Abstract
We identified the cell cycle status of CD34+ cells of steady-state bone marrow (BM) and peripheral blood (PB) obtained from healthy volunteers, and those of apherasis PB samples collected from healthy donors who had been administered granulocyte colony-stimulating factor (G-CSF). More than 10% of CD34+ cells in BM were in S+G2/M phase. In contrast, regardless of whether G-CSF treatment was performed, less than 2% of CD34+ cells in PB were cycling. BM CD34+ cells showed greater VLA-4 expression and adherence to stromal cells than PB CD34+cells. In addition, when cycling and dormant BM CD34+cells were analyzed separately, the cells in S+G2/M phase expressed more VLA-4 and adhered to the stromal cell monolayer more efficiently than the cells in G0/G1 phase. Furthermore, this adhesion of CD34+ cells to the stromal cell layer was almost completely inhibited by anti-VLA-4 antibody. Taken together, these results suggest that CD34+ progenitors in G0/G1 phase of the cell cycle differ from those in S+G2/M phase in adhesiveness mediated by VLA-4 in the hematopoietic microenvironment.
© 1998 by The American Society of Hematology.
FOLLOWING THE FINDINGS that treatment with cytokines, including granulocyte colony-stimulating factor (G-CSF),1-4 granulocyte-macrophage colony-stimulating factor (GM-CSF),5 interleukin-3 (IL-3),6,7 stem cell factor (SCF),8-11 and IL-3/GM-CSF fusion protein PIXY32112-14 could efficiently recruit hematopoietic progenitors and stem cells into the peripheral blood (PB) in murine, primate, and human systems, peripheral blood stem cell transplantation (PBSCT) was introduced to the clinical field. It was then observed that the recovery of hematopoiesis was faster after PBSCT than after bone marrow (BM) and cord blood transplantation. One reason for the accelerated recovery of hematopoiesis after PBSCT could be that PB stem cell samples obtained after apheresis contain approximately 10-fold as many CD34+ cells as do BM samples.2,4 15-17Alternatively, PB progenitor cells are at an active phase in the cell cycle and thus produce mature blood cells more rapidly than do BM progenitors. This issue has not been fully clarified. The mechanism that regulates the cytokine-induced mobilization of hematopoietic progenitors from BM to PB has not been identified either.
During the course of this study, we determine the cell-cycle status of CD34+ cells of BM and PB from healthy donors and from donors who had received G-CSF treatment for stem cell mobilization. Interestingly, the PB CD34+ cells from both the healthy and G-CSF–treated donors were much more quiescent than the BM CD34+ cells. Thus, we identified the cell-cycle status of the nonadherent, adherent, and cobblestone area–forming cells that appeared after a coculture of CD34+ cells with cytokines or stromal cells. We also examined the expression of adhesion molecules and the adhesiveness to stromal cells of CD34+ cells fractionated on the basis of cell-cycle status. We further examined the effects of anti–VLA-4 antibody on the adhesion of CD34+cells and stromal cells.
MATERIALS AND METHODS
Cytokines
Human recombinant IL-3 and human recombinant SCF were provided by Kirin Brewery Co (Tokyo, Japan). The concentration of the cytokines used in culture was 10 ng/mL.
Cells
BM cells were aspirated from the posterior iliac crest of healthy adult volunteers. PB samples were drawn from healthy volunteers. PB samples were also obtained from other healthy volunteers after they underwent a 5-day preadministration of human recombinant G-CSF (5 μg/kg/d; Kirin Brewery Co or Chugai Pharmaceutical Co, Tokyo, Japan) with the use of apheresis equipment (COBE Spectra; COBE Laboratories, Lakewood, CO). We call these samples the “mobilized PB” samples. In some cases, BM cells were collected from the same healthy volunteers right after the apheresis of PB. All donations of blood samples were obtained with informed consent according to the criteria established by the Institutional Review Board at the Hokkaido Red Cross Blood Center. BM, PB, and mobilized PB cells were enriched for low-density (<1.077 g/mL) mononuclear cells with the use of Ficoll-Paque (Pharmacia, Piscataway, NJ), depleted of adherent cells by overnight incubation, and then collected. Nonadherent mononuclear cells were further enriched for CD34+ cells with a MACS CD34 Progenitor Cell Isolation Kit (Miltenyi Biotec, Bergisch-Gladbach, Germany) following the manufacturer's instructions. Briefly, nonadherent mononuclear cells were indirectly magnetically labeled using a hapten-conjugated primary monoclonal antibody (MoAb) against CD34 (QBEND/10) and an antihapten antibody coupled to MACS MicroBeads. The magnetically labeled CD34+ cells were enriched on a positive selection column. The murine BM-derived stromal cell line, MS-5, which can support human myelopoiesis, was a kind gift from Dr Kazuhiro J. Mori (Niigata University, Niigata, Japan).18
Cell-Surface Markers and Cell-Cycle Status Analysis
Enriched CD34+ cells were stained with the fluorescein isothiocyanate (FITC)-conjugated anti-CD34 MoAb (HPCA-2; Nippon Becton Dickinson Co [BD], Tokyo, Japan) or with a cocktail of phycoerythrin (PE)-conjugated anti-CD34 MoAb (BD) and FITC-conjugated MoAbs specific for VLA-4, VLA-5 (Immunotech, Marseille, France) or LFA-1 (Pharmingen, San Diego, CA). FITC-conjugated mouse IgG1 and IgG2b (DAKO, Japan Co, Kyoto, Japan) and PE-conjugated mouse IgG1 (DAKO) were used as isotype controls. After staining with the antibodies, the cells were treated with phosphate-buffered saline (PBS) containing 0.5% paraformaldehyde (Sigma Chemical Co, St Louis, MO) and 0.5% saponin (Sigma) at 4°C for 5 minutes, then incubated with 5 μg/mL propidium iodide (Sigma) in PBS and 1 mg/mL RNase (Sigma) at 4°C for 5 minutes. The stained cells with low to medium forward scatter and low side scatter were analyzed using an Ortho Cytoron (Ortho Diagnostic Systems, Raritan, NJ).
Adhesion Assay for CD34+ Cells
First, 1 × 105 steady-state BM or mobilized PB CD34+ cells were incubated on a monolayer of the murine stromal cell line MS-5 in a medium consisting of α-MEM (Flow Laboratories, Rockville, MD) and 10% fetal calf serum (FCS; Hyclone, Logan, UT) in 24-well tissue culture plates at 37°C in a humidified atmosphere with 5% CO2/95% air. After a 1-hour incubation, nonadherent cells were obtained by gentle agitation and in some cases adherent cells were also harvested after vigorous pipetting. In some experiments, mouse anti-human VLA-4 MoAb (clone:SG/73, mouse IgG1; a gift of Dr Kensuke Miyake, Saga Medical University, Saga, Japan) or isotype-matched control antibody (DAKO) was added to the culture at a concentration of 10 μg/mL. The untreated cells, nonadherent cells, and adherent cells were counted and analyzed for cell-cycle status and the expression of adhesion molecules.
In Vitro Culture of CD34+ Cells With IL-3 and SCF
CD34+ cells, 5 × 105, derived from BM and mobilized PB samples were cultured for 4 days in the presence of IL-3 and SCF in 25-cm2 culture flasks. Cells were obtained, stained with FITC–anti-CD34 MoAb, and then incubated on an MS-5 monolayer for 1 hour. The recovered nonadherent and adherent cells were subjected to cell-cycle analysis on CD34+ cells.
Culture of Cobblestone Area–Forming Cells
First, 1 × 106 steady-state BM or mobilized CD34+ cells were cultured on an MS-5 monolayer in 25-cm2 flasks. On day 4 of the culture, the nonadherent and adherent cells were removed by vigorous pipetting, and the culture was continued for 2 more days. By the end of the culture, cobblestone areas in addition to nonadherent and adherent cells appeared. After collecting the nonadherent population by gentle agitation, the adherent cells were recovered by vigorous pipetting, and then the cobblestone area–forming cells were collected after trypsin treatment. The MS-5 cells were removed from the last cell fraction by culturing the trypsin-treated cells with medium containing α-MEM and 10% FCS for 1 hour in tissue-culture flasks. Most of the MS-5 cells adhered to the culture flasks within 1 hour. The nonadherent, adherent, and cobblestone area–forming cells were then stained with FITC–anti-CD34 MoAb and subjected to cell-cycle analysis.
RESULTS
Cell-Cycle Analysis of CD34+ Cells
Mononuclear cells enriched for CD34+ cells obtained from steady-state BM and PB and mobilized PB were stained with an anti-CD34 antibody (HPCA-2) and propidium iodide to determine the cell-cycle status of CD34+ cells. As shown in Table1, more than 10% of the CD34+cells in the steady-state BM were in S + G2/M phase of the cell cycle, whereas the majority of the CD34+ cells in the PB samples, both from the steady-state volunteers and the G-CSF–treated donors, were cell-cycle dormant (<2% of the cells were in S + G2/M phase).
Cell-Cycle Analysis of CD34+ Cells in BM and PB
| Sample . | S + G2/M (%)-150 . |
|---|---|
| Steady-state BM (N = 9) | 13.8 ± 2.37 |
| Steady-state PB (N = 5) | 1.2 ± 0.61 |
| Mobilized PB (N = 9) | 1.0 ± 0.61 |
| Sample . | S + G2/M (%)-150 . |
|---|---|
| Steady-state BM (N = 9) | 13.8 ± 2.37 |
| Steady-state PB (N = 5) | 1.2 ± 0.61 |
| Mobilized PB (N = 9) | 1.0 ± 0.61 |
BM and PB mononuclear cells were collected from healthy volunteers. Mononuclear cells were also obtained from PB using apheresis equipment. Mononuclear cells were enriched for CD34+ cells and then subjected to cell-cycle analysis.
Data represent the means ± SD.
Difference from the percentage of S + G2/M obtained from steady-state BM is significant at P < .0001 by multiplet-test.
Relationship Between Adhesiveness of CD34+ Cells and Their Cell-Cycle Status
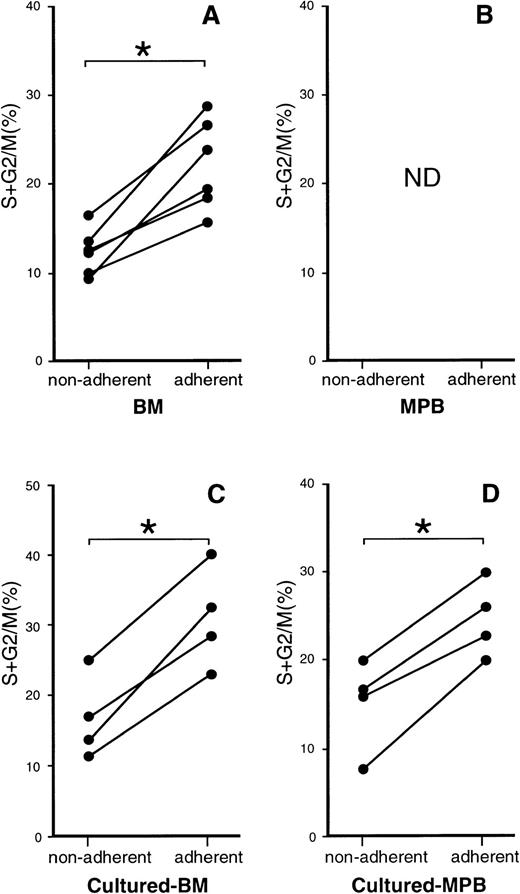
We first compared the adhesiveness to the stromal cell layer of steady-state BM and mobilized PB CD34+ cells. CD34+ cells were incubated on a monolayer of the mouse BM stromal cell line MS-5 for 1 hour. After gentle agitation, nonadherent cells were obtained. The adherent cells were then collected by vigorous pipetting. Approximately twice as many BM CD34+ cells (32%) were recovered as adherent cells as were PB CD34+cells (17%), indicating that BM CD34+ cells adhere to stromal cells more strongly than PB CD34+ cells (Table2). We then analyzed the cell-cycle status of the recovered cells. Figure 1A shows the percentages of the nonadherent and adherent BM CD34+ cells that were in S + G2/M phase. Approximately twice as many adherent cells were in S + G2/M phase compared with the nonadherent cells. PB samples were not analyzed for cell-cycle status (Fig 1B) because most PB CD34+ cells were in G0/G1 phase as shown in Table 1.
Recovery of Steady-State BM and Mobilized PB CD34+ Cells After Coculture on MS-5 Monolayer
| . | Recovery of CD34+Cells (%) . | |
|---|---|---|
| Nonadherent Fraction . | Adherent Fraction . | |
| Steady-state BM (N = 6) | 65 ± 1.4 | 32 ± 2.2 |
| Mobilized PB (N = 4) | 83 ± 2.1 | 17 ± 3.4 |
| . | Recovery of CD34+Cells (%) . | |
|---|---|---|
| Nonadherent Fraction . | Adherent Fraction . | |
| Steady-state BM (N = 6) | 65 ± 1.4 | 32 ± 2.2 |
| Mobilized PB (N = 4) | 83 ± 2.1 | 17 ± 3.4 |
1.0 × 105 steady-state BM and mobilized PB CD34+ cells were cultured on a monolayer of MS-5 cells. After a 1-hour incubation nonadherent cells were recovered by gentle agitation and then adherent cells by vigorous pipetting.
P < .001 by multiple t-test.
Cell-cycle analysis of nonadherent and adherent CD34+ cells from BM and mobilized PB samples. (A and B) CD34+ cells enriched from the BM cells of six donors (A) and from PB cells of four donors (B) were cultured on an MS-5 monolayer. After a 1-hour incubation, nonadherent and adherent cells were obtained for the cell-cycle study. (C and D) BM cells (C) from four donors and mobilized PB cells (D) from four donors were enriched for CD34+ cells and cultured with IL-3 and SCF. On day 4 of the culture recovered cells were stained with CD34 MoAb and incubated on an MS-5 monolayer. One hour later, the nonadherent and adherent cells were collected and stained with FITC–anti-CD34 MoAb. CD34+ cells were analyzed as to cell-cycle status. The percentages of CD34+ cells that were in S + G2/M phase are presented. ND, not done. *P < .005 by paired t-test.
Cell-cycle analysis of nonadherent and adherent CD34+ cells from BM and mobilized PB samples. (A and B) CD34+ cells enriched from the BM cells of six donors (A) and from PB cells of four donors (B) were cultured on an MS-5 monolayer. After a 1-hour incubation, nonadherent and adherent cells were obtained for the cell-cycle study. (C and D) BM cells (C) from four donors and mobilized PB cells (D) from four donors were enriched for CD34+ cells and cultured with IL-3 and SCF. On day 4 of the culture recovered cells were stained with CD34 MoAb and incubated on an MS-5 monolayer. One hour later, the nonadherent and adherent cells were collected and stained with FITC–anti-CD34 MoAb. CD34+ cells were analyzed as to cell-cycle status. The percentages of CD34+ cells that were in S + G2/M phase are presented. ND, not done. *P < .005 by paired t-test.
We next examined cultured CD34+ cells. Steady-state BM and mobilized PB CD34+ cells were cultured with IL-3 and SCF for 4 days. The staining with anti-CD34 MoAb showed that even after 4 days of culture most of the cells still expressed CD34. The cells were then incubated on an MS-5 monolayer to examine their adhesiveness. Nonadherent and adherent cells were then collected after a 1-hour incubation and used for cell-cycle analysis of CD34+ cells. As shown in Fig 1C and D, with both cultured BM and PB samples, more adherent CD34+ cells were in S + G2/M phase than were nonadherent CD34+ cells.
Cell-Cycle Status of Cobblestone Area–Forming Cells and Nonadherent Cells in Culture
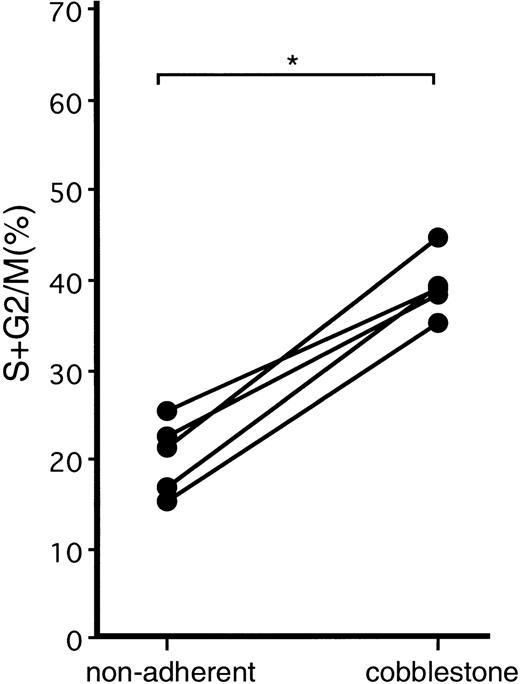
Steady-state BM and mobilized PB CD34+ cells were cultured on a monolayer of MS-5 cells. On day 4 of the culture, the nonadherent and adherent cells were removed as much as possible by vigorous pipetting without disturbing the cells growing underneath the stroma layer, and the culture was continued. On day 6 of the culture, when nonadherent cells, adherent cells, and cobblestone area–forming cells had developed, these three cell populations were obtained by gentle agitation, vigorous pipetting, and trypsin treatment, respectively. The cells were then stained with the anti-CD34 MoAb, and CD34+cells were analyzed for their cell-cycle status. With both BM and PB samples, twice as many CD34+ cobblestone area–forming cells were in S + G2/M phase compared with CD34+ nonadherent cells. The results of mobilized PB samples are presented in Fig 2. The adherent cells recovered were too few for the analysis.
Cell-cycle analysis of nonadherent and cobblestone area–forming cells generated after the coculture of mobilized PB CD34+ cells with stromal cells. Mobilized PB CD34+ cells were cultured on an MS-5 monolayer. On day 4, nonadherent and adherent cells were discarded. On day 6, when nonadherent cells, adherent cells, and cobblestone area–forming cells appeared, the three populations of the cells were collected and stained with anti-CD34 MoAb. CD34+ cells were analyzed as to cell-cycle status. The adherent cells were too few for the analysis. The percentages of CD34+ cells in S + G2/M phase are presented. *P < .0001 by paired t-test.
Cell-cycle analysis of nonadherent and cobblestone area–forming cells generated after the coculture of mobilized PB CD34+ cells with stromal cells. Mobilized PB CD34+ cells were cultured on an MS-5 monolayer. On day 4, nonadherent and adherent cells were discarded. On day 6, when nonadherent cells, adherent cells, and cobblestone area–forming cells appeared, the three populations of the cells were collected and stained with anti-CD34 MoAb. CD34+ cells were analyzed as to cell-cycle status. The adherent cells were too few for the analysis. The percentages of CD34+ cells in S + G2/M phase are presented. *P < .0001 by paired t-test.
Expression of Adhesion Molecules by CD34+ Cells
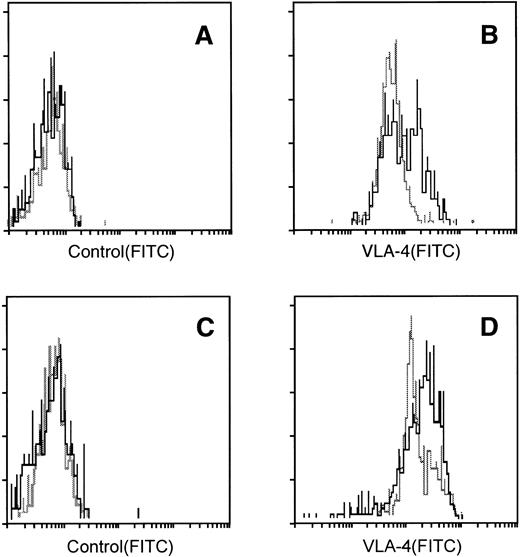
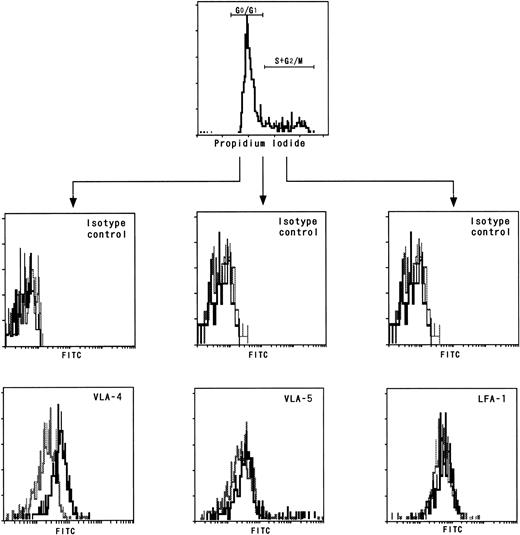
We next examined the expression of the adhesion molecules VLA-4, VLA-5, and LFA-1 by CD34+ cells using freshly prepared BM and mobilized PB CD34+ cells. BM and mobilized PB cells were collected from the same four individuals, purified for CD34+ cells, and then analyzed for the expression of the adhesion molecules. In all four individuals, the mean fluorescence intensities of VLA-4 expressed by the mobilized PB CD34+cells were markedly lower than those expressed by the BM CD34+ cells (Fig 3A and B), while there were no differences in the expressions of VLA-5 and LFA-1 (data not shown). We further investigated the adhesion molecule expression by the nonadherent cultured CD34+ cells and cobblestone area–forming CD34+ cells derived from PB CD34+ cells, the cell-cycle status of which is presented in Fig 2. We used trypsin to obtain cobblestone-forming cells. Because trypsin treatment did not affect the VLA-4 expression of nonadherent cells, it is likely that this treatment did not influence the expression of VLA-4 by cobblestone-forming cells. As shown in Fig 3C and D, the nonadherent cultured CD34+ cells expressed less VLA-4 than the cobblestone area–forming CD34+ cells. We obtained the same results using BM samples. From this relatively lower expression of VLA-4 by the mobilized PB CD34+ cells and nonadherent cultured CD34+ cells compared with the BM CD34+ cells and cobblestone area–forming CD34+cells, respectively, it was suggested that dormant CD34+cells express VLA-4 less than cycling CD34+ cells. To test this possibility, we examined the VLA-4 expression by BM CD34+ cells fractionated on the basis of their cell-cycle status. As shown in Fig 4, the mean fluorescence intensities of VLA-4 expressed by the CD34+cells in G0/G1 phase were significantly lower than those expressed by the CD34+ cells in S + G2/M phase, whereas there were no such differences in the expressions of VLA-5 and LFA-1.
VLA-4 expression by BM and mobilized PB CD34+ cells and cultured CD34+ cells. (A and B) CD34+ cells isolated from BM cells (solid line), and mobilized PB cells (broken line) were stained with control antibody (A) or anti–VLA-4 MoAb (B). (C and D): The cobblestone area–forming cells (solid line) and nonadherent cells (broken line) presented in Fig4 were stained with anti-CD34 MoAb and with control antibody (C) or anti–VLA-4 MoAb (D). VLA-4 expression was analyzed in CD34+ cells.
VLA-4 expression by BM and mobilized PB CD34+ cells and cultured CD34+ cells. (A and B) CD34+ cells isolated from BM cells (solid line), and mobilized PB cells (broken line) were stained with control antibody (A) or anti–VLA-4 MoAb (B). (C and D): The cobblestone area–forming cells (solid line) and nonadherent cells (broken line) presented in Fig4 were stained with anti-CD34 MoAb and with control antibody (C) or anti–VLA-4 MoAb (D). VLA-4 expression was analyzed in CD34+ cells.
Adhesion molecule expression by BM CD34+cells fractionated on the basis of cell-cycle status. CD34+ cells enriched from BM cells were stained with control antibodies (middle) or with MoAbs (bottom) against VLA-4 (right), VLA-5 (middle), or LFA-1 (left) and then subjected to cell-cycle analysis. The expression intensities of the adhesion molecules of CD34+ cells fractionated on the basis of cell-cycle status are presented. (Solid line) CD34+ cells in S + G2/M phase; (broken line) CD34+cells in G0/G1 phase.
Adhesion molecule expression by BM CD34+cells fractionated on the basis of cell-cycle status. CD34+ cells enriched from BM cells were stained with control antibodies (middle) or with MoAbs (bottom) against VLA-4 (right), VLA-5 (middle), or LFA-1 (left) and then subjected to cell-cycle analysis. The expression intensities of the adhesion molecules of CD34+ cells fractionated on the basis of cell-cycle status are presented. (Solid line) CD34+ cells in S + G2/M phase; (broken line) CD34+cells in G0/G1 phase.
Inhibition of Adherence of BM CD34+ Cells to Stromal Cells by Anti–VLA-4 Ab
To clarify the importance of VLA-4 in the cell-cell contact between CD34+ cells and stromal cells, we examined the effects of anti–VLA-4 MoAb on the adhesion of CD34+ cells and stromal cells. BM CD34+ cells were incubated on a monolayer of MS-5 cells in the presence or absence of control or anti–VLA-4 antibody. After 1 hour of incubation nonadherent cells were recovered, counted, and then subjected to cell-cycle analysis. As shown in Table3, the adherence of BM CD34+cells to the stromal cell monolayer was completely inhibited by the addition of anti–VLA-4 antibody. Furthermore, this inhibition was more marked with CD34+ cells in S + G2/M phase than with CD34+ cells in G0/G1 phase of the cell cycle.
Effects of Anti–VLA-4 Antibody on Adherence of BM CD34+ Cells to Stromal Cell Monolayer
| Addition of Antibody . | Recovery of Nonadherent CD34+ Cells (×103) . | ||
|---|---|---|---|
| Total Cells . | Cells in S + G2/M . | Cells in G0/G1 . | |
| None | 80 ± 7.1 | 7.3 ± 0.70 | 73 ± 7.8 |
| Control antibody | 78 ± 5.9 | 6.6 ± 0.87 | 72 ± 5.2 |
| Anti–VLA-4 antibody | 100 ± 2.9 | 11 ± 0.95 | 88 ± 2.8 |
| Addition of Antibody . | Recovery of Nonadherent CD34+ Cells (×103) . | ||
|---|---|---|---|
| Total Cells . | Cells in S + G2/M . | Cells in G0/G1 . | |
| None | 80 ± 7.1 | 7.3 ± 0.70 | 73 ± 7.8 |
| Control antibody | 78 ± 5.9 | 6.6 ± 0.87 | 72 ± 5.2 |
| Anti–VLA-4 antibody | 100 ± 2.9 | 11 ± 0.95 | 88 ± 2.8 |
A total of 1 × 105 steady-state BM cells, 12% ± 1.2% of which were in S + G2/M phase of the cell cycle, were incubated on a monolayer of MS-5 cells in the presence or absence of control antibody or anti–VLA-4 MoAb. After a 1-hour incubation nonadherent cells were obtained by gentle agitation and subjected to cell-cycle analysis. The results of 3 experiments are summarized.
P < .001 by multiple t-test.
DISCUSSION
In this study we identified the cell-cycle status of CD34+cells in steady-state BM, PB, and mobilized PB samples. The majority of the PB CD34+ cells, from both steady-state donors and those treated with G-CSF, were in G0/G1 phase of the cell cycle, whereas more than 10% of the BM CD34+ cells were in S+G2/M phase. Our results are in good agreement with recent studies from several groups. Donahue et al19reported the cell-cycle status of CD34+Thy-1+progenitors in primates treated with a combination of G-CSF and SCF; 9.6% of the CD34+Thy-1+ progenitors in the PB were in S + G2/M phase, as were 36.3% of the progenitors in the BM. Roberts and Metcalf20 performed a tritiated thymidine suicide test using G-CSF–treated mice and found that 47% of the BM hematopoietic progenitors, while only 7% of the PB progenitors, were in S phase. Siegert and Serke21 did not observe any cycling CD34+ cells in PB from patients treated with GM-CSF. More recently, Grzegorzewski et al22 found that the majority of murine PB progenitors mobilized by G-CSF were quiescent. Uchida et al23 reported similar results using more primitive hematopoietic progenitors both in mice and human systems.
Adhesive interactions between hematopoietic progenitors and the microenvironment control the localization, proliferation, and differentiation of hematopoietic stem cells and progenitors.24-26 Investigators have reported that human CD34+ progenitors express several adhesion molecules, especially those of the integrin family, including VLA-4, VLA-5, and LFA-1. Among the adhesion molecules, the importance of VLA-4 has been well demonstrated. For example, Williams et al25 reported significant roles of VLA-4 expressed on murine day 12 colony-forming unit-spleen (CFU-S12) in the attachment to BM stroma cells in vitro and in the formation of CFU-S12–derived spleen colonies and medullary hematopoiesis in vivo. Papayannopoulou et al27 found that anti–VLA4 but not anti-CD18 treatment selectively mobilized progenitors into the bloodstream in primates. Thus, VLA-4 seems to be one of the most important adhesion molecules in progenitor/microenvironment adhesion. We, in this study, and others24 have shown the low expression of VLA-4 by mobilized PB CD34+ cells compared with BM CD34+cells. In addition, we found that the intensity of VLA-4 expressed by CD34+ cells in G0/G1 phase of the cell cycle was lower than that by CD34+ cells in S + G2/M phase, and that among the progenies generated from CD34+ cells after the coculture with cytokines or stromal cells, nonadherent progenies were most quiescent and expressed the lowest level of VLA-4. Thus, there appears to be close relationships among the in vivo and in vitro localizations, the cell-cycle status, and the VLA-4 expression of CD34+ cells. BM CD34+ cells adhered to stromal cells more efficiently than PB CD34+ cells did, and more BM CD34+ cells were in S + G2/M than G0/G1 phase. Furthermore, this adhesion of CD34+ cells was completely blocked by anti–VLA-4 antibody, indicating the importance of VLA-4 in the cell-cell contact between CD34+ cells and stromal cells. Taken together, these results suggest that the lower VLA-4 expression and the reduced adhesiveness to stromal cells of CD34+ cells in G0/G1 phase of the cell cycle contributes to the localization of CD34+progenitor cells in vitro and in vivo. An alternative possibility is that the differences in the cell cycle, VLA-4 expression, and adhesiveness to stromal cells were derived from differentiation- and/or maturation-specific differences of CD34+progenitor cells. Further studies are needed to clarify the mechanisms that regulate the mobilization of hematopoietic progenitors from the microenvironment to peripheral blood.
Address reprint requests to Kenji Ikebuchi, MD, Hokkaido Red Cross Blood Center, 2-2 Yamanote, Nishi-ku, Sapporo 063, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal