Abstract
To approach the goal of consistent long-term erythropoietin (Epo) expression in vivo, we developed an implantation procedure in which transduced autologous vascular smooth muscle was introduced into rats in a chamber created from a polytetrafluoroethylene (PTFE) ring placed under the serosa of the stomach. The implant became vascularized and permitted the long-term survival of smooth muscle cells expressing Epo. Hematocrits of treated animals increased rapidly and monitored over 12 months gave a mean value of 56.0 ± 4.0% (P < .001; n = 9), increased from a presurgery mean of 42.3 ± 1.6%. Hemoglobin levels rose from a presurgery mean of 15.2 ± 0.4 g/dL and for 12 months were significantly elevated with a mean value of 19.5 ± 1.3 g/dL (P < .001; n = 9). The hematocrit and hemoglobin levels of control animals receiving human adenosine deaminase (ADA)–expressing cells were not significantly different from baseline (P > .05; n = 5). In response to tissue oxygenation, kidney, and (to a lesser extent) liver are specific organs that synthesize Epo. Treated animals showed downregulation of endogenous Epo mRNA in kidney over a 12-month period. The PTFE implant provides sustained gene delivery, is safe, and is minimally invasive. It allows easy engraftment of transduced cells and may be applied generally to the systemic delivery of therapeutic proteins such as hormones and clotting factors.
© 1998 by The American Society of Hematology.
ERYTHROPOIETIN (Epo) is a 30-kD glycoprotein hormone that is the regulator of red cell production and maintenance in mammals.1,2 Understanding of the molecular mechanisms of its action was significantly advanced by the cloning of human Epo cDNA.3,4 Epo from many mammals has now been cloned, and a high degree of sequence similarity and genetic structure has been found.1,5 The availability of recombinant human Epo provided a major advance in the treatment of renal failure patients receiving dialysis.6 The attendant dangers of transfusion therapy were eliminated and the quality of life of these patients has significantly increased.2 The administration of recombinant Epo is now widely used for long-term treatment of anemia associated with chronic renal failure, cancer chemotherapy, and human immunodeficiency virus infections.2 Delivery of this hormone by gene therapy rather than by repeated injections would provide substantial clinical and economic benefits and would serve as a model for the expression of other therapeutic proteins.
In adults, Epo is produced primarily in the kidney with the liver as a secondary source.7,8 Tissue oxygen tension regulates the overall level of Epo and red cell production, primarily through the rates of gene transcription in the kidney.1 The hypoxia-responsive cis elements of the Epo gene have been found to be localized to the 3′ untranslated region.9-15 Deletion analysis has shown that a 24-bp portion of the 3′ flanking sequence of the Epo gene was sufficient to give a transcriptional response to hypoxia.12 The oxygen-sensing mechanism involved in the induction of Epo synthesis includes a 120-kD hypoxia-inducible factor 1 (HIF-1) and other transacting factors.16 Most interestingly, the oxygen-sensing system initially identified in Epo-producing cells was found to be present in a wide variety of cell types.11,13 14 Unfortunately these regulatory DNA sequences are too large to be suitable for insertion in a retroviral vector.
Long-term in vivo gene expression requires both target cells and gene delivery vectors that permit continuous vector-encoded activity. Of the three common virus-based methods of gene transfer, retroviral vectors are probably the most useful for ex vivo gene transfer.17-20 Adeno-associated virus (AAV) vectors have many attractive features, such as safety and ability to transduce nonproliferating cells,21-25 but they do not possess advantages over retroviruses for ex vivo gene transduction. Replication-defective retroviral vectors can be made with high titers and will infect a wide variety of cell types. Infection results in stable proviral integration into the host chromosome providing gene expression for the lifetime of the cell and its progeny17-20 Therapeutic genes can be expressed at high level from the viral long terminal repeat (LTR) promoter/enhancer or strong internal promoters. Recently, the incorporation of internal ribosome entry sites from picornaviruses into retroviral vectors has allowed the generation of bicistronic vectors and subsequent advantages in linked-gene selection.26-28
Nonhematopoietic cells studied as vehicles for gene therapy include skin fibroblasts, myoblasts, and vascular smooth muscle cells. Skin fibroblasts are easily obtained, cultured, and transduced but have a major disadvantage of inactivating vector sequences after transplantation.29,30 Myoblasts represent a promising target cell type for gene therapy. Transduced skeletal myoblasts have been used to deliver Epo in mice,31-33 and transplantation of retrovirally transduced skeletal muscle myoblasts has been successfully achieved in dogs with alpha-L-iduronidase deficiency.34 Intramuscular injection of plasmid DNA has produced systemic expression of Epo in mice.35
Smooth muscle cells are present within the vasculature as a multilayered mass of long-lived cells in proximity to the circulation and have been investigated as targets for gene therapy.36-42 We have shown that transduced vascular smooth muscle cells seeded into carotid arteries in the rat will provide sustained expression of both marker and therapeutic genes.36,38,42 However, while showing the potential of smooth muscle cells to provide long-term gene expression of therapeutic proteins, this procedure may not be applied to patients as it requires arterial injury to achieve cell engraftment.36,38 41 As an alternative site for smooth muscle cell implantation, we recognized that the tissue plane between the tunica muscularis and tunica serosa might provide a niche for retention of transduced smooth muscle cells. This tissue is composed of smooth muscle cells, is well vascularized, and is able to provide nutrition for implanted cells. Smooth muscle cells are present at this site, and it is important to the survival of transplanted cells that they are a normal constituent of the targeted area. To examine the potential of this method of cell implantation to provide long-term gene expression we studied the effect of Epo secretion on hematopoiesis in rats.
MATERIALS AND METHODS
Construction of retroviral vectors.
The retroviral vector LrEpSN was made by inserting anEcoRI-BamHI fragment of the rat Epo cDNA into LXSN.38,43 A plasmid containing the rat Epo gene was kindly provided by Drs J.-P.R. Boissel and H.F. Bunn, Boston, MA.5The amphotropic retroviruses and the control retroviral vector LASN, encoding human adenosine deaminase (ADA), were generated as described earlier.44
Cell culture.
Rat smooth muscle cell cultures were prepared by enzymatic digestion of the aorta from a male Fisher 344 rat, and the cells were characterized by positive staining for muscle cell-specific actins with HHF35 antibody37 and staining negative for von Willebrand factor,37 an endothelial cell specific marker. Ecotropic PE501 and amphotropic PA317 retrovirus packaging cell lines,43,45 NIH 3T3 thymidine kinase negative cells,45 and primary cultures of rat smooth muscle cells were grown in Dulbecco/Vogt modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum in humidified 5% CO2 at 37°C. Early passage smooth muscle cells were exposed to 16-hour virus harvests from PA317-LrEpSN or PA317-LASN amphotropic virus-producing cell lines for a period of 24 hours in the presence of polybrene (4 μg/mL). Infected cells were selected in medium containing G418 at 1 mg/mL. Vascular smooth muscle cells infected with LrEpSN and selected in 1 mg/mL G-418 antibiotic secreted 6.7 mU/24 h per 105 cells of Epo.38 In experiments to determine cell distribution in polytetrafluoroethylene (PTFE) implants, LrEpSN-transduced cells were labeled with the fluorescent marker 1,1′-dioctadecyl-3,3,3′3′-tetramethylindocarbocyanine perchlorate (DiI).36
Epo mRNA analysis.
Total RNA was isolated from rat kidney by homogenization in the presence of RNAzol. Using SuperScript reverse transcriptase (GIBCO-BRL, Gaithersburg, MD), 1 μg of total RNA was reverse-transcribed in the presence of random hexamer primers. Polymerase chain reaction (PCR; 30 cycles) was performed with rat Epo-specific primers (5′ AGG CGC GGA GAT GGG GGT GC 3′ and 5′ CCC CGG AGG AAG TTG GAG TAG 3′) to give a 540-bp amplified fragment. An aliquot of the amplified reaction mixture was electrophoresed in a 2% agarose gel and, after Southern transfer, the membrane was hybridized with a 32P-labeled 500-bp Epo cDNA probe. As a control for RNA extraction, integrity, reverse transcription, and amplification, β-actin–specific primers (5′ GTG GGG CGC CCC AGG CAC CA 3′ and 5′ CTC CTT AAT GTC ACG CAC GAT TTC 3′) were used to amplify a 500-bp fragment from the same cDNA preparation. Equal amplification of the samples was confirmed by both ethidium bromide staining and subjecting diluted aliquots of each sample to agarose electrophoresis, Southern transfer, and hybridization of the transferred DNA to a β-actin–specific32P-cDNA probe.
Smooth muscle cell implantation.
Rats were anesthetized by intraperitoneal (IP) injection with 44 mg/kg ketamine, 5 mg/kg xylazine, and 0.5 mg/kg acepromazine. All rats received 0.04 mg dexamethasone IP just before surgery. An area from thoracic inlet to the pubis was prepared for surgery, and a 3-cm midline abdominal incision was made from the xyphoid to the umbilicus. The stomach was temporarily exteriorized and held in place with a mosquito hemostat. A 0.5-cm superficial incision was made in the capsule on the cranial face of the body of the stomach, and a small pocket approximately 0.6 cm in diameter was created under the capsule using blunt dissection. A small PTFE ring (inner diameter 4 mm, outer diameter 6 mm) was inserted into the pocket and sutured in place using 5-0 maxon on a taper needle in a simple continuous pattern. The suture material was drawn tightly to constrict the ring to a final inner diameter of 2 to 3 mm before finishing the knot. The fibrous tunic directly overlying the ring was cryofrozen using a steel probe, and the ring was mechanically elevated to prevent the freezing of the underlying muscular layer to minimize tissue damage. The ring was rinsed with 0.9% saline to remove any blood clots formed during surgery. Cell aliquots of 1 × 106 cells/50 mL media were then introduced into the center of the ring through a 24-g intravenous (IV) catheter. Animals received two rings each containing 1 × 106 transduced vascular smooth muscle cells expressing either Epo or human ADA.
Blood analysis.
Anticoagulated blood samples (300 μL) were obtained from the tail vein and reticulocyte count determined by vital staining with brilliant cresyl blue and cell counting by standard techniques. Hematocrit, hemoglobin, platelet, and white blood cell (WBC) number were measured using a Coulter counter (Coulter Immunology, Hialeah, FL).
RESULTS
To achieve cell implantation we positioned PTFE rings under the serosal plane of the rat stomach to create an area above the muscle layer enclosed by the serosa membrane. The hematocrits of animals implanted with Epo-secreting transduced cells increased steadily over 50 days from a mean of 42.3 ± 1.6% to a maximum of 67.5 ± 3.1% and remained elevated with a mean value of 56.0 ± 4.0% (P < .001) over the 12-month observation period (Fig 1 and Table 1). Hemoglobin levels rose from a presurgery mean of 15.2 ± 0.4 g/dL to a maximum of 22.6 ± 1 g/dL at around 7 weeks and at 12 months were significantly elevated with a mean value of 19.5 ± 1.3 g/dL (P< .001). The hematocrit and hemoglobin levels of control animals treated with transduced cells expressing human ADA were not significantly different from baseline during the experiment (P>.05; Table 1). Greater than 95% of the treated animals showed significant increases in red cell production, indicating this procedure is very reproducible. The control hematological levels were in agreement with normal rat blood values.46 WBC and platelet counts remained within the normal range during the course of the experiment in both the treated and control groups (Table 1). This was expected because previous studies have documented the selective effect of sustained Epo delivery on hematopoiesis with no significant changes in leukocyte or megakaryocyte production.6,38,47,48Reticulocytes were elevated in treated rats and not in controls. The mean value presurgery was 1.9%, with a range of 0.5% to 3.5%, and the postsurgery mean was 3.7%, with a range of 1.3% to 10.3%. The reticulocyte counts peaked between 10 to 30 days, in contrast to implantation of transduced smooth muscle cells on the carotid artery, which gave peak levels at about 8 to 14 days.38 The single administration of dexamethasone at the time of surgery increased the red cell production of treated animals to implantation of Epo-secreting cells. At 2 months animals receiving dexamethasone had a mean hematocrit of 59% compared with a mean hematocrit of 51% recorded from untreated animals (data not shown).
Effect of seeding of transduced vascular smooth muscle cells on hematocrit (Hct). Closed symbols represent animals seeded with LrEpSN-transduced cells, and open symbols are control rats receiving LASN-transduced cells.
Effect of seeding of transduced vascular smooth muscle cells on hematocrit (Hct). Closed symbols represent animals seeded with LrEpSN-transduced cells, and open symbols are control rats receiving LASN-transduced cells.
Treated and Control Rat Blood Cell Values
| . | Presurgery Values . | 1-Year Postsurgery Values . |
|---|---|---|
| LASN Control (n = 5) | ||
| Hct (%) | 45.5 (±1.5) | 46 (±2.5)-150 |
| Hb (g/dL) | 15.7 (±0.4) | 16.4 (±0.5)-150 |
| WBC (×10−3)/μL | 9.8 (±2) | 7.8 (±1.6)-150 |
| Plt (×10−3)/μL | 807 (±109) | 737 (±101)-150 |
| LrEpSN Treated (n = 9) | ||
| Hct (%) | 42.3 (±1.6) | 56 (±4)-151 |
| Hb (g/dL) | 15.2 (±0.4) | 19.5 (±1.3)-151 |
| WBC (×10−3)/μL | 10.7 (±1.5) | 10.7 (±2.3)-150 |
| Plt (×10−3)/μL | 727 (±63) | 687 (±62)-150 |
| . | Presurgery Values . | 1-Year Postsurgery Values . |
|---|---|---|
| LASN Control (n = 5) | ||
| Hct (%) | 45.5 (±1.5) | 46 (±2.5)-150 |
| Hb (g/dL) | 15.7 (±0.4) | 16.4 (±0.5)-150 |
| WBC (×10−3)/μL | 9.8 (±2) | 7.8 (±1.6)-150 |
| Plt (×10−3)/μL | 807 (±109) | 737 (±101)-150 |
| LrEpSN Treated (n = 9) | ||
| Hct (%) | 42.3 (±1.6) | 56 (±4)-151 |
| Hb (g/dL) | 15.2 (±0.4) | 19.5 (±1.3)-151 |
| WBC (×10−3)/μL | 10.7 (±1.5) | 10.7 (±2.3)-150 |
| Plt (×10−3)/μL | 727 (±63) | 687 (±62)-150 |
Abbreviations: Hct, hematocrit; Plt, platelets; WBC, white blood cells; Hb, hemoglobin.
P > .05.
P < .001.
A rat showing an elevated hematocrit of 63% at 12 months after surgery was sacrificed, and the PTFE implant was removed, fixed, and stained with hematoxylin and eosin. A photomicrograph of a cross-section showed tissue within and around the PTFE graft that was fully integrated and well vascularized (Fig2). This suggests that the PTFE structure is well tolerated between the muscle and serosal layers.
Histological cross-sections of stomach PTFE implants containing transduced smooth muscle cells. Tissues in panels 1 and 2 were obtained at 12 months postsurgery from a rat that had a hematocrit of 62%, fixed in formalin, and stained with H&E. PTFE implants containing Epo-secreting vascular smooth muscle cells unlabeled (panel 3) or marked with DiI (panel 4) were removed 2 months postsurgery from a rat with a hematocrit of 67%, frozen, sectioned, and photographed using a Nikon Microphot FXA equipped with a rhodamine filter. PTFE material is denoted P, and mucosal tissue as M. (Panels 1, 2, and 4: ×40 original magnification; panel 3: ×100 original magnification.
Histological cross-sections of stomach PTFE implants containing transduced smooth muscle cells. Tissues in panels 1 and 2 were obtained at 12 months postsurgery from a rat that had a hematocrit of 62%, fixed in formalin, and stained with H&E. PTFE implants containing Epo-secreting vascular smooth muscle cells unlabeled (panel 3) or marked with DiI (panel 4) were removed 2 months postsurgery from a rat with a hematocrit of 67%, frozen, sectioned, and photographed using a Nikon Microphot FXA equipped with a rhodamine filter. PTFE material is denoted P, and mucosal tissue as M. (Panels 1, 2, and 4: ×40 original magnification; panel 3: ×100 original magnification.
To show that transduced vascular smooth muscle cells expressing Epo are contained within the PTFE ring structure, Epo-secreting cells were labeled with DiI, a fluorescent dye, before implantation. Unlabeled Epo-secreting cells were implanted in control rats. At 2 months after surgery a rat with a hematocrit of 68% was sacrificed and tissue cross-sections photographed (Fig 2). A large mass of dye-marked fluorescing cells was evident in the area encompassed by the PTFE ring, and fluorescent cells were not visible in adjacent areas or in the control sections (Fig 2). These data show that transduced vascular smooth muscle cells remain in the PTFE-enclosed area and provide therapeutic Epo expression for at least 12 months.
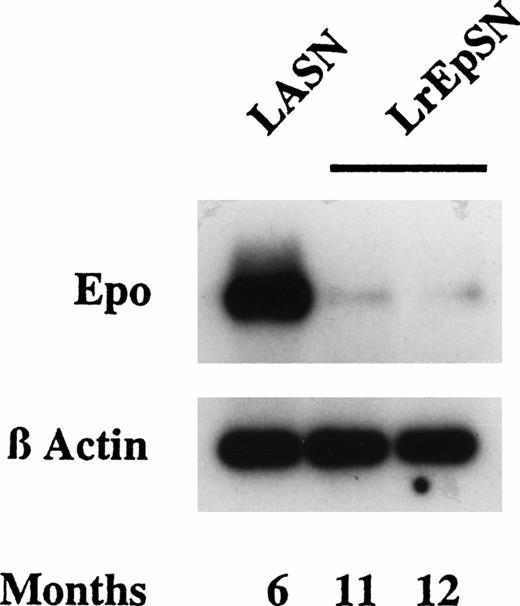
To determine if vector-encoded Epo expression resulted in downregulation of endogenous Epo,8 test rats were sacrificed when elevated hematocrit and hemoglobin levels were established. Control rats that received LASN-transduced cells were sacrificed at similar time points. Because Northern analysis is not sensitive enough to detect endogenous Epo message, RNA obtained from kidney was subjected to reverse transcription (RT)-PCR using rat Epo-specific probes.5 Endogenous Epo mRNA in kidneys of rats seeded with LrEpSN-transduced cells and analyzed at 11 and 12 months was greatly reduced in comparison to a control kidney, suggesting significant downregulation of endogenous Epo production (Fig 3). Epo mRNA in liver was not detectable by this RT-PCR method (data not shown). Southern band intensities from RT-PCR amplification of actin mRNA isolated from test and control tissues were similar, indicating equivalence in RNA isolation and amplification (Fig 3).
Epo mRNA analysis. Total RNA was isolated from kidneys of rats receiving Epo expressing LrEpSN-transduced cells and control rats receiving LASN-transduced cells expressing human ADA. RT-PCR was performed with rat Epo-specific primers to give a 540-bp amplified segment that was subjected to electrophoresis and hybridized with a32P-labeled Epo probe. As a control RT-PCR was performed using β-actin–specific primers to amplify a 500-bp fragment from the same cDNA preparation. Diluted aliquots of each sample were subjected to agarose electrophoresis, Southern transfer, and hybridization of the transferred DNA to a β-actin–specific 32P-cDNA probe.
Epo mRNA analysis. Total RNA was isolated from kidneys of rats receiving Epo expressing LrEpSN-transduced cells and control rats receiving LASN-transduced cells expressing human ADA. RT-PCR was performed with rat Epo-specific primers to give a 540-bp amplified segment that was subjected to electrophoresis and hybridized with a32P-labeled Epo probe. As a control RT-PCR was performed using β-actin–specific primers to amplify a 500-bp fragment from the same cDNA preparation. Diluted aliquots of each sample were subjected to agarose electrophoresis, Southern transfer, and hybridization of the transferred DNA to a β-actin–specific 32P-cDNA probe.
DISCUSSION
We have described a novel site to implant transduced cells for the systemic delivery of proteins. The time from surgery to maximum hematocrit in this study was about 50 days, nearly twice as long as the 3-week induction period we observed using Epo-secreting smooth muscle cells seeded onto denuded rat carotid arteries.38 This difference in achieving maximal hematocrit may be due to arterial seeding providing immediate systemic secretion of Epo, whereas a stomach implant requires time to establish vascularization and a pathway for hormone delivery to the bone marrow. The normal kinetics of red cell production involve a 2- to 3-week period from Epo secretion to mature red cell formation from erythroid precursors.2
The dye-labeling experiment showed that placement of cells within the PTFE ring initiated the creation of a structure that served both to retain and nurture implanted cells that were able to provide sustained, high-level Epo expression. Extrapolation of the increases in red cell production obtained over 12 months suggests that the cell implant would function to deliver potentially therapeutic Epo levels for greater than 3 years, longer than the life time of the rat.
The protocol used in these experiments involved the single administration of dexamethasone at the time of surgery. This was based on initial experiments showing that dexamethasone, a powerful synthetic glucocorticoid, enhanced the response of treated animals to implantation of Epo-secreting cells. The viral LTR promoter, which drives Epo cDNA expression, contains a known steroid-responsive element, but the short serum half-life of dexamethasone (1.8 to 4.7 hours) makes an effect mediated through this promoter element unlikely to be sustained for 12 months.49 Dexamethasone has been used clinically to decrease surgical inflammation and edema and to decrease immune-mediated tissue destruction, making preservation of the transplanted cells by dexamethasone treatment the most probable explanation for increased cell survival and hematocrit.50 51
The analysis of kidney Epo mRNA indicated that the systemic delivery of hormone from the stomach implant caused downregulation of endogenous Epo production, and this was sustained for at least 12 months. These data provide evidence of long-term high-level Epo expression from transplanted cells and, furthermore, indicate that the elevated red cell production we observed was mediated by implanted transduced cells with a minimal contribution from endogenous Epo. Prolonged red cell production in excess of 60% from genetically modified cells is a significant result from this cell transduction and implantation protocol. In these experiments we used the strong viral LTR promoter to achieve unregulated Epo expression. Although multiple cisgenetic elements have been identified that induce hypoxia-mediated Epo expression in kidney and liver, they have yet to be defined in a size suitable for insertion in a retroviral vector9,10 52 and may not function in vascular smooth muscle cells.
The expression of bacterial neomycin phosphotransferase from the virus we used (LrEpSN) did not appear to cause an immune-mediated loss of transplanted cells. This is supported by the longevity of cell survival and the sustained red cell overproduction. The elimination of autologous transduced cells by an immune-mediated mechanism to foreign transgenes has been reported in rats receiving glioma cells53 and human T-cell transplantations.54The smooth muscle cells we targeted for gene expression and implantation are not usually involved in antigen processing and presentation, and the neo gene is expressed in the cytosol and may not be secreted, providing an explanation for the apparent lack of immune-mediated cell loss.
The persistence for at least 12 months of transduced smooth muscle cells contained within a PTFE gastric ring suggests this approach may be useful for human gene therapy. Vascular smooth muscle cells can be obtained from a peripheral vein and can be cultured, transduced, and selected with high efficiency. This method may be applied to patients by using laparoscopic surgery to implant PTFE structures to contain therapeutically relevant cell numbers. We estimate that 108transduced vascular smooth muscle cells would provide a therapeutic supply of Epo to an 80-kg patient,38 and implantation of this cell number is achievable in a PTFE graft. In the absence of a hypoxia-regulated Epo promoter of a size to permit inclusion in a virus, retroviral-mediated red cell production will be controlled by the number of cells implanted. As we know the level of transduced Epo gene expression we can achieve a desired hematocrit by manipulating the number of cells implanted. The use of laparoscopes is now widespread and affords a minimally invasive method to access and perform surgery on the stomach and intestine. This study and others36,38 42have shown that retroviral vectors are not subject to vector inactivation in smooth muscle cells. A patient's cells can be stored frozen for an indefinite period enabling this process to be repeated if desired. Cells can be modified to express and systemically deliver therapeutic proteins such as granulocyte colony-stimulating factor, insulin, clotting factors, and enzymes such as glucocerebrosidase for the treatment of Gaucher's disease. Furthermore, this method is inherently safe because transplanted cells remain within the PTFE structure and can therefore be removed if necessary.
ACKNOWLEDGMENT
We thank Drs D.C. Dale, S.C. Barry, D. Liggitt, and A.M. Gown for much helpful advice, and Drs J.-P.R. Boissel and H.F. Bunn for kindly supplying the rat Epo cDNA.
Supported by Grant Nos. DK 43727, DK 47754, and DK 50686 from the National Institutes of Health.
Address reprint requests to William R.A. Osborne, PhD, Department of Pediatrics, MS 356320, University of Washington, Seattle, WA 98195.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal