Abstract
During the last few years, morphological, immunohistochemical, and genetic findings have placed anaplastic large cell lymphoma (ALCL) as a distinct clinicopathologic entity, and several reports have focused on the existence of different subtypes of the tumor. Particular attention has been paid to the ALCL–Hodgkin's-like (HL) subtype, which seems to be on the border between Hodgkin's disease (HD) and high-grade non-Hodgkin's lymphoma (HG-NHL). From September 1994 to July 1997, during the course of an Italian multicentric trial, 40 ALCL-HLs were randomized to receive as front-line chemotherapy MACOP-B (methotrexate with leucovorin, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin—a third-generation HG-NHL regimen) or ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine—a scheme specific for HD). All patients with bulky disease in the mediastinum at diagnosis underwent local radiotherapy after the chemotherapeutic program. Complete response (CR) was achieved in 17 of the 19 (90%) patients who were treated with MACOP-B, and in 19 of the 21 (91%) patients who were administered ABVD. The probability of relapse-free survival, projected at 32 months, was 94% for the MACOP-B subset and 91% for the ABVD subset. The majority of patients with mediastinal bulky disease obtained CR (evaluated with 67Ga single photon emission computed tomography [SPECT]) after their radiotherapy. The present study suggests that ALCL-HL, in line with its borderline status, responds in an equivalent way to third-generation chemotherapy for HG-NHL and to conventional HD treatment in terms of both CR and relapse-free survival rates. However, as to the latter, a longer follow-up period may be needed before stating the absolute equivalence of the two regimens used.
© 1998 by The American Society of Hematology.
IN 1985, STEIN ET AL1 first reported the expression of the lymphoid activation antigen CD30/Ki-1 in a group of large-cell neoplasms characterized by subtotal effacement of the lymph node structure, prominent sinusal diffusion with spread to the adjacent paracortical areas, and bizarre/anaplastic morphology. These neoplasms turned out to represent a distinct entity, which has been designated as Ki-1/CD30+ anaplastic large cell lymphoma (ALCL), in line with their Ki-1/CD30 expression,2-4morphological features, and lymphoid origin. Although the tumor has also been referred to in other ways, the initial term ALCL is the most appropriate one. The neoplasm was incorporated into the Updated Kiel classification in 1988; subsequently, four histological subtypes were detected in the course of two international workshops: common type (ALCL-CT), Hodgkin's-related (ALCL-HR), giant cell-rich, and lymphohistiocytic.5-8 The ALC-HR subtype has recently been recognized by the Revised European-American Lymphoma (REAL) classification as provisional with just one semantic change: the term Hodgkin's-related was replaced by Hodgkin's-like (ALCL-HL).9
The HL subtype is the most controversial of the varieties, as implied by its inclusion in the REAL classification as a provisional entity. The findings of our previous reports10 11 showed that ALCL-HL has distinctive clinical features slightly different from those of the common type. In particular, our ALCL-HL patients were young adults who most often presented in stage II and had a mediastinal mass, with frequent bulky disease. At present, there is increasing evidence that some cases of Hodgkin's disease (HD) previously diagnosed within the spectrum of the nodular sclerosing and/or lymphocyte depletion variants might represent examples of ALCL-HL.
As regards treatment, our previous data10,11 and those reported by others12-21 have confirmed that ALCL responds to third-generation chemotherapy regimens in a way similar to other aggressive high-grade non-Hodgkin's lymphomas (HG-NHL) in terms of both complete response (CR) and relapse-free survival rates. It is noteworthy that patients with ALCL-HL tended to maintain CR more easily than those with ALCL-CT.11
Despite these encouraging findings, there is no general agreement as to the optimal treatment of the ALCL-HL, and studies are needed to compare the effectiveness of third-generation chemotherapy regimens for HG-NHL with standard protocols for HD. To the best of our knowledge, herein we report on the first randomized, prospective trial comparing the activity of the MACOP-B protocol (methotrexate with leucovorin, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin—specific for conventional HG-NHL) versus ABVD regimen (doxorubicin, bleomycin, vinblastine, and dacarbazine—standard polychemotherapy for HD) in untreated patients with ALCL-HL in terms of CR rate.
MATERIALS AND METHODS
From September 1994 through July 1997, 40 consecutive, unselected adult patients with previously untreated primary ALCL-HL were randomly (ratio 1:1) assigned to receive combination chemotherapy with either MACOP-B or ABVD at 13 Italian institutions. Diagnosis was performed on pathological specimens according to the REAL classification.9 The criteria for eligibility included a confirmed histological diagnosis of ALCL-HL stage II to IV disease according to the Ann Arbor system,22 or stage I with bulky mediastinal tumor, age between 15 and 60 years, an Eastern Cooperative Oncology Group23 performance status less than 3, and human immunodeficiency virus negativity. Informed consent was obtained from all patients in accordance with the ethical policy of the institutes. Staging evaluation in all cases included bone marrow biopsy and hematologic and chemical survey, in addition to chest radiograms, abdominal ultrasonography, and computerized tomography (CT) of the chest and abdomen. The characteristics of all patients are listed in Table 1.
Clinical Characteristics of 40 ALCL-HL Patients
| Characteristics . | MACOP-B Regimen . | ABVD Regimen . | Total (%) . |
|---|---|---|---|
| No. of patients | 19 | 21 | 40 |
| Sex (male/female) | 8/11 | 13/8 | 21/19 |
| Age in years | |||
| Median (range) | 29 (15-52) | 29 (16-55) | 29 (15-55) |
| B symptoms | 7 | 8 | 15 (38) |
| Stage | |||
| I | 1 | — | 1 (2) |
| II | 11 | 15 | 26 (65) |
| III | 4 | 5 | 9 (23) |
| IV | 3 | 1 | 4 (10) |
| Mediastinum | 16 | 18 | 34 (85) |
| Bulky disease | 10 | 9 | 19 (48) |
| Characteristics . | MACOP-B Regimen . | ABVD Regimen . | Total (%) . |
|---|---|---|---|
| No. of patients | 19 | 21 | 40 |
| Sex (male/female) | 8/11 | 13/8 | 21/19 |
| Age in years | |||
| Median (range) | 29 (15-52) | 29 (16-55) | 29 (15-55) |
| B symptoms | 7 | 8 | 15 (38) |
| Stage | |||
| I | 1 | — | 1 (2) |
| II | 11 | 15 | 26 (65) |
| III | 4 | 5 | 9 (23) |
| IV | 3 | 1 | 4 (10) |
| Mediastinum | 16 | 18 | 34 (85) |
| Bulky disease | 10 | 9 | 19 (48) |
Histopathology.
In all cases, the diagnosis was based on the detection of a series of morphological and immunohistochemical findings, as previously described.11 In particular, on morphological grounds the normal nodal structure was effaced by a neoplastic growth, consisting of large cells with variably shaped nuclei (kidney-shaped, round, or oval), prominent nucleoli, and a wide rim of cytoplasm, which usually stained grayish-violet with Giemsa. Tumoral elements showed intrasinusoidal diffusion and tended to give rise to nodular aggregates partially or completely surrounded by sclerotic bands. Immunohistochemistry showed that the cases of the present series were all characterized by T-cell or null phenotype. Tumors with the expression of B-cell markers were excluded, according to the criteria of the REAL Classification, which restricts the term “anaplastic large cell lymphoma” to T-cell or null neoplasms.9 The histological and immunohistologic evaluation was performed by three independent observers (B.F., E.S., and S.A.P.); all interpersonal and intrapersonal differences were recorded. In 15 cases with sufficient pathological material available, the search for the NPM-ALK chimeric fusion protein was performed according to previously reported criteria.24
Chemotherapeutic regimens.
After stratification according to age, stage, performance status, and presence of a mediastinal mass, the patients were randomly assigned to one of two chemotherapy programs: MACOP-B or ABVD. The MACOP-B scheme was administered as originally described by Klimo and Connors.25 The ABVD regimen was administered according to the scheme of the Milan Cancer Institute26; this scheme was repeated every 28 days for a total of six cycles. Patients who presented with mediastinal bulky disease received radiation therapy to the mediastinum at a tumor dose of 36 Gy, after the chemotherapy program. The definition of tumor bulk was represented by a mass/thoracic ratio of greater than 0.33 between the largest transverse diameter of the thorax at the level of T5 or T6 on a standing posteroanterior chest radiograph.
Criteria for response.
Patients were restaged after completion of chemotherapy; clinical and pathological evaluations were made by repeating radiographic investigations and bone marrow biopsy if previous results were positive. In all patients who presented a CT residual mediastinal mass after chemotherapy phase, the radiologic clinical restage included CT and 67Ga single photon emission computed tomography (SPECT). CR was defined as a total disappearance of signs and lymphoma-associated symptoms that was maintained for at least 6 weeks. When a remarkable reduction (>80%) but not complete disappearance of the original bulky mass was observed (a lesion ≥2 cm in diameter as detectable at the CT), when repeated CT scans showed stable residual abnormalities, and 67Ga SPECT had been recorded as negative, the patient was considered to be in CR. Partial response (PR) was defined as the reduction of at least 50% of known disease with disappearance of the systemic manifestations. No response was defined as anything less than a PR. The survival curve was measured from entry into the protocol until death; the relapse-free interval was calculated from the date of response until relapse or death. Survival and relapse-free survival curves were calculated according to the method of Kaplan and Meier.27
RESULTS
All the cases were regarded as examples of ALCL-HL by the three observers, who independently reviewed the histological and immunohistological preparations. In line with the previous reports by Pileri et al10 and Zinzani et al,11 the clinical picture of ALCL-HL turned out to be associated with stage I-II disease (26 of 40; 68%), mediastinal masses (34 of 40; 85%), and bulky disease (19 of 49; 48%). Among the 40 evaluable cases, 19 were treated with MACOP-B regimen and 21 with the ABVD scheme. Nineteen patients who presented bulky disease (17 on the mediastinum, 1 on the left axilla, and 1 on the left inguinal lymph node) after the chemotherapy program received radiotherapy on the site of bulky disease at diagnosis.
Responses to therapy are listed in Table 2. In particular, 36 of 40 (90%) patients obtained a CR and 1 of 40 (3%) a PR, with a major response (CR plus PR) rate of 92.5%. No significant differences were observed between patients of the MACOP-B arm and ABVD arm, because 17 of 19 (90%) and 19 of 21 (91%) obtained a CR, respectively. The remaining 3 patients (8%) were resistant to first-line therapy (2 in the ABVD and 1 in MACOP-B arm). It is noteworthy that 16 of 19 (84%) of the patients with mediastinal bulky disease (8 in the ABVD subset and 8 in the MACOP-B group) obtained only a PR after the chemotherapy. All these 16 patients subsequently achieved a CR with radiotherapy (when repeated CT scans showed stable residual abnormalities, and 67Ga SPECT was recorded as negative).
Overall Response Rate to Treatment of ALCL-HL Patients
| . | MACOP-B (n = 19) . | ABVD (n = 21) . | Overall (n = 40) . |
|---|---|---|---|
| Response | No. (%) | No. (%) | No. (%) |
| CR | 17 (90) | 19 (91) | 36 (90) |
| PR | 1 (5) | — | 1 (3) |
| None | 1 (5) | 2 (9) | 3 (7) |
| . | MACOP-B (n = 19) . | ABVD (n = 21) . | Overall (n = 40) . |
|---|---|---|---|
| Response | No. (%) | No. (%) | No. (%) |
| CR | 17 (90) | 19 (91) | 36 (90) |
| PR | 1 (5) | — | 1 (3) |
| None | 1 (5) | 2 (9) | 3 (7) |
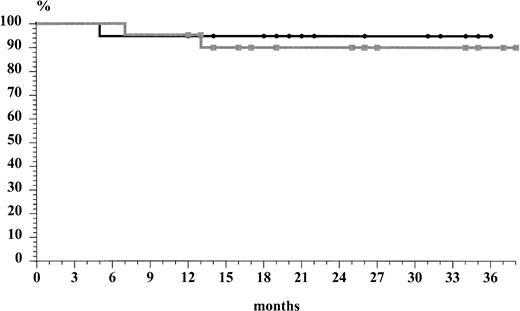
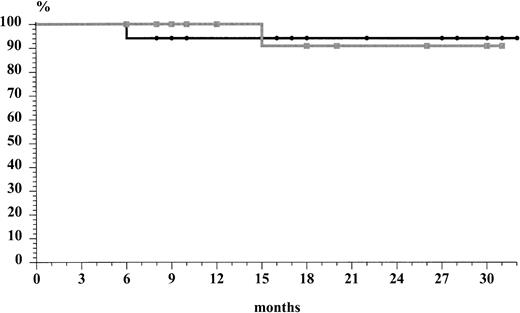
The two chemotherapeutic subsets did not show differences in terms of relapse rate. In fact, 1 of 21 (5%) of the patients treated with ABVD relapsed after 15 months, whereas 1 of 19 (6%) in the MACOP-B group relapsed after 6 months. The overall actuarial risk of relapse of the 36 patients who obtained a CR was 7% at 32 months (median follow-up of 25 months [range, 7 to 37] for ABVD subset and median follow-up of 22 months [range, 5 to 36] for MACOP-B subset, respectively). Both patients who relapsed presented without mediastinal bulky disease at the time of diagnosis. The first-line ABVD patient has obtained a second CR after MACOP-B regimen and is still alive with a second relapse-free survival of 6 months. On the other hand, the patient who relapsed after the MACOP-B CR did not respond completely to the second-line treatment, obtaining only a PR. The overall survival rate (Fig 1) at 37 months was 95% for the MACOP-B patients and 90% for ABVD patients; the median follow-up time for all patients was 24 months. The probability of relapse-free survival (Fig 2), projected at 32 months (median, 18; range, 6 to 32), was 94% and 91% for the MACOP-B and ABVD patients, respectively. As to the search for the NPM-ALK chimeric fusion protein, only 2 of 15 (13%) cases tested turned out to be positive.
DISCUSSION
The close morphological and phenotypical similarities2-4between the neoplastic cells of ALCL and those of HD have recently raised two crucial questions involving possible pathogenetic relationships between the two entities and the histological criteria that would permit a precise differential diagnosis. At present, the borders between grade II nodular sclerosis HD, syncytial nodular sclerosis HD, and ALCL-HL seem to be rather vaguely defined.5,10 It might be that this situation is largely due to the application of subjective criteria for the distinction of the two diseases and will be overcome by the systematic introduction of more refined tools, such as the search for the t(2;5) or the NPM/ALK product, which seem to exclusively occur in ALCL.24 In this respect, the observation of the NPM/ALK hybrid gene product in 2 of 15 cases tested is of interest and supports the need of maintaining ALCL-HL as a provisional entity: further clinicopathologic studies are required before any definitive conclusions can be drawn as to whether it should be included among NHL or in the context of HD. The limited number of cases with sufficient material available for further immunohistochemical studies does not allow us to speculate on the clinical relevance of the ALK protein in the present series.28
To our knowledge, this report involves the largest series of ALCL-HL patients yet studied. In line with the findings collected during our previous trial,11 in the present study the ALCL-HL subtype showed distinctive clinical features that differed slightly from those of the common type. In particular, our ALCL-HL patients were young adults (mean age, 29 years) who most often presented in stage I-II (68%), mostly with a mediastinal mass (85%), and frequently with bulky disease (48%). These data on a large series of patients confirm the clinical identikit of ALCL-HL patients. In this light, it is obvious that certain cases diagnosed as nodular sclerosing grade II29 and most if not all examples of syncytial nodular sclerosis HD30 could also easily be assigned to the category of ALCL-HL.
Our previous study11 confirmed that third-generation HG-NHL chemotherapy regimens are effective in the treatment of ALCL cases. The present study is, to our knowledge, the first to assess the effectiveness of a standard protocol for HD versus a third-generation chemotherapy regimen designed for the treatment of HG-NHL (with the addition, when necessary, of local radiotherapy on the bulky site at diagnosis). Despite the relatively short follow-up and limited number of patients, it is interesting to note how the two chemotherapeutic approaches have achieved remarkably similar degrees of success. In particular, the CR rates showed similarly high levels of success in both the chemotherapeutic subsets: 90% in MACOP-B patients versus 91% in ABVD patients. Furthermore, on the basis of our limited follow-up data, the MACOP-B and ABVD groups seem to show equivalent relapse rates after CR induction: 1 of 17 (6%) patients successfully treated with MACOP-B relapsed, as compared with 1 of 19 (5%) in the ABVD subgroup. One of the relapsed patients has obtained a second CR after a crossover to the MACOP-B regimen after first-line treatment with ABVD. At 32 months, with a median follow-up time of 18 months, the rates of relapse-free survival were 94% and 91% for patients with MACOP-B and ABVD, respectively. It should be noted that in terms of CR rate, our ALCL-HL patients fared better with respect to those of other HG-NHL subtypes studied with the same MACOP-B regimen.31 However, it must be remembered that according to the criteria of the International Prognostic Factor Index,32 the majority of our patients belonged to low or low-intermediate prognostic subgroups. Concerning the role of autologous bone marrow transplantation as consolidation phase described by Fanin et al,19 on the basis of our data a sequential intensive treatment could be avoided for the treatment of ALCL-HL patients.
Another significant finding was that ALCL-HL patients with bulky disease in the mediastinum often had apparent minimal residual disease, as shown by CT scan. The serial use of SPECT showed that in 16 of 17 (8 MACOP-B and 8 ABVD) patients residual active disease was present after the chemotherapy phase. However, all these patients obtained a CR with SPECT negativity after the additional radiation therapy as by protocol. These data suggest a probable useful contribution played by radiotherapy in obtaining the eradication of this lymphoma when there is bulky disease involvement in the mediastinum.
In summary, these preliminary data suggest three considerations: (1) third-generation chemotherapy for HG-NHL (MACOP-B) and conventional HD treatment (ABVD) have so far shown equivalent levels of success in terms of CR and relapse-free survival rates (the latter data being extremely preliminary due to the short follow-up time) in the frontline treatment of ALCL-HL; (2) the probable use of radiation therapy in the bulky mediastinum and the consequent role that nuclear imaging techniques such as SPECT and positron emission tomography are needed for evaluating the real CR in patients with residual mediastinal abnormalities; and (3) on therapeutic grounds, ALCL-HL again seems to reflect its hypothesized status—as revealed by histological9 and serological33 studies—of borderline entity lying midway between HG-NHL and HD.
ACKNOWLEDGMENT
We are grateful to Robin M.T. Cooke for his helpful suggestions regarding the manuscript.
Supported in part by “TRENTA ORE PER LA VITA 1996.”
Address reprint requests to Pier Luigi Zinzani, MD, Istituto di Ematologia “L. e A. Seràgnoli,” Policlinico S. Orsola, Via Massarenti 9, I-40138 Bologna, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal