5 days every 4 weeks on a current practice research protocol (FLU-CP) and are classified as FLU together with the phase I-II protocol patients. In the phase I-II protocol, the number of courses was not specified. In the latter 2 protocols, all patients were projected to receive 6 cycles of therapy and continue therapy until complete response or treatment failure. However, some patients discontinued treatment after achieving complete remission in less than 6 courses. Some patients who had not achieved a complete remission after 6 courses but were continuing to improve their clinical status had additional therapy. The median number of courses was 6 (range, 2 to 11). Patients were eligible for treatment if they fulfilled the National Cancer Institute (NCI) recommendations for therapy, having advanced stage disease (Rai III and IV) or progressive stage I and II disease.24
One hundred seventy-four patients fulfilled the criteria for treatment with Rai stage I-IV disease. The median age of the 174 Rai III-IV patients was 61 years (Table 1). Two-thirds of the patients were male and 38% had Rai stage III and IV disease. The range of times from diagnosis to treatment was wide, but the median time was 9 months. Few patients had B symptoms, and the majority of patients had performance status 0 to 1 using the Zubrod performance scale. Less than 10% of patients had a history of prior infection (Table 1).
Characteristics of 174 Fludarabine-Treated CLL Patients
| Median age (yr) (range) | 61 (25-84) |
| Male/female | 110/64 |
| Rai | |
| I/II | 59/49 (108) |
| III/IV | 38/28 (66) |
| Time from diagnosis to Rx | 9 mo (0-346) |
| B symptoms | 16 (9%) |
| Performance status (0, 1, 2) | (93, 75, 6) |
| WBC ×103/mL (range) | 61.7 (5.1-430.0) |
| Prior infection | 12 (7%) |
| Median age (yr) (range) | 61 (25-84) |
| Male/female | 110/64 |
| Rai | |
| I/II | 59/49 (108) |
| III/IV | 38/28 (66) |
| Time from diagnosis to Rx | 9 mo (0-346) |
| B symptoms | 16 (9%) |
| Performance status (0, 1, 2) | (93, 75, 6) |
| WBC ×103/mL (range) | 61.7 (5.1-430.0) |
| Prior infection | 12 (7%) |
Patients were started on treatment between 1986 and 1993. Informed consent was obtained according to institutional guidelines. All patients had a workup including history and physical examination; complete blood counts; differential and platelet counts; sequential multiple analysis-12 (SMA-12), including liver and renal function studies; bone marrow aspiration and biopsy; and blood and marrow samples for immunophenotyping and molecular studies. The criteria required for a confirmation of the diagnosis were a monotypic expansion of lymphoid cells ≥5 × 103/μL morphologically consistent with CLL (small lymphocytes) in the blood for 2 months before treatment and greater than 30% lymphocytes in the bone marrow. One hundred fifty patients were proven to be CD5+, 18 were CD5− (<20%), and 6 had no immunophenotyping studies performed. Normal renal and hepatic functions (creatine <2 mg% and bilirubin <2 mg%) were required. Patients were evaluated for marrow response after each 3 courses. NCI Working Group criteria for response were used.24 Complete remission (CR) required disappearance of all palpable disease, a neutrophil count greater than 1,500/μL, a platelet count greater than 100,000/μL, a hemoglobin level greater than 11 g/dL, and a bone marrow aspirate lymphocyte percentage of less than 30%. Patients fulfilling the criteria noted above but with persistent lymphoid aggregates or nodules in the bone marrow biopsy were classified within the partial remission (PR) group as PR-Nod. Other PRs required ≥50% decrease in palpable disease as well as ≥50% improvement of all abnormal blood parameters. No bone marrow evaluation was required for determination of PR. Computerized tomography scans were not required to stage patients or evaluate response.
STATISTICAL CONSIDERATIONS
Associations between patient characteristics and response to outcome were evaluated using the χ2 test. Cut-points for quantitative variables were those defining abnormal levels or other cut-points in common use. Distributions of survival and time to progression were estimated by the method of Kaplan-Meier. Survival intervals were measured from the first day of chemotherapy to death from any cause. Time to progression was measured from the first day of chemotherapy to the first detection of relapse from CR (defined as >10,000 lymphocytes/μL in the peripheral blood, development of anemia or thrombocytopenia, or more than 50% lymphocytes in the bone marrow aspirate), reappearance of lymphadenopathy, hepatomegaly, splenomegaly, or extramedullary disease. Development of Richter’s transformation was considered to be a relapse. Time to progression in PR patients was defined as ≥50% increase in size of residual abnormalities in liver, spleen, or lymph nodes, a consistently increasing lymphocyte count to a level of at least greater than 10,000/μL, or development of anemia or thrombocytopenia.
RESULTS
Response to induction regimen.
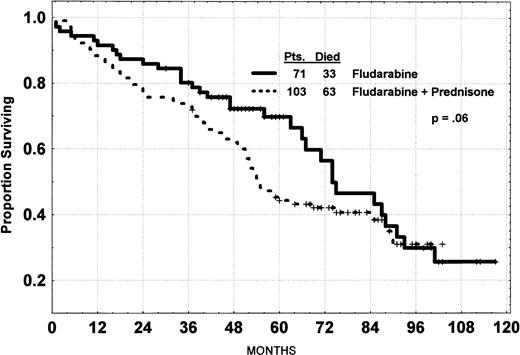
The overall response rate for 174 patients treated with fludarabine regimens was 78% (29% CR, 32% PR-Nod, and 17% PR), and the overall median survival was 63 months. Five patients were considered to be early deaths, having died without time to recover from at least 3 courses of chemotherapy. There was no significant difference in the overall response rate for the regimens, FLU (57/71 [80%]) compared with the FLU+Pred group (79/103 [77%]). However, the CR rate for FLU+Pred (24/103 [23%]) was significantly less than for FLU (27/71 [38%]; P = .04). The vast majority of the 38 patients classified as nonresponders had a significant response in the peripheral blood lymphocytes (69%), nodes (57%), spleen (67%), liver (60%), and bone marrow (36%). Two of 8 patients who had a tumor CR or PR but failed to respond to treatment because of persistent cytopenia are still alive at 48 and 68 months. The survival curves show no significant difference in survival for the 3 regimens, with the median values being 74, 55, and 47+ months. All patients receiving FLU-PhI-II have completed 98 months of follow-up; the median follow-up of the FLU+Pred group is 62 months and that of the FLU-CP protocol is 39 months. The curve for FLU+Pred versus the other 2 protocols combined is shown in Fig 1.
Survival of CLL patients treated with fludarabine alone or with prednisone. P value, log rank; NB median survival, 63 months.
Survival of CLL patients treated with fludarabine alone or with prednisone. P value, log rank; NB median survival, 63 months.
Time to progression.
The median time to progression for CR, PR-Nod, and PR patients was 31 months, being significantly longer for the true CR patients (37 months) compared with PR-Nod (30 months; Fig 2). No other pretreatment characteristic (shown in Table 2) was associated with the time to progression. There is no indication of a plateau developing on the curve of any group to suggest a cured fraction.
Time to progression of CLL patients by response.P value, log rank.
Time to progression of CLL patients by response.P value, log rank.
Response to Fludarabine Regimens and Survival in Previously Untreated CLL by Pretreatment Characteristics
| Characteristics . | Value . | No. of Patients . | Median Survival (mo) . | P Value Log Rank . |
|---|---|---|---|---|
| Total | — | 174 | 67 | |
| Regimen | No Pred | 71 | 74 | NS |
| With Pred | 103 | 54 | ||
| Age (yr) | <60 | 84 | 75 | |
| 60-69 | 64 | 67 | <.001 | |
| ≥70 | 26 | 32 | ||
| Rai stage | I-II | 108 | 74 | .02 |
| III-IV | 66 | 51 | ||
| Binet stage | A | 53 | 74 | |
| B | 75 | 66 | NS | |
| C | 46 | 52 | ||
| Platelets (×103/μL) | <100,000 | 28 | 71 | NS |
| ≥100,000 | 146 | 63 | ||
| Hemoglobin (G%) | <11 | 54 | 47 | <.001 |
| ≥11 | 120 | 74 | ||
| White blood cell count (×103/μL) | <100,000 | 122 | 69 | .10 |
| ≥100,000 | 52 | 54 | ||
| Hepatomegaly | Yes | 24 | 47 | NS |
| No | 150 | 66 | ||
| Splenomegaly | Yes | 94 | 56 | NS |
| No | 80 | 67 | ||
| Node sites | 0 | 23 | 49 | |
| 1-2 | 69 | 67 | NS | |
| 3 | 81 | 58 | ||
| Blood urea nitrogen (mg%) | <23 | 145 | 71 | |
| ≥23 | 27 | 33 | <.001 | |
| LDH | Normal | 71 | 66 | |
| Elevated | 102 | 75 | NS | |
| β2M (mg%) | <3 | 36 | 75+ | |
| 3.0-3.9 | 21 | 55 | ||
| ≥4 | 26 | 56 | .05 | |
| IgG (mg%) | <650 | 41 | 67 | |
| ≥650 | 98 | 61 | NS | |
| IgA (mg%) | <75 | 65 | 56 | |
| ≥75 | 74 | 74 | NS | |
| IgM (mg%) | <30 | 28 | 54 | |
| ≥30 | 111 | 67 | NS | |
| Marrow cellularity (clot section) | <50 | 36 | 60 | |
| 50-84 | 68 | 67 | ||
| ≥85 | 57 | 49 | NS | |
| Marrow lymphocytes (%) | <70 | 43 | 55 | |
| 70-89 | 92 | 74 | ||
| ≥90 | 34 | 44 | .09 | |
| Cytogenetics* | Diploid | 100 | 83 | |
| Other | 34 | 53 | .02 | |
| Insufficient | 27 | 59 |
| Characteristics . | Value . | No. of Patients . | Median Survival (mo) . | P Value Log Rank . |
|---|---|---|---|---|
| Total | — | 174 | 67 | |
| Regimen | No Pred | 71 | 74 | NS |
| With Pred | 103 | 54 | ||
| Age (yr) | <60 | 84 | 75 | |
| 60-69 | 64 | 67 | <.001 | |
| ≥70 | 26 | 32 | ||
| Rai stage | I-II | 108 | 74 | .02 |
| III-IV | 66 | 51 | ||
| Binet stage | A | 53 | 74 | |
| B | 75 | 66 | NS | |
| C | 46 | 52 | ||
| Platelets (×103/μL) | <100,000 | 28 | 71 | NS |
| ≥100,000 | 146 | 63 | ||
| Hemoglobin (G%) | <11 | 54 | 47 | <.001 |
| ≥11 | 120 | 74 | ||
| White blood cell count (×103/μL) | <100,000 | 122 | 69 | .10 |
| ≥100,000 | 52 | 54 | ||
| Hepatomegaly | Yes | 24 | 47 | NS |
| No | 150 | 66 | ||
| Splenomegaly | Yes | 94 | 56 | NS |
| No | 80 | 67 | ||
| Node sites | 0 | 23 | 49 | |
| 1-2 | 69 | 67 | NS | |
| 3 | 81 | 58 | ||
| Blood urea nitrogen (mg%) | <23 | 145 | 71 | |
| ≥23 | 27 | 33 | <.001 | |
| LDH | Normal | 71 | 66 | |
| Elevated | 102 | 75 | NS | |
| β2M (mg%) | <3 | 36 | 75+ | |
| 3.0-3.9 | 21 | 55 | ||
| ≥4 | 26 | 56 | .05 | |
| IgG (mg%) | <650 | 41 | 67 | |
| ≥650 | 98 | 61 | NS | |
| IgA (mg%) | <75 | 65 | 56 | |
| ≥75 | 74 | 74 | NS | |
| IgM (mg%) | <30 | 28 | 54 | |
| ≥30 | 111 | 67 | NS | |
| Marrow cellularity (clot section) | <50 | 36 | 60 | |
| 50-84 | 68 | 67 | ||
| ≥85 | 57 | 49 | NS | |
| Marrow lymphocytes (%) | <70 | 43 | 55 | |
| 70-89 | 92 | 74 | ||
| ≥90 | 34 | 44 | .09 | |
| Cytogenetics* | Diploid | 100 | 83 | |
| Other | 34 | 53 | .02 | |
| Insufficient | 27 | 59 |
Abbreviation: NS, not significant.
Thirteen not performed.
Prognostic factors for survival.
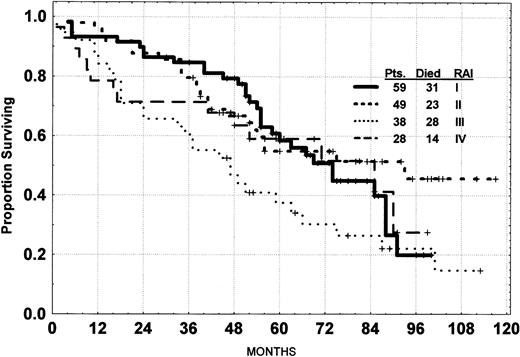
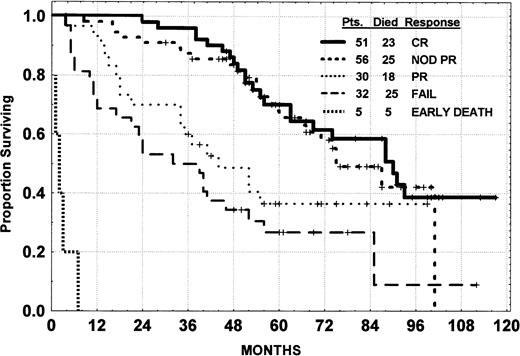
Survival of patients with advanced Rai stage III and IV was modestly inferior to that for Rai stage I and II (Fig 3). There was no significant difference in survival according to whether patients had a true CR or PR-Nod (Fig 4). The survival of the CR and PR-Nod groups was significantly superior to that of the PR patients (P < .01), who, in turn, had a significantly longer survival than the resistant patients.
Survival of CLL patients treated with fludarabine by Rai stage.
Survival of CLL patients treated with fludarabine by Rai stage.
Survival of CLL patients treated with fludarabine with or without prednisone by response.
Survival of CLL patients treated with fludarabine with or without prednisone by response.
Rai stage was significantly associated with survival. A hemoglobin level of less than 11 g% was significantly associated with a shorter survival, but no such association was noted for platelet count. There was no significant association of measures of tumor burden such as enlargement of liver or spleen, number of involved node sites, white blood cell count, bone marrow cellularity, or lymphocyte percentage with survival. Patients more than 70 years of age survived for a shorter time than did younger patients. The level of blood urea nitrogen in the serum was significantly associated with survival, as was the serum β2-microglobulin. Cytogenetic analysis was performed on 161 patients before therapy, but 27 patients had insufficient metaphases for analysis. The 100 patients with a diploid karyotype had a significant shorter survival than the aneuploid patients (P< .01). Abnormalities (abn) in chromosome 11q were noted in 7 patients, trisomy 12 in 7 patients, abn 13q in 3 patients, abn 14q in 6 patients, and other changes in 11 patients. The small number of patients in each group prevented meaningful statistical analysis for response or survival. None of the other factors had a significant impact on survival (Table 2).
Mortality.
The survival of patients on fludarabine and prednisone is slightly inferior to the survival of patients on fludarabine as a single agent (P = .06; Fig 1). Ninety-six patients have died. Nine of these patients died during remission induction: 6 of infection, 1 of progressive uncontrolled CLL, 1 of cardiac failure, and 1 with a stroke. Five patients died in remission. Two patients died of pulmonary infection with unknown pathogen. One patient had a myocardial infarction. Another patient developed Hodgkin’s disease and died of this disease without recurrence of the CLL. One patient died of liver cancer. Eighty-two patients died after progression of their disease or failing to respond to remission induction therapy. Thirty-five of these patients died of infection and 7 died of CLL, according to their local physician. Five patients died of unrelated causes, being either myocardial infarctions, cardiac arrests, or stroke. Nine patients had developed Richter’s syndrome (large-cell lymphoma) at 13 to 62 months after the initiation of treatment and 8 have died. The cumulative incidence of large-cell lymphomatous transformation is projected to be 8%. In addition, 3 patients developed and died from Hodgkin’s disease. Five other patients have developed cancer: 2 with lung cancer, 1 with ovarian cancer, 1 with colon cancer, and 1 with head and neck cancer. Four patients died after receiving a transplantation, 4 more died of hemorrhage, and 2 died with renal failure. No cause of death was established on follow-up in 9 patients.
Retreatment.
Ninety-one patients who have come off study have received salvage therapy at MDACC. Sixty-three patients have been rechallenged with a fludarabine-containing regimen and 41 (67%) have responded (Table 3). For patients receiving other regimens, the response rate was 7 of 28 (25%). A number of different treatment regimens were used, as shown in Table 3. No response was noted for investigational agents such as Taxol or Topotecan. One patient achieved a PR-Nod with chlorodeoxyadenosine (2-CDA) and 2 patients responded to allogeneic bone marrow transplantation (both CRs). The CR rates were higher in patients who had achieved a true CR (11/29 [38%]) on their initial fludarabine treatment than for PR-Nod patients (4/30 [13%]). Seven of 16 (44%) patients who achieved a PR on their initial fludarabine regimen responded again when challenged, but none obtained a CR. No patient who had initially failed to respond to fludarabine had a response when retreated with a fludarabine combination. It is interesting that 4 of the 5 patients who failed to have an initial response to fludarabine regimens but were treated with CHOP obtained CRs (2) or PRs (2).
Response to Salvage Therapy in Patients Retreated After Failing or Relapsing From Fludarabine Regimens
| . | Fludarabine Retreatment CR + PR/Total (%) . | Other Treatment CR + PR/Total (%) . |
|---|---|---|
| Initial response | ||
| Overall | 13 + 28/63 (67) | 4 + 3/28 (25) |
| CR | 10 + 8/25 (72) | 1 + 0/4 (25) |
| PR-Nod | 3 + 13/23 (64) | 1 + 1/7 (28) |
| PR | 0 + 7/11 (70) | 0/5 (—) |
| Fail | 0/4 (—) | 2 + 2/12 (33) |
| Time to progression (CR, PR-Nod, PR) | ||
| <18 mo | 2 + 5/11 (64) | 0/8 (—) |
| 18-35.9 mo | 8 + 16/31 (77) | 2 + 1/5 (60) |
| ≥36 mo | 3 + 7/17 (59) | 0/3 (—) |
| Regimen | ||
| Fludarabine (3 or 5 days) | 8 + 18/35 (74) | |
| Fludara + Mitoxantrone | 0 + 3/9 (33) | |
| FLAP | 1 + 0/4 (25) | |
| Flud. + Ara-C + CDDP | 0/2 (—) | |
| Flud. + Cyclophosphamide | 4 + 7/13 (85) | |
| 2CDA | — | 0 + 1/2 (50) |
| CHOP | — | 2 + 2/7 (57) |
| Chlorambucil | — | 0/2 (—) |
| Allogeneic BMT | — | 2 + 0/4 (50) |
| VP-16 | — | 0/3 (—) |
| Taxol | — | 0/2 (—) |
| ASHAP | — | 0/1 (—) |
| Topotecan | — | 0/7 (—) |
| . | Fludarabine Retreatment CR + PR/Total (%) . | Other Treatment CR + PR/Total (%) . |
|---|---|---|
| Initial response | ||
| Overall | 13 + 28/63 (67) | 4 + 3/28 (25) |
| CR | 10 + 8/25 (72) | 1 + 0/4 (25) |
| PR-Nod | 3 + 13/23 (64) | 1 + 1/7 (28) |
| PR | 0 + 7/11 (70) | 0/5 (—) |
| Fail | 0/4 (—) | 2 + 2/12 (33) |
| Time to progression (CR, PR-Nod, PR) | ||
| <18 mo | 2 + 5/11 (64) | 0/8 (—) |
| 18-35.9 mo | 8 + 16/31 (77) | 2 + 1/5 (60) |
| ≥36 mo | 3 + 7/17 (59) | 0/3 (—) |
| Regimen | ||
| Fludarabine (3 or 5 days) | 8 + 18/35 (74) | |
| Fludara + Mitoxantrone | 0 + 3/9 (33) | |
| FLAP | 1 + 0/4 (25) | |
| Flud. + Ara-C + CDDP | 0/2 (—) | |
| Flud. + Cyclophosphamide | 4 + 7/13 (85) | |
| 2CDA | — | 0 + 1/2 (50) |
| CHOP | — | 2 + 2/7 (57) |
| Chlorambucil | — | 0/2 (—) |
| Allogeneic BMT | — | 2 + 0/4 (50) |
| VP-16 | — | 0/3 (—) |
| Taxol | — | 0/2 (—) |
| ASHAP | — | 0/1 (—) |
| Topotecan | — | 0/7 (—) |
Abbreviations: CHOP, cyclophosphamide + doxorubicin + vincristine + prednisone; FLAP, fludarabine + doxorubicin + prednisone; Ara-C, cytosine arabinoside; CDDP, cis-platinum; BMT, bone marrow transplantation.
Immune reconstitution.
Thirty-one patients had an IgG level less than 650 mg% (the lower limit of normal for our laboratory) and had at least 2 follow-up values performed to evaluate the response of the Ig level to fludarabine therapy. The mean of all IgG values from start of fludarabine until the patients commenced on another regimen was established. Of the 31 patients with a low IgG level before fludarabine, 12 (39%) returned to a normal level. Sixteen (51%) had an increase of more than 100 mg% from their pretreatment level and 2 patients had a decrease of 100 mg% or more from their pretreatment value. Thirteen patients had increases or decreases in the IgG level of lower amplitude. Fifty-three patients had an IgA level less than 75 mg%. Fourteen (26%) had a return of the IgA level to normal, 15 had an increase of 20 mg% or more (28%), and 2 (4%) had a decrease of 20 mg% or more. The other 36 patients had no substantial change. The IgM level was less than 30 mg% in 23 patients. Twelve (52%) had an increase to the normal range, 13 had an increase of 10 mg% (56%), and 1 (4%) had a decrease of more than 10 mg%. The other 9 had no substantial change. No significant correlation appears to exist with the response of the patients. Patients who failed to respond had a change in their Ig levels equivalent to the CR, PR-Nod, and PR patients.
T-cell levels.
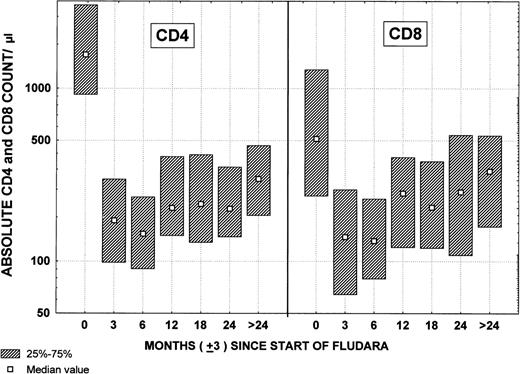
Pretreatment CD4 and CD8 lymphocyte counts were available on 127 patients. The median CD4 count was 1,562/μL and the median CD8 count was 510/μL. Ninety-seven patients had CD4 and CD8 estimates after the third course and 44 patients had estimates after the sixth course of treatment (Fig 5). The median CD4 count after 3 courses was 172/μL and after 6 courses was 163/μL. The median CD8 counts after the third course and after the sixth course were 138/μL and 133/μL, respectively.
Changes in CD4 and CD8 lymphocyte counts by time after fludarabine therapy.
Changes in CD4 and CD8 lymphocyte counts by time after fludarabine therapy.
Because of concern over the low CD4 and CD8 counts, the incidence of infections that occurred while patients were in remission off treatment until they showed evidence of progressive disease or needed other treatment was examined. The number of episodes has been divided by the number of patient years at risk, which were computed by summing the time from recovery of the last course of chemotherapy until the patient went off study because of progressive disease or moved to some other treatment regimen (remission time off therapy). Of the 137 patients who achieved a CR, PR-Nod, or PR, 94 episodes occurred during remission (Table 4). The most common single event was dermatomal herpes zoster, occurring in 19 patients. Upper respiratory, sinus, and bronchial infections and pneumonia (a total of 40 episodes); 6 cases of influenza (diagnosed clinically); 5 episodes of reactivation of herpes simplex; and 10 urinary tract infections were noted. One episode of septicemia was caused by Listeria monocytogenes. No episode of Pneumocystis carinii pneumonia was noted during remission; 1 case of cytomegalovirus infection was seen. These 94 episodes of fever or infection occurred in 313.2 patient years at risk, giving an overall incidence of episodes of infection or fever of approximately 1 for each 3.3 patient years at risk.
Incidence of Infections While in Remission by Response to Fludarabine
| Infection . | CR (51 patients) . | PR-Nod (56 patients) . | PR (30 patients) . | Total (137 patients) . |
|---|---|---|---|---|
| Pneumonia | 2 (0.01) | 5 (0.04) | 5 (0.13) | 12 (0.04) |
| Septicemia | 0 (—) | 0 (—) | 1 (0.03) | 1 (0.003) |
| Sino-bronchial | 3 (0.02) | 6 (0.05) | 6 (0.16) | 15 (0.05) |
| Upper respiratory | 3 (0.02) | 6 (0.05) | 4 (0.10) | 13 (0.04) |
| Influenza | 3 (0.02) | 3 (0.03) | 0 (—) | 6 (0.02) |
| Minor/FUO | 6 (0.04) | 3 (0.03) | 4 (0.10) | 13 (0.04) |
| Herpes simplex | 2 (0.01) | 3 (0.03) | 0 (—) | 5 (0.02) |
| Herpes zoster | 10 (0.06) | 8 (0.07) | 1 (0.03) | 19 (0.06) |
| Urinary | 5 (0.03) | 4 (0.03) | 1 (0.03) | 10 (0.03) |
| Total | 34 (0.22) | 38 (0.32) | 22 (0.57) | 94 (0.30) |
| Patient years at risk | 157.44 | 117.33 | 38.55 | 313.32 |
| Infection . | CR (51 patients) . | PR-Nod (56 patients) . | PR (30 patients) . | Total (137 patients) . |
|---|---|---|---|---|
| Pneumonia | 2 (0.01) | 5 (0.04) | 5 (0.13) | 12 (0.04) |
| Septicemia | 0 (—) | 0 (—) | 1 (0.03) | 1 (0.003) |
| Sino-bronchial | 3 (0.02) | 6 (0.05) | 6 (0.16) | 15 (0.05) |
| Upper respiratory | 3 (0.02) | 6 (0.05) | 4 (0.10) | 13 (0.04) |
| Influenza | 3 (0.02) | 3 (0.03) | 0 (—) | 6 (0.02) |
| Minor/FUO | 6 (0.04) | 3 (0.03) | 4 (0.10) | 13 (0.04) |
| Herpes simplex | 2 (0.01) | 3 (0.03) | 0 (—) | 5 (0.02) |
| Herpes zoster | 10 (0.06) | 8 (0.07) | 1 (0.03) | 19 (0.06) |
| Urinary | 5 (0.03) | 4 (0.03) | 1 (0.03) | 10 (0.03) |
| Total | 34 (0.22) | 38 (0.32) | 22 (0.57) | 94 (0.30) |
| Patient years at risk | 157.44 | 117.33 | 38.55 | 313.32 |
Incidence is the number of episodes per patient years at risk. Values are the number of episodes with the incidence in parentheses.
There was a strong association with the quality of the remission and the probability of patients developing these episodes (Table 4). Patients who achieved PR had a greater likelihood of developing infections or febrile episodes than did patients who had PR-Nod, whereas the least morbidity occurred in CR patients who achieved a CR (P < .01). No relationship was found between the CD4 count at the end of therapy and the risk of fever/infection during remission (Table 5). In addition, infection or febrile episodes decreased as length of remission increased, whereas the return to normal CD4 counts was more gradual (Table6 and Fig 5). This may explain the lower incidence of episodes in the FLU+Pred patients who have shorter median follow-up (Table 5).
Infections/Febrile Episodes During Remission of CLL Patients Receiving Fludarabine Regimens as Initial Therapy
| Characteristic . | Value . | No. of Patients . | No. of Episodes . | Years at Risk . | Incidence . |
|---|---|---|---|---|---|
| Regimen | No Pred | 57 | 28 | 142.98 | 1/5.11 |
| Pred | 80 | 66 | 170.34 | 1/2.58 | |
| CD4 count at end of Rx | <100/μL | 31 | 27 | 82.05 | 1/3.03 |
| 100-200/μL | 31 | 21 | 68.06 | 1/3.24 | |
| >200/μL | 30 | 21 | 61.39 | 1/2.92 | |
| Total | — | 137 | 94 | 313.32 | 1/3.33 |
| Characteristic . | Value . | No. of Patients . | No. of Episodes . | Years at Risk . | Incidence . |
|---|---|---|---|---|---|
| Regimen | No Pred | 57 | 28 | 142.98 | 1/5.11 |
| Pred | 80 | 66 | 170.34 | 1/2.58 | |
| CD4 count at end of Rx | <100/μL | 31 | 27 | 82.05 | 1/3.03 |
| 100-200/μL | 31 | 21 | 68.06 | 1/3.24 | |
| >200/μL | 30 | 21 | 61.39 | 1/2.92 | |
| Total | — | 137 | 94 | 313.32 | 1/3.33 |
Incidence of Major and Minor Infections/FUO or Herpes Zoster During Remission After Fludarabine Therapy
| . | Time From Discontinuation of Treatment (mo) . | |||||
|---|---|---|---|---|---|---|
| 0-6 . | 7-12 . | 13-18 . | 19-24 . | 25-30 . | 30-36 . | |
| Patients at risk | 137 | 133 | 125 | 113 | 102 | 95 |
| Major* | 4 (2.9%) | 1 (0.8%) | 4 (3.22%) | 3 (2.7%) | 0 (—) | 0 (—) |
| Minor/FUO | 24 (17.5%) | 18 (13.5%) | 12 (9.6%) | 3 (2.7%) | 3 (2.9%) | 4 (4.2%) |
| Herpes zoster | 6 (4.4%) | 6 (4.5%) | 3 (2.4%) | 2 (1.8%) | 1 (1%) | 0 (—) |
| Total | 34 (24.8%) | 25 (18.8%) | 19 (15.5%) | 8 (7.1%) | 4 (3.9%) | 4 (4.2%) |
| . | Time From Discontinuation of Treatment (mo) . | |||||
|---|---|---|---|---|---|---|
| 0-6 . | 7-12 . | 13-18 . | 19-24 . | 25-30 . | 30-36 . | |
| Patients at risk | 137 | 133 | 125 | 113 | 102 | 95 |
| Major* | 4 (2.9%) | 1 (0.8%) | 4 (3.22%) | 3 (2.7%) | 0 (—) | 0 (—) |
| Minor/FUO | 24 (17.5%) | 18 (13.5%) | 12 (9.6%) | 3 (2.7%) | 3 (2.9%) | 4 (4.2%) |
| Herpes zoster | 6 (4.4%) | 6 (4.5%) | 3 (2.4%) | 2 (1.8%) | 1 (1%) | 0 (—) |
| Total | 34 (24.8%) | 25 (18.8%) | 19 (15.5%) | 8 (7.1%) | 4 (3.9%) | 4 (4.2%) |
*Pneumonia and/or septicemia.
DISCUSSION
This analysis focuses on long-term outcome for patients who receive fludarabine or fludarabine plus prednisone as initial therapy for progressive or advanced CLL. No difference was noted for the addition of prednisone to fludarabine in terms of response rate or survival. Three recently described studies confirm a higher response rate for fludarabine in comparison with chlorambucil, CAP, and French CHOP, but no survival advantage.21-23 Smaller studies suggest a similar response rate to 2-CDA19,25,26 without adequate follow-up data.
Our data demonstrate a long time to progression in CR (30 to 37 months) and PR patients (27 months) that is similar to a multicenter report for fludarabine (35+ months) and superior to CAP (<12 months).21 The duration of response reported for chlorambucil and prednisone was 2 years and for CVP was 1.9 years in an ECOG study.5 To date, neither of the fludarabine comparative trials has defined a survival advantage for fludarabine.21,23 Comparison of the data in this report with our previous 2 studies of CAP and POACH (median survival of 5 years in both) shows only a modest increase in overall survival.7 8
The only component of the Rai and Binet staging systems associated with survival was the hemoglobin level. The 28 Rai stage IV patients had an excellent survival in this study (71+ months). A high blood urea nitrogen (BUN) level was associated with a short survival and high early death rate. Because fludarabine is excreted largely by the kidneys, modification of dosage may be recommended, perhaps using a 3-day schedule.12 Unfortunately, the β2-microglobulin level before therapy was not available on all patients, because it appears to be the characteristic most strongly associated with survival.27
The risk of administering fludarabine as initial therapy for CLL has been addressed, and deaths in the first year were associated with well-known adverse features such as age, stage, tumor burden, and myelosuppression.10,11 There was no increase in toxicity or early death reported in comparative studies with chlorambucil or CAP.21 23 Thus, fludarabine used with appropriate levels of caution is safe and effective as induction therapy in previously untreated patients with CLL.
Depression of CD4 and CD8 counts was marked in this study, occurring mainly during the first 3 courses of therapy, as reported in other studies.10-12 No difference in these values was noted between patients treated with or without prednisone. Recovery from this effect after fludarabine was discontinued was slow. Despite the persistent T-cell suppression, infections and febrile episodes were uncommon during remission and decreased with time of follow-up. No association was noted between infection rate and the CD4 level at the end of fludarabine treatment. Thus, the risk of infection appears to be low during remission, but vigilance for opportunistic infections is always necessary.28 The improvement of IgG, IgA, and IgM levels in many patients after therapy was encouraging and may contribute to the low incidence of opportunistic infections.
Concern has been expressed about the responsiveness to salvage therapy after relapse in patients who receive fludarabine as initial therapy. As we have shown, most patients achieve a second remission. Second remissions are more likely in patients achieving CR with initial therapy than in patients achieving PR or failing to respond to induction therapy. Surprisingly, the salvage response rate was not associated with the length of the first remission. Most patients were rechallenged with a fludarabine-based regimen. Combination therapies tended to be administered to patients with the shortest initial response and to those with evidence suggestive of transformation. Some fludarabine-resistant patients responded to CHOP-like regimens but not to new agents such as Taxol or Topotecan.
Responsiveness of patients to salvage therapy raises the question of the optimum duration of fludarabine treatment. If patients are still responding without toxicity, should patients be treated until there is no evidence of disease using techniques such as two-parameter flow cytometry or polymerase chain reaction29,30? Should maintenance therapy with fludarabine (perhaps an oral formulation), alkylating agents, or biologic agents be attempted? These are appropriate questions for subsequent clinical trials. In conclusion, fludarabine is effective and relatively safe as initial therapy for CLL with no evidence yet for a survival advantage over previous regimens. Future studies should be planned to increase the incidence of true CRs in an attempt to prolong survival in patients with this disease.
Address reprint requests to M.J. Keating, MD, Department of Hematology, Box 92, The University of Texas, M.D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal