Abstract
The occurrence of large cell transformation has been well documented in a subgroup of patients with mycosis fungoides/Sezary syndrome (MF/SS). However, because of the rarity of MF/SS, little is known about the influence of clinicopathologic features in predicting large cell transformation and about outcome in the transformed cases. We evaluated all patients with MF/SS who were registered in our clinic during the study period and for whom pathologic slides for review were available or could be obtained. Disease was classified as transformed if biopsy showed large cells (≥4 times the size of a small lymphocyte) in more than 25% of the infiltrate or if they formed microscopic nodules. Twenty-six patients with transformation were identified from a total of 115 evaluable cases with a diagnosis of MF/SS. The actuarial cumulative probability of transformation reached 39% in 12 years. The median time from diagnosis of MF/SS to transformation was 12 months (range, 0 to 128 months). Thirty-one percent of all patients with stage IIB-IV disease at presentation eventually transformed versus 14% of those with stage I-IIA (P= .03), with transformation being especially common in patients with tumors (T3), 46% of whom transformed. Combining elevated β2 microglobulin and lactic dehydrogenase (neither elevated v one or both elevated) was also predictive for transformation (P = .009). The median survival from initial diagnosis of MF/SS for the transformed patients was 37 months versus 163 months for the untransformed group (P = .0029). The median survival from transformation was 19.4 months (range, 2+ to 138 months). The following characteristics were associated with an inferior survival in transformed patients: (1) early transformation (<2 years from the diagnosis v ≥2 years; P = .011) and (2) advanced stage (IIB-IV v I-IIA; 2-year survival, 23% v 86%;P = .0035). We conclude that MF/SS patients with stages IIB-IV disease and, in particular, those with tumors have a high incidence of large-cell transformation. Patients with transformation have a relatively poor survival, especially if transformation occurs early (within 2 years) in the course of disease or if they are staged as IIB or higher.
© 1998 by The American Society of Hematology.
MYCOSIS FUNGOIDES/Sezary syndrome (MF/SS) is characterized pathologically by epidermotropic infiltrates of clonal malignant T lymphocytes with cerebriform nuclei.1-4Although the skin is always involved, disease may occur in extracutaneous sites such as peripheral blood, lymph nodes, or viscera.1-4 The clinical course of MF/SS is usually indolent.5 However, occasionally individuals with MF/SS develop a morphologic change (transformation) from small- to intermediate-sized cerebriform cells to a large cell variant, a condition that may be difficult to differentiate pathologically from certain other lymphoproliferative disorders such as lymphomatoid papulosis and Ki-1–positive anaplastic large-cell lymphoma.6-13 The presence of large cells exceeding 25% of the total lymphoid infiltrate has been used to define large-cell transformation.11 The incidence of such transformation has been reported to range between 8% and 55%.7,11-13Molecular mechanisms responsible for large-cell transformation have not yet been elucidated. However, immunologic and molecular evidence based on T-cell receptor analysis indicates that transformed MF/SS represents evolution of the original malignant clone.14 15
Because of the rarity of MF/SS there are few studies addressing the value of specific characteristics for predicting which MF/SS patients will later undergo large-cell transformation. Therefore, to determine these characteristics as well as the clinical implications and outcome of large-cell transformation in MF/SS, we reviewed the course of 115 patients with MF/SS.
PATIENTS AND MATERIALS
We evaluated all patients with MF/SS who were registered in our clinic between June 1975 and September 1995 and for whom pathologic slides for review were available or could be obtained. Patients had a clinical diagnosis compatible with MF/SS. A pathologic diagnosis of MF/SS was confirmed by our pathologist (M.C.-G.) according to criteria described previously.1 In each case, all pathological slides obtained from referral and through the patients’ entire course were reviewed to determine if evidence existed for morphologic transformation.
Clinical evaluation.
Twenty-six patients with transformation were identified from a total of 115 evaluable cases of confirmed MF/SS seen at the Mycosis Fungoides Clinic at M.D. Anderson Cancer Center during the study period. (A total of 131 patients were seen, but slides were not available for review on 16 of these individuals.) For classification and staging, patients received a complete physical examination, complete blood cell count with examination of the peripheral smear for Sezary/lymphoma cells, serology for human T-cell lymphotropic virus (HTLV-1) and human immunodeficiency virus (HIV), renal and liver function tests, lactic dehydrogenase (LDH), β2 microglobulin (β2M), chest roentgenogram (CXR), and skin biopsy. (Patients with elevated serum creatinine that could account for an elevated serum β2M level were not counted.) Patients with clinically significant adenopathy had their nodes evaluated by fine needle aspiration or lymph node biopsy. When indicated clinically, patients had an extensive staging evaluation, including bone marrow aspirate and biopsy, and appropriate radiologic studies to determine visceral involvement.
After the initial evaluation, staging criteria were used according to the Mycosis Fungoides Cooperative Group (MFCG).16 The extent of the skin involvement is referred to as the clinical T category: T1, limited patch/plaques less than 10% of total skin surface; T2, generalized patch/plaques ≥10% of the total skin surface; T3, tumors; and T4, generalized erythroderma. Lymph nodes were evaluated by physical exam, and biopsy or fine needle aspirate was performed if nodes were enlarged. Adenopathy was considered present if the lymph nodes were palpable and abnormally enlarged (N1). N2 refers to clinically negative, histologically positive nodes. N3 refers to adenopathy with pathological involvement. Patients with Sezary syndrome (SS) were defined as those with erythroderma (T4, stage III) and circulating Sezary cells (lymphoma cells) ≥5%. Finding lymphoma cells in the peripheral blood did not alter the stage of the disease in accordance with the MFCG staging criteria. Stage I patients had plaque disease (<10% involvement of the skin = IA; ≥10% involvement of the skin = IB); stage II patients had clinical adenopathy (IIA) and/or tumors (IIB); stage III patients had erythroderma without (IIIA) or with (IIIB) clinical adenopathy; and stage IV patients had pathological evidence of lymph node (IVA) or visceral involvement (IVB).16
Patients had repeat skin biopsies performed at the time of clinical relapse after treatment. In addition, if adenopathy, tumors, or visceral disease developed or were suspected during the course of follow-up, a fine-needle aspiration or biopsy was performed.
Histopathologic and immunologic analysis.
In accordance with previously defined criteria, cases of MF/SS were classified as having large-cell transformation if at least one biopsy showed large cells exceeding 25% of the infiltrate throughout or if they formed microscopic nodules.11,12 A large cell was defined as a cell that was 4 times or more the size of a small lymphocyte.17 The infiltrate was classified as diffuse, lichenoid, or patchy, and the thickness of the infiltrate was measured. Lichenoid indicated a band-like infiltrate of mononuclear cells in the upper dermis in close proximity to the epidermis; diffuse indicated an interstitial/perivascular/periadnexal mononuclear cell infiltrate throughout the dermis; and patchy indicated a predominantly perivascular and/or periadnexal mononuclear cell infiltrate throughout the dermis.18
The thickness of the infiltrate was measured from the granular cell layer of the epidermis to the bottom of the infiltrate using a micrometer if the measurement was less than 1 mm or using a ruler if greater than 1 mm. The entire thickness of the infiltrate was measured in the cases with a diffuse pattern. Only the thickness of the lichenoid component was measured in the cases with a lichenoid infiltrate. The small foci of lymphocytes around the vessels and/or adnexa in the deep dermis present in some of the lichenoid cases were excluded from the measurement of the thickness. In the cases with a patchy infiltrate, only the upper clearly denser pattern of the infiltrate was included in the measurement of the thickness. The small foci of lymphocytes around vessels and adnexa in the deep dermis were excluded from the measurement of the thickness. The thickness of the infiltrate was not measured in 2 cases because the initial transformation was in the liver and lymph node, respectively.
Immunohistochemical studies were performed on fresh frozen tissue sections in 22 of the 26 cases using the avidin-biotin-peroxidase complex method as previously described.19 We used anti–T-cell antibodies to T-cell–associated antigens [CD2, CD3, CD4, CD5, CD7, CD8, UCHL-1 (CD45RO), and CD43], B-cell–associated antigens (CD19, CD20, and CD22), and antibodies directed against CD25 (interleukin-2 [IL-2] receptor) and CD30 (Ki-1).
Data analysis.
Statistical analyses were performed using Statistica, software version 5.0 for Microsoft windows (StatSoft, Tulsa, OK). The association of transformation with other characteristics at referral was assessed by χ2 or Fisher exact test as appropriate.20Survival was calculated from the date of diagnosis to the date of death or last follow-up evaluation. Survival after transformation was calculated from the time of the first biopsy that established transformation to the date of the death or last follow-up evaluation. Survival and actuarial curves were estimated by the method of Kaplan and Meier.20 Differences between survival curves were tested by the log-rank test21 and generalized Wilcoxon test of Gehan,22 as appropriate . All causes of death were included in the survival analysis.
The following characteristics were evaluated for prognostic value: age, sex, stage, peripheral blood and bone marrow involvement, transformation, type of skin infiltration at transformation (lichenoid, diffuse, patchy), thickness of the infiltrate at transformation, site of transformation (skin, lymph node), response to initial and subsequent treatments before transformation, high LDH, and high β2M. High LDH was defined as ≥10% above the upper limit of the normal range (225 U/L before November 1988 and 618 U/L after November 1988). For β2M, the cut-off value for normal range was 2 mg/L (>2v ≤2; Pharmacia β2M microradioimmunoassay; Pharmacia Diagnostic, Uppsala Sweden).
RESULTS
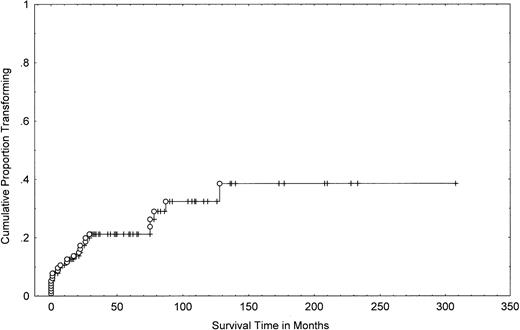
One-hundred fifteen patients with MF/SS were seen at the M.D. Anderson Cancer Center during the study period and had pathologic slides available for review. One-hundred thirteen had been followed for ≥2 years. Twenty-six of the 115 patients (23%) demonstrated morphologic transformation to a large-cell variant at some point during their disease. These patients included 13 women and 13 men. Their median age at transformation was 65 years (range, 29 to 80 years). Serology for HTLV-1 and HIV testing was negative in all cases. The actuarial (cumulative probability) of transformation was 21% (95% confidence interval [CI], 13% to 29%) at 4 years, 32% (95% CI, 20% to 44%) at 8 years, and 39% (95% CI, 23% to 55%) at 12 years (Fig 1).
Cumulative probability of transformation in 115 patients with MF/SS. A Kaplan-Meier curve shows the number of patients who transformed (○) as a function of time, beginning with the diagnosis of MF/SS. Tick marks represent last follow-up of patients without transformation.
Cumulative probability of transformation in 115 patients with MF/SS. A Kaplan-Meier curve shows the number of patients who transformed (○) as a function of time, beginning with the diagnosis of MF/SS. Tick marks represent last follow-up of patients without transformation.
The majority of patients (N =18) had transformation of MF/SS within the first 2 years from diagnosis (Fig 1). Nine patients were diagnosed with transformed MF/SS at (or within 1 month) of initial presentation (Table 1). However, these patients had a long history of dermatitis compatible with clinical MF that preceded the pathological diagnosis of transformed MF/SS.
Phenotypic Features of 26 Patients With Large-Cell Transformation
| Patient No. . | Age-150 . | Interval From Dx of MF/SS to Transformation (mo) . | Stage at Diagnosis . | Stage at Transformation . | Site of Transformation . |
|---|---|---|---|---|---|
| 1 | 56 | 1 | IB (T2N0M0) | IB (T2N0M0) | Skin |
| 2 | 66 | 1 | IIB (T3N0M0) | IIB (T3N0M0) | Skin |
| 3 | 65 | 87 | IIIA (T4N0M0) | IIIA (T4N0M0) | Skin |
| 4 | 71 | 0 | IIA (T2N1M0) | IIA (T2N1M0) | Skin |
| 5 | 69 | 75 | IB (T2N0M0) | IB (T2N0M0) | Skin |
| 6 | 51 | 75 | IB (T2N0M0) | IB (T2N0M0) | Skin |
| 7 | 33 | 5 | IIA (T2N1M0) | IIA (T2N1M0) | Skin |
| 8 | 72 | 128 | IIB (T3N0M0) | IIB (T3N0M0) | Skin |
| 9 | 50 | 29 | IIB (T3N1M0) | IIB (T3N1M0) | Skin |
| 10 | 65 | 17 | IIB (T3N0M0) | IIB (T3N0M0) | Skin |
| 11 | 29 | 5 | IIB (T3N1M0) | IIB (T3N1M0) | Skin |
| 12 | 72 | 0 | IVA (T4N3M0) | IVA (T4N3M0) | Skin, lymph node |
| 13 | 64 | 0 | IVA (T3N3M0) | IVA (T3N3M0) | Skin, lymph node |
| 14 | 65 | 12 | IVA (T3N3M0) | IVA (T3N3M0) | Skin |
| 15 | 65 | 0 | IIB (T3N0M0) | IIB (T3N0M0) | Skin |
| 16 | 59 | 12 | IIB (T3N0M0) | IIB (T3N0M0) | Skin |
| 17 | 62 | 21 | IIIA (T4N0M0) | IIIA (T4N0M0) | Skin |
| 18 | 69 | 22 | IVA (T3N3M0) | IVA (T3N3M0) | Skin |
| 19 | 75 | 0 | IVB (T4N1M1) | IVB (T4N1M1) | Skin |
| 20 | 68 | 1 | IIB (T3N0M0) | IIB (T3N0M0) | Skin |
| 21 | 80 | 78 | IIB (T3N0M0) | IIB (T3N0M0) | Skin |
| 22 | 53 | 26 | IIB (T3N0M0) | IIB (T3N0M0) | Skin |
| 23 | 78 | 0 | IB (T2N0M0) | IB (T2N0M0) | Skin |
| 24 | 60 | 7 | IIA (T2N1M0) | IIA (T2N1M0) | Skin |
| 25 | 62 | 26 | IB (T2N0M0) | IVB (T2N1M1) | Liver |
| 26 | 40 | 22 | IIIA (T4N0M0) | IVA (T4N1M0) | Lymph node |
| Patient No. . | Age-150 . | Interval From Dx of MF/SS to Transformation (mo) . | Stage at Diagnosis . | Stage at Transformation . | Site of Transformation . |
|---|---|---|---|---|---|
| 1 | 56 | 1 | IB (T2N0M0) | IB (T2N0M0) | Skin |
| 2 | 66 | 1 | IIB (T3N0M0) | IIB (T3N0M0) | Skin |
| 3 | 65 | 87 | IIIA (T4N0M0) | IIIA (T4N0M0) | Skin |
| 4 | 71 | 0 | IIA (T2N1M0) | IIA (T2N1M0) | Skin |
| 5 | 69 | 75 | IB (T2N0M0) | IB (T2N0M0) | Skin |
| 6 | 51 | 75 | IB (T2N0M0) | IB (T2N0M0) | Skin |
| 7 | 33 | 5 | IIA (T2N1M0) | IIA (T2N1M0) | Skin |
| 8 | 72 | 128 | IIB (T3N0M0) | IIB (T3N0M0) | Skin |
| 9 | 50 | 29 | IIB (T3N1M0) | IIB (T3N1M0) | Skin |
| 10 | 65 | 17 | IIB (T3N0M0) | IIB (T3N0M0) | Skin |
| 11 | 29 | 5 | IIB (T3N1M0) | IIB (T3N1M0) | Skin |
| 12 | 72 | 0 | IVA (T4N3M0) | IVA (T4N3M0) | Skin, lymph node |
| 13 | 64 | 0 | IVA (T3N3M0) | IVA (T3N3M0) | Skin, lymph node |
| 14 | 65 | 12 | IVA (T3N3M0) | IVA (T3N3M0) | Skin |
| 15 | 65 | 0 | IIB (T3N0M0) | IIB (T3N0M0) | Skin |
| 16 | 59 | 12 | IIB (T3N0M0) | IIB (T3N0M0) | Skin |
| 17 | 62 | 21 | IIIA (T4N0M0) | IIIA (T4N0M0) | Skin |
| 18 | 69 | 22 | IVA (T3N3M0) | IVA (T3N3M0) | Skin |
| 19 | 75 | 0 | IVB (T4N1M1) | IVB (T4N1M1) | Skin |
| 20 | 68 | 1 | IIB (T3N0M0) | IIB (T3N0M0) | Skin |
| 21 | 80 | 78 | IIB (T3N0M0) | IIB (T3N0M0) | Skin |
| 22 | 53 | 26 | IIB (T3N0M0) | IIB (T3N0M0) | Skin |
| 23 | 78 | 0 | IB (T2N0M0) | IB (T2N0M0) | Skin |
| 24 | 60 | 7 | IIA (T2N1M0) | IIA (T2N1M0) | Skin |
| 25 | 62 | 26 | IB (T2N0M0) | IVB (T2N1M1) | Liver |
| 26 | 40 | 22 | IIIA (T4N0M0) | IVA (T4N1M0) | Lymph node |
Abbreviation: Dx, diagnosis.
Age of the patients at transformation.
With regard to site of transformation of MF/SS, it presented initially in skin in 24 cases, in lymph nodes in 3 cases (in 2 of these cases, transformation presented in both skin and lymph nodes), and in liver in 1 case (Table 1).
Histopathologic analysis.
Before transformation, the cells retain the hyperchromatic, cerebriform nuclei of malignant T cells, whereas after transformation, the malignant cells have larger, more vesicular nuclei (Fig 2A and B). Histologic evaluation of the skin lesions in transformed cases demonstrated that most of them (N = 15) had a diffuse lymphoid infiltrate. Five cases had a lichenoid and 4 had a patchy lymphoid infiltrate in the skin. The median thickness of the infiltrate was 2.15 mm (range, 0.5 to 10 mm; 95% CI, 1.875 to 4.174 mm). Patients with tumor stage (T3) disease had thicker lymphoid infiltrates compared with patients with T2 or T4 stage disease, as well as an increased incidence of diffuse pattern of infiltration (P= .0123 and P = .03, respectively).
(A) MF before transformation. The Pautrier’s microabscesses show intermediate-sized lymphocytes with hyperchromatic cerebriform nuclei. (B) MF after transformation. There is a micronodule in the papillary dermis composed of large lymphoid cells with clear vesicular nuclei and prominent nucleoli characteristic of large-cell transformation.
(A) MF before transformation. The Pautrier’s microabscesses show intermediate-sized lymphocytes with hyperchromatic cerebriform nuclei. (B) MF after transformation. There is a micronodule in the papillary dermis composed of large lymphoid cells with clear vesicular nuclei and prominent nucleoli characteristic of large-cell transformation.
Immunophenotypic analysis.
Immunohistochemical results were reported in 22 of the 26 cases of transformed MF/SS. In all cases, transformed large cells were typed as being of T-cell origin. All cases except 1 (case no. 24) examined for CD4 (T-helper cell) were positive for this marker. This case was CD4− and CD8−. Two cases were CD8+ (T-suppressor cell marker) as well as CD4+(cases no. 1 and 11). Immunologic typing was performed on pretransformation and posttransformation biopsies in 4 cases and showed no differences. At the time of transformation, CD25 (IL-2 receptor) marker was weakly positive in all of the 4 cases in which it was tested. CD30 (Ki-1) expression was present in 7 cases and weakly expressed in 1 additional case of a total of 15 cases analyzed for this marker.
Correlation between characteristics at referral and eventual transformation.
There was no difference in age group or in sex distribution between patients with and without eventual transformation (Table 2). There was also no statistical difference between the incidence of peripheral blood (≥5% of Sezary/lymphoma cells) or lymph node involvement in patients with and without eventual transformation.
Characteristics at Referral in Patients With and Without Eventual Transformation
| Characteristic . | No. of Patients . | No. With Transformation . | P Value* . |
|---|---|---|---|
| Age | |||
| <60 yr | 60 | 11 | .25 |
| ≥60 yr | 55 | 15 | |
| Sex | |||
| Male | 65 | 13 | .48 |
| Female | 50 | 13 | |
| Stage | |||
| I-II v | 78 | 18 | .94 |
| III-IV | 37 | 8 | |
| I-IIA v | 58 | 8 | .03 |
| IIB-IV | 57 | 18 | |
| T staging | |||
| T1 | 36 | 0 | .000005 |
| T2 | 24 | 8 | |
| T3 | 28 | 13 | |
| T4 | 27 | 5 | |
| β2M (mg/L) | |||
| Low (≤2) | 54 | 11 | .080 |
| High (>2) | 38 | 14 | |
| LDH (U/L) | |||
| Low | 74 | 16 | .52 |
| High† | 37 | 10 | |
| LDH + β2M‡ | |||
| No v | 46 | 7 | .009 |
| 1 and/or 2 | 46 | 18 | |
| Lymph node involvement | |||
| No | 79 | 16 | .37 |
| Yes | 36 | 10 | |
| Peripheral blood involvement | |||
| No | 93 | 21 | .95 |
| Yes | 22 | 5 | |
| Bone marrow involvement | |||
| No | 108 | 25 | .95 |
| Yes | 7 | 1 |
| Characteristic . | No. of Patients . | No. With Transformation . | P Value* . |
|---|---|---|---|
| Age | |||
| <60 yr | 60 | 11 | .25 |
| ≥60 yr | 55 | 15 | |
| Sex | |||
| Male | 65 | 13 | .48 |
| Female | 50 | 13 | |
| Stage | |||
| I-II v | 78 | 18 | .94 |
| III-IV | 37 | 8 | |
| I-IIA v | 58 | 8 | .03 |
| IIB-IV | 57 | 18 | |
| T staging | |||
| T1 | 36 | 0 | .000005 |
| T2 | 24 | 8 | |
| T3 | 28 | 13 | |
| T4 | 27 | 5 | |
| β2M (mg/L) | |||
| Low (≤2) | 54 | 11 | .080 |
| High (>2) | 38 | 14 | |
| LDH (U/L) | |||
| Low | 74 | 16 | .52 |
| High† | 37 | 10 | |
| LDH + β2M‡ | |||
| No v | 46 | 7 | .009 |
| 1 and/or 2 | 46 | 18 | |
| Lymph node involvement | |||
| No | 79 | 16 | .37 |
| Yes | 36 | 10 | |
| Peripheral blood involvement | |||
| No | 93 | 21 | .95 |
| Yes | 22 | 5 | |
| Bone marrow involvement | |||
| No | 108 | 25 | .95 |
| Yes | 7 | 1 |
Abbreviations: N/E, not evaluable; T1, limited patch/plaque (<10% of total skin surface); T2, generalized patch/plaque (≥10% of total skin surface); T3, tumors; T4, generalized erythroderma.
By χ2 or Fisher exact test, as appropriate.
≥10% above the upper limit of the normal range.
No elevation of LDH or β2M versus elevation of LDH and/or β2M.
Neither initial β2M nor LDH was significantly higher in the patients with eventual transformation. However, when both these variables were examined together (both normal v one or both elevated), the number of patients who eventually transformed was significantly elevated in the latter group (P = .009).
Outcome of transformed versus nontransformed patients.
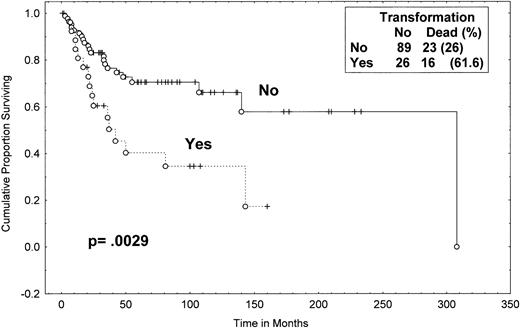
One-hundred twelve of the total of 115 MF/SS patients have been observed for at least 2 years. The median time from the initial diagnosis of MF/SS to the documentation of large-cell transformation was 12 months (range, 0 to 128 months; Table 1). The median follow-up time of surviving patients was 55 months (from the time of diagnosis) for the transformed group. Of the 89 patients without transformation, 23 (26%) died during the follow-up period as compared with 16 (62%) of the 26 patients with transformation. The median survival from initial diagnosis of MF/SS for patients with transformation was 37 months; the median survival from diagnosis for the group that have not transformed was 163 months (P = .0029; Fig 3). The percentage of patients alive at 4 years from diagnosis was 45% (95% CI, 24% to 66%) in the patients who eventually transformed versus 73% (95% CI, 62% to 84%) in patients who have not transformed (P = .01).
Survival (from diagnosis) of MF/SS patients with and without eventual transformation. Data were analyzed by the method of Kaplan-Meier. A log-rank P value was determined. Tick marks indicate points at which there are 1 or more patients who were still alive at the time of the analysis. (○) Patients who have died.
Survival (from diagnosis) of MF/SS patients with and without eventual transformation. Data were analyzed by the method of Kaplan-Meier. A log-rank P value was determined. Tick marks indicate points at which there are 1 or more patients who were still alive at the time of the analysis. (○) Patients who have died.
Half of all the transformed patients had tumor stage (T3) disease and 46% of all tumor stage patients eventually underwent transformation. The median survival time (from diagnosis) in transformed (N = 13) versus untransformed (N = 15) tumor stage (T3) patients was 24.5 months versus 35 months (P = .61). Similarly, the median survival time from diagnosis in stage IIB patients who eventually transformed did not differ from that of those who did not transform (P = .73). The median survival time from diagnosis in transformed stage III and IV patients was 24.4 months (95% CI, 0 to 44 months); the median survival time has not been reached in the untransformed stage III-IV patients at a median follow-up time for surviving patients of 44 months (P = .13).
Prognostic variables within the tranformed group of patients.
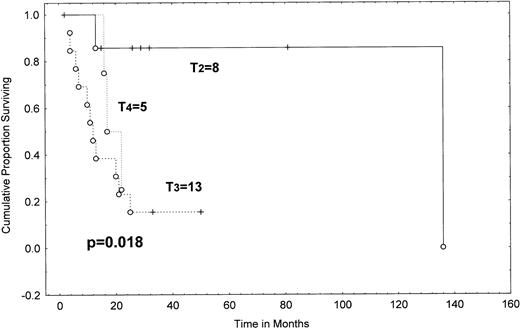
The median survival from transformation was 19.4 months (range, 2+ to 138 months; Table 3 and Fig 4). The patients who transformed within the first 2 years after diagnosis (N = 18) had a median survival of 23.5 months; the median survival has not been reached in those with late (≥2 years) transformation and 57% of this group are alive at 81 months (95% CI, 20% to 94%; P = .011). The stage of skin disease (T stage) at the time of pathologically confirmed transformed MF/SS was also an important prognostic indicator for survival. The actuarial survival from diagnosis of patients with generalized (T2) plaque disease was 85% at 2 years, compared with only 23% for patients with tumor stage (T3) disease and 25% for patients with erythroderma (T4), a difference that is statistically significant (P = .0182; Table 4 and Fig 5).
Response to Therapy of 26 Patients With Large-Cell Transformation
| Patient No. . | Stage at Transformation . | Initial Therapy After Transformation* . | Response to Therapy . | Response Duration (mo) . | Survival After Transformation (mo) . | Previous Chemotherapy . | Patient Status . |
|---|---|---|---|---|---|---|---|
| 1 | IB | Comb. modal. 1† | CR | 14+ | 32+ | No | NED |
| 2 | IIB | Comb. modal. 2‡ | CR | 42+ | 51+ | No | NED |
| 3 | IIIA | Photopheresis | PR | 11 | 22+ | Yes | AWD |
| 4 | IIA | Comb. modal. 1 | CR | 55+ | 82+ | No | NED |
| 5 | IB | PUVA | PR | 14+ | 26+ | No | AWD |
| 6 | IB | Photopheresis | PR | 8 | 29+ | No | AWD |
| 7 | IIA | Comb. modal. 1 | CR | 7+ | 15+ | No | NED |
| 8 | IIB | DCF | PR | 7 | 34+ | No | AWD |
| 9 | IIB | XRT | PR | 6 | 21 | Yes | DOD |
| 10 | IIB | XRT | PR | 1 | 4 | Yes | DOD |
| 11 | IIB | IFN + Accutane | NR | 0 | 6 | No | DOD |
| 12 | IVA | CVP | PR | 2 | 22 | No | DOD |
| 13 | IVA | Comb. modal. 2 | NR | 0 | 11 | No | DOD |
| 14 | IVA | TBEB | PR | 12 | 13 | Yes | DOC |
| 15 | IIB | CHOP + BLEO | NR | 0 | 25 | No | DOD |
| 16 | IIB | Photopheresis | NR | 0 | 12 | Yes | DOD |
| 17 | IIIA | H65MoAb | PR | 4 | 16 | Yes | DOD |
| 18 | IVA | XRT + IFN + NM | PR | 12 | 20 | Yes | DOD |
| 19 | IVB | IFN + Accutane | NR | 0 | 17 | No | DOD |
| 20 | IIB | Comb. modal. 2 | CR | 2 | 7 | No | DOC |
| 21 | IIB | XRT | NR | 0 | 4 | Yes | DOD |
| 22 | IIB | CHOP + BLEO | PR | 4 | 10 | No | DOD |
| 23 | IB | NM | PR | 5 | 13 | No | DOD |
| 24 | IIA | TBEB | MR | 7 | 138 | No | DOD |
| 25 | IVB | CMED | PR | 1+ | 2+ | No | AWD |
| 26 | IVA | Photopheresis | PR | 1+ | 2+ | No | AWD |
| Patient No. . | Stage at Transformation . | Initial Therapy After Transformation* . | Response to Therapy . | Response Duration (mo) . | Survival After Transformation (mo) . | Previous Chemotherapy . | Patient Status . |
|---|---|---|---|---|---|---|---|
| 1 | IB | Comb. modal. 1† | CR | 14+ | 32+ | No | NED |
| 2 | IIB | Comb. modal. 2‡ | CR | 42+ | 51+ | No | NED |
| 3 | IIIA | Photopheresis | PR | 11 | 22+ | Yes | AWD |
| 4 | IIA | Comb. modal. 1 | CR | 55+ | 82+ | No | NED |
| 5 | IB | PUVA | PR | 14+ | 26+ | No | AWD |
| 6 | IB | Photopheresis | PR | 8 | 29+ | No | AWD |
| 7 | IIA | Comb. modal. 1 | CR | 7+ | 15+ | No | NED |
| 8 | IIB | DCF | PR | 7 | 34+ | No | AWD |
| 9 | IIB | XRT | PR | 6 | 21 | Yes | DOD |
| 10 | IIB | XRT | PR | 1 | 4 | Yes | DOD |
| 11 | IIB | IFN + Accutane | NR | 0 | 6 | No | DOD |
| 12 | IVA | CVP | PR | 2 | 22 | No | DOD |
| 13 | IVA | Comb. modal. 2 | NR | 0 | 11 | No | DOD |
| 14 | IVA | TBEB | PR | 12 | 13 | Yes | DOC |
| 15 | IIB | CHOP + BLEO | NR | 0 | 25 | No | DOD |
| 16 | IIB | Photopheresis | NR | 0 | 12 | Yes | DOD |
| 17 | IIIA | H65MoAb | PR | 4 | 16 | Yes | DOD |
| 18 | IVA | XRT + IFN + NM | PR | 12 | 20 | Yes | DOD |
| 19 | IVB | IFN + Accutane | NR | 0 | 17 | No | DOD |
| 20 | IIB | Comb. modal. 2 | CR | 2 | 7 | No | DOC |
| 21 | IIB | XRT | NR | 0 | 4 | Yes | DOD |
| 22 | IIB | CHOP + BLEO | PR | 4 | 10 | No | DOD |
| 23 | IB | NM | PR | 5 | 13 | No | DOD |
| 24 | IIA | TBEB | MR | 7 | 138 | No | DOD |
| 25 | IVB | CMED | PR | 1+ | 2+ | No | AWD |
| 26 | IVA | Photopheresis | PR | 1+ | 2+ | No | AWD |
Abbreviations: AWD, alive with disease; BLEO, bleomycin; CHOP, cyclophosphamide, adriamycin, vincristine, prednisone; CMED, cyclophosphamide, methotrexate, etoposide, decadron; comb. modal, combined modality; CVP, cyclophosphamide, vincristine, prednisone; CR, complete response; DCF, deoxycoformycin; DOC, dead of other causes; DOD, dead of disease; Dx, diagnosis; H65MoAb, anti-CD5 antibody coupled to the ricin A chain toxin (H65-RTA); IFN, interferon; MR, mixed response; NED, no evidence of disease; NM, nitrogen mustard; NR, no response; PR, partial response; PUVA, psoralen (8-methoxypsoralen) and UVA light; XRT, local radiation.
First regimen after transformation.
Combined modality 1 = interferon-α + cis-retinoid acid, followed by TBEB, followed by interferon-α and topical nitrogen mustard maintenance (stages I-II).
Combined modality 2 = interferon-α and cis-retinoic acid followed by multiagent chemotherapy, followed by TBEB, followed by interferon-α and topical nitrogen mustard maintenance (stage III-IV).23
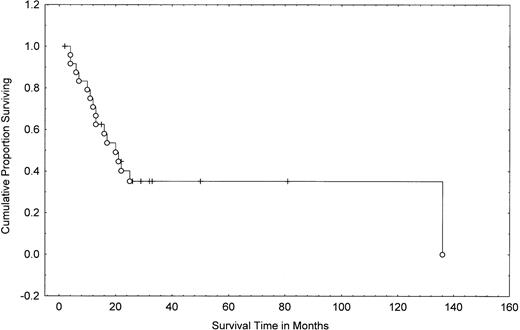
Overall survival from transformation in MF/SS patients. Data were analyzed by the method of Kaplan-Meier. Tick marks indicate points at which there are 1 or more patients who were still alive at the time of the analysis. (○) Patients who have died.
Overall survival from transformation in MF/SS patients. Data were analyzed by the method of Kaplan-Meier. Tick marks indicate points at which there are 1 or more patients who were still alive at the time of the analysis. (○) Patients who have died.
Survival (From Transformation) According to Prognostic Variables in 26 Patients With Transformed MF/SS (Univariate Analysis)
| Grouping . | No. . | Median Survival (mo) . | % of Patients Alive at 2 yr (95% CI) . | P Value . |
|---|---|---|---|---|
| Age | ||||
| <60 yr | 8 | 15.3 | 38 (0-78) | .96 |
| ≥60 yr | 18 | 18.5 | 41 (17-65) | |
| Stage | ||||
| I-II | 17 | 22.4 | 50 (26-74) | .34 |
| III-IV | 9 | 16 | 17 (0-47) | |
| I-IIA | 7 | Not reached3-150 | 86 (60-100) | |
| IIB | 10 | 10 | 30 (1-59) | .04 |
| III | 2 | Not evaluable | Not evaluable | |
| IV | 7 | 15 | 3-151 | |
| I-IIA v | 7 | Not reached | .0035 | |
| IIB-IV | 19 | 14.5 | 86 (60-100) | |
| 23 (3-43) | ||||
| T staging | ||||
| T1 | 0 | Not applicable | Not applicable | .0182 |
| T2 | 8 | Not reached | 85 (58-100) | |
| T3 | 13 | 11.5 | 23 (0-46) | |
| T4 | 5 | 17 | 25 (0-68) | |
| Site of initial transformation | ||||
| Skin | ||||
| Yes | 24 | 17 | 39 (18-60) | .18 |
| No | 2 | 4 | 100 (not applicable) | |
| Lymph nodes | ||||
| Yes | 3 | 22 | 50 (0-100) | .46 |
| No | 23 | 17 | 39 (18-60) | |
| Depth of invasion | ||||
| >2.15 mm | 12 | 12 | 30 (6-54) | .15 |
| ≤2.15 mm | 12 | 20 | 50 (22-78) | |
| Histologic distribution | ||||
| Diffuse | 15 | 12.5 | 31 (7-55) | .17 |
| Lichenoid | 5 | Not reached | 60 (16-100) | |
| Patchy | 4 | 20 | 50 (10-100) | |
| LDH (U/L) | ||||
| High3-152 | 5 | 11 | 0 (0-55) | .10 |
| Low | 10 | 32.7 | 55 (22-88) | |
| β2M (mg/L) | ||||
| High (>2) | 9 | 16 | 50 (16-84) | .093 |
| Low (≤2) | 5 | 6 | 253-153 (0-68) |
| Grouping . | No. . | Median Survival (mo) . | % of Patients Alive at 2 yr (95% CI) . | P Value . |
|---|---|---|---|---|
| Age | ||||
| <60 yr | 8 | 15.3 | 38 (0-78) | .96 |
| ≥60 yr | 18 | 18.5 | 41 (17-65) | |
| Stage | ||||
| I-II | 17 | 22.4 | 50 (26-74) | .34 |
| III-IV | 9 | 16 | 17 (0-47) | |
| I-IIA | 7 | Not reached3-150 | 86 (60-100) | |
| IIB | 10 | 10 | 30 (1-59) | .04 |
| III | 2 | Not evaluable | Not evaluable | |
| IV | 7 | 15 | 3-151 | |
| I-IIA v | 7 | Not reached | .0035 | |
| IIB-IV | 19 | 14.5 | 86 (60-100) | |
| 23 (3-43) | ||||
| T staging | ||||
| T1 | 0 | Not applicable | Not applicable | .0182 |
| T2 | 8 | Not reached | 85 (58-100) | |
| T3 | 13 | 11.5 | 23 (0-46) | |
| T4 | 5 | 17 | 25 (0-68) | |
| Site of initial transformation | ||||
| Skin | ||||
| Yes | 24 | 17 | 39 (18-60) | .18 |
| No | 2 | 4 | 100 (not applicable) | |
| Lymph nodes | ||||
| Yes | 3 | 22 | 50 (0-100) | .46 |
| No | 23 | 17 | 39 (18-60) | |
| Depth of invasion | ||||
| >2.15 mm | 12 | 12 | 30 (6-54) | .15 |
| ≤2.15 mm | 12 | 20 | 50 (22-78) | |
| Histologic distribution | ||||
| Diffuse | 15 | 12.5 | 31 (7-55) | .17 |
| Lichenoid | 5 | Not reached | 60 (16-100) | |
| Patchy | 4 | 20 | 50 (10-100) | |
| LDH (U/L) | ||||
| High3-152 | 5 | 11 | 0 (0-55) | .10 |
| Low | 10 | 32.7 | 55 (22-88) | |
| β2M (mg/L) | ||||
| High (>2) | 9 | 16 | 50 (16-84) | .093 |
| Low (≤2) | 5 | 6 | 253-153 (0-68) |
Prognostic variables were determined at the time of transformation. Survival was calculated by Kaplan-Meier; Pvalue by log-rank or Gehan-Wilcoxon test, as appropriate.
Abbreviations: β2M, β2 microglobulin; CR, complete response; MF/SS, mycosis fungoides/Sezary syndrome; LDH, lactic dehydrogenase; PR, partial response; T1, limited patch/plaque (<10% of total skin surface); T2, generalized patch/plaque (≥10% of total skin surface); T3, tumors; T4, generalized erythroderma.
Five patients alive at median follow-up of 29 months.
Two of the patients were still alive, but the follow-up was less than 2 years (22 months).
≥10% above the upper limit of the normal range.
25% alive at 20 months.
Survival from transformation in MF/SS according to clinical T category at transformation (Kaplan-Meier). T2, more than 10% of skin surface involved by plaque (8 patients); T3, cutaneous tumors (13 patients); T4, erythroderma (5 patients). There were no patients with clinical T1 category. A P value (shown) was determined by the Gehan-Wilcoxon test. Tick marks indicate points at which there are 1 or more patients who are still alive at the time of the analysis. (○) Patients who have died.
Survival from transformation in MF/SS according to clinical T category at transformation (Kaplan-Meier). T2, more than 10% of skin surface involved by plaque (8 patients); T3, cutaneous tumors (13 patients); T4, erythroderma (5 patients). There were no patients with clinical T1 category. A P value (shown) was determined by the Gehan-Wilcoxon test. Tick marks indicate points at which there are 1 or more patients who are still alive at the time of the analysis. (○) Patients who have died.
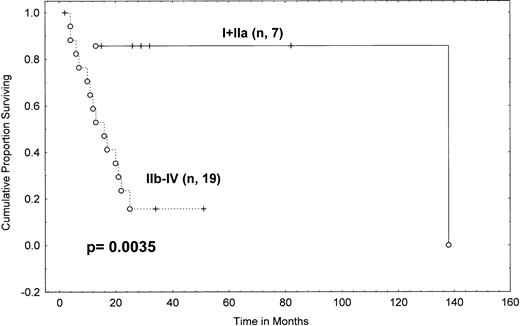
Comparing survival from transformation in patients staged as I-II versus III-IV showed no difference (P = .34; Table 4). However, when patients with histopathologically proven stage IA, IB, and IIA disease were combined in one group, they had a 2-year actuarial survival of 86% (95% CI, 60% to 100%) from transformation, whereas patients with stage IIB disease had a median survival of only 10 months, with only 30% of them alive at 2 years (95% CI, 1% to 59%), and stage IV patients had a median survival of 15 months from transformation (P = .04; Table 4). When we combined stages IIB-IV, the survival curve remained similar to that of stage IV patients alone (14.5 months median, with 23% of stage IIB-IV patients alive at 2 years). The overall survival difference between these two groups (I-IIA v IIB-IV) was statistically significant (P = .0035; Table 4 and Fig 6). These results indicate that transformed stage IIB patients did as poorly or worse than transformed stage IV patients but that transformed stage I-IIA patients did much better.
Survival from transformation in MF/SS patients according to Mycosis Fungoides Cooperative Group clinical stage at transformation (Kaplan-Meier). Tick marks indicate points at which there are 1 or more patients who are still alive. (○) Patients who have died. A log rankP value is shown. N = 7 for stages I-IIA; N = 19 for stages IIB-IV.
Survival from transformation in MF/SS patients according to Mycosis Fungoides Cooperative Group clinical stage at transformation (Kaplan-Meier). Tick marks indicate points at which there are 1 or more patients who are still alive. (○) Patients who have died. A log rankP value is shown. N = 7 for stages I-IIA; N = 19 for stages IIB-IV.
Advanced age (≥60 years old) and the presence of elevated LDH and/or β2M at the time of transformation was not associated with an inferior survival in the transformed MF/SS patients (Table 4). Differences in the depth of invasion (thickness of infiltrate; >2.15v ≤2.15 mm) or in the histologic distribution of the lymphoid infiltrate (diffuse [15] v patchy [4] or lichenoid [5]) also did not influence the prognosis in the transformed patients (P = .15 and P = .17, respectively; Table4).
Treatment of patients with tranformation.
Sixteen patients (62%) received therapy before transformation. The treatments were diverse, but most commonly included a combination of systemic (usually interferon, retinoids, and/or chemotherapy) and topical (usually nitrogen mustard, PUVA, or total body electron beam) treatment, with only 4 patients receiving only topical treatment. Type of response to previous treatment (complete remission [CR] versus less than CR) failed to show any influence on survival after transformation (P = .7). The median survival from transformation in patients who had prior chemotherapy (N = 8) was 13 months versus 24 months for those without prior systemic chemotherapy (N = 18; P = .068; Table 3).
Similarly, a wide variety of treatments were used after transformation. Although the response to the first treatment after the transformation (CR = 5, other = 21) was associated with a trend towards a better survival, this did not reach statistical significance (P = .078), perhaps because of the small number of patients. Only 5 patients achieved a CR after transformation. Among the patients who attained a CR, 3 had early stage I-IIA disease and 2 had IIB (tumor stage T3) disease. All those who achieved a CR were treated with a combined modality approach: interferon-α + cis-retinoid acid, followed by total body electron beam (TBEB), followed by interferon-α and topical nitrogen mustard maintenance in stage I-IIA; multiagent chemotherapy (cyclophosphamide, methotrexate, etoposide, and dexamethasone [CMED] alternating with doxorubicin, bleomycin, and vinblastine [ABV]) was added to the above in stage IIB-IV patients.23 None of these patients had prior systemic treatment. In addition, a CR was achieved with CMED (N = 2) and with DCF (N = 1) in some patients failing to respond to initial therapy. Overall, CMED was the most common treatment used. Of a total of 12 patients who received it, either by itself or as part of a combined modality approach administered immediately after transformation or as salvage, 8 (66%) attained a CR or partial remission (PR). The median duration of response in these 8 patients was 6 months (range, 1+ to 42+ months).
DISCUSSION
Morphologic transformation has been seen in various hematopoietic malignancies and is often associated with a more aggressive clinical course and shorter survival.6 Over the last few years, several investigators have recognized a large-cell variant of MF/SS.6-8,11-13 Transformed MF/SS may be difficult to differentiate from Ki-1 anaplastic large-cell lymphoma and lymphomatoid papulosis, because all can be CD30+ and display anaplastic large cells.9,10 Distinguishing features may be the presence of small, intermediate, and large atypical lymphocytes in addition to the large anaplastic cells in transformed MF/SS11 and pertinent clinical features such as spontaneous regression of disease in lymphomatoid papulosis9 or a history of extensive skin plaques in MF/SS.4 Molecular studies have demonstrated that the large-cell infiltrate in transformed MF/SS represents evolution from the original clone.14,15Because of the uncommon nature of MF/SS, the exact incidence of transformed MF/SS is difficult to estimate from the literature. Ranges of 8% to 55% have been reported.7,11-13 In our study of 115 patients, 26 (23%) showed transformation, and the 4-, 8-, and 12-year cumulative probabilities of transformation were 21%, 32%, and 39%, respectively (Fig 1). However, the relatively high actuarial probability of transformation could reflect referral bias to a tertiary care institute. The wide range of reported frequencies for transformation of MF/SS may be due, at least in part, to different definitions of transformation, and/or to criteria for patient selection. Dmitrovsky et al7 reported an 8% incidence of transformation in their patients, but they defined transformation as the presence of more than 50% large cells. Cerroni et al12used the criteria for large cell transformation defined by Salhany et al11 (the presence of large cells exceeding 25% of the lymphoid infiltrate throughout or the formation of microscopic nodules by the large cells), but included only advanced tumor stage disease in their selected population. They reported an incidence of transformation approaching 55%.12 In our study, we included all stages of MF/SS and used a definition similar to that given by Salhany et al,11 and the overall incidence of transformation was 23% in our study and 18% in the study by Salhany et al.11 If we included only tumor stage disease, the incidence of transformation would be 46%, a figure similar to that reported by Cerroni et al12 and Vonderheid et al24 (55% and 52%, respectively).
Clinical features at diagnosis of MF/SS, such as age, sex, and lymph node, peripheral blood, or bone marrow involvement, were not associated with a statistically significant increased risk for subsequent transformation (Table 2). However, although elevated LDH and β2M as individual factors did not predict for transformation, combining these two factors did (Table 2). In addition, advanced disease (especially tumor stage) was strongly associated with transformation. Indeed, half of the patients with transformation had tumor stage (T3) disease and 46% of all tumor stage patients eventually transformed.
Transformation is generally considered an event occuring late in the course of a malignancy. However, in transformed MF/SS series, the median time from diagnosis to transformation is usually less than 2 years, with ranges from 16 months13 to 21.5 months.7 In our current study, the median time from diagnosis to transformation was 12 months (range, 0 to 128 months). Occasionally, transformation to a large cell variant can occur at the time of the diagnosis. In a study by Greer et al13 of 113 patients, 9 patients had transformation at diagnosis (7.9%). This is very similar to the 9 of 115 patients (7.8%) in our study who had transformed at diagnosis.
Transformation in MF/SS to a large-cell variant is associated with a change in the clinical aggressiveness of the malignancy; survival is often measured in months.7,11-13,24 Indeed, some investigators have suggested that transformed MF/SS may represent an additional stage of the disease (accelerated form).25 The median survival from transformation has been reported to be as low as 2 months in the study by Dmitrovsky et al7 and 12 months in the studies by Greer et al13 and Salhany et al.11 In our current report, the median survival from transformation was 19.4 months and the 2-year survival was 40% (95% CI, 20% to 60%; Fig 4).
Despite the relatively small numbers of individuals with transformation, several features with important prognostic ramifications emerged. Factors associated with a poor outcome in our patients were transformation within 2 years after diagnosis (P= .011) and more advanced disease at transformation (stages IIB-IVv I-IIA; P = .0035). Indeed, transformed patients with tumor stage (T3) disease had a very poor median survival of 11.5 months from transformation, with only 23% of them alive at 2 years (Fig 5). Outcome in these patients was comparable with that of patients with erythroderma (T4; median survival, 17 months, with 25% of patients alive at 2 years). Patients with stage T2 plaque disease did much better; median survival was not reached at a median follow-up of 29 months and 85% were alive at 2 years after transformation (P = .018). Similarly, when we did the analysis of survival according to stage at transformation, patients with IIB disease had a median survival of only 10 months from transformation (with 30% of these patients alive at 2 years); patients with stages III and IV disease had a median survival of 16.5 months (P = .87). Therefore, transformed patients with stage IIB disease do at least as poorly as stage III-IV patients. In contrast, the median survival of stage I-IIA transformed patients was not reached and the 2-year survival was 86% (P = .04; Table 4 and Fig 6). Extracutaneous sites of transformation have previously been shown to confer a poor prognosis when compared with transformation confined to the skin.7,8,11 13 However, in our study, the vast majority of patients transformed within the skin.
The median survival from diagnosis was 37 months in our patients who eventually transformed, whereas the median survival in the group who have not transformed was 163 months (P = .0029). Similar differences have been reported by Greer et al13 (27 months for the transformed group compared with 53 months for the nontransformed group).11 A question that arises is whether the poor median survival of MF/SS patients who transformed is due to transformation or to the fact that transformation generally takes place in patients with advanced disease. Within individual stages, survival was consistently lower in our patients who eventually underwent transformation versus those who did not. However, these differences were not statistically significant, perhaps because of the small number of individuals in each subgroup. Therefore, evaluation of a larger cohort will probably be needed to address this question.
Immunologic analysis demonstrated expression of T-cell markers in all transformed cases tested. All cases but 1 retained a T-helper phenotype. The 1 exception was CD4− and CD8−. Two of the cases had both a CD4 and a CD8 phenotype. Similar patterns of aberrant T subtypes have been seen by other investigators.11,13 Association of more aggressive behavior with the expression of activation markers [CD25 and Ki-1(CD30)] developing after the diagnosis in MF/SS patients has been suggested.26 27 In our series, CD25 was tested in only 4 cases. CD30 was positive in 7 of the 15 cases tested, and the median survival for these patients was 21 months.
In patients with transformation of low-grade follicular lymphoma, Yuen et al28 have demonstrated that long-term survival can be achieved in individuals with limited disease and/or no prior chemotherapy. Patients were treated with radiotherapy alone (generally in limited disease) or with combination chemotherapy. In MF/SS, Kaye et al29 have shown that aggressive combined therapy (radiation and adriamycin-based chemotherapy) achieves a higher response rate than does conservative topical therapy, but it has no impact in survival. However, the investigators did not specifically address patients with transformation in their report. In our study, diverse treatments were also used, precluding a definitive analysis of impact. However, CRs were for the most part restricted to patients who received multimodal therapy: interferon and cis-retinoic acid, TBEB, and a multiagent chemotherapy regimen including methotrexate.
In summary, of our group of 115 patients with MF/SS, 23% underwent transformation. The cumulative probability of transformation was 39% at 12 years. Advanced disease (stage IIB and higher) and especially the presence of tumors as well as elevation of both LDH and β2M were associated with an increased incidence of transformation. Outcome was poor in transformed patients if transformation took place within 2 years from diagnosis or if they had advanced stage at presentation. The median overall survival from transformation was 19.4 months, and transformed patients with tumors had a median survival of less than 1 year. Considering the poor outlook of these patients, a more systematic approach to managing individuals with transformed MF/SS appears warranted.
Address reprint requests to Razelle Kurzrock, MD, Department of Bioimmunotherapy, Box 302, 1515 Holcombe Blvd, University of Texas, M.D. Anderson Cancer Center, Houston, TX 77030.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal